Abstract
Background
Chordomas are locally invasive neoplasms, arising from notochordal remnants and can appear anywhere along the axial skeleton. Local recurrences are common, and distant metastases may occur years after the initial presentation.
Methods
Literature review of current treatment strategies for chordomas of the skull base.
Results
Surgery is the mainstay of treatment and complete resection has paramount importance for prognosis.
When complete resection is not achieved recurrent disease is common. The anatomical complexity of the skull base makes resection complex. Endonasal endoscopic approaches to the clivus has become increasingly favored in recent years although addressing reconstruction of the skull base to prevent CSF leak may be challenging.
Evidence suggests that radiotherapy should not be considered as a primary single modality when trying to achieve cure of the disease. Nonetheless, immediate post-operative radiotherapy improves survival. Many strategies have been suggested to preserve sensitive vital structures in the skull base during treatment but as for survival there is no evidence of advantage when comparing adjuvant therapy with photon radiotherapy, gamma knife surgery, proton beam therapy, and carbon ion radiation therapy.
There is no evidence to support cytotoxic chemotherapy in the treatment of chordomas but targeted therapies have started to show promise. Several optional molecular targets exist. Brachyury is overexpressed in 95% of chordomas but not in other mesenchymal neoplasms. However, its precise role in chordoma pathogenesis is currently unclear, and its cellular location in the nucleus makes it difficult to target. The inhibition of brachyury in chordoma cell lines induces growth arrest and apoptosis. This does not have clinical application to date. There are retrospective results with different molecular targeted therapies for advanced chordomas with some effectiveness.
Conclusion
Despite improvements made in the past 10 years in our knowledge of chordoma biology, available therapies still offer a limited benefit. There is an unmet need for new therapeutic options for patients with advanced disease. Therefore, patients with advanced disease should be encouraged to participate in clinical trials when and where available.
Keywords: Skull Base, Chordoma, Surgery, Review, Targeted therapy
Background
Chordomas are locally invasive neoplasms, arising from notochordal remnants. The notochord is an early-forming midline structure in the trilaminar embryo mesoderm layer initially ventral to the ectoderm, then the neural plate, and finally the neural tube. This is a transient embryonic anatomy structure, not existing in adults, required for patterning the surrounding tissues. In humans, the notochord forms in week 3, and is eventually lost from vertebral regions and contributes the entire nucleus pulposus of the intervertebral disc during the formation of the vertebral column.1
Notochord remnants can occur anywhere along the axial skeleton. Historically, it was presumed that chordomas present in the sacrum more than in the skull base; however, evidence suggests an almost equal distribution in the skull base (32%), mobile spine (32.8%), and sacrum (29.2%).2, 3, 4 Chordomas usually occur in the fifth to seventh decades and shows a male predominance. Local recurrences are common, and distant metastases may occur years after the initial presentation.
Negative prognostic factors include preoperative visual deficit, older patient age and nontotal or intralesional tumor resection. However, adjunctive radiotherapy and chondroid chordoma type portended a favorable progression free survival.5
Chordomas were first characterized microscopically by Virchow in 1857. He described unique, intracellular, bubble-like vacuoles that were referred to as physaliferous, a term now synonymous with their histopathology. These physaliferous features of chordoma remain a distinguishing, if not pathognomonic, feature. Virchow hypothesized that chordomas were derived from cartilage; however, more recent evidence suggests that they are derived from undifferentiated notochordal remnants that reside within the vertebral bodies and throughout the axial skeleton. There is little direct evidence that cells transform to chordoma. Molecular phenotyping of these primitive rests compared with neoplastic lesions suggest they are the likely source for transformation.6,7 Perhaps the most compelling evidence of the notochordal hypothesis was the discovery of gene duplication in the transcription factor brachyury gene in familial chordoma.8
The brachyury gene, also referred to as the T gene, is located on chromosome 6q27, and encodes a transcription factor that is essential for the generation of mesoderm and the regulation of mesodermal differentiation to notochord during embryogenesis.9 Brachyury is highly and specifically expressed in mesoderm and notochord, and is also overexpressed in chordoma.6,10,11 Previous reports showed that brachyury expression is useful for distinguishing chordoma from other sometimes histologically indistinguishable tumors, such as chondrosarcoma. Knocking down brachyury expression in a chordoma cell line suppresses growth in vitro12 and the immunohistochemical detection of brachyury protein is associated with shorter time to progression in patients with skull base chordoma.13 Experimental models targeting brachyury have started to show promise for developing new drugs to treat advanced chordoma.14
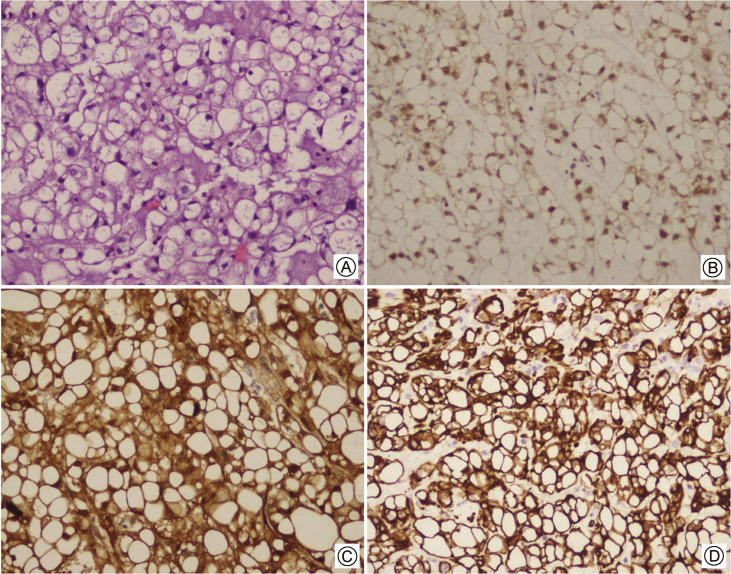
Chordomas are divided into 3 subtypes, based on microscopic morphology: (1) conventional, (2) chondroid, and (3) dedifferentiated. Conventional chordomas are slowly growing tumors, which account for approximately 1%–4% of malignant tumors arising in bone. Macroscopically, they are soft, tan, myxoid masses that frequently show areas of hemorrhage. Histologically, chordomas characteristically show large cells with vacuolated cytoplasm (physaliphorous cells) arranged in nests, cords, or sheets within a myxoid stroma (Fig. 1A). Immunohistochemically, they show reactivity for brachyury (Fig. 1B), S-100 protein (Fig. 1C), epithelial markers including cytokeratins (Fig. 1D), and epithelial membrane antigen. However, they are usually negative for carcinoembryonic antigen. The myxoid or mucoid appearing stroma may contain lakes of extracellular mucin containing hyaluronidase resistant, sulfated mucopolysaccharides. Chondroid chordoma is a variant showing foci of chondroid differentiation in close proximity to areas of conventional chordoma. Dedifferentiated chordoma is a biphasic tumor showing areas of high-grade sarcoma and conventional or chondroid sarcoma. Chondroid chordomais associated with the most favorable survival rates followed by conventional chordoma. Dedifferentiated, whereas patients with dedifferentiated chordomais associated with a very poor prognosis.15
Figure 1.
Skull base chordoma histology A: Large cells with vacuolated cytoplasm (physaliphorous cells) arranged in nests, cords, or sheets within a myxoid stroma (H&E); B: Immunohistochemical staining for brachyury demonstrating strong nuclear expression; C: Immunohistochemical staining for S-100 protein; D: Immunohistochemical staining for Keratin.
Imaging
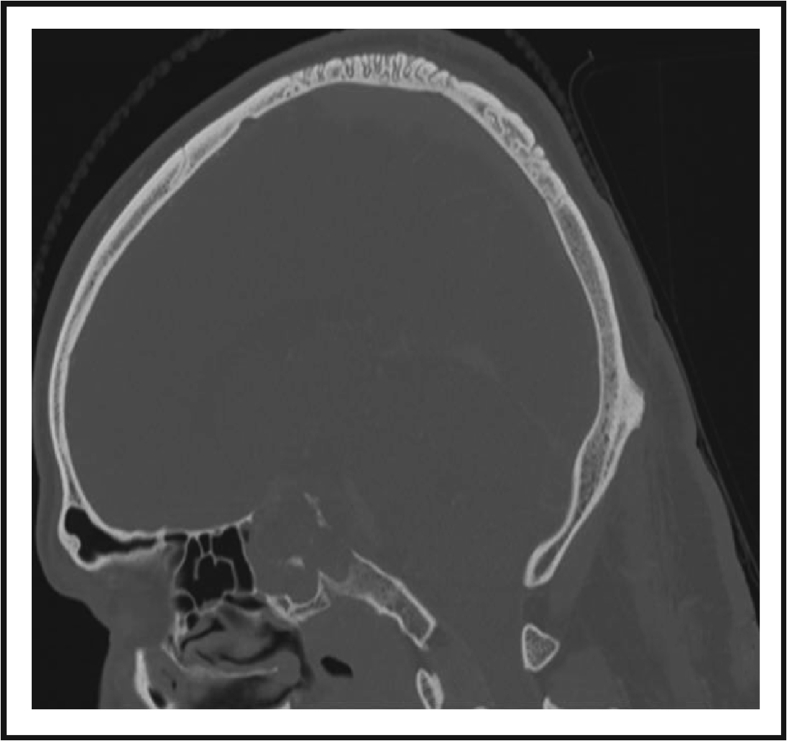
Chordomas and chondrosarcomas constitute the majority of primary skull base tumors. Both tumor entities are rare, slowly growing, locally aggressive malignant tumors invading the bone. Due to their similar appearance in computed tomography (CT) and magnetic resonance imaging (MRI) scans, it is difficult to differentiate them from one another. In a CT scan, both entities display destruction of the osseous skull base and isolated intratumoral calcifications. They appear homogeneous on CT scans and are hypodense (Fig. 2).16,17 MRI is the method of choice for evaluating tumor expansion and localization of skull base tumors due to good soft tissue contrast.18
Figure 2.
CT imaging of skull base chordoma; The lesion displays destruction of the osseous skull base and isolated intratumoral calcifications (thought to represent sequestra of normal bone). Usually hyper-attenuating relative to adjacent brain; however, inhomogenous areas may be seen due to necrosis or hemorrhage.
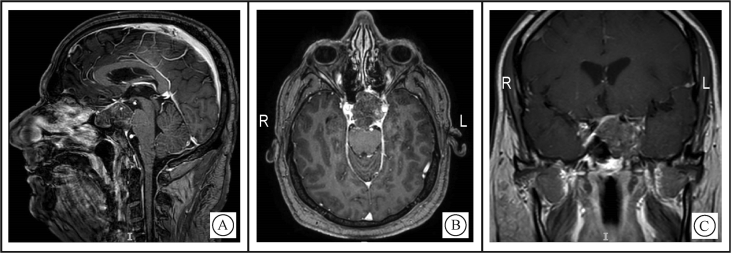
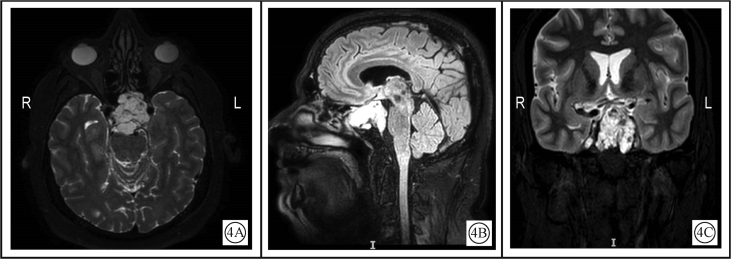
Chordomas are isointense (75%) to hypointense on T1-weighted images (T1WI) (Fig. 3).19 On T2-weighted images (T2WI), they are of high signal with heterogeneous signal noted in 79% (Fig. 4).20
Figure 3.
T1WI MRI of skull base chordoma demonstrating heterogeneous enhancement with a honeycomb appearance corresponding to low T1 signal areas within the tumor A: Sagittal view; B: Axial view; C: Coronal view.
Figure 4.
T2WIMRI of skull base chordoma, demonstrating high signal with heterogeneity and displacement of the optic chiasm upwards A: Axial view; B: Sagittal view, FLAIR sequence, sagittal; C: Coronal view.
Diffusion-weighted imaging (DWI) has been established as an important new method for assessing tumors. The advantage of DWI is the non-invasive assessment of tumor cellularity. Several studies demonstrated that the apparent diffusion coefficient (ADC) correlates well with the tumor cellularity in histological examinations.21,22 It has been shown that increased cellular density of tumors results in restricted water diffusion and decreased ADC values. Several studies have demonstrated that skull base chondrosarcomas generally have higher mean, minimum, maximum, and normalized ADC values than skull base chordomas.23,24
Enhancement of skull base chordomason MRI T1WI with gadolinium was shown to be a risk factor for tumor progression/recurrence following surgical resection in a study examining results following surgical treatment in comparison to pre-operative MRI. Progression/recurrence was seen in 78.6% of patients with enhancing tumors pre-operatively and in zero of the patients with non-enhancing tumors.25
Surgical treatment
In 2015, the Chordoma Foundation published a global consensus based on the input of experts from the fields of medical oncology, radiation oncology, neurosurgery, and orthopedic surgery during the 2013 European Society for Medical Oncology annual meeting.26 These guidelines should be referred to when initially seeing a patient with chordoma, but an effort by a dedicated multidisciplinary team with experience in this rare disease is vitally important. Surgery is the mainstay of treatment for primary and/or recurrent chordoma when feasible. The goal is to achieve a complete tumor excision with clear margins which is associated with best survival outcomes.
When complete resection is not achieved recurrent disease is common. In a study including 47 patients who underwent primary surgery and 27 patients who had previously undergone surgery or radiotherapy, Tzortzidis et al27 achieved gross total removal in 53 (71.6%) of patients, and subtotal resection was accomplished in 21 (28.4%) of the patients, and found recurrence-free survival at 10 years to be 31%.
The anatomical complexity of the skull base makes resection complex. Transphenoidal, transmaxillary, transnasal, high anterior cervical retropharyngeal, and transoral approaches have been well documented in the literature, as have endoscopic techniques.28,29 Although achieving complete tumor resection is the goal of surgery, subtotal resection may be acceptable to avoid debilitating neurological damage (cranial nerve deficits, cerebrovascular injury). Small tumor remnants can be managed effectively with radiotherapy. In a series published by Polturi et al30 high-dose radiotherapy was found to be an effective management strategy for low volume residual tumor.
Endonasal endoscopic approaches to the clivus have become increasingly favored in recent years. In a large series of 60 patients, reported by the Pittsburgh group, gross total resection was achieved in 66.7%.31 The most critical limitations for gross total resection were tumor volume greater than 20 cm3, tumor location in the lower clivus with lateral extension, and a previously treated disease.31
Reconstruction of dural defects following intradural tumor resections is a significant challenge in these cases, as clival chordomas have been found to be associated with high flow intra operative cerebrospinal fluid (CSF). Multi layered reconstruction including pedicled mucosal flaps from the inferior turbinate or septum32 are warranted. Post-operative CSF leak rate in these cases has been reported to be as high as 30%, despite these measures. Use of perioperative lumbar drains has not been reported to statistically decrease the rate of post-operative CSF leak.33
Radiation therapy
Evidence suggests that radiotherapy should not be considered as a primary single modality in the treatment of chordomas when trying to achieve cure of the disease.34 Nonetheless, immediate post-operative radiotherapy showed a 10-year survival rate of 65% versus 0% in patients treated with RT at the time of recurrence.35
The recommended radiation dose in these radio-resistant tumors is higher than the standard (56–70 Gy) doses usually administered. Higher doses of at least 74 Gy using conventional fractionation (1.8–2 Gy per fraction) that are beyond the tolerance of several critical structures (brainstem, optic pathways) are recommended.36,37
Luckily, advances in photon irradiation techniques, such as intensity-modulated radiation therapy, three-dimensional conformal radiation therapy, and tomotherapy, are permitting delivery of higher dose to tumor while sparing critical normal structures from radiation exposure above tolerable levels.
Particle beams such as Proton Beam Therapy (PBT) and Carbon Ion Radiation Therapy (CIRT) have different physical and biological characteristics with better dose distribution. They deliver a lower entry dose, depositing the majority of their energy at the end of their path, yielding atypical narrow dose energy peak called “Bragg peak”. This steep fall-off allows for delivery of high doses and sparing of tissue beyond the tumor. PBT was shown to be superior to photons, delivering higher doses to the tumor while keeping lower doses to normal tissues in the clival region.38 PBT was also used safely and effectively in the pediatric population.39
Still, photon therapy with modern and precise techniques is being used to treat skull base chordomas. Intensity modulated radiation therapy (IMRT) is the new paradigm of treatment in radiotherapy and has been reported in some series.40
Stereotactic radiation therapy as the primary, adjuvant, or salvage management was reported by the North American Gamma Knife Consortium,41 treating 71 patients with a median follow-up of 5 years. 23 had died of tumor progression. Overall survival at 5 years was 93% for patients who had not received prior radiation therapy and 43% for those who had received prior therapy. Recurrent tumors can be controlled with gamma knife radio surgery mainly in case of residual lesions localized and small after initial aggressive resection.42
In a meta-analysis including 23 case series including pooled 807 patients, Di Maio et al43 found no significant differences in 5-year overall survival when comparing adjuvant therapy with photon radiotherapy, gamma knife surgery, PBT, and CIRT. Nevertheless, the retrospective nature of the included studies prevents achieving solid conclusions.
Cytotoxic chemotherapy
There is no evidence to support cytotoxic chemotherapy in the treatment of chordomas. The only available phase II prospective study using chemotherapy did not show positive results.44 A topoisomerase I inhibitor, Irinotecan, was given to 15 patients who were found to have a median time to progression of 10 months, with only 1 patient showing objective response lasting at least 8 months.
Several case reports with different chemotherapy agents have been published to date. Dhall et al45 published a retrospective study with 6 cases of pediatric clival chordomas treated Ifosfamide and etoposide (VP-16); they found the combination of these chemotherapy agents after surgery to have some effect on tumor progression. Four out of 6 patients were alive, with stable radiographic abnormalities at median follow-up 9 years from diagnosis. Another anecdotal report of chemotherapy use in pediatric chordoma, which consisted of cycles of Vincristine/Cyclophosphamide/Doxorubicin alternating with Etoposide/Ifosfamide in a 7-monthold infant with a clival chordoma responded to combination chemotherapy; the patient had durable response 2 years post-chemotherapy.46
Still, there is no large good quality body of evidence suggesting cytotoxic chemotherapy as an effective treatment in chordomas.
Targeted therapy
Many molecular targets have been identified in chordomas. Brachyury is over expressed in 95% of chordomas but not in other mesenchymal neoplasms. However, its precise role in chordoma pathogenesis is currently unclear, and its cellular location in the nucleus makes it difficult to target. Although no mutations account for over expression of brachyury in chordomas, the brachyury gene shows minor allelic gains and is amplified in some sporadic cases.47 In addition, germline tandem duplication of the brachyury gene can be associated with familial chordoma.48 The inhibition of brachyury in chordoma cell lines induces growth arrest and apoptosis.49
Other potential targets, include epidermal growth factor receptor (EGFR), PDGFRB, PI3K/mTOR, MAPK, STAT, FGFR, MET, CDK4, VEGF, and INI1, are increasingly being identified in chordomas.50 Notably, none of them have shown recurrent mutations or gene rearrangements, but available data point to their activation and, sometimes, to copy number amplification. An updated list of all published available molecular, preclinical and clinical data on therapeutic targets in chordomas is available at the Chordoma Foundation's website.51
Lebellec et al52 treated 80 patients with advanced chordoma; 18 patients had skull base chordomas and were treated with molecular targeted therapies (MTTs). Their outcomes were reported retrospectively, but it is still one of the largest cohorts of patients receiving molecular targeted treatments, with data on the subject. The first line of MTTs consisted of Imatinib in 62 patients (77.5%), Sorafenib in 11 patients (7%), Erlotinib in 5 patients (6.3%), Sunitinib in 1 patient (1.2%), and Temsirolimus in 1 patient (1.2%). The reported responses were as follows: partial response in 5 patients (6.3%), stable disease in 58 patients (72.5%), or progressive disease, which was found in 10 patients (12.5%). The progression-free and overall survival did not significantly differ between the MTTs. It is important to note that skull base primary tumor location was significantly correlated with poor prognosis and shorter progression-free survival.
Conclusions
Despite improvements made in the past 10 years in our knowledge of chordoma biology, available therapies still offer a limited benefit. There is an unmet need for new therapeutic options for patients with advanced disease. Therefore, patients with advanced disease should be encouraged to participate in clinical trials when and where available.
Funding
This research was funded in part through the NIH/NCI Cancer Center Support Grant, P30 CA008748.
Declaration of Competing Interest
None.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Shapiro Irving M., Risbud Makarand V. Transcriptional profiling of the nucleus pulposus: say yes to notochord. Arthritis Res Ther. 2010;12:117. doi: 10.1186/ar3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heffelfinger M.J., Dahlin D.C., MacCarty C.S. Chordomas and cartilaginous tumors at the skull base. Cancer. 1973;32:410–420. doi: 10.1002/1097-0142(197308)32:2<410::aid-cncr2820320219>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.O'Neill P., Bell B.A., Miller J.D., Jacobson I., Guthrie W. Fifty years of experience with chordomas in southeast Scotland. Neurosurgery. 1985;16:166–170. doi: 10.1227/00006123-198502000-00007. [DOI] [PubMed] [Google Scholar]
- 4.McMaster M.L., Goldstein A.M., Bromley C.M., Ishibe N., Parry D.M. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control. 2001;12:1–11. doi: 10.1023/a:1008947301735. [DOI] [PubMed] [Google Scholar]
- 5.Zou M.X., Lv G.H., Zhang Q.S., Wang S.F., Li J., Wang X.B. Prognostic factors in skull base chordoma: a systematic literature review and meta-analysis. World Neurosurg. 2018;109:307–327. doi: 10.1016/j.wneu.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Vujovic S., Henderson S., Presneau N. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209:157–165. doi: 10.1002/path.1969. [DOI] [PubMed] [Google Scholar]
- 7.Shen J., Li C.D., Yang H.L. Classic chordoma coexisting with benign notochordal cell rest demonstrating different immunohistological expression patterns of brachyury and galectin-3. J Clinneurosci. 2011;18:96–99. doi: 10.1016/j.jocn.2010.03.066. [DOI] [PubMed] [Google Scholar]
- 8.Yang X.R., Ng D., Alcorta D.A. T (brachyury) gene duplication confers major susceptibility to familial chordoma. Nat Genet. 2009;41:1176–1178. doi: 10.1038/ng.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nibu Y., José-Edwards D.S., Gregorio A.D. From notochord formation to hereditary chordoma: the many roles of Brachyury. Rev Biomed Res Int. 2013:826435. doi: 10.1155/2013/826435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jambhekar N.A., Rekhi B., Thorat K., Dikshit R., Agrawal M., Puri A. Revisiting chordoma with brachyury, a “new age” marker: analysis of a validation study on 51 cases. Arch Pathol Lab Med. 2010;134:1181–1187. doi: 10.5858/2009-0476-OA.1. [DOI] [PubMed] [Google Scholar]
- 11.Oakley G.J., Fuhrer K., Seethala R.R. Brachyury, SOX-9, and podoplanin, new markers in the skull base chordoma vs chondrosarcoma differential: a tissue microarray-based comparative analysis. Mod Pathol. 2008;21:1461–1469. doi: 10.1038/modpathol.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu W., Mohyeldin A., Shah S.R. Generation of chordoma cell line JHC7 and the identification of Brachyury as a novel molecular target. J Neurosurg. 2011;115:760–769. doi: 10.3171/2011.5.JNS11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitamura Y., Sasaki H., Kimura T. Molecular and clinical risk factors for recurrence of skull base chordomas: gain on chromosome 2p, expression of brachyury, and lack of irradiation negatively correlate with patient prognosis. J Neuropathol Exp Neurol. 2013;72:816–823. doi: 10.1097/NEN.0b013e3182a065d0. [DOI] [PubMed] [Google Scholar]
- 14.Magnaghi P., Salom B., Cozzi L. Afatinib is a new therapeutic approach in chordoma with a unique ability to target EGFR and Brachyury. Mol Cancer Ther. 2018;17:603–613. doi: 10.1158/1535-7163.MCT-17-0324. [DOI] [PubMed] [Google Scholar]
- 15.Fechner R.E., Mills S.E. Tumors of the bones and joints. In: Fascicle, Rosai J., Sobin L., editors. Atlas of Tumor Pathology. 3rd ed. Armed Forces Institute of Pathology; Washington, DC: 1993. pp. 239–243. [Google Scholar]
- 16.Meyer J.E., Oot R.F., Lindfors K.K. CT appearance of clival chordomas. J Comput Assist Tomogr. 1986;10:34–38. doi: 10.1097/00004728-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Pamir M.N., Ozduman K. Analysis of radiological features relative to histopathology in 42 skull-base chordomas and chondrosarcomas. Eur J Radiol. 2006;58:461–470. doi: 10.1016/j.ejrad.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Razek A.A., Mossad A., Ghonim M. Role of diffusion -weighted MR imaging in assessing malignant versus benign skull-base lesions. Radiol Med. 2011;116:125–132. doi: 10.1007/s11547-010-0588-y. [DOI] [PubMed] [Google Scholar]
- 19.Sze G., Uichanco L.S., 3rd, Brand-Zawadzki M.N. Chordomas: MR Imaging Radiol. 1988;166:187–191. doi: 10.1148/radiology.166.1.3336677. [DOI] [PubMed] [Google Scholar]
- 20.Meyers S.P., Hirsch W.L., Jr., Curtin H.D., Barnes L., Sekhar L.N., Sen C. Chordomas of the skull base: MR features. AJNR Am J Neuroradiol. 1992;13:1627–1636. [PMC free article] [PubMed] [Google Scholar]
- 21.Provenzale J.M., Mukundan S., Barboriak D.P. Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology. 2006;239:632–649. doi: 10.1148/radiol.2393042031. [DOI] [PubMed] [Google Scholar]
- 22.Yamasaki F., Kurisu K., Satoh K. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235:985–991. doi: 10.1148/radiol.2353031338. [DOI] [PubMed] [Google Scholar]
- 23.Welzel T., Meyerhof E., Uhl M. Diagnostic accuracy of DW MR imaging in the differentiation of chordomas and chondrosarcomas of the skull base: a 3.0-T MRI study of 105 cases. Trial Eur J Radiol. 2018;105:119–124. doi: 10.1016/j.ejrad.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Müller U., Kubik-Huch R.A., Ares C. Is there a role for conventional MRI and MR diffusion-weighted imaging for distinction of skull base chordoma and chondrosarcoma. Acta Radiol. 2016;57:225–232. doi: 10.1177/0284185115574156. [DOI] [PubMed] [Google Scholar]
- 25. Lin E., Scognamiglio T., Zhao Y., Schwartz T.H., Phillips C.D. Prognostic implications of gadolinium enhancement of skull base chordomas. AJNR Am J Neuroradiol. 2018;39:1509–1514. doi: 10.3174/ajnr.A5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stacchiotti S., Sommer J., Chordoma Global Consensus Group Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol. 2015;16:e71–e83. doi: 10.1016/S1470-2045(14)71190-8. [DOI] [PubMed] [Google Scholar]
- 27.Holzmann D., Reisch R., Krayenbühl N., Hug E., Bernays R.L. The transnasal transclival approach for clivus chordoma. Minim Invasive Neurosurg. 2010;53:211–217. doi: 10.1055/s-0030-1267929. [DOI] [PubMed] [Google Scholar]
- 28.Tzortzidis F., Elahi F., Wright D., Natarajan S.K., Sekhar L.N. Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chordomas. Neurosurgery. 2006;59:230–237. doi: 10.1227/01.NEU.0000223441.51012.9D. [DOI] [PubMed] [Google Scholar]
- 29.Singh H., Harrop J., Schiffmacher P., Rosen M., Evans J. Ventral surgical approaches to craniovertebral junction chordomas. Neurosurgery. 2010;66:96–103. doi: 10.1227/01.NEU.0000365855.12257.D1. [DOI] [PubMed] [Google Scholar]
- 30.Potluri S., Jefferies S.J., Jena R. Residual postoperative tumour volume predicts outcome after high-dose radiotherapy for chordoma and chondrosarcoma of the skull base and spine. Clin Oncol (R Collradiol) 2011;23:199–208. doi: 10.1016/j.clon.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Koutourousiou M., Gardner P.A., Tormenti M.J. Endoscopic endonasal approach for resection of cranial base chordomas: outcomes and learning curve. Neurosurgery. 2012;71:614–625. doi: 10.1227/NEU.0b013e31825ea3e0. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann T.K., Hindy N.E., Müller O.M. Vascularised local and free flaps in anterior skull base reconstruction. Eur Arch Otorhinolaryngol. 2013;270:899–907. doi: 10.1007/s00405-012-2109-1. [DOI] [PubMed] [Google Scholar]
- 33.Stapleton A.L., Tyler-Kabara E.C., Gardner P.A., Snyderman C.H., Wang E.W. Risk factors for cerebrospinal fluid leak in pediatric patients undergoing endoscopic endonasal skull base surgery. Int J Pediatr Otorhinolaryngol. 2017;93:163–166. doi: 10.1016/j.ijporl.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 34.Boriani S., Chevalley F., Weinstein J.N. Chordoma of the spine above the sacrum. Treatment and outcome in 21 cases. Spine. 1996;21:1569–1577. doi: 10.1097/00007632-199607010-00017. [DOI] [PubMed] [Google Scholar]
- 35.Carpentier A., Polivka M., Blanquet A., Lot G., George B. Suboccipital and cervical chordomas: the value of aggressive treatment at first presentation of disease. J Neurosurg. 2002;97:1070–1077. doi: 10.3171/jns.2002.97.5.1070. [DOI] [PubMed] [Google Scholar]
- 36.Amichetti M., Amelio D., Cianchetti M., Giacomelli I., Scartoni D. The treatment of chordoma and chondrosarcoma of the skull base with particular attention to radiotherapy. Clin Oncol. 2017;2:1195. [Google Scholar]
- 37.Amichetti M., Cianchetti M., Amelio D., Enrici R.M., Minniti G. Proton therapy in chordoma of the base of the skull: a systematic review. Neurosurg Rev. 2009;32:403–416. doi: 10.1007/s10143-009-0194-4. [DOI] [PubMed] [Google Scholar]
- 38.Munzenrider J.E., Liebsch N.J. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175:57–63. doi: 10.1007/BF03038890. [DOI] [PubMed] [Google Scholar]
- 39.Rombi B., Ares C., Hug E.B. Spot-scanning proton radiation therapy for pediatric chordoma and chondrosarcoma: clinical outcome of 26 patients treated at Paul Scherrer institute. Int J Radiat Oncol Biol Phys. 2013;86:578–584. doi: 10.1016/j.ijrobp.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 40.Jiang W.H., Zhao S.P., Xie Z.H. Endoscopic resection of chordomas in different clival regions. Acta Otolaryngol. 2009;129:71–83. doi: 10.1080/00016480801995404. [DOI] [PubMed] [Google Scholar]
- 41.Hauptman J.S., Barkhoudarian G., Safaee M. Challenges in linear accelerator radiotherapy for chordomas and chondrosarcomas of the skull base: focus on complications. Int J Radiat Oncol Biol Phys. 2012;83:542–551. doi: 10.1016/j.ijrobp.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Ito E., Saito K., Okada T., Nagatani T., Nagasaka T. Long-term control of clivalchordoma with initial aggressive surgical resection and gamma knife radiosurgery for recurrence. Acta Neurochir (Wien) 2010;152:57–67. doi: 10.1007/s00701-009-0535-7. [DOI] [PubMed] [Google Scholar]
- 43.Di Maio S., Temkin N., Ramanathan D., Sekhar L.N. Current comprehensive management of cranial base chordomas: 10-year meta-analysis of observational studies. J Neurosurg. 2011;115:1094–1105. doi: 10.3171/2011.7.JNS11355. [DOI] [PubMed] [Google Scholar]
- 44.Chugh R., Dunn R., Zalupski M.M. Phase II study of 9-nitro-camptothecin in patients with advanced chordoma or soft tissue sarcoma. J Clin Oncol. 2005;23:3597–3604. doi: 10.1200/JCO.2005.02.170. [DOI] [PubMed] [Google Scholar]
- 45.Dhall G., Traverso M., Finlay J.L. The role of chemotherapy in pediatric clivalchordomas. J Neurooncol. 2011;103:657–662. doi: 10.1007/s11060-010-0441-0. [DOI] [PubMed] [Google Scholar]
- 46.Al-Rahawan M.M., Siebert J.D., Mitchell C.S., Smith S.D. Durable complete response to chemotherapy in an infant with a clivalchordoma. Pediatr Blood Cancer. 2012;59:323–325. doi: 10.1002/pbc.23297. [DOI] [PubMed] [Google Scholar]
- 47.Colia V., Stacchiotti S. Medical treatment of advanced chordomas. Eur J Cancer. 2017;83:220–228. doi: 10.1016/j.ejca.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 48.Kelley M.J., Korczak J.F., Sheridan E. Familial chordoma, a tumor of notochordal remnants, is linked to chromosome 7q33. Am J Hum Genet. 2001;69:454–460. doi: 10.1086/321982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Presneau N., Shalaby A., Ye H. Role of the transcription factor T (brachyury) in the pathogenesis of sporadic chordoma: a genetic and functional-based study. J Pathol. 2011;223:327–335. doi: 10.1002/path.2816. [DOI] [PubMed] [Google Scholar]
- 50.Flanagan A.M., Chordoma T.Y., Fletcher C.D.M. Lyon: Pathology and Genetics, IARC Press; 2013. World Health Organization (WHO) classification of tumours of soft tissue and bone; pp. 328–329. [Google Scholar]
- 51.Chordoma Foundation. 2019. https://www.chordomafoundation.org [Google Scholar]
- 52.Lebellec L., Chauffert B., Blay J.Y. Advanced chordoma treated by first-line molecular targeted therapies: outcomes and prognostic factors. A retrospective study of the French Sarcoma Group (GSF/GETO) and the Association des Neuro-Oncologuesd'Expression Française (ANOCEF) Eur J Cancer. 2017;79:119–128. doi: 10.1016/j.ejca.2017.03.037. [DOI] [PubMed] [Google Scholar]