Abstract
The oxidative stress leading to degenerative changes in the brain of Alzheimer’s disease (AD) is evident. Our aim was to evaluate the therapeutic and protective effects of pomegranate extract (PE) and pomegranate extract-loaded nanoparticles (PE nano) in an AlCl 3-induced AD rat model. Nanoparticles were synthesized with a PE load of 0.68% w/w, and 70 male Wistar rats were divided into 7 groups: Group I was the control, Group II received PE., Group III received PE nano for 2 weeks, Group IV received AlCl 3 (50 mg/kg) daily orally for 4 weeks, Group V received PE for 2 weeks, Group VI received PE nano for 2 weeks, and Groups V and VI were started after AlCl 3 administration was stopped. Group VII received PE for 2 weeks and was stopped before AlCl 3 was administered. The Results revealed that the discrimination index in the novel object recognition test was the least in AD rat model but increased in cases protected with PE treated with PE nano. Similar results were shown based on calculating the brain weight/body weight percent. The biomarkers of antioxidant activity (catalase, glutathione and total antioxidant activity) in brain homogenate were significantly increased in groups treated with either PE or PE nano. The thiobarbituric acid reactive substance measured to estimate lipid peroxidation was significantly increased in AD rat model and decreased in groups protected with PE or treated with PE nano. Histopathological studies using hematoxylin and eosin, cresyl violet, and silver stains revealed hyaline degeneration, chromatolysis, and hallmarks of AD; neurofibrillary tangles and the senile plaques in brains of AD rat model. Restoration of the histological architecture, Nissl granules, and minimal appearance of hallmarks of AD characterized brains treated with PE or PE nano. In conclusion, PE was more effective as a protectant than a therapeutic measure in alleviating the antioxidant, lipid peroxidative effects and histopathological hallmarks in AD brains. But, the therapeutic PE-loaded nanoparticles increased the efficacy of active components and produced similar results as the protective PE.
Keywords: Alzheimer's, Pomegranate extract, Nanoformulations, Histopathology, Antioxidant markers, Lipid peroxidation, Novel object recognition test
1. Introduction
Millions of people are affected by Alzheimer’s disease (AD), which has become a chief medical and social burden worldwide (Brookmeyer et al., 2007). The degenerative changes of the brain leading to loss of neurons is correlated to the cognitive impairment of the patient (Price and Morris, 1999).
Oxidative stress results from an imbalance between antioxidative defense and the free radical production of reactive oxygen species (ROS) which are factors playing a fatal in age-related cognitive deterioration and neurodegeneration. There is ample evidence to demonstrate that AD patients brain tissue is exposed to oxidative stress (Sayre et al. 2008) as indicated by the presence of oxidized lipids, DNA and proteins (Abd El Mohsen et al. 2005). AD pathogenesis reveals the presence persistent neuropil threads, amyloid plaques, dystrophic neuritis, astrogliosis and neurofibrillary tangles (Crews and Masliah 2010). As a consequence of the generation of ROS by Aβ peptide, both functional and structural damage may result to the cell membranes via lipid peroxidation (Valko et al. 2007). In the brains of those with AD, ROS leads to the modification of lipids whereby a significant association between lipid peroxides, neurofibrillary tangles (NFTs) amyloid plaques, and antioxidant enzymes (Arlt et al. 2002).
The possible routes for treatment of AD are either neurotransmitter-based, glial modulating drugs, anti-tau and tau modulators, or neuroprotection as antioxidants (Aprahamian et al., 2013). In the search for antioxidant natural nutritional agents, the pomegranate plant, Punica granatum L, widely distributed in the Middle Eastern countries, was chosen for this study (Xie et al. 2008).
Pomegranate has been used by various civilizations to treat fever, ulcers, diarrhea, dysentery, hemorrhage, microbial infections, parasites, and respiratory disorders (Lee et al. 2010), thus preventing cancer and arteriosclerosis and lowering high cholesterol levels (Viuda-Martos et al., 2010). The useful effects of pomegranate are related to its extensive spectrum of phytochemicals, such as tannins, alkaloids, and dyes (Zarei et al., 2011). Vegetables and fruits phytochemical extracts showed a powerful anti-proliferative and antioxidant potential (Temple 2000). Research proposed that nutritional intake of antioxidants affects not only the development of the disease but also can slow or stop its progression (Gillette-Guyonnet et al. 2013).
In the present study, aluminum chloride (AlCl3) was used to induce AD in a rat model. The antioxidant effect and antilipid peroxidation of pomegranate extract (PE) were evaluated by measuring catalase, glutathione, total antioxidant capacity, and thiobarbituric acid reactive substances in brain homogenate. Moreover, to increase the efficacy of active components and enhance delivery to the brain, PE-loaded nanoparticles were synthesized and assessed in the rat AD model.
2. Materials and methods
2.1. Materials
AlCl3 was purchased from Sigma Aldrich (San Louis, Missouri, USA). Pomegranate - nanoparticles (PE-NPs), stearic acid, cholesterol, lecithin, and chitosan are the main components of the nanoformulations, and Tween 80 were all purchased from Sigma-Aldrich.
2.2. Animals
Seventy male adult Wistar albino rats, weighing between 200 and 250 g each, were fed Purina chow diet and allowed drinking water ad libitum. Animal were housed at animal facility of Faculty of Pharmacy, King Abdulaziz University (KAU), Jeddah, Saudi Arabia. The protocol of the current study followed the guidelines of animal care at KAU following “Principles of Laboratory Animal Care”. The study was approved by KAU ethical committee. It was performed in strict compliance with the ethical guidelines for humane treatment of animals as defined by the university ethical committee (Reference number 330-19).
2.2.1. Nanoparticle synthesis
Lecithin (150 mg), stearic acid (100 mg), cholesterol (80 mg), and pomegranate extract (PE) (40 mg) were mixed together in ethanolic solution (10 ml) and the solution was heated for 10 min at 70 °C. In another vial 2% of Tween 80 (5 ml) and 5 ml of an aqueous solution containing chitosan (25 mg) were added and then heated at about 70 °C for 10 min. In the next step, both solutions were mixed under constant magnetic stirring. Then the solution was stirred for about 30 min at 70 °C. Finally, to this solution 40 ml of water was added and the solution was sonicated in a probe sonicator for about 5 min intermittently (with an interval of 30 s). Then it underwent constant magnetic stirring for about 1 h at 70 °C in an open beaker to evaporate the ethanol. The entire sample was dialyzed for about 6–8h to remove the free PE (not encapsulated in the solid lipid nanoparticle (SLNPs) and the other impurities. The dialyzed samples were lyophilized using 5% sucrose as a cryoprotectant. Lyophilized powder was re-dispersed in DI water/PBS for further use.
2.3. Size determination
Malvern zeta sizer will be used to reveal the size distribution of the PE-NPs nanoparticles in aqueous dispersions using a ~40 mg of the lyophilized nanoparticles which will be re-suspended in 2 ml of DI water, and this solution will be placed in a clear plastic cuvette, and measured.
2.4. Entrapment/Loading efficiency
The amount of pomegranate extract found in the nanoparticles will be measured by breaking the nanoparticles open and determining the amounts of EA by using UV–Vis spectroscopy (PE; absorbance at λ 270 nm) against a standard curve using various known PE concentrations.
The entrapment efficiency will be evaluated as shown below:
Where [Drug]f is PE concentration, and [Drug]t is the theoretical level of PE (referring to all PE added initially).
The loading (w/w) will be computed by recording the total weight of the nanoformulation and evaluating the corresponding level of PE using UV/VIS spectroscopy. The overall loading of the drug in nanoparticles will be assayed as w/w.
2.4.1. Experimental design
Rats were randomly divided into seven groups (10 rats in each). The dose of PE was calculated based on the nanoparticles’ loading (0.68% w/w) per kg of rat and the calculated dose was 1.47 mg/kg body weight. Group I (control) was a negative control and received same amount of water and food. Group II (PE) received PE dissolved in water, for 14 days. Group III (PE nano) received nanoparticles loaded with PE, for 2 weeks. Group IV (AD) received AlCl3 daily as oral administration of AlCl3 at a dose of 50 mg/kg dissolved in water, for 4 successive weeks (Ouafa, and Noor 2008). Group V (AD + PE) therapeutic PE was administered for 2 weeks after AlCl3 administration was stopped. Group VI (AD + PE nano) received therapeutic PE nanoformulation for 2 weeks after AlCl3 administration was stopped. Group VII (PE + AD) received PE as protective measure for 2 weeks and then PE was stopped before AlCl3 was administered.
2.4.1.1. Novel object recognition test
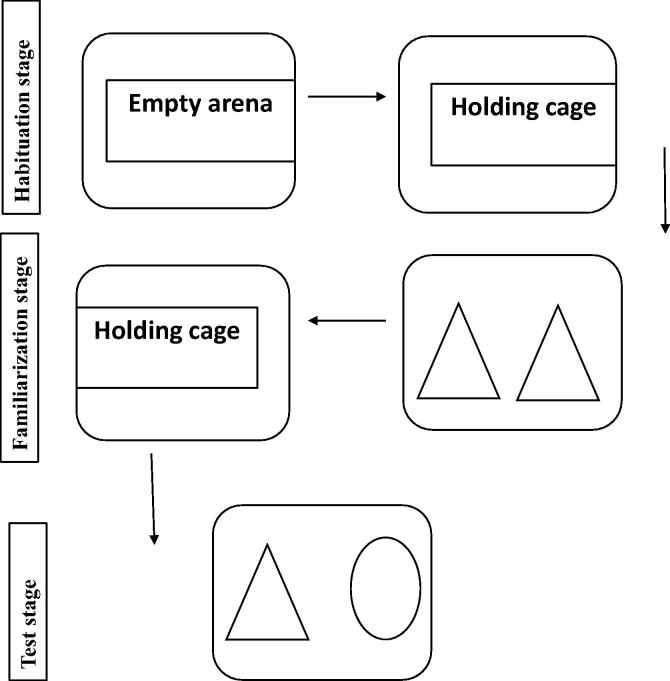
Animals in this study were subjected to the novel object recognition test before and after induction of AD and after treatment. This procedure evaluated the ability of a rat to recognize a novel object in the arena and consists of three stages: habituation, familiarization and test. In the habituation stage, each animal was allowed to explore the empty arena for 5 min and then the animal was returned to its holding cage. In the familiarization stage, two identical objects were placed in an open-field arena, and a single rat was allowed to explore them for 5 min and then returned to its holding cage. During the test stage, the rat returned to the opened-field arena with two objects of which one was the same and the other was a new object. A normal rat is expected to spend more time at the new object. The time exploring the new object was measured with Etho Vision video tracking software, version xt8 (Lueptow, 2017). The discrimination index (DI) was measured, and it indicates the variation in the exploration time and it is expressed as a proportion of the total time used to explore the two objects in a test trial.
The total exploration time was determined according to the discrimination index (DI) (Grayson et al. 2007) as shown in Fig. 1:
Fig. 1.
A diagram showing Novel object recognition test.
2.4.2. Brain tissue sampling
The experimental rats were killed at the end of the experiment after fasting for 12 h by decapitation. Immediately after that, brains from each rat were dissected. After that, isotonic saline solution was used to thoroughly wash the brains and then they were dried and their weight was recorded. After that, each brain will be sagitally split into two portions. The first portion was used for the preparation of the brain homogenate and the other one was used for histopathological examination (Kumar and Kumar 2009).
2.4.3. Biomarkers for antioxidant activity
-
1.
Estimation of catalase activity (CAT)
Catalase activity was measured according to the method of (Maehly and Chance 1954). The activity of catalase was expressed as % activity.
-
2.
Estimation of glutathione (GSH)
GSH in brain was estimated according to the method described by (Habig et al. 1974). The concentration of glutathione in the supernatant expressed as μmol per mg protein.
-
3.
Estimation of total antioxidant capacity (TAC)
The total antioxidant capacity in brain homogenate was determined by comparison with the uric acid standards using Total Antioxidant Capacity Assay Kit (ab65329) TAC Assay Cambridge, UK. The reaction was read with a standard 96-well spectrophotometric microplate reader at 490 nm.
2.4.4. Estimation of lipid peroxidation assay: Thiobarbituric acid reactive substance (TBARS)
The assay for lipid peroxidation was carried out following the protocol of OXLtek TBARS assay kit (thiobarbituric acid reactive substances) ZMC Catalog #: 0801192.
2.4.5. Histopathological examination
The second portion of each brain was fixed in formalin buffer (10%) for 24 h. The brains were washed with tap water and then dehydrated using serial dilutions of alcohol. Specimens were processed and embedded in paraffin in a hot air oven at 561C for 24 h. Paraffin bees wax blocks were sectioned at 4 μm using a microtome. The obtained tissue sections were collected on glass slides, deparaffinized, and stained with hematoxylin and eosin stains, Cresyl violet and Silver (Bancroft and Stevens, 1996). Sections were examined and photographed using Olympus light microscope (model: BX51TF- Tokoyo, Japan).
2.4.6. Statistical analysis
Data was analyzed using SPSS 22. ANOVA, followed by LSD for post hoc analysis will be applied when multiple comparisons exist. Statistical significance will be acceptable at the level of P ≤ 0.05.
3. Results
3.1. PE-nanoparticle preparation
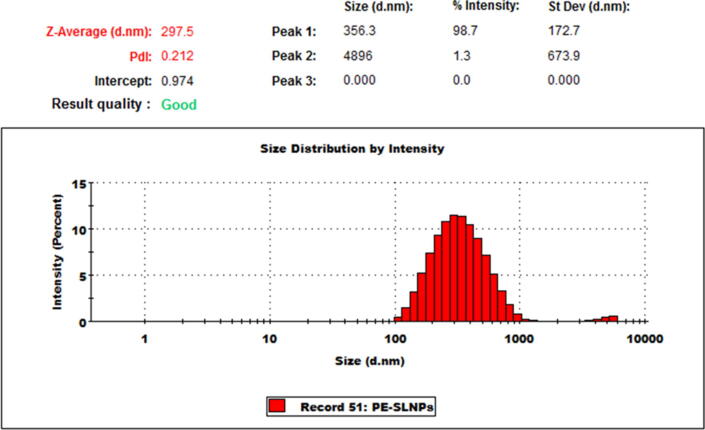
The size distribution of the PE-SLNPs is shown in Fig. 2.
Fig. 2.
Size measurement of PE-SLNPs using Dynamic Light Scattering (DLS). Average particle size is around 297 nm in diameter; PDI = 0.212. The entrapment efficiency was found to be around 45%.
The overall loading of PE in the nanoparticles was found be around 0.68% w/w. The relatively low loading efficiency is due to the presence of excessive cryoprotectant in the nanoformulations. The cryoprotectant can be removed by resuspending the nanoformulations in DI/sterile water and dialyzing (with 12 KDa cutoff membrane) for about 3 hrs as previously described (Nagla Abd El-Aziz El-Shitany, 2019).
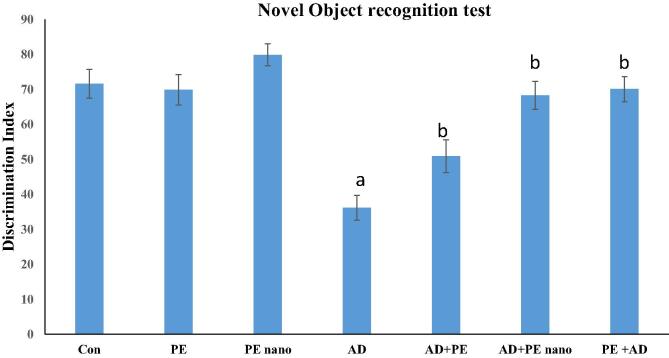
3.2. Novel object recognition test (NORT)
In the testing stage, and in comparison to the control, the DI of rats administered PE showed no significant difference while the DI of rats administered PE nano formulation increased by 11.56% more than the control, indicating a better cognitive function. On the other hand, AD mice had a significantly lower DI of 45. This is 50%, lower than the control. Treatment with PE enhanced the cognitive function and increased the DI by 40.70% while the PE nano treatment significantly increased the DI by 88.93% in comparison to the AD group. The protective role of PE was also statistically significant due to the increased time spent in recognition of the novel object and increasing the DI by 93.88% (Fig. 3).
Fig. 3.
Bar graph showing DI calculated in NORT in all groups. The one-way analysis of variance (ANOVA) test was used. When equal variance could be assumed, the Fisher’s least significant difference (LSD) t-test was applied. Data are presented as means ± standard deviation (SD). (a) Significantly different from the control, PE, PE Nano at P ≤ 0.05. (b) Significantly different AD group at P ≤ 0.05.
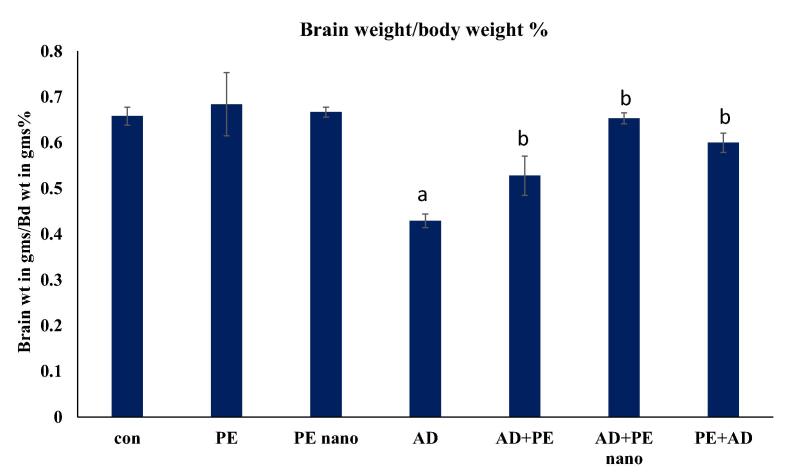
3.3. Brain weight/body weight
Brain weight/body weight percent of the AD rat model revealed a statistically significant decrease (0.429 ± 0.015) (P < 0.05) in comparison to the control (0.658 ± 0.02), while in rats treated with PE nano (0.653 ± 0.012) no significant difference was noted compared to control. Moreover, it appears that the protective effect of PE (0.600 ± 0.021) was better than its therapeutic effect (0.528 ± 0.021) in comparison to the AD model group. On the other hand, treatment with PE nano was the best treatment to regain the brain weight/body weight ratio and did not show any significant difference when compared to control (P > 0.05) (Fig. 4).
Fig. 4.
Bar graph showing brain weight /body weight percent in all groups. The one-way analysis of variance (ANOVA) test was used. When equal variance could be assumed, the Fisher’s least significant difference (LSD) t-test was applied. Data are presented as means ± standard deviation (SD). (a) Significantly different from the control, PE, PE Nano at P ≤ 0.05. (b) Significantly different AD group at P ≤ 0.05.
3.4. Biomarkers for antioxidant activity
Administration of PE nano to the AD rat model and PE as therapeutic or protective measure increased significantly the levels of CAT, GSH, and TAC (P < 0.05). Moreover, when PE nano was given to the control healthy rats, it enhanced the TAC and increased the levels of CAT and GSH in brain homogenate. Though the PE alone did not reveal any effect on the catalase level in brain homogenate, it significantly increased levels of GSH and TAC (P < 0.05) (Table1).
Table 1.
The content of antioxidant biomarkers in brain homogenate. Catalase (CAT), Glutathione (GSH) and total antioxidant capacity (TAC) in different groups.
| CAT(% activity) Mean ± SD |
GSH(µmol/mg) Mean ± SD |
TAC(CRE) Mean ± SD |
|
|---|---|---|---|
| Control | 423.42 ± 5.79 | 55 ± 3.26 | 893.71 ± 4.15 |
| PE | 403.14 ± 5.33 | 74 ± 5.59 | 1370.57 ± 3.04 |
| PE nano | 1324.14 ± 6.93 | 444.57 ± 4.35 | 4131.71 ± 4.23 |
| AD | 118.42 ± 4.50* | 25.14 ± 4.37* | 387.57 ± 5.91* |
| AD + PE | 392.42 ± 5.38** | 91.57 ± 5.38** | 936.71 ± 3.72** |
| AD + PE nano | 433.16 ± 7.78** | 120.57 ± 4.64** | 1227.14 ± 5.72** |
| PE + AD | 455.71 ± 6.71** | 108.42 ± 4.85** |
|
Groups: control, PE (pomegranate), PE nano (pomegranate nano), AD (Alzheimer rat model), AD + PE (therapeutic treatment with pomegranate), AD + PE nano (therapeutic treatment with pomegranate nanoparticle), PE + AD (protective pomegranate).
(*) indicates significant from the control, PE, PE Nano at P ≤ 0.05.
(**) indicates significant from AD group at P ≤ 0.05.
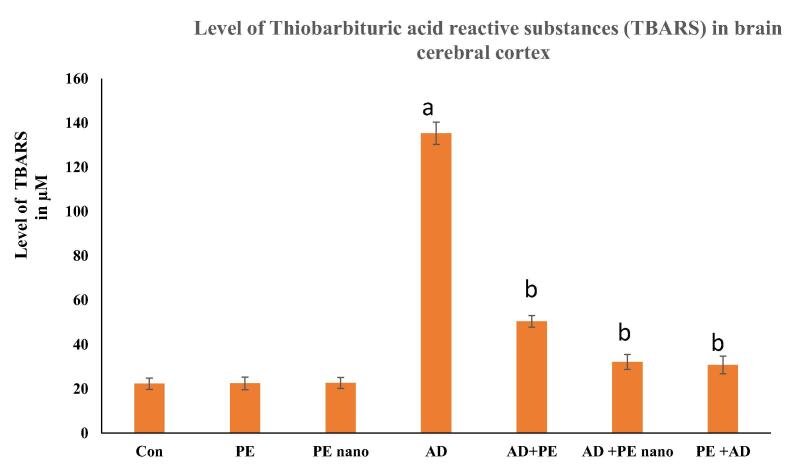
3.5. Estimation of lipid peroxidation (TBARS)
The level of TBARS resulting from lipid peroxidation in brain homogenate revealed a significant increase in AD rat models (135.28 ± 5.05) in comparison to control, PE, and PE nano groups. The levels of TBARS significantly decreased on therapeutic administration of PE by 62.72% and PE nano by 76.28%. Moreover, the protective effect of PE administered before induction of the AD model significantly decreased TBARS by 77.29% (Fig. 5).
Fig. 5.
Bar graph showing the mean concentration of TBARS µM in brain homogenate of rats of all groups. The one-way analysis of variance (ANOVA) test was used. When equal variance could be assumed, the Fisher’s least significant difference (LSD) t-test was applied. Data are presented as means ± standard deviation (SD). (a) Significantly different from the control, PE, PE Nano at P ≤ 0.05. (b) Significantly different AD group at P ≤ 0.05.
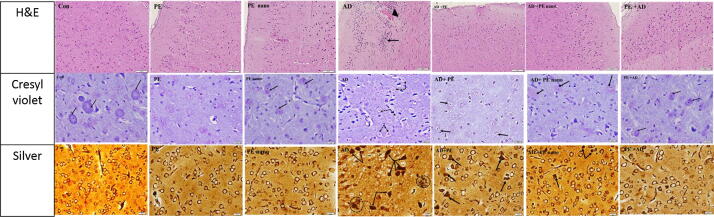
3.6. Histopathological results
H&E stained sections of the cerebral cortex of control rats revealed well organized structure in which neurons had rounded pale nuclei and basophilic cytoplasm and the neuroglial cells filled the neuropil. The structure of the cerebral cortex of rats administered PE or PE nano did not show any difference as compared to control. In case of the AD rat model, the cerebral cortex revealed marked disturbed structure with shrunken neurons and condensed pyknotic nuclei, microvacuolation of the neuropil and areas of hyaline degeneration. Treatment with PE alone or PE nano restored the disturbed structure in AD cerebral cortex and neurons regained features like the control. Similarly, the PE administered before induction of AD effectively protected the AD cerebral cortex from pathological alteration and retained characteristic features of neurons.
Using the cresyl violet stain, prominent basophilic Nissl’s granules in the cytoplasm of neurons were revealed in section of cerebral cortex of the control, PE, and PE nano groups. In sections of AD model, all neurons showed loss of Nissl’s granules and prominent chromatolysis was evident. Treatment with PE alone partially regained the granules and the use of therapeutic PE nano, or protective PE, were more effective in recovering Nissl’s granules as compared to the control section.
The silver stain was specifically used to reveal the hallmarks of AD; neurofibrillary tangles (NFT) and senile plaques (SP). Sections of cerebral cortex of the AD rat model revealed darkly stained fibrillary tangles involving the neurons and focal interneuronal spherical neuritic plaques. These characteristic features appeared to decrease in sections treated with PE nano or protected with PE and to a lesser extent in cases treated with PE only where neurofibrillary tangles were still noted (Fig. 6).
Fig. 6.
Photomicrograph of sections of cerebral cortex of rats. H&E reveals well organized structure with neurons having rounded pale nuclei and basophilic cytoplasm in Con, PE and PE Nano. Disturbed structure, shrunk neurons with condensed pyknotic nuclei (arrow) and areas of hyaline degeneration (head arrow) noted in AD. Sections of AD treated with PE and PE Nano formulation or PE + AD reveals restoration of the well-organized structure of the cortex and the neurons. The cresyl violet stain shows prominent basophilic Nissl’s granules in the cytoplasm of neurons (arrows) present in Con, PE and PE Nano formulation. In AD, chromatolysis is evident (dashed arrows). Nissle granules is restored in sections of PE Nano formulation or PE + AD but partly in AD + PE (arrows). Silver stain shows numerous NFT (arrows) and SP (circles) in AD sections. NFT (arrows) appeared to decrease in AD + PE or PE nano but disappeared in PE + AD. (H&E X200, Cresyl violet and silver X600).
4. Discussion
AlCl3 intoxication to the brain has been shown to induce an AD-like model (Stevanovic et al. 2010). In the present study, the novel object recognition test proved cognitive impairment in an AD rat model that was concomitant with positive histopathological features: NFT and SP, karyopyknosis, and chromatolysis. This was reflected by the biomarkers of antioxidation in brain homogenate where a decrease in levels of CAT, GSH, and TAC was revealed. On the other hand, the level of lipid peroxidation products (TBARS) was significantly increased. The loss of Nissl granules, a condition known as chromatolysis, results in stopping the protein synthesis in the neuron, which results in its consequent death (Steward 2000).
The NFT deposits compromise the cellular functions via the displacement of organelles and by the distortion of the spacing between microtubules. They also result in the impairment of the axonal transport thus influencing the nutrition of axon terminals and dendrites. Aβ, the main component of SP, is toxic to neurons. It results in a long-term loss of potentiation, damages synapses, and kills neurons (Nelson et al. 2009).
Hawkins and Ahmad (2016) postulated that neurons of various cellular layers in the neocortex implements a variation of a common sequence memory algorithm. And such neurons use sequence memory for different purposes (Hawkins and Ahmad 2016). Moreover, Preston and Eichenbaum (2013) explained that when encoding an experience into memory, the internally and externally perceived aspects of the experience are initially processed in the cortex and then integrated by the hippocampus into a cohesive memory (Preston and Eichenbaum 2013).
In addition, Bota et al (2015) reported that hippocampus acts as a major network hub during retrieval. Based on animal and human studies, they reported that hippocampus ultimately receives input from most regions of the brain via the entorhinal cortex (Bota et al. 2015).
Ample evidence shows that the brain of AD patients is subjected to oxidative stress and that the memory deficit is proportional to the decrease in brain and plasma antioxidant factors (Berr 2000). Moreover, lipid peroxidation disrupts membrane integrity, which enhances age-related neurodegenerative disorders (Uttara et al. 2009).
It is documented that CAT and GSH, antioxidant enzymes present in all living cells, can effectively halt H2O2 concentration in cells, which puts them as a first-line antioxidant defense enzyme (Ighodaro and Akinloye 2017).
PE, rich in antioxidants polyphenol, was evaluated as a therapeutic and protective measure on the structural and cognitive function in an AD rat model. It was also compared to a PE nanoparticle formulation. The results revealed that PE as a protective measure caused the rats to regain brain weight/body weight ratio better than its therapeutic effect while the PE-loaded nanoformulation therapeutic action revealed the best results. The protective role of PE proved to be the best in enhancing the cognitive function by 93.88%.
The brain levels of CAT, GSH, and TAC showed significant increase in cases of protective PE and the therapeutic PE nano. Moreover they alleviated the pathological hallmarks in cerebral cortex structure, restored Nissl’s granules, and decreased brain lipid peroxidation products (TBARS).
Bookheimer et al. (2013) used functional magnetic resonance imaging (fMRI) to study the effect of pomegranate juice in older human adults with mild memory complaints. Their results revealed that 8 oz of pomegranate juice taken daily over one month improved a sensitive measure of verbal memory and alter neural activity during a visual source memory task. They also confirmed that the presence of polyphenol metabolites validated compliance with the experimental regimen and efficacy of pomegranate juice in releasing polyphenols (Bookheimer et al. 2013).
Antioxidants have been proved to stop formation of Aβ deposits or modify their structure resulting in their decrease (Shoda et al. 1997). Antioxidants are likely to scavenge intracellular and extracellular superoxide radicals and H2O2 before these radicals damage cell constituents or activate microglia through their action as intracellular second messengers (Staehelin 2005).
Essa et al. (2012) indicated that the target specificity, lipophilicity, and charge of the polyphenol in natural compounds as PE is the reason why it crosses the blood brain barrier. The mechanism behind the neuroprotective action is enhancement of the expression of antioxidant enzymes and attentuation of apoptotic genes’ expressions.
It was reported that exogenous antioxidants at physiologic doses can sustain or re-establish the redox homeostasis, a vital factor in keeping sound biological systems (Bouayed and Bohn, 2010, Valko et al., 2007). However, instability and poor bioavailability of PE is a main problem associated with its use. This could be overcame by the use of nanoformulations like the ones we used in this study that protect the active ingredient within the nanoparticulate network and thus prevent its degradation, and thereby enhances its bioavailability (Li et al. 2011). It also rarely poses any toxicity to normal cells (Zhang et al. 2008). Hence, the use of nanoparticulate technology is encouraged to enhance the therapeutic effectiveness of natural agents.
Here we have shown why the protective effect of PE exceeded its therapeutic effect. Moreover, the PE nanoformulation therapeutic outcome succeeded in alleviating pathological features by elevating brain antioxidant enzymes and enhancing cognitive function in this AD rat model.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgement
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. G: 1544-140-1440. The authors, therefore, acknowledge with thanks the DSR for their technical and financial support.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohammed S. Almuhayawi, Email: msalmuhayawi@kau.edu.sa.
Wafaa S. Ramadan, Email: wramadhan@kau.edu.sa.
Steve Harakeh, Email: sharakeh@gmail.com.
Dhruba J. Bharali, Email: dhruba.bharali@acphs.edu.
Shaker A. Mousa, Email: shaker.mousa@acphs.edu.
References
- Abd El Mohsen M.M., Iravani M.M., Spencer J.P., Rose S., Fahim A.T., Motawi T.M., Ismail N.A., Jenner P. Age-associated changes in protein oxidation and proteasome activities in rat brain: modulation by antioxidants. Biochem. Biophys. Res. Commun. 2005;336(2):386–391. doi: 10.1016/j.bbrc.2005.07.201. [DOI] [PubMed] [Google Scholar]
- Aprahamian I., Stella F., Forlenza O.V. New treatment strategies for Alzheimer's disease: is there a hope? Indian J. Med. Res. 2013:449–460. [PMC free article] [PubMed] [Google Scholar]
- Arlt S., Beisiegel U., Kontush A. Lipid peroxidation in neurodegeneration: new insights into Alzheimer's disease. Curr. Opin. Lipidol. 2002;13(3):289–294. doi: 10.1097/00041433-200206000-00009. [DOI] [PubMed] [Google Scholar]
- Bancroft, J.D., Stevens, A., 1996. The haematoxylin and eosin. Theory and Practice of Histological Techniques. In, edited by Churchill Livingstone, 99-113. London, New York & Tokyo.
- Berr C. Cognitive impairment and oxidative stress in the elderly: results of epidemiological studies. BioFactors. 2000;13(1–4):205–209. doi: 10.1002/biof.5520130132. [DOI] [PubMed] [Google Scholar]
- Bookheimer S.Y., Renner B.A., Ekstrom A., Li Z., Henning S.M., Brown J.A., Jones M., Moody T., Small G.W. Pomegranate juice augments memory and FMRI activity in middle-aged and older adults with mild memory complaints. Evid. Based Complement Alternat. Med. 2013;2013:946298. doi: 10.1155/2013/946298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota M., Sporns O., Swanson L.W. Architecture of the cerebral cortical association connectome underlying cognition. Proc. Natl. Acad. Sci. USA. 2015;112(16):E2093–E2101. doi: 10.1073/pnas.1504394112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouayed J., Bohn T. Exogenous antioxidants–Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010;3(4):228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Crews L., Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer's disease. Hum. Mol. Genet. 2010;19(R1):R12–R20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essa M.M., Vijayan R.K., Castellano-Gonzalez G., Memon M.A., Braidy N., Guillemin G.J. Neuroprotective effect of natural products against Alzheimer's disease. Neurochem. Res. 2012;37(9):1829–1842. doi: 10.1007/s11064-012-0799-9. [DOI] [PubMed] [Google Scholar]
- Gillette-Guyonnet S., Secher M., Vellas B. Nutrition and neurodegeneration: epidemiological evidence and challenges for future research. Br. J. Clin. Pharmacol. 2013;75(3):738–755. doi: 10.1111/bcp.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson B., Idris N.F., Neill J.C. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav. Brain Res. 2007;184(1):31–38. doi: 10.1016/j.bbr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- Hawkins J., Ahmad S. Why neurons have thousands of synapses, a theory of sequence memory in neocortex. Front. Neural Circuits. 2016;10:23. doi: 10.3389/fncir.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2017 doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- Kumar P., Kumar A. Neuroprotective effect of cyclosporine and FK506 against 3-nitropropionic acid induced cognitive dysfunction and glutathione redox in rat: possible role of nitric oxide. Neurosci. Res. 2009;63(4):302–314. doi: 10.1016/j.neures.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Lee Chia-Jung, Chen Lih-Geeng, Liang Wen-Li, Wang Ching-Chiung. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010;118(2):315–322. doi: 10.1016/j.foodchem.2009.04.123. [DOI] [Google Scholar]
- Li Z., Percival S.S., Bonard S., Gu L. Fabrication of nanoparticles using partially purified pomegranate ellagitannins and gelatin and their apoptotic effects. Mol. Nutr. Food Res. 2011;55(7):1096–1103. doi: 10.1002/mnfr.201000528. [DOI] [PubMed] [Google Scholar]
- Lueptow, L.M., 2017. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. (126). doi: 10.3791/55718. [DOI] [PMC free article] [PubMed]
- Maehly A.C., Chance B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- El-Shitany Nagla Abd El-Aziz, Abbas Aymn Tallat, Ali Soad Shaker, Eid Basma, Harakeh Steve, Neamatallah Thikryat, Al-Abd Ahmed, Mousa Shaker. Nanoparticles ellagic acid protects against cisplatin-induced hepatotoxicity in rats without inhibiting its cytotoxic activity - SciAlert responsive version. Int. J. Pharmacol. 2019;15:465–477. doi: 10.3923/ijp.2019.465.477. [DOI] [Google Scholar]
- Nelson P.T., Braak H., Markesbery W.R. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J. Neuropathol. Exp. Neurol. 2009;68(1):1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston A.R., Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 2013;23(17):R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Morris J.C. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann. Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Sayre L.M., Perry G., Smith M.A. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2008;21(1):172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- Shoda H., Miyata S., Liu B.F., Yamada H., Ohara T., Suzuki K., Oimomi M., Kasuga M. Inhibitory effects of tenilsetam on the Maillard reaction. Endocrinology. 1997;138(5):1886–1892. doi: 10.1210/endo.138.5.5151. [DOI] [PubMed] [Google Scholar]
- Staehelin H.B. Micronutrients and Alzheimer's disease. Proc. Nutr. Soc. 2005;64(4):565–570. doi: 10.1079/pns2005459. [DOI] [PubMed] [Google Scholar]
- Stevanovic I.D., Jovanovic M.D., Colic M., Jelenkovic A., Bokonjic D., Ninkovic M. Nitric oxide synthase inhibitors protect cholinergic neurons against AlCl3 excitotoxicity in the rat brain. Brain Res. Bull. 2010;81(6):641–646. doi: 10.1016/j.brainresbull.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Steward, 2000. Functional Neuroscience. Edited by Springer –Verlag. Vol. 73. New York.
- Temple, Norman J., 2000. Antioxidants and disease: more questions than answers. 20:449–459. doi: http://hdl.handle.net/2149/1192.
- Uttara B., Singh A.V., Zamboni P., Mahajan R. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M., Fernandez-Lopez J., Perez-Alvarez J.A. Pomegranate and its many functional components as related to human health: a review. Compr. Rev. Food Sci. Food Saf. 2010;9(6):635–654. doi: 10.1111/j.1541-4337.2010.00131.x. [DOI] [PubMed] [Google Scholar]
- Xie Y., Morikawa T., Ninomiya K., Imura K., Muraoka O., Yuan D., Yoshikawa M. Medicinal flowers. XXIII. New taraxastane-type triterpene, punicanolic acid, with tumor necrosis factor-alpha inhibitory activity from the flowers of Punica granatum. Chem. Pharm. Bull. (Tokyo) 2008;56(11):1628–1631. doi: 10.1248/cpb.56.1628. [DOI] [PubMed] [Google Scholar]
- Zarei M., Azizi M., Bashir-Sadr Z. Evaluation of physicochemical characteristics of pomegranate (Punica granatum L.) fruit during ripening | Fruits | Cambridge Core. Fruits. 2011;66(2):121–129. doi: 10.1051/fruits/2011021. [DOI] [Google Scholar]
- Zhang L., Gu F.X., Chan J.M., Wang A.Z., Langer R.S., Farokhzad O.C. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]