Graphical abstract
Keywords: Bassia muricata, Volatile oils, Hexahydrofarnesyl acetone, Bioherbicides, Phytotoxicity
Abstract
The essential oil (EO) of Bassia muricata shoots was extracted via hydro-distillation and then investigated by gas chromatography-mass spectrometry. Thirty-four compounds were recognized for the first time from this plant, representing 100% of the total mass. Terpenoids represented the major components with 69.17% of the total mass, containing oxygenated sesquiterpenes (53.18%), oxygenated monoterpenes (9.77%), sesquiterpene hydrocarbons (5.03%), and diterpenes (1.19%). Additionaly, 6-methoxy-1-acetonaphthone was the only aromatic compound represented in a high percentage of the total identified compounds with 22.35%. Additionally, a percent of 8.48% of the total mass was hydrocarbons. Only one oxygenated sesquiterpene namely hexahydrofarnesyl acetone representing 47.35% of the total mass was identified. It was followed by methoxy-1-acetonaphthone (19.92%), n-dotriacontane (3.58%), endo-borneol (3.24%), 6-methy-α-ionone (3.04%), and α-gurjunene (2.65%). The EO exhibited moderate antioxidant activity comparable with ascorbic acid as a standard, where it attained IC50 value of 20.70 µL L−1 and 16.32 µL L−1, for DPPH and ABTS. The EO of B. muricata significantly reduces the germination and seedling development of the weed Chenopodium murale. The EO showed an IC50 value of 175.60 µL L−1, 246.65 µL L−1, and 308.33 µL L−1 for root growth, shoot growth, and germination, respectively. Therefore, this EO could be a good green resource for the control of weeds.
1. Introduction
By the time the human population increases, and thereby, the human needs for food, feed and medicines become greater than before. In this context, using natural eco-friendly resources from plants attract the attention of researcher and scientists all over the world. Essential oils (EOs) are one of the important enriched resources among different bioactive metabolites. They have been used in ancient Egypt for remediation of various diseases. The EOs have been reported to have several biological activities such as antifungal, antiviral, antibacterial (Perricone et al., 2015, Elshamy et al., 2019b), antioxidant, allelopathic, and insecticidal activities (Abd El-Gawad, 2016, Abd El-Gawad et al., 2016, Abd El-Gawad et al., 2018b, Abd El-Gawad et al., 2018a, Abd El-Gawad et al., 2019, Elshamy et al., 2019b). In addition, the EOs are intergraded in food preservation industries, aromatherapy, fragrance industries, pharmaceutical, cosmetic, and agricultural industries (Ali et al., 2015, Zeng et al., 2015).
EOs as natural antioxidants attract the attention of researchers and scientists worldwide, and many studies incorporated the EOs as an eco-friendly alternative for the synthetic antioxidants. Moreover, the EOs are considered as an important class of the allelopathy agents as they are very diverse, biodegradable and have low resistance from organisms (Dudai et al., 1999, Abd El-Gawad et al., 2018b, Abd El-Gawad et al., 2019). Allelopathy is a phenomenon where a plant releases chemical compounds; secondary metabolites called allelochemicals to its environment that possess stimulatory or inhibitory activity against other organisms (Rice, 1984). The EOs as allelopathy agents play different roles where they act as a chemical defense mechanism against pathogens and herbivorous organism as well as they play a specific role in plant signaling due to their dispersal and aroma characteristics (Kessler and Baldwin, 2001).
Bassia muricata (L.) Asch. is an annual plant of Chenopodiaceae family, Syns. Salsola muricata L. or Kochia muricata (L.) Schrad. It is growing in sandy habitats in North Africa, East Mediterranean region, Sinai, Saudi Arabia, and Iran (Boulos, 1999). It is one of the important plants that used in traditional medicine where it used as analgesic, antipyretic, and nephritic as well as it has several biological activities such as antioxidant, antibacterial (Chemsa et al., 2016) molluscicidal, (El-Sayed, 1993) and insecticidal activities (El-Sayed et al., 1998). The extract of B. muricata has been reported to reduce the white blood cells counts, increase the prothrombin time as well as to have hypotension effect and antimicrobial activities (Al-Yahya et al., 1990, Chemsa et al., 2016). Some phytochemical constituents were isolated and identified from B. muricata such as flavonoid glycosides, (El-Sayed et al., 1998, El Hossary et al., 2000, Kamel et al., 2001, Shaker et al., 2013) saponins, (El-Sayed, 1993) and phenolic compounds (Shaker et al., 2013). Up to our knowledge, there is not any report concerning the EO of B. muricata or their bioactivity. Therefore, the present study aimed to (i) determine of the chemical composition of the EO from B. muricata shoots (ii) evaluate the antioxidant activity of its EO, and (iii) determine its allelopathic effect against the weed, Chenopodium murale.
2. Materials and methods
2.1. Plant material
Healthy shoots of B. muricata were collected throughout the flowering stage in March 2017 from sandy habitat in Gamsa City, northern Egypt (31°30′14.1″N 31°21′43.9″E). The plant was identified according to Boulos (1999) by Dr. Ahmed Abd-ElGawad, Associate Professor of Plant Ecology, Botany Dept, Mansoura University, Egypt. A voucher specimen with code Mans.030213002 was dropped in the Faculty of Science herbarium, Mansoura University, Egypt.
2.2. Extraction, GC–MS analysis, and identification of the EO
The EO (0.01% w/w) was extracted from 300 g of air-dried shoots of B. muricata through hydrodistillation for three hours via a Clevenger-type apparatus. The oil layer was separated and dried with 0.5 g of anhydrous sodium sulfate. This extraction was repeated two times using two samples and collected oils were stored in sealed air-tight glass vials at 4 °C until further GC–MS analysis.
The samples of EOs were analysed by GC–MS analysis using gas chromatography-mass spectrometry instrument at the Medicinal and Aromatic Plants Research Department, National Research Center of Egypt. The details of the GC–MS analysis and identification of chemical compounds were mentioned in detail in our previously work (Abd El-Gawad et al., 2019).
2.3. Antioxidant activity of the EO
2.3.1. DPPH radical scavenging activity
The reduction in the color of stable radical, 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma-Aldrich, Germany) by the EO was determined as scavenging activity following the methods of Miguel (2010) and as described in (Abd El-Gawad et al., 2019).
2.3.2. ABTS-free radical scavenging activity
To confirm the antioxidant activity of the B. muricata EO, the ability of the EO to scavenge the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical (Sigma-Aldrich, Germany) was determined according to Re et al. (1999) and as described in our reported work (Abd El-Gawad et al., 2019).
2.4. Allelopathic activity of the EO
The allelopathic activity of the extracted EO from B. muricata was tested against the germination and seedling growth of the weed C. murale. The seeds of this weed were harvested from the garden of Agriculture College, Mansoura University, Egypt (31°02′35.4″N 31°21′01.5″E). Homogenous seeds with uniform color and size were selected and sterilized using 0.3% sodium hypochlorite.
Concentrations of 25, 50, 100, 150, 200, and 250 µL L−1 of the EO were prepared using 1% (v/v) Tween®80 (Sigma-Aldrich, Germany), as an emulsifying agent. To test the allelopathic activity, 20 seeds were moved to a sterilized Petri plate (90 mm) lined with filter papers (Whatman No. 1). Subsequently, 4 mL of each concentration of the EO was poured to the plates, and sealed by a Parafilm® tape (Sigma, USA) and kept at 25 °C in a growth chamber. (Abd El-Gawad et al., 2016) Control plates were prepared as previously mentioned but using Tween 1% alone instead of EO. After eight days of incubation, the germinated seeds were counted and the lengths of the root and shoot were measured. The inhibition of seed germination or seedling growth was calculated as follows:
2.5. Statistical analysis
Bioassay of the allelopathic activity of EO of B. muricata was conducted in a completely randomized design, and the experiment was repeated two times with five replicates per each treatment. The data were subjected to one-way ANOVA, followed by Duncan's test at the probability level of 0.05. The data from the antioxidant experiment in triplicates was also subjected to one-way ANOVA using CoStat software program (CoHort Software, Monterey, CA, USA).
3. Results and discussion
3.1. Chemical composition of the EO
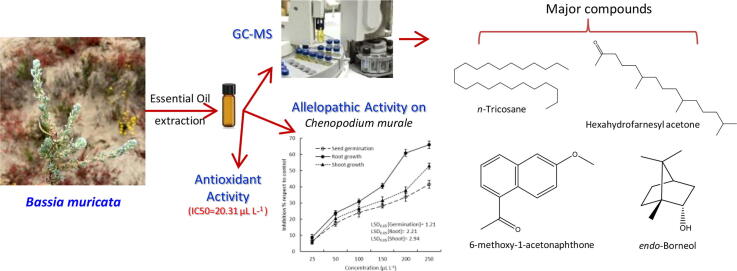
The study of EO from Bassia plant species around overall the world is rare. For the first time, the EO of the aerial parts of B. muricata collected from Egypt was isolated by hydro-distillation using a Clevenger-type apparatus for three hours. The EO was analyzed by GC–MS (Fig. 1). Thirty-four compounds were identified representing 100% of the total mass. The identified compounds based on GC–MS analysis showed that the EO of this plant is very rich in terpenoid compounds, which attained 69.17% of the total identified compounds (Table 1).
Fig. 1.
GC–MS chromatogram of the essential oil of Bassia muricata areial parts.
Table 1.
Essential oil constituents extracted from the aerial parts of Bassia muricata.
| No | RT[a] | KILit[b] | KIExp[c] | Compound Name | Conc. (%)[d] | Identification[e] |
|---|---|---|---|---|---|---|
| Oxygenated monoterpenes | ||||||
| 1 | 11.66 | 1146 | 1148 | 2-Bornanone | 1.60 ± 0.02 | KI, MS |
| 2 | 12.61 | 1139 | 1140 | endo-Borneol | 3.24 ± 0.02 | KI, MS |
| 3 | 21.22 | 1351 | 1354 | α-Damascenone | 1.08 ± 0.01 | KI, MS |
| 4 | 31.87 | 1480 | 1477 | Methy-α-Ionone | 3.04 ± 0.03 | KI, MS |
| 5 | 28.00 | 1142 | 1146 | P-Menth-3-en-8-ol | 0.81 ± 0.01 | KI, MS |
| Sesquiterpene hydrocarbons | ||||||
| 6 | 20.36 | 1363 | 1359 | Cyclosativene | 0.16 ± 0.02 | KI, MS |
| 7 | 20.68 | 1377 | 1372 | α-Copaene | 1.19 ± 0.02 | KI, MS |
| 8 | 21.80 | 1408 | 1411 | α-Gurjunene | 2.65 ± 0.04 | KI, MS |
| 9 | 24.25 | 1460 | 1457 | Rotundene | 0.43 ± 0.01 | KI, MS |
| 10 | 26.88 | 1517 | 1495 | trans-calamenene | 0.34 ± 0.01 | KI, MS |
| 11 | 25.84 | 1467 | 1474 | α-Muurolene | 0.26 ± 0.02 | KI, MS |
| Oxygenated sesquiterpenes | ||||||
| 12 | 26.32 | 1687 | 1683 | Nerolidyl acetate | 1.36 ± 0.04 | KI, MS |
| 13 | 32.11 | 1645 | 1646 | Torreyol | 0.41 ± 0.01 | KI, MS |
| 14 | 32.85 | 1876 | 1879 | Longifolenaldehyde | 0.57 ± 0.03 | KI, MS |
| 15 | 29.15 | 1580 | 1544 | Caryophyllene oxide | 0.30 ± 0.02 | KI, MS |
| 16 | 29.03 | 1608 | 1591 | Spathulenol | 0.21 ± 0.01 | KI, MS |
| 17 | 35.36 | 1631 | 1602 | Alloaromadendrene oxide-2 | 0.35 ± 0.03 | KI, MS |
| 18 | 35.70 | 1675 | 1681 | Z-α-Bisabolene epoxide | 0.52 ± 0.02 | KI, MS |
| 19 | 36.30 | 1749 | 1753 | Driminol | 1.11 ± 0.02 | KI, MS |
| 20 | 36.71 | 1603 | 1608 | Longiborneol | 0.26 ± 0.03 | KI, MS |
| 21 | 38.62 | 1845 | 1846 | Hexahydrofarnesyl acetone | 47.35 ± 0.05 | KI, MS |
| 22 | 39.61 | 2005 | 2045.9 | Isochiapin B | 0.74 ± 0.03 | KI, MS |
| Diterpenes | ||||||
| 23 | 40.26 | 1811 | 1810 | Phytan | 1.19 ± 0.02 | KI, MS |
| Aromatics | ||||||
| 24 | 30.86 | 1621 | 1618 | 6-methoxy-1-acetonaphthone | 19.92 ± 0.06 | KI, MS |
| 25 | 26.58 | – | 1669 | 4-(2′,3′,4′-Trimethylphenyl)-but-3(E)-en-2-one | 2.00 ± 0.02 | KI, MS |
| 26 | 28.81 | 1237 | 1241 | Chavicol | 0.43 ± 0.03 | KI, MS |
| Non-terpene compounds | ||||||
| 27 | 24.16 | 1555 | 1550 | 2,6,10-Trimethyltetradecane | 0.64 ± 0.02 | KI, MS |
| 28 | 25.02 | 1993 | 1995 | 10-Octadecenal | 0.92 ± 0.03 | KI, MS |
| 29 | 33.02 | 2100 | 2098 | n-Heneicosane | 0.89 ± 0.02 | KI, MS |
| 30 | 37.08 | 2200 | 2198 | n-Docosane | 0.35 ± 0.05 | KI, MS |
| 31 | 42.03 | 2300 | 2300 | n-Tricosane | 0.33 ± 0.01 | KI, MS |
| 32 | 46.81 | 2700 | 2703 | n-Heptacosane | 0.67 ± 0.04 | KI, MS |
| 33 | 48.00 | – | 2872 | 13-Heptadecyn-1-ol | 1.10 ± 0.02 | KI, MS |
| 34 | 50.08 | 3200 | 3203 | n-Dotriacontane | 3.58 ± 0.03 | KI, MS |
[a] retention time, [b] Kovats retention index from literature reviews, [c] Experimental Kovates retention index, [d] Values are mean ± SD, and [e] The identification of essential oil components was established depending upon the mass spectral data of compounds (MS) and Kovats indices (RI) with those of Wiley spectral library collection and NIST (he National Institute of Standards and Technology) library databases.
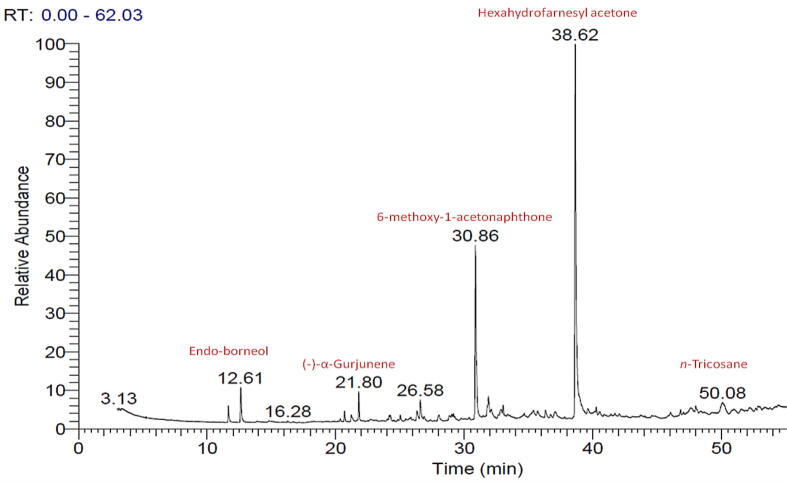
The aerial parts EO of B. muricata was characterized by its richness in terpenoids including sesquiterpenes (58.21%), monoterpenes (9.77%), and diterpenes (1.19%) as well as considerable concentrations of aromatic compounds (21.92%) and hydrocarbons (8.91) (Fig. 2). Terpenoids, especially mono and sesquiterpenes as well as rarely diterpenoids, were reported as the major constituents of EOs of most of the plants. (Abd El-Gawad et al., 2019, Elshamy et al., 2019b)
Fig. 2.
Essential oil classes from Bassia muricata aerial parts.
The sesquiterpenoids were the major components of B. muricata EO of which the oxygenated sesquiterpenes constituted 53.18%, and sesquiterpene hydrocarbons were 5.03%. Hexahydrofarnesyl acetone was the main oxygenated sesquiterpenes (47.34%) followed by nerolidyl acetate (1.36%) and driminol (1.11%), while longiborneol represented the minor compound (0.26%). Hexahydrofarnesyl acetone was documented and deduced as widely distributed and preponderance compound in EOs of several plants such as several plants belonging to, Launaea, (Elshamy et al., 2019a) Phlmis, (Liolios et al., 2007) Cyperus, (Nassar et al., 2015) and other genera. The EO composition of the present study is more or less similar to that for Bassia indica EO, where the later is also rich in the terpenoids. (El-Shamy et al., 2012) However, the major compound in the EO of B. indica was α-thujaplicin.
Monoterpenes were the second major terpenoid components with a concentration of 9.77% from the total. All the identified monoterpenes were characterized as oxygenated type. Endo-borneol (3.24%) and 6-methyl-α-ionone (3.04%) represented the main oxygenated monoterpene by around 64.27% from overall identified monoterpenes. Otherwise, p-menth-3-en-8-ol was characterized as the minor one. The diterpenes are considered as rare compounds in the EOs of most plants, while few plants have high content of diterpenes such as Lactuca serriola, (Abd-ElGawad et al., 2019b) Araucaria cunninghamii, and Araucaria heterophylla. (Verma et al., 2014) In our study, diterpenes were represented as a minor class of compounds (1.19%). Only one very common diterpene hydrocarbon, phytan, was characterized. (Liolios et al., 2007, Abd El-Gawad et al., 2019, Elshamy et al., 2019a) In this context, El-Shamy et al. (2012) reported the presence of phytan with comparable content (1.22%) in the EO of B. indica.
The aromatic compounds were also characterized in the EO of B. muricata with fairy concentration (22.35%) and they are represented by two compounds; 6-methoxy-1-acetonaphthone (19.92%) and 4-(2′,3′,4′-Trimethylphenyl)-but-3(E)-en-2-one (2.0%). Additionally, nine compounds were identified as hydrocarbons with a percentage of 8.48% from the total mass in which n-dotriacontane (3.58%), and 13-heptadecyn-1-ol (1.10%) were the majors, while n-tricosane was a minor (Table 1).
3.2. Antioxidant activity of B. muricata EO
The EO of the aerial parts of B. muricata exhibited considerable antioxidant activity comparable with ascorbic acid as a standard reference, where it attained an IC50 value of 20.70 µL L−1 and 16.32 µL L−1 for DPPH and ABTS, respectively (Table 2). The antioxidant activity of the present EO was higher than those reported for the EOs of other plants such as Xanthium strumarium, (Abd El-Gawad et al., 2019) Cleome droserifolia, (Abd El-Gawad et al., 2018b) Cullen plicata, (Abd El-Gawad, 2016) and Euphorbia heterophylla. (Elshamy et al., 2019b)
Table 2.
Scavenging activity of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) as well as the IC50 values by Bassia muricata essential oil.
| Treatment | Concentration (µL L−1) | DPPH |
ABTS |
||
|---|---|---|---|---|---|
| Scavenging (%)* | IC50 (µL L−1) | Scavenging (%) | IC50 (µL L−1) | ||
| Bassia muricata (Essential oil) | 5 | 2.89 ± 0.11E | 20.70 | 4.95 ± 0.19E | 16.32 |
| 10 | 24.62 ± 1.57D | 30.46 ± 1.22D | |||
| 15 | 31.09 ± 1.84C | 48.65 ± 1.53C | |||
| 20 | 50.34 ± 2.00B | 65.04 ± 1.05B | |||
| 25 | 63.53 ± 2.01A | 74.11 ± 1.98A | |||
| Ascorbic acid | 0.40 | 0.33 | |||
Values are means of triplicate ± SE, IC50: the concentration of the sample that required to reduce the DPPH or ABTS absorbance by 50%. Different superscript letters within the column means values significant variation at p ≤ 0.05.
The antioxidant activity may be correlated to the high percentage of sesquiterpenoids, especially oxygenated ones (53.18%). The oxygenated sesquiterpenoids were reported to have radical scavenging activity. (Barbieri et al., 2016, Abd El-Gawad et al., 2019) The free hydroxy groups in the oxygenated sesquiterpenoids were documented to play a significant role to increase antioxidant abilities due to the highly donating of hydrogen atoms. (Ruberto and Baratta, 2000). Hexahydrofarnesyl acetone, with around 50%from the total mass in our study, might act a leading antioxidant agent as described in previous studies. As example, EOs derived from Heliotropium curassavicum (Abd-ElGawad et al., 2019a), Launaea mucronata, and Launaea nudicaulis (Elshamy et al., 2019a) were described to have significant antioxidant activities due to the aboundance of hexahydrofarnesyl acetone. Also, the EO of B. muricata consisted of a high percentage of aromatics especially phenolics. The phenolic compounds were deduced to be promising antioxidant agents especially with the presence of free hydroxyl groups that have highly donating of hydrogen atoms. (El-Kashak et al., 2017) Many reports described the high impact of the phenolic compounds as antioxidant agents (Hammad et al., 2013). However, no data for the antioxidant activity of 6-methoxy-1-acetonaphthone are reported till now, thus further study is recommended to evaluate the biological activity of this aromatic compound. Furthermore, the synergetic effect of the interaction of these significant factors that increased by increasing the concentrations of these oxygenated sesquiterpenes and phenolic compounds could briefly play an important role as antioxidant agents.
3.3. Allelopathic activity of B. muricata EO
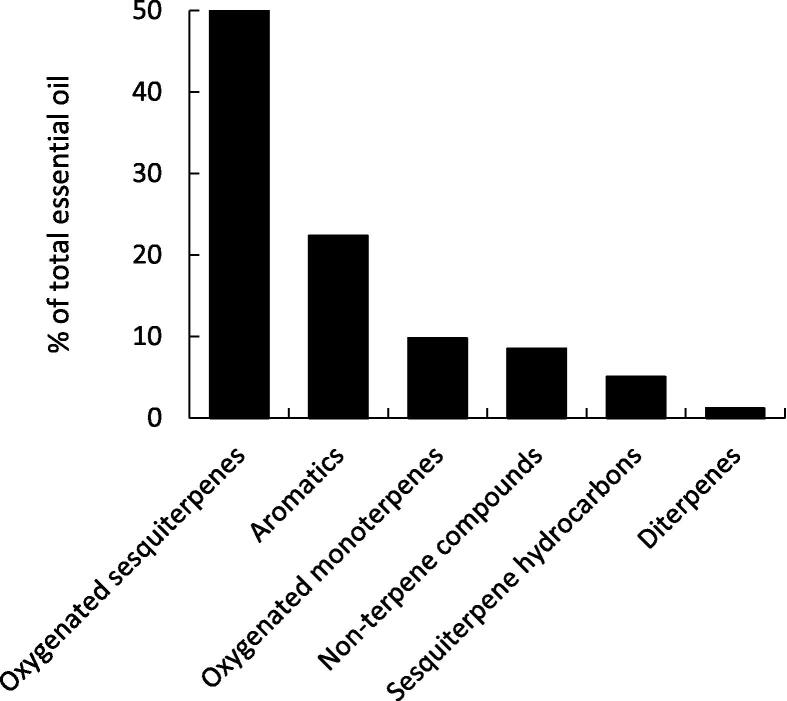
The EO from the aerial parts of B. muricata exhibited significant phytotoxic effect (P ≤ 0.05) on both seed germination and seedling growth of Chenopodium murale in a dose-dependent manner (Fig. 3). However, the seedling growth was more affected than the seed germination. At high concentration of the EO (250 µL L−1), the germination of seeds was inhibited by 41.51% compared to control, while the shoots and roots were reduced by 52.89% and 65.98%, respectively (Fig. 3). The EO attained IC50 value of 175.60 µL L−1, 246.65 µL L−1, and 308.33 µL L−1 for root growth, shoot growth, and germination, respectively. By comparison of the inhibitory activity of the EO with common herbicides, the 2,4-dichlorophenoxyacetic acid (2, 4-D), 2,4,5-trichlorophenoxyacetic acid (2, 4, 5-T), and 2-methyl-4-chlorophenoxyacetic acid (MCPA) were reported to inhibited the seed germination of C. murale by 78.82%, 44.71% and 35.29%, respectively at concentration of 250 ppm. (Agarwal, 2009). This means that the activity of the EO from B. muricata showed a comparable effect to that for the synthetic herbicide 2, 4, 5-T and higher activity than MCPA. It is worth mentioning herer that C. murale is considered as winrer weed in the cultivated crops as well as the roadsides habitat. It is imported to the cultivated land as contaminants with the seeds and it has cabability to compete with the crops via allelopathy (Al-Johani et al., 2012).
Fig. 3.
Inhibitory effect of different concentrations of the essential oil from the aerial parts of Bassia muricata on the germination, root, and shoot growth of Chenopodium murale.
The roots of C. murale were more sensitive to the phytotoxic effect of the EO than shoots. This sensitivity could be correlated to the direct contact with the allelochemicals (EO) as well as the membrane permeability of the root cells. (Abd El-Gawad, 2014, El-Shora and Abd El-Gawad, 2015a, El-Shora and Abd El-Gawad, 2015b, Abd El-Gawad, 2016, Abd El-Gawad et al., 2019)
The EOs from various plants were tested against the weed C. murale such as Tagetes minuta (Arora et al., 2017) and Cleome droserifolia. (Abd El-Gawad et al., 2018b) The EO from C. droserifolia attained the IC50 value of 157.5, 147.1, and 169.4 µL L−1, for the germination, root growth, shoot growth of C. murale, respectively. (Abd El-Gawad et al., 2018b) However, the EO from T. minuta showed less effective germination inhibition on the C. murale, where it attained an IC50 value of 490 µL L−1.
The oxygenated terpenes are considered to be more biologically active than non-oxygenated ones. (Dambolena et al., 2016) In the present study, the majority of compounds are oxygenated, so this could explain the activity of our EO. The hexahydrofarnesyl acetone was reported as predominant compounds of the EOs from Hildegardia barteri, (Balogun et al., 2017) Equisetum arvense, (Radulović et al., 2006) Geranium columbinum, (Radulovic et al., 2011) Tilia tomentosa, (Fitsiou et al., 2007) Ecballium elaterium, (Razavi and Nejad-Ebrahimi, 2010) Sagittaria trifolia, (Xiangwei et al., 2006) Leontopodium longifolium, (Qian et al., 2018) Phlomis bucharica, P. salicifolia and P. sewerzowii. (Mamadalieva et al., 2019) In addition, the EOs from these plants exhibited antimicrobial, (Xiangwei et al., 2006, Radulovic et al., 2011, Balogun et al., 2017, Mamadalieva et al., 2019) antioxidant, (Qian et al., 2018) and allelopathic activities. (Razavi and Nejad-Ebrahimi, 2010)
The amount of hexahydrofarnesyl acetone our EO was higher (47.35%) than all these plants (11.0-19.1%), except for S. trifolia, which comprises 62.3%. Thereby, the allelopathic activity of B. muricata EO could be ascribed to this major compound. However, the allelopathic activity of the other major compound 6-methoxy-1-acetonaphthone still is not known.
Hexahydrofarnesyl acetone is a very common identified sesquiterpene in EOs derived from numerous plant species. This compound was reported as major compound in EOs of several plants such as Atriplex cana, (Wei et al., 2019), Launaea lanifera, (Abd El-Gawad and El-Shora, 2017), Cyperus leavigatus, (Nassar et al., 2015) Trianthema portulacastrum, (Abd El-Gawad et al., 2016) Launaea mucronata and Launaea nudicaulis, (Verma et al., 2014). Elshamy et al. (2019a) ascribed the phytotoxic activity of the EO from Launea species to hexahydrofarnesyl acetone as major compound. In addition, Wei et al. (2019) deduced that the significant phytotoxic activity of Atriplex cana EO was correlated with the major compounds especially hexahydrofarnesyl acetone.
On the other hand, C. murale has been reported to have various allelochemicals such as benzoic acid, p-coumaric acid, ferulic acid, and vanillic acid. (Batish et al., 2007b) C. murale is a noxious weed as it interferes negatively with wheat, (Batish et al., 2007a, Majeed et al., 2012) barley, (Al-Johani et al., 2012) rice, (Alam and Shaikh, 2007) pea, and chickpea (Batish et al., 2007b) through its allelopathic activity.
Summing up, our results revealed promising allelopathic activity against weeds, at least the noxious weed C. murale compared to other EOs reported from other wild plants. Therefore, the EO from B. muricata aerial parts could be integrated into the control tools of the C. murale as green, biodegradable, and eco-friendly resource.
4. Conclusions
The very rich EO with terpenoids from the aerial parts of B. muricata was found to have 34 compounds. Sesquiterpenes represented the major class (58.21%) with aboundance of hexahydrofarnesyl acetone, 6-methoxy-1-acetonaphthone, n-tricosane, endo-borneol, and methyl-α-ionone. The EO of B. muricata exhibited considerable antioxidant activity; thus, it could be used as a green antioxidant. The present study revealed that EO from B. muricata has potential allelopathic activity against the weed C. murale. Therefore, this EO could be a promising food supplement and natural, green, eco-friendly resource to control this noxious weed. However, further studies are required to separate the chemical compounds from the EO of B. muricata, and evaluate their allelopathic activities individually or in combination against C. murale or maybe other weeds, and evaluate its valuable economic use on a large scale.
Author contributions
Conceptualization, A.A-E. and A.E.; Data curation, A.A-E. and A.E.; Formal analysis, A.A-E., A.E-G., A.G. and A.E.; Investigation, A.A-E., Y.E., A.G. and A.E.; Methodology, A.A-E., A.E-G. and Y.E.; Resources, A.A-E. and A.E.; Writing – original draft, A.A-E., Y.E. and A.E.; Writing – review & editing, A.A-E., S.O., S.A-R., A.A., Y.D., and A.E.
Acknowledgments
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, Saudi Arabia for funding this work through research group No (RG-1441-302). Further, the authors like to sincerely thanks the National Research Centre, and Department of Botany, Faculty of Science, Mansoura University, Egypt.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd-ElGawad A.M., Elshamy A.I., Al-Rowaily S.L., El-Amier Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants. 2019;7:482. doi: 10.3390/plants8110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-ElGawad A.M., Elshamy A., El Gendy A.G., Al-Rowaily S.L., Assaeed A.M. Preponderance of oxygenated sesquiterpenes and diterpenes in the volatile oil constituents of Lactuca serriola L. revealed antioxidant and allelopathic activity. Chem. Biodivers. 2019;16:e1900278. doi: 10.1002/cbdv.201900278. [DOI] [PubMed] [Google Scholar]
- Abd El-Gawad A.M. Ecology and allelopathic control of Brassica tournefortii in reclaimed areas of the Nile Delta. Egypt. Turk. J. Bot. 2014;38:347–357. [Google Scholar]
- Abd El-Gawad A.M. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crops Prod. 2016;80:36–41. [Google Scholar]
- Abd El-Gawad A.M., El-Amier Y.A., Bonanomi G. Allelopathic activity and chemical composition of Rhynchosia minima (L.) DC. essential oil from Egypt. Chem. Biodivers. 2018;15:e1700438. doi: 10.1002/cbdv.201700438. [DOI] [PubMed] [Google Scholar]
- Abd El-Gawad A.M., El-Amier Y.A., Bonanomi G. Essential oil composition, antioxidant and allelopathic activities of Cleome droserifolia (Forssk.) Delile. Chem. Biodivers. 2018;15:e1800392. doi: 10.1002/cbdv.201800392. [DOI] [PubMed] [Google Scholar]
- Abd El-Gawad A.M., El-Shora H.M. Assessment of allelopathic potential of Hyoscyamus muticus L. on antioxidant system and nucleic acids of purslane. Fresen. Environ. Bull. 2017;36:2147–2155. [Google Scholar]
- Abd El-Gawad A.M., El Gendy A.G., Elshamy A.I., Omer E.A. Chemical composition of the essential oil of Trianthema portulacastrum L. Aerial parts and potential antimicrobial and phytotoxic activities of its extract. J. Essent. Oil Bear. Plants. 2016;19:1684–1692. [Google Scholar]
- Abd El-Gawad A.M., Elshamy A.I., El Gendy A.G., Gaara A., Assaeed A.M. Volatiles profiling, allelopathic activity, and antioxidant potentiality of Xanthium strumarium leaves essential oil from Egypt: Evidence from chemometrics analysis. Molecules. 2019;24:584. doi: 10.3390/molecules24030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S.K. APH Publishing; New Delhi: 2009. Pesticide Pollution. [Google Scholar]
- Al-Johani N.S., Aytah A.A., Boutraa T. Allelopathic impact of two weeds, Chenopodium murale and Malva parviflora on growth and photosynthesis of barley (Hordeum vulgare L.) Pak. J. Bot. 2012;44:1865–1872. [Google Scholar]
- Al-Yahya M., Al-Meshal I., Mosa J., Al-Badr A., Tariq M. King Saud University Press; Riyadh, KSA: 1990. Saudi Plants, a Phytochemical and Biological Approach. [Google Scholar]
- Alam S., Shaikh A. Influence of leaf extract of nettle leaf goosefoot (Chenopodium murale L.) and NaCl salinity on germination and seedling growth of rice (Oryza sativa L.) Pak. J. Bot. 2007;39:1695–1699. [Google Scholar]
- Ali B., Al-Wabel N.A., Shams S., Ahamad A., Khan S.A., Anwar F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015;5:601–611. [Google Scholar]
- Arora K., Batish D., Kohli R., Singh H. Allelopathic impact of essential oil of Tagetes minuta on common agricultural and wastedland weeds. Innovare J. Agric. Sci. 2017;5:1–4. [Google Scholar]
- Balogun O.S., Ajayi O.S., Adeleke A.J. Hexahydrofarnesyl acetone-rich extractives from Hildegardia barteri. J. Herbs Spices Med. Plants. 2017;23:393–400. [Google Scholar]
- Barbieri N., Costamagna M., Gilabert M., Perotti M., Schuff C., Isla M.I., Benavente A. Antioxidant activity and chemical composition of essential oils of three aromatic plants from La Rioja province. Pharm. Biol. 2016;54:168–173. doi: 10.3109/13880209.2015.1028077. [DOI] [PubMed] [Google Scholar]
- Batish D., Lavanya K., Pal Singh H., Kohli R. Root-mediated allelopathic interference of nettle-leaved goosefoot (Chenopodium murale) on wheat (Triticum aestivum) J. Agron. Crop Sci. 2007;193:37–44. [Google Scholar]
- Batish D.R., Lavanya K., Singh H.P., Kohli R.K. Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea. Plant Growth Regul. 2007;51:119–128. [Google Scholar]
- Boulos, L., 1999. Flora of Egypt, Vol. I (Azollaceae-Oxalidaceae). Al-Hadara Publishing, Cairo, Egypt.
- Chemsa A.E., Derdouri S., Labbi Z., Acila S., Amara D.G., Chouikh A., Kherraz K., Allali A., Zellagui A. Total phenolic and total flavonoid contents of different solvent extracts of Bassia muricata (L.) Asch. and evaluation of antibacterial and antioxidant activities. J. Chem. Pharm. Res. 2016;8:1317–1321. [Google Scholar]
- Dambolena J.S., Zunino M.P., Herrera J.M., Pizzolitto R.P., Areco V.A., Zygadlo J.A. Terpenes: Natural products for controlling insects of importance to human Health—A structure-activity relationship study. Psyche J. Entomol. 2016;2016:4595823. [Google Scholar]
- Dudai N., Poljakoff-Mayber A., Mayer A., Putievsky E., Lerner H. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999;25:1079–1089. [Google Scholar]
- El-Kashak W.A., Elshamy A.I., Mohamed T.A., El Gendy A.G., Saleh I.A., Umeyama A. Rumpictuside A: Unusual 9, 10-anthraquinone glucoside from Rumex pictus Forssk. Carbohydr. Res. 2017;448:74–78. doi: 10.1016/j.carres.2017.05.023. [DOI] [PubMed] [Google Scholar]
- El-Sayed M.M. Molluscicidal saponins from Bassia muricata. Zagazig J. Pharm. Sci. 1993;2:92–102. [Google Scholar]
- El-Sayed N., Mogahed M., Haron A., Mabry T. Flavonoids and other constituents from Bassia muricata and their insecticidal activities. Rev. Latinoam. Quim. 1998;26:81–85. [Google Scholar]
- El-Shamy A.-S.I., El-Beih A.A., Nassar M.I. Composition and antimicrobial activity of essential oil of Kochia scoparia (L.) Schrad. J. Essent. Oil Bear. Plants. 2012;15:484–488. [Google Scholar]
- El-Shora H.M., Abd El-Gawad A.M. Physiological and biochemical responses of Cucurbita pepo L. mediated by Portulaca oleracea L. allelopathy. Fresen. Environ. Bull. 2015;24:386–393. [Google Scholar]
- El-Shora H.M., Abd El-Gawad A.M. Response of Cicer arietinum L. to allelopathic effect of Portulaca oleracea L. root extract. Phyton Ann. Rei Bot. 2015;55:215–232. [Google Scholar]
- El Hossary G., Selim M., Sayed A.E., Khaleel A. Study of the flavonoid content of Bassia muricata and Bauhinia racemosa. Bull. Fac. Pharm. Cairo Univ. 2000;38:93–97. [Google Scholar]
- Elshamy A., Abd-ElGawad A.M., El-Amier Y.A., El Gendy A.G., Al-Rowaily S. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019;34:316–328. [Google Scholar]
- Elshamy A.I., Abd El-Gawad A.M., El Gendy A.G., Assaeed A.M. Chemical characterization of Euphorbia heterophylla L. essential oils and their antioxidant activity and allelopathic potential on Cenchrus echinatus L. Chem. Biodivers. 2019;16:e1900051. doi: 10.1002/cbdv.201900051. [DOI] [PubMed] [Google Scholar]
- Fitsiou L., Tzakou O., Hancianu M., Poiata A. Volatile constituents and antimicrobial activity of Tilia tomentosa Moench and Tilia cordata Miller oils. J. Essent. Oil Res. 2007;19:183–185. [Google Scholar]
- Hammad H.M., Albu C., Matar S.A., Litescu S.-C., Al Jaber H.I., Abualraghib A.S., Afifi F.U. Biological activities of the hydro-alchoholic and aqueous extracts of Achillea biebersteinii Afan. (Asteraceae) grown in Jordan. Afr. J. Pharm. Pharmacol. 2013;7:1686–1694. [Google Scholar]
- Kamel M.S., Mohamed K.M., Hassanean H.A., Ohtani K., Kasai R., Yamasaki K. Acylated flavonoid glycosides from Bassia muricata. Phytochemistry. 2001;57:1259–1262. doi: 10.1016/s0031-9422(01)00240-0. [DOI] [PubMed] [Google Scholar]
- Kessler A., Baldwin I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Liolios C., Laouer H., Boulaacheb N., Gortzi O., Chinou I. Chemical composition and antimicrobial activity of the essential oil of Algerian Phlomis bovei De Noé subsp. bovei. Molecules. 2007;12:772–781. doi: 10.3390/12040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed A., Chaudhry Z., Muhammad Z. Allelopathic assessment of fresh aqueous extracts of Chenopodium album L. for growth and yield of wheat (Triticum aestivum L.) Pak. J. Bot. 2012;44:165–167. [Google Scholar]
- Mamadalieva N.Z., Youssef F.S., Ashour M.L., Akramov D.K., Sasmakov S.A., Ramazonov N.S., Azimova S.S. A comparative study on chemical composition and antimicrobial activity of essential oils from three Phlomis species from Uzbekistan. Nat. Prod. Res. 2019;33:1–6. doi: 10.1080/14786419.2019.1591400. [DOI] [PubMed] [Google Scholar]
- Miguel M.G. Antioxidant activity of medicinal and aromatic plants. Flavour Fragr. J. 2010;25:291–312. [Google Scholar]
- Nassar M.I., Yassine Y.M., Elshamy A.I., El-Beih A.A., El-Shazly M., Singab A.N.B. Essential oil and antimicrobial activity of aerial parts of Cyperus leavigatus L. (Family: Cyperaceae) J. Essent. Oil Bear. Plants. 2015;18:416–422. [Google Scholar]
- Perricone M., Arace E., Corbo M.R., Sinigaglia M., Bevilacqua A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015;6:76. doi: 10.3389/fmicb.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Zhang W., He Y., Li G., Shen T. Chemical composition, antioxidant and antimicrobial activities of essential oil from Leontopodium longifolium Ling. J. Essent. Oil Bear. Plants. 2018;21:175–180. [Google Scholar]
- Radulovic N., Dekic M., Stojanovic-Radic Z., Palic R. Chemical composition and antimicrobial activity of the essential oils of Geranium columbinum L. and G. lucidum L. (Geraniaceae) Turk. J. Chem. 2011;35:499–512. [Google Scholar]
- Radulović N., Stojanović G., Palić R. Composition and antimicrobial activity of Equisetum arvense L. essential oil. Phytother. Res. 2006;20:85–88. doi: 10.1002/ptr.1815. [DOI] [PubMed] [Google Scholar]
- Razavi S.M., Nejad-Ebrahimi S. Phytochemical analysis and allelopathic activity of essential oils of Ecballium elaterium A. Richard growing in Iran. Nat. Prod. Res. 2010;24:1704–1709. doi: 10.1080/14786410902884933. [DOI] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rice E. Academic Press; New York, USA: 1984. Allelopathy. [Google Scholar]
- Ruberto G., Baratta M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69:167–174. [Google Scholar]
- Shaker K.H., Al Jubiri S.M., El-Hady F.A., Al-Sehemi A.G. New compounds from Bassia muricata and Fagonia indica. Int. J. Pharm. Res. 2013;23:231–236. [Google Scholar]
- Verma R.S., Padalia R.C., Goswami P., Verma S.K., Chauhan A., Darokar M.P. Chemical composition and antibacterial activity of foliage and resin essential oils of Araucaria cunninghamii Aiton ex D. Don and Araucaria heterophylla (Salisb.) Franco from India. Ind. Crops Prod. 2014;61:410–416. [Google Scholar]
- Wei C., Zhou S., Li W., Jiang C., Yang W., Han C., Zhang C., Shao H. Chemical composition and allelopathic, phytotoxic and pesticidal activities of Atriplex cana Ledeb. (Amaranthaceae) essential oil. Chem. Biodivers. 2019;16:e1800595. doi: 10.1002/cbdv.201800595. [DOI] [PubMed] [Google Scholar]
- Xiangwei Z., Xiaodong W., Peng N., Yang Z., JiaKuan C. Chemical composition and antimicrobial activity of the essential oil of Sagittaria trifolia. Chem. Nat. Compd. 2006;42:520–522. [Google Scholar]
- Zeng Z., Zhang S., Wang H., Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015;6:7. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]