Abstract
Objective
There has been a significant shift from open craniofacial resection of the anterior skull base to endoscopic approaches that accomplish the same outcomes in tumor ablation. However, when open resection is required, free flap reconstruction is often necessary to provide sufficient well-vascularized tissue for optimal wound healing as well as providing adequate tissue bulk for cosmesis. This articleaims to providea focused review of free flaps most commonly used in anterior skull base reconstruction.
Methods
This is a state-of-the-art review based on expert opinion and previously published reviews and journal articles, queried using PubMed and Google Scholar.
Results & conclusion
Anterior skull base reconstruction via free tissue transfer is imperative in limiting complications and promoting healing, particularly with large defects, post-radiation, and in at-risk patients. The type of free flap utilized for a particular anterior skull base reconstruction should be tailored to the patient and nature of the disease. This review offers insight into the numerous reconstructive options for the free flap surgeon.
Keywords: Free tissue transfer, Anterior skull base, Head and neck microvascular, Reconstruction
Introduction
In 1907 Dr. Hermann Schloffer described the first transnasal transsphenoidal approach to the pituitary utilizing a transfacial lateral rhinotomy incision.1 Nearly one century later Casiano et al published their experience and outcomes of endoscopic anterior skull base resection in a series of esthesioneuroblastoma patients demonstrating that the endoscopic approach was safe and effective.2 Additionally, endoscopic resection has been demonstrated to have fewer complications and shorter hospital stays.3,4 Popularity of this approach over open craniofacial resection coupled with technological advances over the past twenty years has lead to the now widespread use of this technique to resect both benign and malignant tumors of the skull base. Frequently endoscopic ablations still often require some form of skull base reconstruction based on the defect created.5 The majority of these reconstructions lie in the middle of the reconstructive ladder including free mucosal grafts and pedicled vascularized flaps such as the nasoseptal flap, middle turbinate and inferior turbinate flaps.6,7 There have been a number of papers that have comprehensively reviewed the endoscopic reconstruction algorithm of the anterior skull base.6,8, 9, 10 Unfortunately some tumors based on size, unfavorable location, or invasion of surrounding structures require a larger open craniofacial approach for adequate extirpation necessitating the use of free tissue transfer for reconstruction. In this paper, we seek to provide a review of free tissue reconstructive options for anterior craniofacial resection and the subsequent defects created.
The most crucial aspect in skull base reconstruction, whether the defect was created endoscopically or from an open approach, is to adequately separate the intracranial contents from the sinonasal cavity. This is critical to control cerebrospinal fluid leak as well as create a physical barrier to deter mucous and air from tracking intracranially.11 If an adequate seal is not created the complications can be disastrous including intracranial infection or even death.12 In addition to this consideration, the free tissue reconstruction should also attempt to recreate the volume and projection lost in the resection for best possible functional outcome and cosmesis.13,14 The considerations in selecting a free tissue donor site are many. It is important to think about whether the patient has had prior radiation, prior surgery and the location and distance of recipient vessels. The reconstructive surgeon needs to evaluate the size of the defect and determine how much skin, muscle or fat will need to be harvested to sufficiently restore soft tissue loss. This assessment of volume will likely affect which donor site the surgeon selects. An additional question is whether there is a need for osseous reconstruction to assist with projection or orbital support.13 Acceptable projection can be accomplished without the use of an osseous flap when there is satisfactory soft tissue bulk present; however, this may not be in line with the patient's ultimate goals i.e. if the patient has the expectation for future osseointegrated implants. This highlights the fact that each reconstruction is unique and has its own patient centered considerations that must be taken into account.
Discussion
Rectus abdominis free flap
In earlier reviews of skull base reconstruction, the rectus abdominis was the most frequently utilized flap due to the large amount of soft tissue bulk that it provides, allowing for better dead space obliteration.15,16 Benefits of the rectus include consistent landmarks when elevating the flap, reliable vascular anatomy with long vascular pedicle of up to 15 cm with the deep inferior epigastric artery and primary closure of the donor site with an up to 8–10 cm wide skin paddle.17 This flap also allows for simultaneous harvesting in a two team setting. The large volume of tissue that can be harvested is a benefit but in the obese patient may be a drawback. A careful history should be taken regarding prior abdominal surgery to determine if the vascular supply may be compromised. There is a risk of accidently entering the peritoneal cavity during flap elevationand eventual development of ventral hernias after healing of the donor site.18 Currently at our institution, the anterolateral thigh flap has largely replaced use of the rectus abdominis as it has many of the benefits of the rectus without the donor site morbidity.
Anterolateral thigh free flap
As mentioned above, the anterolateral thigh flap is now used more frequently than the rectus when a free flap with both muscle and adipose bulk is desired.19 The anterolateral thigh flaphas reliable landmarks for flap skin paddle design including the anterior superior iliac spine and lateral patella. These are typically palpable even in the obese patient. The flap site allows for a two-team approach. A large skin paddle up to 10 cm wide by 20–25 cm in length can be taken with primary closure of the donor site. The pedicle length can be up to 8–12 cm in length which is another benefit of the flap making it similar to the rectus.20 One pitfall when raising the anterolateral thigh flap is the variability in vascular supply to the vastus lateralis and skin paddle. The flap has been described to have both septocutaneous and musculocutaneous perforators. In addition, the flap is supplied by the lateral circumflex femoral arterial system with 75% of perforators branching from the descending branch and 25% of perforators branching from the transverse branch.21 Being aware of these potential variations can prevent damage to the vascular system during flap elevation.
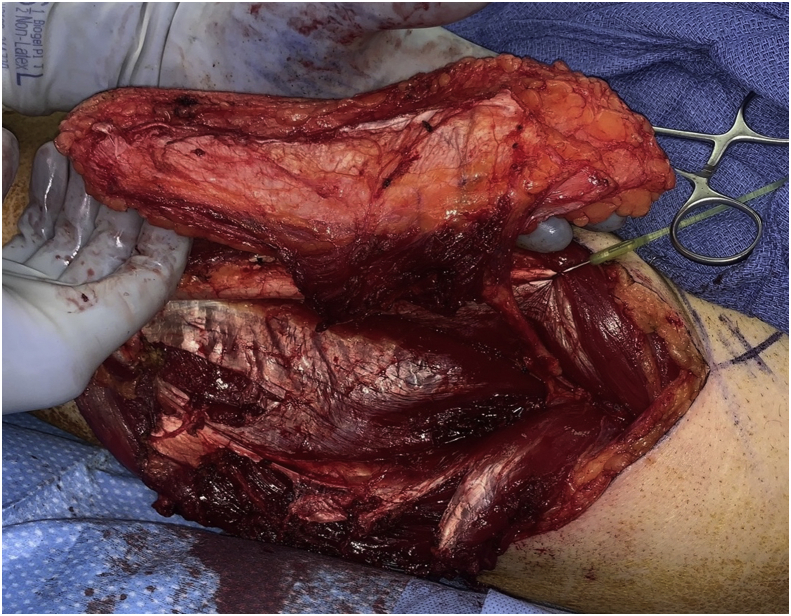
Another benefit of this flap is that it does offer versatility in the components taken when harvesting the flap as well as creative inset options (Fig. 1). It can be raised as either a fasciocutaneous flap or as a myofasciocutaneous flap when more bulk is required.20 The tensor fascia lata is divided when harvesting the vastus lateralis in a myofasciocutaneous flap elevation. The fascial strip can either be left attached to the vastus lateralis or a free fascial graft can be taken. In skull base reconstruction the attached fascia can be used to suspend a bulky flap to the remaining bony construct using non-resorbable suture to help prevent flap sag. The attached vascularized fascia is valuable as a second layer repair for dural defects as well.22 The flap can also be used in a chimeric fashion. The perforators can be traced separately to the skin paddle and to the vastus lateral is muscle, separating the two components and allowing for separate arcs of rotation when needed for more complex defects.23
Figure 1.
Anterolateral Thigh Free Flap Harvest. When harvested as a myofasciocutaneous flap, the volume of muscle harvested can be tailored to the defect size, with the attached fascia providing added support and versatility support for complex reconstructions.
Radial forearm free flap
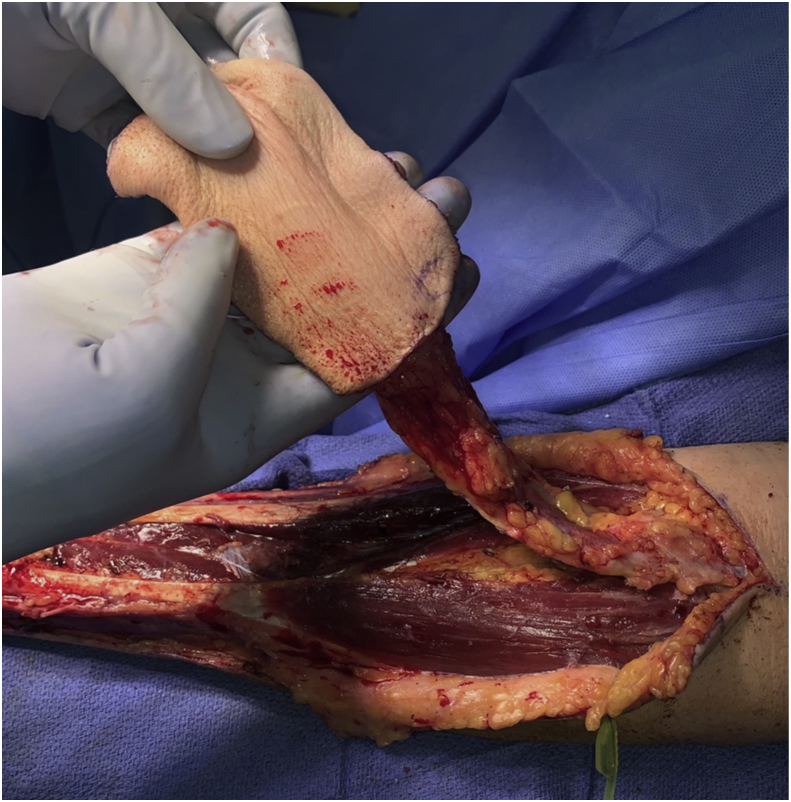
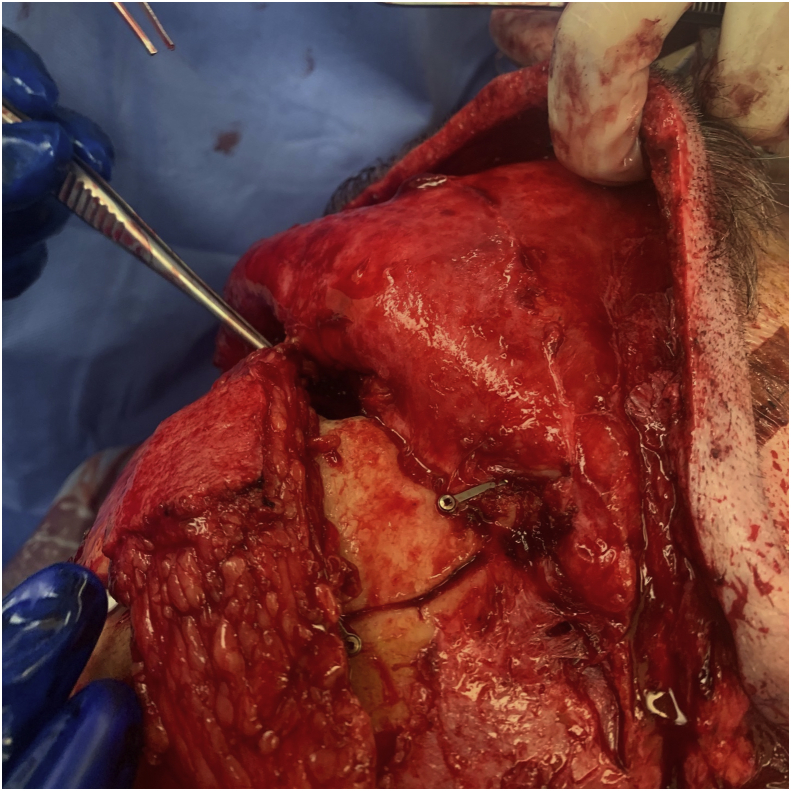
The radial forearm free flap was first described in 1978 and has been shown to be a dependable free flap when used for both head and neck as well as skull base reconstruction.24 It has reliable landmarks and the flap harvest is straightforward. It can be raised in a two-team approach and can have up to a 15 cm long pedicle depending on the forearm length (Fig. 2). The size of the skin paddle can be limited by forearm surface area, but large skin paddles can be designed, up to 10 by 14 cm. The flap is raised as a fasciocutaneous flap making it thin and pliable. This makes it the ideal choice of flap when the defect is small from a volume perspective, but reliable skull base or dural closure is required (Fig. 3).25 It can also be folded back on itself to create more volume where needed.26 There are some drawbacks to this flap. A pre-operative Allen test must be performed to ensure collateral flow from the ulnar system to the radial surface of the hand. Unfortunately, the donor site requires reconstruction with a split thickness skin graft to cover exposed tendons and nerves. This creates a second donor site, typically on the thigh in our practice, which can be a secondary source of pain and skin discoloration on healing. When this flap is used for skull base reconstruction it typically requires de-epithelialization so that epithelium is not buried. In this case the split thickness skin graft can be taken from the forearm skin paddle after the tourniquet has been placed. This is left attached distal at the wrist crease and then can be re-draped over the flap donor site for closure. This has been described by Boahene et al and we have had good results using this technique in our practice.27 Regardless of where the split thickness skin graft is taken from, the forearm site can still be cosmetically unappealing after healing which is important to counsel the patient on pre-operatively.28
Figure 2.
Radial Forearm Free Flap Harvest. The length of the pedicle can be up to 15 cm, with a thin, pliable skin paddle.
Figure 3.
Reconstruction of Anterior Skull Base Defect with Radial Forearm Free Flap. The flap is ideal for small volume defects necessitating reliable skull base or dural closure.
Fibula free flap
As mentioned above, restoring the facial contour based on the concept of facial buttress reconstruction can improve cosmetic outcome.13 This can often be accomplished with soft tissue bulk alone, but when the orbit and frontal bone are resected osseous reconstruction may be required.29 The fibula free flap was first described in 1986 and since that time has become the primary workhorse flap for osseous reconstruction of the head and neck.30 This flap has the benefit of a large skin paddle, reported up to 14 cm by 24 cm, that is usually thin with minimal subcutaneous fat and a long bone segment up to 25 cm depending on calf length.31 It is important to ensure three vessel patency in the lower extremity prior to free flap harvest. It is our practice to order lower extremity CT angiography to evaluate this, but Doppler ultrasonography can also be used. If there is a viable facial skin flap present, then the fibula skin paddle can be de-epithelialized and buried to close a skull base defect with bone overlying to reconstruct the frontal bar or orbital rim. The skin paddle can also be used to close an external skin defect overlying the osseous reconstruction. Cervical facial vessels can be routinely used for vascular anastomosis due to the long fibula pedicle length without need for vein grafting.32
Subscapular system flaps: scapula, lateral border, scapula tip, and latissimus dorsi flaps
The primary benefit of the scapula free flap over the fibula free flap is that it provides healthy bone stock as well as greater tissue bulk. The flap can also be harvested as a chimeric flap with bone, muscle and skin all harvested on separate branches from the subscapular system. Additionally the bone stock can be taken as a longer lateral border section based on the circumflex artery or as the scapular tip based on the angular artery.33 The flap is robust and the subscapular arterial system is usually protected from atherosclerotic disease as compared to the fibular free flap, which is another advantage.34 The lateral border can be used to reconstruct the frontal and orbital bar with the skin paddle either de-epithelialized to line the skull base defect or externalized. The scapular tip has been demonstrated to work well for both mandibular and anterior maxillary wall reconstruction.35 In addition to the fascio cutaneous component the latissimus dorsi muscle can also be harvested based on the thoracodorsal pedicle. This can be rotated as additional soft tissue where needed to line the skull base, fill a maxillectomy defect, or externalized with coverage of a split thickness skin graft for cutaneous defects.36 In rare cases a patient may not be a candidate for other fasciocutaneous or myofascio cutaneous donor sites due to prior surgery or extremity trauma. A fasciocutaneous skin paddle can be raised from the subscapular system without taking vascularized bone. This can then be used and inset like any other soft tissue flap. The primary drawback to this flap is the positioning required which typically necessitates a single team approach. This also adds to the total ischemia time as the donor site is closed prior to repositioning for microvascular anastomosis and inset.
Conclusion
Open craniofacial resection has become less common over the past twenty years; however, the art of anterior skull base reconstruction using free tissue is a critical skill for a reconstructive surgeon. Free tissue reconstruction is key to decrease the number of associated complications with skull base resection.25 As reviewed there are multiple free flaps that can be used for anterior skull base reconstruction. Each has its own benefits and drawbacks that are both defect and patient dependent. Regardless of the donor site selected all of the flaps reviewed in this article have been successfully utilized for skull base reconstruction, and it is imperative for the reconstructive surgeon to be familiar with them.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Schmidt R.F., Choudhry O.J., Takkellapati R., Eloy J.A., Couldwell W.T., Liu J.K. Hermann Schloffer and the origin of transsphenoidal pituitary surgery. Neurosurg Focus. 2012;33:E5. doi: 10.3171/2012.5.FOCUS12129. [DOI] [PubMed] [Google Scholar]
- 2.Casiano R.R., Numa W.A., Falquez A.M. Endoscopic resection of esthesioneuroblastoma. Am J Rhinol. 2001;15:271–279. [PubMed] [Google Scholar]
- 3.Meccariello G., Deganello A., Choussy O. Endoscopic nasal versus open approach for the management of sinonasal adenocarcinoma: a pooled-analysis of 1826 patients. Head Neck. 2016;38(Suppl 1):E2267–E2274. doi: 10.1002/hed.24182. [DOI] [PubMed] [Google Scholar]
- 4.Zimmer L.A., Theodosopoulos P.V. Anterior skull base surgery: open versus endoscopic. Curr Opin Otolaryngol Head Neck Surg. 2009;17:75–78. doi: 10.1097/moo.0b013e328325a525. [DOI] [PubMed] [Google Scholar]
- 5.Yano T., Okazaki M., Tanaka K. A new concept for classifying skull base defects for reconstructive surgery. J Neurol Surg B Skull Base. 2012;73:125–131. doi: 10.1055/s-0032-1301402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuniga M.G., Turner J.H., Chandra R.K. Updates in anterior skull base reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2016;24:75–82. doi: 10.1097/MOO.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 7.Boyce D.E., Shokrollahi K. Reconstructive surgery. BMJ. 2006;332:710–712. doi: 10.1136/bmj.332.7543.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakravarthi S., Gonen L., Monroy-Sosa A., Khalili S., Kassam A. Endoscopic endonasal reconstructive methods to the anterior skull base. Semin Plast Surg. 2017;31:203–213. doi: 10.1055/s-0037-1607274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil Z., Abergel A., Leider-Trejo L. A comprehensive algorithm for anterior skull base reconstruction after oncological resections. Skull Base. 2007;17:25–37. doi: 10.1055/s-2006-959333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadad G., Bassagasteguy L., Carrau R.L. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 11.Gullane P.J., Lipa J.E., Novak C.B., Neligan P.C. Reconstruction of skull base defects. Clin Plast Surg. 2005;32:391–399. doi: 10.1016/j.cps.2005.02.001. [vii] [DOI] [PubMed] [Google Scholar]
- 12.Chang D.W., Langstein H.N., Gupta A. Reconstructive management of cranial base defects after tumor ablation. Plast Reconstr Surg. 2001;107:1346–1355. doi: 10.1097/00006534-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Bluebond-Langner R., Rodriguez E.D. Application of skeletal buttress analogy in composite facial reconstruction. Craniomaxillofac Trauma Reconstr. 2009;2:19–25. doi: 10.1055/s-0028-1098966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmalbach C.E., Webb D.E., Weitzel E.K. Anterior skull base reconstruction: a review of current techniques. Curr Opin Otolaryngol Head Neck Surg. 2010;18:238–243. doi: 10.1097/MOO.0b013e32833a4706. [DOI] [PubMed] [Google Scholar]
- 15.Disa J.J., Pusic A.L., Hidalgo D.H., Cordeiro P.G. Simplifying microvascular head and neck reconstruction: a rational approach to donor site selection. Ann Plast Surg. 2001;47:385–389. doi: 10.1097/00000637-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Teknos T.N., Smith J.C., Day T.A., Netterville J.L., Burkey B.B. Microvascular free tissue transfer in reconstructing skull base defects: lessons learned. Laryngoscope. 2002;112:1871–1876. doi: 10.1097/00005537-200210000-00032. [DOI] [PubMed] [Google Scholar]
- 17.Spector M.E., Kim J.C. The latissimus dorsi, pectoralis minor, and rectus abdominis free flaps for dynamic reconstruction of the paralyzed face. Op Tech Oto. 2012;23:268–274. [Google Scholar]
- 18.Nussenbaum B., Pipkorn P. Rectus abdominis flap for head and neck reconstruction. Open Access Atlas of Otolaryngology, Head & Neck Operative Surgery. http://www.entdev.uct.ac.za/guides/open-access-atlas-of-otolaryngology-head-neck-operative-surgery/
- 19.Kwon D., Iloreta A., Miles B., Inman J. Open anterior skull base reconstruction: a contemporary review. Semin Plast Surg. 2017;31:189–196. doi: 10.1055/s-0037-1607273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali R.S., Bluebond-Langner R., Rodriguez E.D., Cheng M.H. The versatility of the anterolateral thigh flap. Plast Recon Surg. 2009;124 doi: 10.1097/PRS.0b013e3181bcf05c. 395e-407. [DOI] [PubMed] [Google Scholar]
- 21.Cormack G., Lamberty B.G.H. 2nd ed. Churchill Livingstone; New York: 1994. The Arterial Anatomy of Skin Flaps; pp. 228–243. [Google Scholar]
- 22.Malata C.M., Tehrani H., Kumiponjera D., Hardy D.G., Moffat D.A. Use of anterolateral thigh and lateral arm fasciocutaneous free flaps in lateral skull base reconstruction. Ann Plast Surg. 2006;57:169–175. doi: 10.1097/01.sap.0000218490.16921.c2. discussion 176. [DOI] [PubMed] [Google Scholar]
- 23.Karonidis A., Chang L.R. Using the distal part of vastus lateralis muscle as chimeric anterolateral thigh free flap is a more flexible tool for head and neck reconstruction. Eur J Plast Surg. 2010;33:1–5. [Google Scholar]
- 24.Soutar D.S., Scheker L.R., Tanner N.S., McGregor I.A. The radial forearm flap: a versatile method for intra-oral reconstruction. Br J Plast Surg. 1983;36:1–8. doi: 10.1016/0007-1226(83)90002-4. [DOI] [PubMed] [Google Scholar]
- 25.Wang W., Vincent A., Sokoya M., Kohlert S., Kadakia S., Ducic Y. Free-flap reconstruction of skull base and orbital defects. Semin Plast Surg. 2019;33:72–77. doi: 10.1055/s-0039-1677881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin A.C., Lin D.T. Reconstruction of lateral skull base defects with radial forearm free flaps: the double-layer technique. J Neurol Surg B Skull Base. 2015;76:257–261. doi: 10.1055/s-0035-1548551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boahene K., Richmon J., Byrne P., Ishii L. Hinged forearm split-thickness skin graft for radial artery fasciocutaneous flap donor site repair. Arch Facial Plast Surg. 2011;13:392–394. doi: 10.1001/archfacial.2011.65. [DOI] [PubMed] [Google Scholar]
- 28.de Bree R., Hartley C., Smeele L.E., Kuik D.J., Quak J.J., Leemans C.R. Evaluation of donor site function and morbidity of the fasciocutaneous radial forearm flap. Laryngoscope. 2004;114:1973–1976. doi: 10.1097/01.mlg.0000147931.29261.18. [DOI] [PubMed] [Google Scholar]
- 29.Heredero S., Solivera J., García B., Dean A. Osteomyocutaneous peroneal artery perforator flap for reconstruction of the skull base. Br J Oral Maxillofac Surg. 2016;54:99–101. doi: 10.1016/j.bjoms.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Wei F.C., Chen H.C., Chuang C.C., Noordhoff M.S. Fibular osteoseptocutaneous flap: anatomic study and clinical application. Plast Reconstr Surg. 1986;78:191–200. doi: 10.1097/00006534-198608000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Al D.N.F., Kao H.K., Wei F.C. The fibula osteoseptocutaneous flap: concise review, goal-oriented surgical technique, and tips and tricks. Plast Reconstr Surg. 2018;142:913–923. doi: 10.1097/PRS.0000000000005065. [DOI] [PubMed] [Google Scholar]
- 32.Macía G., Picón M., Nuñez J., Almeida F., Alvarez I., Acero J. The use of free flaps in skull base reconstruction. Int J Oral Maxillofac Surg. 2016;45:158–162. doi: 10.1016/j.ijom.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Fairbanks G.A., Hallock G.G. Facial reconstruction using a combined flap of the subscapular axis simultaneously including separate medial and lateral scapular vascularized bone grafts. Ann Plast Surg. 2002;49:104–108. doi: 10.1097/00000637-200207000-00016. discussion 108. [DOI] [PubMed] [Google Scholar]
- 34.Brown J., Bekiroglu F., Shaw R. Indications for the scapular flap in reconstructions of the head and neck. Br J Oral Maxillofac Surg. 2010;48:331–337. doi: 10.1016/j.bjoms.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Tracy J.C., Brandon B., Patel S.N. Scapular tip free flap in composite head and neck reconstruction. Otolaryngol Head Neck Surg. 2019;160:57–62. doi: 10.1177/0194599818791783. [DOI] [PubMed] [Google Scholar]
- 36.Urken M.L., Bridger A.G., Zur K.B., Genden E.M. The scapular osteofasciocutaneous flap: a 12-year experience. Arch Otolaryngol Head Neck Surg. 2001;127:862–869. [PubMed] [Google Scholar]