Abstract
The present study aimed in green synthesis and characterization of silver nanoparticles (AgNPs) using the leaves of Cleistanthus collinus. The NPs showed various absorption peaks between 3402 cm−1 and 1063 cm−1. FTIR spectrum revealed the presence of OH group, alkene, aromatic hydrocarbon, aliphatic fluro compound and aliphatic chloro compounds. Scanning electron microscopic analysis revealed the particle size ranged from 30 to 50 nm. The biosynthesized NPs have potent activity against Shigella dysentriae, Staphylococcus aureus and Bacillus subtilis and the zone of inhibition was 21 ± 1, 20 ± 2, 16 ± 2 mm, respectively. Toxicity of the synthesized NPs was tested on green gram (Vigna radiata) seed at various concentrations (20–100%) and germination was induced by NPs treated seeds. Shoot length and root length was higher in NPs treated plant than control plant (p < 0.01). Elevated level of catalase (CAT) and superoxide dismutase (SOD) and about 13% CAT and 7% SOD activity registered than control. Superoxide dismutase activity of root and shoot varied based on the dosage of AgNPs (p < 0.01). Also, the NPs (1%) showed significant larvicidal activity on Aedes aegypti and 100% mortality was achieved after 24 h treatment. The green synthesized NPs reduced methylene blue and 4-nitrophenol significantly (p < 0.01). The colouration of methylene blue and 4-nitrophenol were considerably reduced after 60 min showed the potential of dye degrading ability.
Keywords: Cleistanthus collinus, Nanoparticles, Antibacterial, Larvicidal, Dye degradation, Waste management
1. Introduction
Metal nanoparticles (NPs) are widely reported due to their catalytic, electrical and optical properties. To optimize and largely utilize physical and chemical properties of NPs, wide spectrum of research has been focused to control the shape and size, which is very important to determine the optical, chemical and physical properties (Coe et al., 2002). Many methods including, heat evaporation (Smetana et al., 2005), photochemical reduction (Mallick et al., 2005), electrochemical (Liu and Len et al., 2004) and chemical reduction (Yu, 2007). Generally, the surface passivator reagents are frequently used to control the size of NPs and to prevent NPs aggregation. However, these chemical passivators such as, thiourea, thiophenol are highly toxic to the environment, if large amount of NPs are synthesized (Lin et al., 2000). In recent years biosynthesis of NPs has attained much more attention due to the growing need to identify eco-friendly technologies in NPs synthesis. Much effort has been paid into the biosynthesis of NPs using various microorganisms (Basavaraja et al., 2008). Both dead and live microbes are gaining much more importance in biosynthesis of NPs. While organisms such as, fungi, actinomycetes and bacteria frequently used, the application of plant parts also have similar effect in the biosynthesis of metal NPs. Although synthesis of gold NPs are mainly considered as biocompatible method, chemical synthesis of NPs may still lead to the source of various toxic substances adsorbed on the surface of the material and may have serious side effects in various applications. NPs synthesis using plants can effectively eliminate these problems by preparing the NPs more bio-compatible. The application of plant extracts from various parts for the biosynthesis of NPs could be more advantageous than other biological methods by using simple biosynthesis process (Gardea-Torresdey et al., 2003).
Various naturally available sources such as, leguminous shrub (Sesbania drummondii) (Shankar et al., 2007), lemongrass leaves extract (Chandran et al., 2006), natural rubber (Abu Bakar et al, 2007), leaf broth (Shiv Shankar et al., 2004), neem (Azadirachta indica) and green tea (Camellia sinensis) (Vilchis et al., 2008) have been used for the synthesis of nanomaterials. In a study, Berchmans and Hirata (2008) used Jatropha curcas latex for the biosynthesis of NPs. Also, the biomedical and eco-friendly applications of plant parts and plants were well known. Plant based synthesis of NPs are economic, cost effective and are alternative to costly chemical synthesis (Iravani 2011). Many studies reported the uses of inorganic NPs as bacteriostatic agents (Vaseeharan et al., 2012), prevent or control various parasites, inhibiting fungal growth and interaction with HIV-I virus (Marimuthu et al., 2011). In a study, Shankar et al., (2003) used Geranium leaf extract for the biosynthesis of Ag NPs with 16 to 40 nm particle size. Krishnaraj et al., (2010) used the extract of Acalypha indica for the biosynthesis of Ag NPs with the particle size between 20 and 30 nm. The morphology and size of the synthesized NPs are very much related to the interaction between metal atoms and biomolecules (Merisko-Liversidge et al., 2003). Cleistanthus collinus is a toxic plant and this plant contains prime phytochemicals, Cleistanthin B and Cleistanthin A, Genin and Collinusin. Cleistanthin A was isolated from the leaves of the C. collinus plant and tested for its anticancer property (Pradeepkumar and Shanmugam 1999). These plant parts are used in traditional medicine in China (Donald and Barceloux 2008). This toxic plant was used as homicidal, fish poison, cattle poison and the alcoholic extract of this toxic plant was used to treat gastro intestinal disorders and also showed anticancer activity. Medicinal plants also have larvicidal and insecticidal properties against various larvae and pests (Harwarsh et al., 2010). Arivoli and Samuel (2012) analyzed the larvicidal property of C. collinus against Anopheles stephensi. In this study, an attempt was made to use C. collinus extract for the biosynthesis of silver NPs for environmental and biomedical applications.
2. Materials and methods
2.1. Sample preparation
Fresh leaves of Cleistanthus collinus were collected from Vellore, Tamilnadu, India and rinsed carefully using double distilled water, and sterilized by 0.02% (w/v) mercuric chloride solution. These leaves were dried and finely powdered using a mixer grinder. Plant extract was prepared by mixing 50 g leaves powder dissolved in 200 ml double distilled water and the mixture was boiled about 80 °C for 5 min.
2.2. Green synthesis of silver NPs from C. Collinus leaves extract
Green synthesis of NPs was performed by the reduction of silver ions. About 5 ml of leaves extract was carefully added drop by drop into the aqueous solution of 1 mM silver ions solution (90 ml). Initially the colour of the extract was observed as milky white, later it turned to yellowish brown. The synthesized NPs were centrifuged at 4 °C for 15 min at 10,000 rpm and were lyophilized.
2.3. Characterization of silver NPs
The green synthesized NPs were characterized by UV-Visible spectrophotometer, FT-IR analysis and SEM analysis. UV-Visible spectrum of the green synthesized AgNPs was analyzed between 300 and 700 nm using a UV-Visible spectropohotometer. The synthesized NPs were subjected to FT-IR spectroscopy analysis in the range of 4000–4500 cm−1 at a resolution of 4 cm−1. SEM analysis was performed using a 4500 SEM machine. Thin film of the NPs was prepared on a carbon coated copper grid by gently dropping a very small quantity of NPs on the grid. Excess solution was carefully removed by blotting and the film on the SEM grid was allowed to dry under a mercury lamp for 5 min.
2.4. Antimicrobial properties of NPs
The bacteria such as, Shigella sonnei, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis and Shigella dysentriae were used as the test organisms. All these isolates were obtained from Microbial Type Culture Collection, Pune, India. All organisms were sub cultured using LB Agar (Himedia, Mumbai, India). Then, the organisms were cultured individually in 100 ml Erlenmeyer flask containing nutrient broth medium (Himedia, Mumbai, India) and was grown in an rotary shaker incubator at 37 °C. The culture was centrifuged and the pellet was harvested, then it was washed twice in water, followed by Phosphate Buffered Saline (PBS) and the broth was diluted appropriately (106 CFU/ml). Antibacterial activity test was performed to analyze the biological activity of green synthesized silver NPs against these tested bacterial strains as suggested by Cheesbrough (2000). For the determination of antibacterial activities of the green synthesized NPs, 20 µg of sample was loaded into the wells and the plate was kept in refrigerator for about 2 h for sample diffusion. Then all plates were incubated in an incubator at 37 °C for overnight and antibacterial activity was recorded. The zone of inhibition was tabulated.
2.5. Toxicity analysis
The synthesized silver NPs was prepared at various concentrations (20, 40, 60, 80 and 100%). Petri dishes (12 cm diameter) were used for the determination of germination test. In this study, green gram (Vigna radiata) was placed in a Petri dish and the seed were carefully sterilized using HgCl2 solution (0.1%, w/v). Then the seed was washed thrice with double distilled water and NPs were added at various concentrations. Then the Petri dishes were maintained in a seed germinator in dark condition, optimum humidity and temperature (25 °C) was maintained to ensure germination. The seeds with 1 mm root tip of green gram were considered as complete germination. Then germination (%), root (mm) and shoot length (mm) for every 24 h for three days after seed germination was registered and calculated. The root (200 mg) and shoot tip (200 mg) were dissected out from the seed and was homogenized with phosphate buffer (pH 7.0, 0.1 M) and the sample was centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant was retained and used for enzyme assay. Total protein content of the sample was performed (Lowry, 1951) using standard method. For the determination of catalase (CAT), hydrogen peroxide (15 mM) was used as the substrate. The sample was mixed with buffered substrate (sodium phosphate buffer, pH 7.0, 0.05 M) and the kinetic assay was performed. The declined absorbance at 240 nm was performed for 60 s and the result was expressed as μmol of hydrogen peroxide consumed per min per mg protein. Superoxide dismutase (SOD) assay was performed using nitroblue tetrazolium (NBT) as the standard substrate in the presence of optimum level of riboflavin. Reaction was initiated by adding crude enzyme sample with the substrate and an increased optical density was recorded for five min at 470 nm and the results were expressed as ΔOD/min/mg protein.
2.6. Larvicidal bioassay of NPs
Mosquito larvicidal activity assay of the green synthesized NPs was performed as described by WHO (2005)). Larvicidal bioassay experiment was carried out at room temperature (27 ± 2 °C). Twenty five 4th instar mosquito larvae of A. aegypti were introduced into 250 ml of filter sterilized tap water and the photoperiod was adjusted as 18:6h light and dark cycle. The larvicidal activity of the silver NPs was determined after incubation of 24 h. NPs were mixed in aqueous solution and diluted and a control experiment (aqueous plant extract) was also performed in triplicate analysis. After every 4 h, mortality of larvae was observed and dead larvae were removed from the experimental container. The mortality of larvae was finally corrected as suggested by Abbott (1925).
2.7. Dye reduction properties of green synthesized silver NPs
Methylene blue and 4-nitrophenol were used for dye reduction experiments. In order to evaluate the activity of NPs on dye reduction, experiment was performed at various concentrations and the reduction was characterized by absorbing the decreased colouration at regular intervals. In the experiments methylene blue and 4-nitrophenol solution (1 × 10−3 M) was incubated with the synthesized silver NPs and incubated for 20, 40 and 60 min. Then, the decreased colouration was observed and the absorbance of the sample was recorded.
3. Results and discussion
3.1. Green synthesis of silver NPs
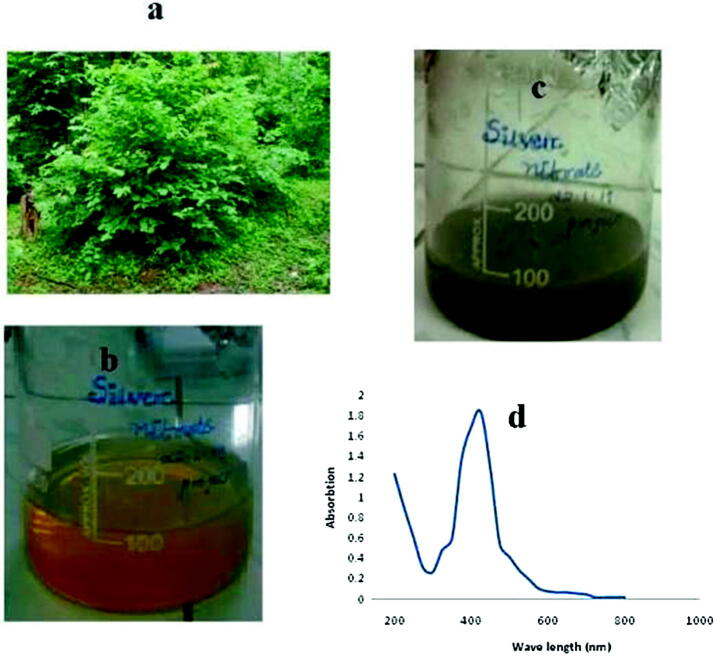
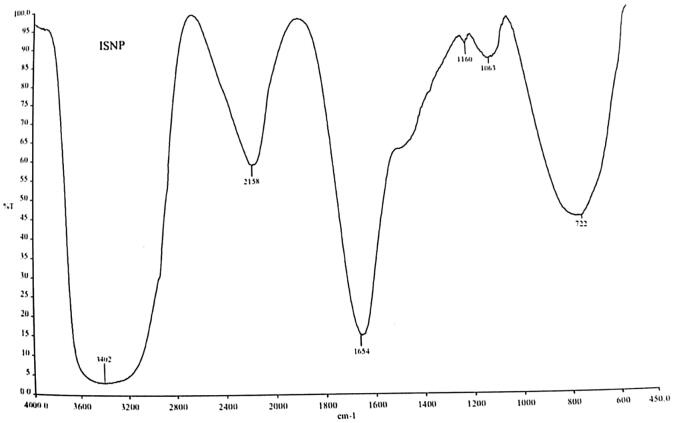
Green synthesis of silver NPs was performed using the leaves extract of C. collinus. Development of AgNPs from the silver nitrate solution (1 mM) was clearly visualized by the colour change from green to yellowish brown in colour. UV–Visible spectroscopic analysis revealed a major peak at 420 nm (Fig. 1). In aqueous solution, AgNPs shows a yellowish brown in colour and this colour development from excitation of surface plasmon vibrations in the NPs, mainly metal NPs (Henglein et al., 1993). Plant extract has been frequently used for the biosynthesis of silver NPs to enhance the stability. The functional group of metal NPs was analyzed in this study using FT-IR. The intense bands were observed with standard values to explore the functional groups. The FTIR spectrum shows prominent absorption peaks at 3402 cm−1 which corresponds to OH group H-bonded OH stretch alcohols and OH compound group, 2153 cm−1 corresponds to C C stretch thiols and thio-substitute compound. The absorption peaks 1654 cm−1, 1160 cm−1, 1063 cm−1, 1722 cm−1 corresponds to Alkenyl C C stretch alkene group, Aromatic C-H in plane bend, Aliphatic fluro compound C—F stretch, Aliphatic chloro compound C-CI stretch (Fig. 2). Haung et al., (2007) concluded that the availability of proteins as legend on NPs surface critically enhances its stability. SEM analysis was performed to analyze the particle size of NPs. The particle size was uniform and was ranged between 30 and 50 nm (Fig. 3).
Fig. 1.
Green synthesis of silver NPs from C. collinus(a) Cleisanthuscollinus plant, (b) leaves extract, (c) plant extract reduced silver nitrate solution, (d) absorption spectra of silver NPs.
Fig. 2.
FT-IR spectrum of green synthesized NPs.
Fig. 3.
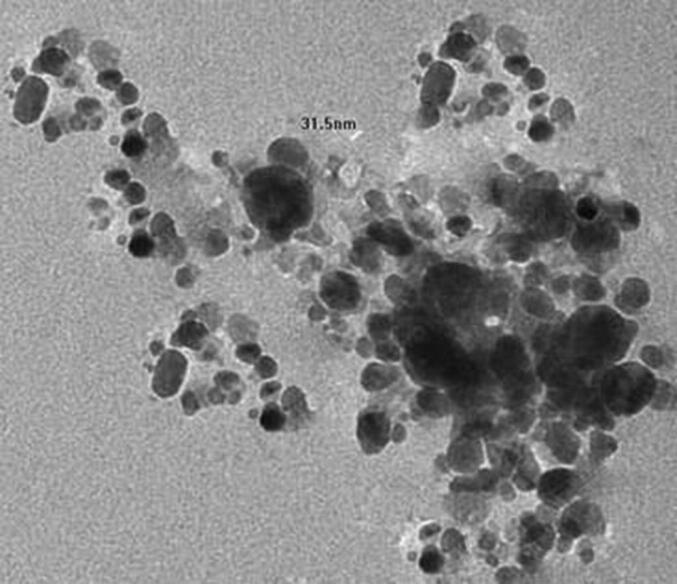
Scanning electron microscopy image of green synthesized NPs.
3.2. Antimicrobial activity of synthesized NPs
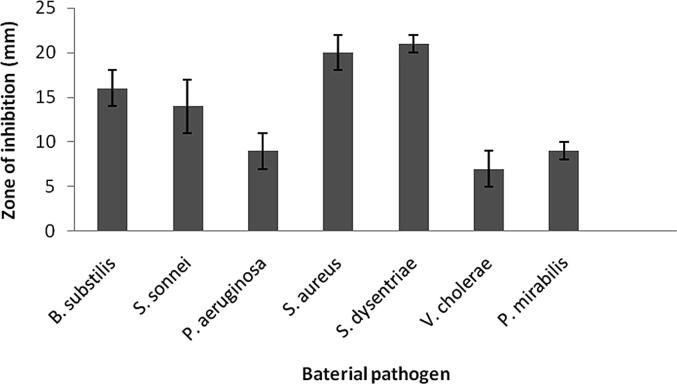
Antibacterial activity of NPs were performed by using Shigella sonnei, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis and Shigella dysentriae colonies on Meuller Hinton Agar plates loaded with NPs. NPs were highly active against S. dysentriae, S. aureus and B. subtilis and the zone of inhibition was 21 ± 1, 20 ± 2, 16 ± 2 mm, respectively (Fig. 4a, Fig. 4b). Antibacterial activity of silver NPs was reported previously. Recently, Namratha (2013) reported antibacterial efficacy of silver NPs against various pathogenic bacteria. The synthesized NPs were highly active against Shigella dysentriae and the zone of inhibition was 21 mm, whereas 20 mm zone of inhibition was observed against multi drug resistant Staphylococcus aureus. Siver NPs and silver ions were well known to have potent antibacterial and antifungal properties (Furno et al., 2004). The antibacterial property of the synthesized NPs in this study clearly revealed that both Gram negative and Gram positive bacteria were effectively inhibited by various degrees. These findings agreed with earlier work reported by Bindhu and Umadevi, 2013, Li et al., 2011, Kim et al., 2007. The synthesized NPs have least activity (9 ± 1 mm) against Pseudomonas aeruginosa. The Gram’s positive Bacillus subtilis showed less activity than S. aureus. This result was similar to the report of Sondi and Salopek-Sondi (Feng et al., 2000). The mechanism of action of Ag-NPs on bactericidal activity was proposed. Ag-NPs interact with sulfur containing proteins and may attach to the surface of the bacterial cell membrane, affecting respiration and permeability functions of the bacterial cell resulting in cell death (Morones et al., 2005). Ag-NPs interacted with the bacterial membrane surface and also penetrate the cytoplasm of the bacteria (Sondi and Salopek-Sondi 2004). It is predicted that nanomaterials affect the microbes responsible for the respiratory infections (Li et al., 2011, Klasen, 2000). However, Li et al., (2011) reported that Ag-NPs entered into the cells of bacteria and affected DNA replication process affect multiplication of bacteria.
Fig. 4a.
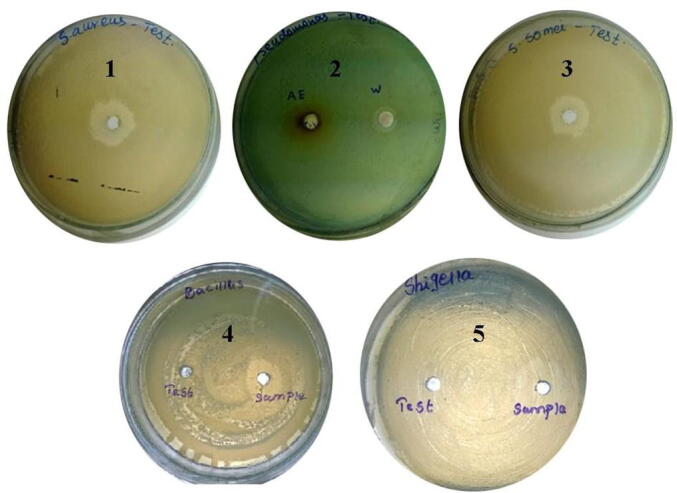
Antibacterial activity of green synthesized NPs. 20 µg NPs were loaded into each well and incubated for 24 h and zone of inhibition was obtained. (1) S. aureus, (2) P. aeruginosa, (3) S. sonnei, (4) B. subtilis and (5) S. dysentriae.
Fig. 4b.
Activity of silver NPs against bacterial pathogens. Error bar: standard deviation.
3.3. Toxicity of Ag-NPs analysis
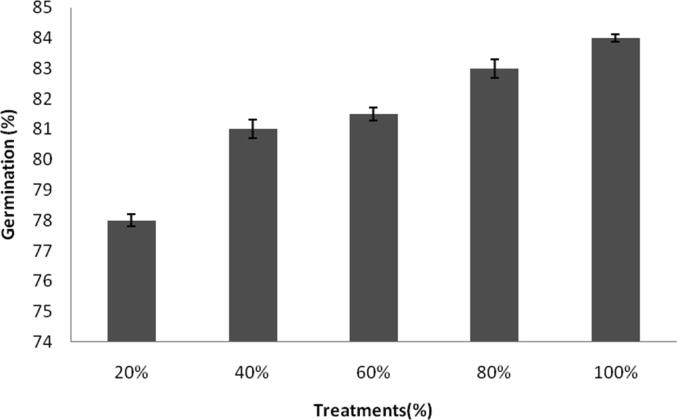
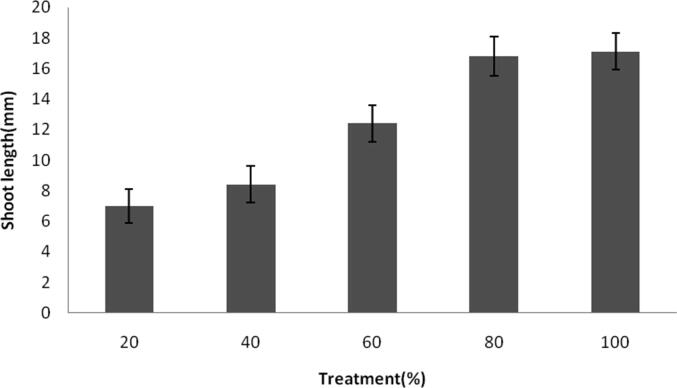
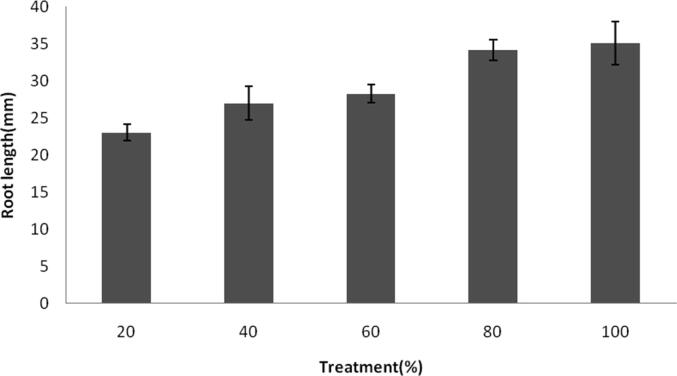
Ag-NPs toxicity towards green gram seed was performed and the final shoot and root length were registered. Germination of seeds was found to be maximum after 24 h and the results were statistically significant (p < 0.05) which was significantly higher than control seeds. At 20% NPs concentrations, germination percentage was 78 ± 0.2% and it increased as 84 ± 0.12% at 100% treatment (Fig. 5a). Shoot length and root length of green gram seedlings were higher than controls which were treated with tap water. The results were depicted in Fig. 5b and Fig. 5c. Generally, ionic Ag was highly toxic to plants and algae; however, toxic effect of ionic Ag was not clearly reported in the case of Cucurbita pepo and Arabidopsis thaliana (Geisler-Lee et al., 2013, Stampoulis et al., 2009). Also, the interactions of AgNPs and plants critically improved the release of Ag ion from the AgNPs, thus greatly enhance the uptake of Ag and enhance AgNPs’ toxicity (Navarro et al., 2008). In our study, after exposure of plants to silver NPs showed various changes in the morphology were observed. The root and shoot length increased than control experiments. The green synthesized AgNPs did not show any toxicity to the seeds, however toxicity stress and retarded growth was reported in some cases previously. To access phototoxicity, analysis of seed germination, leaf surface area, biomass and growth potential are commonly used (Tripathi et al., 2017). It was previously demonstrated that the exposure of NPs could significantly affected root growth and shoot growth in Spirodela polyrrhiza (Jiang et al., 2012). In a study Kaveh et al. (2013) recorded decreased biomass in Arabidopsis when AgNPs was applied at higher concentrations. Dimkpa et al., (2013) reported decreased roots and shoots length of wheat and the reduced shoot and root length was due to the effect of AgNPs. Also, it was previously revealed that AgNPs effectively reduced root fresh weights, shoot fresh weights and root elongation (Nair and Chung, 2014). In C. pepo, AgNPs (>100 mg/L) affected reduced biomass and inhibited seed germination (Stampoulis, 2009). Oxidative stress of the plant was analyzed by measuring enzyme activity in the root and shoot (Table 1a, Table 1b). SOD activity was 0.031 ± 0.001 U/ml after 24 h of treatment and it increased as 0.074 ± 0.003 U/ml after 72 h. POD activity was 15.2 ± 0.5 U/ml after 24 h and increased exponentially at 39.2 ± 1.6 U/ml after 72 h. CAT activities of root and shoot were decreased, however SOD activity was found to be higher than control plant. Enzyme activity of root and shoot varied based on the dosage of AgNPs. The increased activity of SOD and decreased level of CAT was associated with the inhibition of enzyme synthesis (Malik and Singh, 1980). Activity of POD increased in dose dependent AgNPs. In the treated seed, POD activity was higher than control seed. Peroxidases catalyses dehydrogenation of various organic compounds such as, amines, phenols etc (Bashir et al., 2007).
Fig. 5a.
Influence of germination inducing activity of silver NPs. Germination power was increased at higher NPs concentration. Error bar: standard deviation.
Fig. 5b.
Influence of NPs on the growth of shoot at various concentrations. Error bar: standard deviation.
Fig. 5c.
Influence of NPs on the growth of root at various concentrations. Error bar: standard deviation.
Table 1a.
Effect of NPs on superoxide dismutase, catalase and peroxidase activity from the shoot of the plant.
| Enzyme activity (U/ml) | |||||
|---|---|---|---|---|---|
| Enzymes | NPs (%) | 24 h | 48 h | 72 h | 96 h |
| SOD | 20 | 0.002 ± 0.001 | 0.029 ± 0.002 | 0.048 ± 0.001 | 0.059 ± 0.001 |
| 40 | 0.023 ± 0.001 | 0.031 ± 0.001 | 0.057 ± 0.002 | 0.063 ± 0.002 | |
| 60 | 0.024 ± 0.001 | 0.033 ± 0.002 | 0.054 ± 0.001 | 0.069 ± 0.001 | |
| 80 | 0.028 ± 0.002 | 0.034 ± 0.002 | 0.0057 ± 0.003 | 0.071 ± 0.003 | |
| 100 | 0.031 ± 0.001 | 0.036 ± 0.001 | 0.061 ± 0.002 | 0.074 ± 0.003 | |
| CAT | 20 | 0.0129 ± 0.001 | 0.0120 ± 0.002 | 0.010 ± 0.002 | 0.008 ± 0.0006 |
| 40 | 0.0122 ± 0.002 | 0.014 ± 0.001 | 0.009 ± 0.0006 | 0.007 ± 0.0007 | |
| 60 | 0.0132 ± 0.001 | 0.017 ± 0.001 | 0.007 ± 0.0005 | 0.004 ± 0.0004 | |
| 80 | 0.017 ± 0.001 | 0.018 ± 0.002 | 0.004 ± 0.0002 | 0.005 ± 0.0003 | |
| 100 | 0.013 ± 0.001 | 0.011 ± 0.001 | 0.009 ± 0.0006 | 0.004 ± 0.0002 | |
| POD | 20 | 12.8 ± 0.6 | 18.4 ± 0.9 | 24.9 ± 1.1 | 32.1 ± 1.4 |
| 40 | 13.1 ± 0.7 | 22.1 ± 1.1 | 26.1 ± 1.2 | 34.8 ± 1.2 | |
| 60 | 13.4 ± 0.8 | 25.2 ± 1.2 | 27.1 ± 1.3 | 35.1 ± 1.5 | |
| 80 | 13.9 ± 0.6 | 26.6 ± 1.1 | 28.3 ± 1.1 | 36.9 ± 1.4 | |
| 100 | 15.2 ± 0.5 | 27.7 ± 1.2 | 29.8 ± 1.3 | 39.2 ± 1.6 |
Table 1b.
Effect of NPs on superoxide dismutase, catalase and peroxidase activity from the root of the plant.
| Enzyme activity (U/ml) |
|||||
|---|---|---|---|---|---|
| Enzymes | NPs(%) | 24 h | 48 h | 72 h | 96 h |
| sod U/ml | 20 | 0.014 ± 0.001 | 0.022 ± 0.001 | 0.031 ± 0.003 | 0.046 ± 0.003 |
| 40 | 0.016 ± 0.001 | 0.024 ± 0.002 | 0.033 ± 0.002 | 0.048 ± 0.002 | |
| 60 | 0.017 ± 0.002 | 0.027 ± 0.002 | 0.038 ± 0.001 | 0.049 ± 0.001 | |
| 80 | 0.019 ± 0.001 | 0.023 ± 0.001 | 0.042 ± 0.003 | 0.054 ± 0.004 | |
| 100 | 0.022 ± 0.002 | 0.047 ± 0.003 | 0.054 ± 0.002 | 0.063 ± 0.005 | |
| CAT U/ml | 20 | 0.012 ± 0.001 | 0.019 ± 0.001 | 0.024 ± 0.002 | 0.31 ± 0.002 |
| 40 | 0.0111 ± 0.001 | 0.017 ± 0.002 | 0.021 ± 0.001 | 0.029 ± 0.001 | |
| 60 | 0.01 ± 0.002 | 0.017 ± 0.003 | 0.019 ± 0.001 | 0.020 ± 0.001 | |
| 80 | 0.009 ± 0.0004 | 0.015 ± 0.001 | 0.017 ± 0.002 | 0.018 ± 0.001 | |
| 100 | 0.008 ± 0.0006 | 0.013 ± 0.001 | 0.014 ± 0.001 | 0.012 ± 0.002 | |
| POD U/ml | 20 | 10.1 ± 1.1 | 10.7 ± 1.2 | 11.2 ± 1.2 | 15.8 ± 1.3 |
| 40 | 11.4 ± 1.2 | 14.4 ± 1.2 | 15.8 ± 1.5 | 19.3 ± 1.5 | |
| 60 | 11.7 ± 1.4 | 17.2 ± 1.1 | 21.3 ± 2.1 | 24.5 ± 2.1 | |
| 80 | 12.3 ± 1.3 | 19.3 ± 1.4 | 24.6 ± 2.3 | 26.4 ± 1.6 | |
| 100 | 12.8 ± 1.4 | 21.3 ± 2.1 | 27.1 ± 2.4 | 29.5 ± 2.2 | |
3.4. Larvicidal activity of silver NPs
Silver NPs are mainly considered to be efficient for many biological functions and also used as a mosquito control agents. The larvicidal property of green synthesized silver NPs from C. collinus is described in Table 2. The present finding clearly indicated that the green synthesized silver NPs showed significant larvicidal activity on A. aegypti. Mortality rate was significantly increased in the larvae treated with silver NPs at higher concentrations. NPs at 1% concentration effectively decreased the survival of larvae to 15 ± 2.5% after 4 h of exposure, however, 100% mortality of the total larval population was registered at the same concentration after 24 h of treatment. The NP at 5% killed about 85 ± 2.5% larvae after 24 h of exposure. Larvicidal activity was maximum at 5% silver NPs concentrations. The treated NPs penetrate through the membrane of mosquito larvae and cause death to the organism. The green synthesized silver NPs specifically bind with phosphorus containing compounds such as, DNA or sulphur containing proteins, resulting to the denaturation of various enzymes and organelles (Rai et al., 2009). AgNPs easily permeate into the body of invertebrates through the exoskeleton and finally penetrate into the cell of insects and the NPs were bind with DNA, proteins and other molecules, modifying their structure and hence functional changes also happened (Benelli 2016). It could be noted that, plant based green synthesized AgNPs which is highly toxic to various mosquito larvae and have no or very little effect on other non-target organisms, including, fishes and other aquatic arthropods (Subramaniam et al., 2015).
Table 2.
Survivial of A. aegypt larvae after exposure to various concentrations of Ag NPs.
| NPs (%) | Mortality (%) |
|||||
|---|---|---|---|---|---|---|
| 4 h | 8 h | 12 h | 16 h | 20 h | 24 h | |
| 1 | 15 ± 2.5 | 17.5 ± 2.5 | 17.5 ± 2.5 | 25 ± 2.5 | 65 ± 5 | 100 ± 2.5 |
| 2 | 20 ± 2.5 | 35 ± 2.5 | 47.5 ± 2.5 | 52.5 ± 2.5 | 67.5 ± 1.25 | 100 ± 2.5 |
| 3 | 20 ± 2.5 | 42.5 ± 2.5 | 55 ± 2.5 | 80 ± 2.5 | 100 ± 5 | 100 ± 1.5 |
| 4 | 25 ± 3.5 | 55 ± 2.5 | 80 ± 2.5 | 100 ± 5 | 100 ± 2.5 | 100 ± 1.5 |
| 5 | 85 ± 2.5 | 90 ± 2.5 | 100 ± 5.0 | 100 ± 2.5 | 100 ± 7.5 | 100 ± 1.6 |
3.5. Dye degrading activity of silver NPs
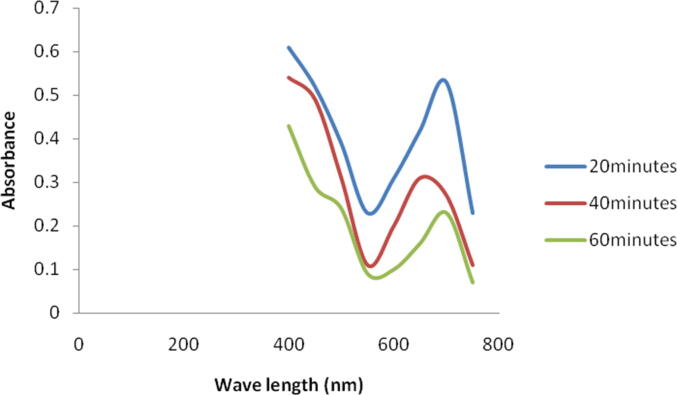
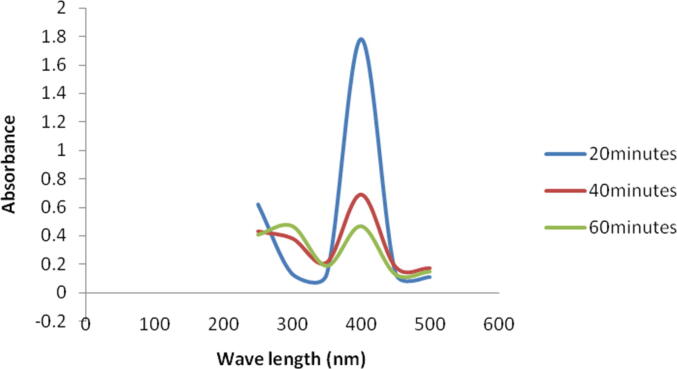
The present finding revealed marked reduction of methylene blue by green synthesized silver NPs. The absorbance maximum was high at 664 nm. After 20 min of treatment, the absorbance of the dye was considerably reduced at this nanometer and absorbance maxima were shifter to higher wavelength. After 20 min the absorbance was 0.42 at 664 nm and it decreased continuously as 0.31 and 0.16, respectively (Fig. 6a). It was reported that the synthesized metal NPs have potent catalytic properties because of high surface area to volume ratio and high rate of surface adsorption (Grogger et al., 2004). AgNPs catalyzed 4-nitrophenol into 4-aminophenol, which is one of the useful compound and widely used in dye industries and pharmaceutical industries. Addition sodium hydroxide involved deprotonation of 4-NP and the generation of nitrophenolate ion as an intermediate (Christopher et al., 2011). In this study, the colour of 4-NP decreased considerably after 60 min of incubation (Fig. 6b). It was previously reported the fact that silver NPs and the composites of NPs showed good catalytic property in the process of dye removal and reduction. During dye degradation process the catalysis was mainly occurs on the metals surface, hence enhancing the availability of the surface area will critically improve the capacity of the catalyst. Also, decreasing the particle size of the NPs will generally enhance the catalytic property; however there is a low size also decreased the reaction (Pradhan et al., 2002). Generally metal NPs assist the electron relay and also act as a critical substrate for the reaction of electron transfer. In the process of electron transfer, the reactants (dye) are mainly adsorbed on the metal surface and further, the dye (reactants) gains an electron and further reduced. Hence, AgNPs generally acts as potential catalyst through electron transfer mechanism in dye degrading mechanism (Domingos et al., 2009).
Fig. 6a.
Effect of NPs on the degradation of methylene blue. At 60 min incubation, colour intensity was less considerably due to degradation of methylene blue.
Fig. 6b.
Effect of NPs on the degradation of 4-nitrophenol. At 60 min incubation, colour intensity was less considerably due to degradation of methylene blue.
4. Conclusion
For sustainable nanotechnology, green chemistry should be adopted for the synthesis of NPs using various plant sources other than microbial origin. In recent years, colloid based nanoparticle synthesis has been well established to control the particle shape, size, functional properties and uniformity. In this study NPs were biosynthesized from the leaves of C. collinus in a single step. The applied strategy proved to be useful and has the potential against various bacterial pathogens and also larvicidal activity. The biosynthesized AgNPs showed tremendous degradation potential of methylene blue and 4-nitrophenol. The synthesized NPs showed less toxic and can be used to treat wastewater from dye industry.
Acknowledgement
The authors thank Department of Biotechnology, Sathyabama Institute of Science and Technology for providing the facility and fund for this work. This work was supported by the Deanship of Scientific Research at King Saud University for its funding this Research work through Research Group No. (RGP-VPP-304).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbott W.S. A Method of computing the effectiveness of an insecticide. J. Eco. Entomol. 1925;18(2):265–267. [PubMed] [Google Scholar]
- Abu Bakar N.H.H., Ismail J., Abu Bakar M. Synthesis and characterization of silver nanoparticles in natural rubber. Mater. Chem. Phys. 2007;104(2–3):276–283. [Google Scholar]
- Arivoli S., Samuel T. Antifeedant Activity of plant extracts against Spodoptera litura (Fab.) (Lepidoptera: Noctuidae) American-Eurasian J. Agric. Environ. Sci. 2012;12(6):764–768. [Google Scholar]
- Basavaraja S., Balaji S.D., Lagashetty A., Rajasab A.H., Venkataraman A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater. Res. Bull. 2008;43(5):1164–1170. [Google Scholar]
- Bashir F., Mahmooduzzafar S.T.O., Iqbal M. The antioxidative response system in Glycine max (L.) Merr: exposed to Deltamethrin, a synthetic pyrethroid insecticide. Environ. Poll. 2007;147:94–100. doi: 10.1016/j.envpol.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Benelli G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res. 2016;115(1):23–34. doi: 10.1007/s00436-015-4800-9. [DOI] [PubMed] [Google Scholar]
- Berchmans H.J., Hirata S. Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresour. Technol. 2008;99(6):1716–1721. doi: 10.1016/j.biortech.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Bindhu M.R., Umadevi M. Synthesis of mono dispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity. Spectrochimica Acta. Part A - Mol. & Bimol. Spectro. 2013;101:184–190. doi: 10.1016/j.saa.2012.09.031. [DOI] [PubMed] [Google Scholar]
- Chandran S.P., Chaudhary M., Pasricha R., Ahmad A., Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using aloevera plant extract. Biotechnol. Prog. 2006;22(2):577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- Cheesbrough M. Cambridge University Press; Cambridge, UK: 2000. District Laboratory Practice in Tropical Countries, Part 2; p. 434. [Google Scholar]
- Christopher P., Xin H., Linic S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat Chem. 2011;3:467–472. doi: 10.1038/nchem.1032. [DOI] [PubMed] [Google Scholar]
- Coe S., Woo W.K., Bawendi M., Bulovic V. Electroluminescence from single monolayer of nanocrystals in molecular organic devices. Nature. 2002;420(6917):800–803. doi: 10.1038/nature01217. [DOI] [PubMed] [Google Scholar]
- Dimkpa C.O., McLean J.E., Martineau N., Britt D.W., Haverkamp R., Anderson A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013;47(2):1082–1090. doi: 10.1021/es302973y. [DOI] [PubMed] [Google Scholar]
- Domingos R.F., Baalousha M.A., Nam Y.J., Reid M.M., Tufenkji J.R., Lead G., Leppard G., Wilkinson K.J. Characterizing manufactured nanoparticles in the environment: Multimethod determination of particle sizes. Environ. Sci. Technol. 2009;43(19):7277–7284. doi: 10.1021/es900249m. [DOI] [PubMed] [Google Scholar]
- Donald, G., Barceloux., Medical toxicology of natural substances, Cleistanthin, diterphene esters and the spurge Family. John Wiley & Sons. Chap, 2008, 123.
- Feng G., Mellor R.H., Bernstein M., Keller-Peck C., Nguyen Q.T., Wallace M., Nerbonne J.M., Lichtnman J.W., Sanes J.R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Furno F.S., Morley K.S., Wong B., Sharp B.L., Arnold P.L., Howdle S.M., Bayston R., Paul D., Brown P.D., Winship P.D., Reid H.J. Silver Nanoparticles and Polymeric Medical Devices: A New Approach to Prevention of Infection? J. Antimic. Chemotherapy. 2004;54(6):1019–1024. doi: 10.1093/jac/dkh478. [DOI] [PubMed] [Google Scholar]
- Gardea-Torresdey J.L., Gomez E., Peralta-Videa J., Parsons J.G., Troiani H.E., Jose- Yacaman M. Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir. 2003;19(4):1357–1361. [Google Scholar]
- Geisler-Lee J., Wang Q., Yao Y., Zhang W., Geisler M., Li K., Haung Y., Chen Y., Kolmakov Y., Ma X. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicol. 2013;7(3):322–337. doi: 10.3109/17435390.2012.658094. [DOI] [PubMed] [Google Scholar]
- Grogger C., Fattakhov S.G., Jouikov V.V., Shulaeva M.M., Reznik V.S. Primary steps of oxidation and electronic interactions in anodic cleavage of α, ω- diisocyanurate substituted dialkyl disulfides. Electrochimica. Acta. 2004;49(19):3185–3194. [Google Scholar]
- Harwansh R.K., Akhlaquer Rahman M., Dangi J.S. Microemulsion System for Transdermal Delivery of Diclofenac Sodium for Bioavailability Enhancement. J. Pharm. Res. 2010;3(9):2182–2185. [Google Scholar]
- Henglein B. Physicochemical properties of small metal particles in solution: ‘‘microelectrode’’ reactions, chemisorption, composite metal particles, and the atom- to-metaltransition. J. Phys. Chem. B. 1993;97:5457–5471. [Google Scholar]
- Huang J., Li Q., Sun D., Lu Y., Su Y., Yang X., Wang H., Wang Y., Shao1 W., He N., Hong J., Chen C. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnol. 2007;18(16):105104–105115. [Google Scholar]
- Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–2650. [Google Scholar]
- Jiang H.S., Li M., Chang F.Y., Li W., Yin L.Y. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ. Toxicol. Chem. 2012;31(8):1880–1886. doi: 10.1002/etc.1899. [DOI] [PubMed] [Google Scholar]
- Kaveh R., Li Y.S., Ranjbar S., Tehrani R., Brueck C.L., Van Aken B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013;47(18):10637–10644. doi: 10.1021/es402209w. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Kuk E., Yu K.N., Kim J.H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwang C.Y., Kim Y.K., Lee Y.S., Jeong D.H., Cho M.H. Antimicrobial effect of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007;3(1):95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Klasen H.J. A historical review of the use of silver in the treatment of burns. Part I. Burns. 2000;26(2):117–130. doi: 10.1016/s0305-4179(99)00108-4. [DOI] [PubMed] [Google Scholar]
- Krishnaraj C., Jagan E.G., Rajasekar S., Selvakumar P., Kalaichelvan P.T., Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B: Biointerf. 2010;76(1):50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Li W.R., Xie X.B., Shi Q.S., Duan S.S., Ou-Yang Y.S., Chen Y. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals. 2011;24(1):135–141. doi: 10.1007/s10534-010-9381-6. [DOI] [PubMed] [Google Scholar]
- Lin S.M., Lin F.Q., Guo H.Q., Zhang Z.H., Wang Z.G. Surface states induced photoluminescence from Mn2+ doped CdS nanoparticles. Solid State Commun. 2000;115:615–618. [Google Scholar]
- Liu Y.C., Lin L.H. New pathway for the synthesis of ultrafine silver nanoparticles from bulk silver substrates in aqueous solutions by sonoelectrochemical methods. Electrochem. Commun. 2004;6(11):1163–1168. [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Malik C.P., Singh M.B. Kalyani Publishers; New Delhi, India: 1980. Plant enzymology and histoenzymology. [Google Scholar]
- Mallick K., Witcombb M.J., Scurrella M.S. Self-assembly of silver nanoparticles in a polymer solvent: formation of a nanochain through nanoscale soldering. Mater. Chem. Phys. 2005;90(2–3):221–224. [Google Scholar]
- Marimuthu S., Rehuman A.A., Rajakumar G., Santhoshkumar T., Kirthi A.V., Jayaseelan C. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol, Res. 2011;108(6):1541–1549. doi: 10.1007/s00436-010-2212-4. [DOI] [PubMed] [Google Scholar]
- Merisko-Liversidge E., Liversidge G.G., Cooper E.R. A Formulation Approach for Poorly- water soluble compounds. Eur. J. Pharm Sci. 2003;18(3):113–120. doi: 10.1016/s0928-0987(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J., Ramirez J.T., Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- Nair P.M.G., Chung M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere. 2014;112:105–113. doi: 10.1016/j.chemosphere.2014.03.056. [DOI] [PubMed] [Google Scholar]
- Namratha N., Monica P.V. Synthesis of silver nanoparticles using Azadirachta indica (Neem) extract and usage in water purification. Asian. J. Pharm. Tech. 2013;3(4):170–174. [Google Scholar]
- Navarro E., Piccapietra F., Wagner B., Marconi F., Kaegi R. Toxicity of Silver Nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008;42(23):8959–8964. doi: 10.1021/es801785m. [DOI] [PubMed] [Google Scholar]
- Pradeepkumar C.P., Shanmugam M. Anticancer potentials of cleistanthin A isolated from the toxic plant Cleistanthus collinus. Oncol Res. 1999;11(5):225–232. [PubMed] [Google Scholar]
- Pradhan N.A., Pal A., Pal T. Silver nanoparticle catalyzed reduction of aromatic nitro compounds. Colloids Surf. A. 2002;196(2–3):247–257. [Google Scholar]
- Rai P., Balakrishnan P., Muhammed P., Safeena P., Karunasekar I. Simultaneous presence of infectious hypodermal and hematopoietic necrosis virus (IHHNV) and Type A virus-related sequence in Penaeus monodon from India. Aquacul. 2009;295(3):168–174. [Google Scholar]
- Shankar K., Gopal K.M., Haripriya E.P., Sorachon Y., Oomman K.V., Craig A.G. Highly- ordered TiO2 nanotube arrays up to 220 µm in length: use in water photoelectrolysis and dye- sensitized solar cells. Nanotech. 2007;18(6):1–6. [Google Scholar]
- Shankar S.S., Ahmad A., Sastry M. Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. Biotechnol. Prog. 2003;19(6):1627–1631. doi: 10.1021/bp034070w. [DOI] [PubMed] [Google Scholar]
- Shiv Shankar S., Rai A., Ahmad A., Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J. Colloid Interf. Sci. 2004;275(2):496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Smetana A.B., Klabunde K.J., Sorensen C.M. Synthesis of spherical silver nanoparticles by digestive ripening, stabilization with various agents, and their 3-D and 2-D superlattice formation. J. Colloid Interf. Sci. 2005;284(2):521–526. doi: 10.1016/j.jcis.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interf. Sci. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Stampoulis D., Sinha S.K., White J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Enviro. Sci. Technol. 2009;43(24):9473–9479. doi: 10.1021/es901695c. [DOI] [PubMed] [Google Scholar]
- Subramaniam J., Murugan K., Panneerselvam C., Kovendan K., Madhiyazhagan P., Kumar P.M. Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: towards an integrative approach? Environ. Sci. Pollut. Res. Int. 2015;22(24):20067–20083. doi: 10.1007/s11356-015-5253-5. [DOI] [PubMed] [Google Scholar]
- Tripathi D.K., Tripathi A., Singh S., Singh Y., Vishwakarma K., Yadav G., Sharma S., Singh V.K., Mishra R.K., Upadhyay R.G. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017;8(7) doi: 10.3389/fmicb.2017.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseeharan B., Sargunar C.G., Lin Y.C., Chen J.C. Green synthesis of silver nanoparticles through Calotropis gigantea leaf extracts and evaluation of antibacterial activity against Vibrio alginolyticus. Nanotech Develop. 2012;2:12–16. [Google Scholar]
- Vilchis-Nestor A.R., Sanchez-Mendieta V., Camacho-Lopez M.A., Gomez- Espinosa R.M., Camacho-Lopez M.A., Arenas-Alatorre J.A. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett. 2008;62:3103–3105. [Google Scholar]
- World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. World Health Organization, 2005, https://apps.who.int/iris/handle/10665/69101.
- Yu D.G. Formation of colloidal silver nanoparticles stabilized by Na+-poly (-q glutamic acid) silver nitrate complex via chemical reduction process. Colloids Surf. B. 2007;59(2):171–178. doi: 10.1016/j.colsurfb.2007.05.007. [DOI] [PubMed] [Google Scholar]