Abstract
The emergence of drug-resistant organisms have been increasing globally; therefore, it is a burning need to find an alternative drug to get rid of the diseases caused by resistant strains. This study aims to evaluate the antimicrobial and wound healing activities of Loranthus acacia, Cassia obtusifolia and Cymbopogon proximus plants. All the plants were collected and extracted — by maceration method. Antimicrobial activities determined using standard ATCC strain for Gram-positive bacteria (Bacillus subtilis, Bacillus crew, Methicillin-resistant Staphylococcus aureus, Staphylococcus aureus) and Gram-negative bacteria (Shigella sonnnei, Salmonella Typhimurium, Salmonella typhi, Klebsiella pnuemoniae, Escherichia coli and Pseudomonas aeruginosa) following agar well diffusion method. Plants extracts were prepared as gel and investigated for in vivo wound healing activities in rats. Histological studies were performed on animals’ skin. The results showed that all tested plants have various antimicrobial and wound healing activities. Out of these plants, L. acacia exhibited the best result; it revealed a significant result for antimicrobial activities counter to all Gram-positive, Gram-negative bacteria and wound healing activities in comparing with the reference drug. Thus, it is essential to consider L. acacia as a prospective source in progress in the synthesis of a new antimicrobial drug for the treatment of infectious diseases.
Keywords: Loranthus acacia, Cassia obtusifolia, Cymbopogon proximus, Antimicrobial, Wound healing, Sudan
1. Introduction
Sudan is a country located in Africa, where folklore remedy is a unique mixture of Islamic, Arabic and African philosophies and cultures (Khalid et al., 2012). Moreover, medicinal plants serve as nature’s gift to humans to help them pursue better health by their bioactive compounds, which have been used in traditional practices since ancient times (Moglad et al., 2019). Sudanese people commonly used these medicinal plants products as well as in Africa are extensively consumed (Maroyi, 2013). 90% of Sudan’s population relies primarily on traditional medication because getting the present synthetic drugs are expensive, inadequate and limited since a large number of people are travelers (Mohammed and Babikir, 2013, Karar and Kuhnert, 2017).
In the current study, our focus on three Sudanese medicinal plants, namely; Cassia obtusifolia, Cymbopogon proximus and Loranthus acacia belonging to tree families; Fabaceae, Poaceaea and Loranthaceae, respectively. C. obtusifolia, locally known as Kawal, traditionally used for diuretic and Jaundice (Elkhalifa et al., 2006). C. proximus locally recognized as Mahareb which it leaves used as a decoction for renal colic, fever, spasm, prostate inflammation and helminthiasis (El-Askary et al., 2003, Khalid et al., 2012). L. acacia’s traditional uses in folk medicine are in the treatment of numerous infections like smallpox, diarrhea, hookworms infections, tonsillitis, otitis media (Moreno-Salazar et al., 2008, Noman et al., 2019).
Furthermore, there are limited records of wound healing activities for these medicinal plants. According to a published review article about traditional uses and phytoconstituents of herbal drugs from Sudan, they stated that out of 48 medicinal plants from Sudan only four plants investigated for wound healing activities and 27 of them screened for antibacterial activities (Karar and Kuhnert, 2017). Besides, in the recently published article, recommended to formula ethanolic extracts of C. proximusas topical ointment for in vivo wound healing activities after examined it for antibacterial activity towards multidrug-resistant bacteria isolated from wound infection (Moglad et al., 2020). Since there was a lacuna of information about antimicrobial and wound healing activities of these selected plants and so consequently, our work aimed to investigate the antimicrobial and wound healing activities of L. acacia, C. obtusifolia, and C. proximus has grown in Sudan.
2. Material and methods
2.1. Plant collection and authentication
The leaves and stems of selected plants were collected during March 2018 from the South Kurdufan State, Sudan. The plants were authenticated by Dr Haider Abedelgadir, a plant taxonomist from the herbarium of the Medicinal and Aromatic Plants Research Institute (MAPRI), National Research Center, Khartoum-Sudan. The plant identified as Cassia obtusifolia L., Cymbopogon proximus (Hochst. ex A. Rich) Stapf. and Loranthus acaciae Zucc. A voucher specimen (No. MAP/2018/10-8) was deposited in MAPRI herbarium. The leaves and stems together were ground to powder for preparation of the extract.
2.2. Preparation of extracts
The shade dried and ground aerial part (1 kg) from each plant was extracted with 2000 ml 96% ethanol using cold maceration technique. The obtained extract was filtered and concentrated under reduced pressure using a rotary flash evaporator to obtain crude alcoholic extracts.
2.3. Antimicrobial activity
Antimicrobial activities of the plants' extracts were determined using standard ATCC strain; for Gram-negative bacteria (Shigella sonnnei ATCC 11060, Salmonella Typhimurium ATCC 14025, Salmonella typhi ATCC 13311, Klebsiella pnuemoniae NCTC 9633, E. coli ATCC 13706 and Pseudomonas aeruginosa ATCC 27853). For Gram-positive bacteria used Bacillus subtilis ATCC 11774, Bacillus crew ATCC10876, Methicillin-resistant Staphylococcus aureus ATCC 35501, Staphylococcus aureus ATCC 33862 following agar well diffusion method (Balouiri et al., 2016, Magaldi et al., 2004). Bacterial suspensions were prepared in physiological saline solution (0.9%). The suspension turbidity compared with a 0.5 McFarland turbidity standard, number of bacterial cells approximately 1–2 × 108 CFU/ mL for each of the tested bacteria.1 ml from each organism suspension of was taken and poured onto sterile Petri plates above which 20 ml of Muller and Hinton agar was poured. A sterile Cork borer of 8 mm size was used to create wells in the agar plates. Stock solution for the antimicrobial activity test was prepared using 100% dimethylsulfoxide (DMSO) at 100 mg/mL. The resultant solution was subsequently diluted using two-fold dilution method to 50 mg/mL, 25 mg/mL, 12.5 mg/mL, 6.25 mg/mL, 3.125 mg/mL, and 1.56 mg/mL using methanol as solvent. Using a micropipette, 100 μL of each extracts concentrations were poured into each of three wells on all plates. Methanol was run as a negative control in well four. Then in order to dry solution, the plates were left uncovered in a sterile safety cabinet for 20 min (Dkhil et al., 2020). After incubation at 35 °C ± 2 °C for 24 h, the zone of inhibition’s size was measured. The test was done in duplicates and performed thrice, and the result presented as mean of inhibition zone. Antibiotics sensitivity profile for commercially a viable drug against tested bacteria were performed to compare their efficacies with the bacterial spectrum and susceptibility profile of tested plants.
2.4. Preparation of gel with carbopol 940 from plants ethanolic extracts
A measured quantity of carbopol 940 was taken in a beaker and dispersed in 50 ml of distilled water. The beaker was kept aside to allow the swelling of carbopol for half an hour and then stirring was done on a magnetic stirrer at 1200 rpm for 30 min. In another beaker, 5 ml of Propylene glycol was added to 10 ml of water and added weighed quantities of methylparaben and stirred properly. After all, Carbopol dispersed weighed amounts (5 g for 5% and 10 g for 10%) of extracts were added with constant stirring. Finally, the volume made up to 100 ml with distilled water. Triethanolamine was added as drops to the formulation to adjust the pH from 6.5 to 7, which the skin pH also to obtain the gel at required consistency (Niyogi et al., 2012).
2.5. Experimental animals
Mice (20 ± 5 g) and Male Wistar rats weighing (150 ± 20)g were obtained from the animal house of the Department of Pharmacology and Toxicology, College of Pharmacy, Prince Sattam Bin Abdulaziz University and used for the investigation. The animals were housed under standard controlled conditions (24 ○C and a 12 h light/dark cycle). The animals were provided with standard pellet diet and water ad libitum.
2.5.1. Acute Toxicity studies
The acute toxicity experiments for selected plants were carried out according to the test guideline of OECD (Organization for Economic Cooperation and Development) guideline No: 423 for testing of chemicals (OECD, 2001). Forty-Five mice were used for the acute toxicity study (five for each group). Various doses of the extract (0.5, 1, and 2 g/kg/po) were prepared, and it was suspended in 0.5% aqueous Tween 80 and administered orally. The animals were constantly observed for the signs and symptoms of toxicity for 12 h including like central nervous system, somatomotor and behavioral changes like tremors, convulsions, salivation, diarrhea, lethargy, sleep, etc., along with the mortality was observed for 72 h.
2.5.2. Wound healing activity
Grouping and Dosing of Animals
Male rats weighing (150–180 g) divided into eight groups of six animals in each group. The animals were adapted to the laboratory conditions for a week before the start of the study and provided with standard food and water ad libitum.
Group I - animals induced with a wound not treated with any drug
Group II - animals treated with the standard silver sulphadiazine (1% w/w)
Ointment
Group III - animals treated with 5% of C. Proximus gel.
Group IV - animals treated with 10% of C. Proximus gel.
Group V - animals treated with 5% of L. acacia gel.
Group VI - animals treated with 10% of L. acacia gel.
Group VII - animals treated with 5% of C. obtusifolia gel.
Group VIII - animals treated with 10% C. obtusifolia gel.
Group IX - control animals (normal animals without induction of wound)
Excision Wound Model
The wound was created and excised on the rats using i.v. injection of ketamine (120 mg/kg body weight) as anesthesia. The back of the mice was shaved, and the wound was created on the sides of the central trunk using a scalpel and sharp scissors and sterilized with ethanol (Akkol et al., 2009). The skin was removed from the marked area to obtain a wound measuring a maximum of 135 mm2. After removing the skin, the wound was cleaned with a cotton swab soaked in saline, and then the animals were placed individually in cages. After 24 h to the wound-induced animals, the ointments were gently applied to cover the wounded area once per day until reaching complete healing. The wound diameter of each animal was measured at 0, 7, 14 and 21 days using a transparent ruler every week until epithelialization and complete wound closure was recorded. The wound area assessed wound healing activity, and percent wound contraction rate (Verma et al., 2012). Percent wound contraction was calculated using the following formula;
2.6. Histopathological studies
Skin tissue samples (3–5cm) from each group of animals were submerged immediately in the appropriate amount of 10% formalin and processed in automatic tissue processing machine (ASP300s, Leica Biosystems, IL, USA) and embedded in paraffin wax and 5 µm thickness section was prepared using rotary microtome (SHUR/Cut 4500, TBS, NC, USA) (Hamad and Ahmed, 2018). Two sections of each sample were taken for staining as one was stained by hematoxylin and eosin technique as general staining method and the other was stained by Masson tricrome as unique stain method for connective tissue fibres (Hamad and Ahmed, 2016). Hematoxylin and Eosin method was as following: Dewax sections, rehydrate through descending grades of alcohol to water. If needed excess fixation pigments were removed. Stain in hematoxylin (HX082464, MERK, Darmstadl, Germany) for 10 min. Wash thoroughly in running tap water until sections 'blue' for 5–10 min or less. Stain in 1% eosin Y for 10 min. Wash in running tap water for 1–5 min. Dehydrate through alcohols, clear, and mount in DPX (Kim et al., 2018). Masson trichrome technique for connective tissue fibres demonstration (mainly collagen) was performed using the following method: Stain nuclei with Weigert's iron hematoxylin for 10 min, wash with water, stain in an acid fuchsin solution for 5 min, rinse rapidly in water, differentiate in 1% phosphomolybdic acid for approximately 5 min, drain and counterstain with methyl blue, dehydrate, clear and mount sections in DPX (Hamad and Ahmed, 2016).
2.7. Statistical analysis
Microsoft excel version 2016 used to analyze the antimicrobial activity of plant extracts and fractions. All assays were carried out in triplicates. The experimental results were presented as mean ± standard deviation.
3. Results
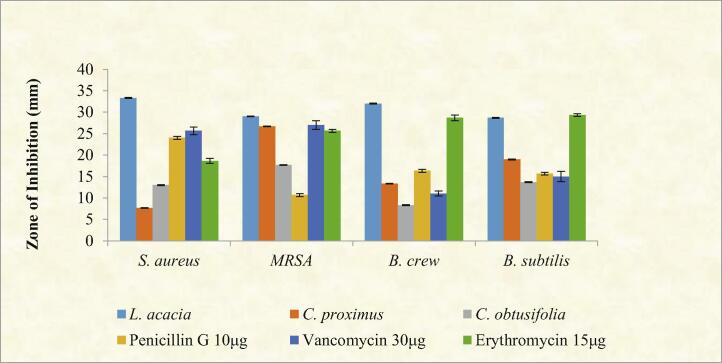
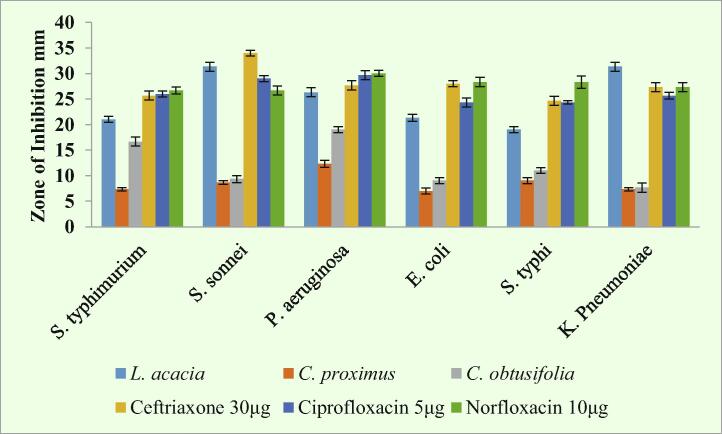
Antimicrobial results revealed that L. acacia was highly active against all tested bacteria. Interestingly, it was more active than standard drugs (Penicillin G 10 µg, Vancomycin 30 µg and Erythromycin 15 µg). While, C. proximus and C. obtusifolia were exhibited moderate activities towards some of the tested bacteria, as shown in Fig. 1, Fig. 2.
Fig. 1.
Evaluation of the antimicrobial activity of selected plants and standard drug against Gram-positive bacteria: S. aureus, Methicillin-Resistant S. aureus, B. cereus and B. subtilis. All plants extract at concentration 10 mg\ml, and the diameter of the zone of inhibition was measured. Values are expressed as mean ± STD of three independent trials.
Fig. 2.
Antimicrobial activity of selected plants and standard drug against Gram-negative bacterial pathogens: Shigella sonnnei, Salmonella Typhimurium, Salmonella typhi, Klebsiella pnuemoniae, E. coli and Pseudomonas aeruginosa. Dilutions similar to those used in Fig. 1 were applied, and the diameter of zone of inhibition was measured.
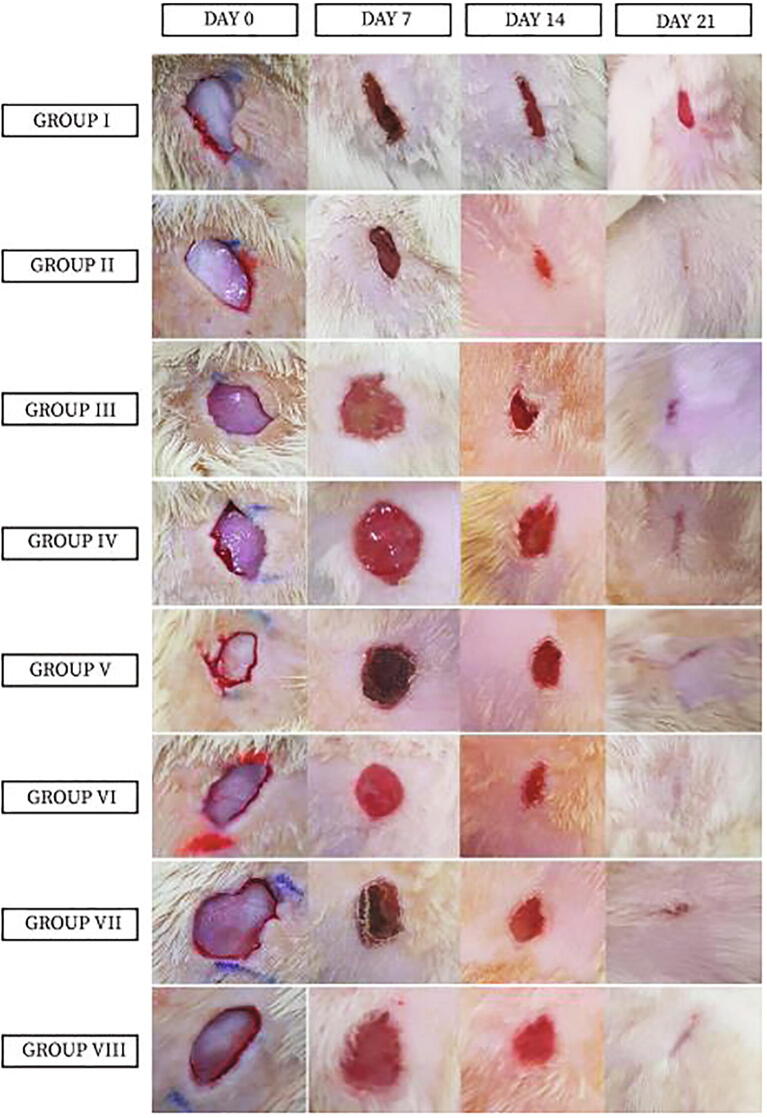
In the wound healing activities showed that the best gel for wound healing was compared with the positive control (Group II) were group V and group VI which treated with 5% and 10% of L. acacia gel, respectively. Also, group VIII, which treated with 10% C. obtusifolia gel (Table 1 and Figs. 3 and 4).
Table 1.
Percentage of wound healing reduction (mean ± STD).
| Animal Group | Day7 | Day 14 | Day 21 |
|---|---|---|---|
| GROUP I | 23.31 ± 3.92 | 67.13 ± 3.12 | 92.45 ± 0.69 |
| GROUP II | 48.65 ± 4.63 | 75.68 ± 3.16 | 99.34 ± 0.65 |
| Group III | 36.40 ± 5.55 | 73.24 ± 2.97 | 97.48 ± 0.45 |
| GROUP IV | 39.73 ± 4.02 | 79.47 ± 0.70 | 98.65 ± 0.01 |
| GROUP V | 45.55 ± 4.82 | 79.48 ± 3.95 | 97.99 ± 0.655 |
| GROUP VI | 40.21 ± 4.52 | 73.74 ± 5.13 | 97.51 ± 0.95 |
| GROUP VII | 40.90 ± 2.64 | 70.22 ± 7.34 | 97.11 ± 1.02 |
| GROUP VIII | 45.8 ± 2.32 | 80.2 ± 8.33 | 98.20 ± 1.04 |
Note: Percentage of wound healing reduction calculated according to above formula for all tested plants in two concentration (5 and 10%) in day 7, 1, 21. The result expressed as mean ± STD.
Fig. 3.
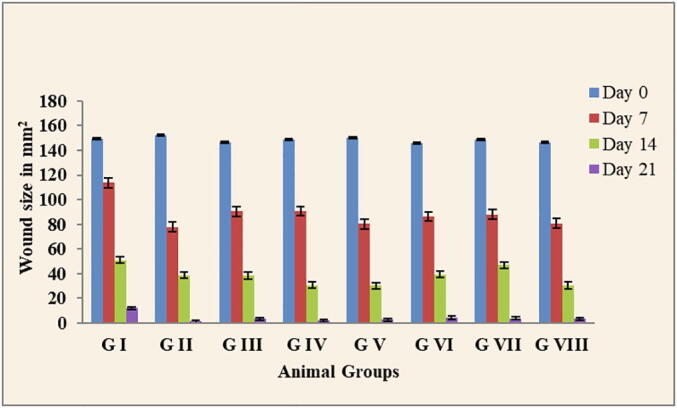
Reduction in wound size by tested plant extracts. G I = animals induced with a wound not treated with any drug, G II = animals treated with the standard silver sulphadiazine (1% w/w) Ointment, G III = animals treated with 5% of C. Proximus, G IV = animals treated with 10% of C. Proximus, G V = animals treated with 5% of L. acacia, G VI = animals treated with 10% of L. acacia, G VII = animals treated with 5% of C. obtusifolia, G VIII = animals treated with 10% C. obtusifolia. The result expressed as mean ± STD.
Fig. 4.
Photographs of excision wounds of all animal groups on treatment day 0, 7, 14 and 21.
In the Acute toxicity activity, the extract-treated animals did not show any symptoms and signs of toxic reactions or behavioral changes during the 24 h period. No mortality was found up to the highest dose of 2000 mg/Kg after the completion of 48 h. As per the ranking system used by the European Economic Community (EEC) for acute oral toxicity, the LD50 dose of 2000 mg/kg and above are categorized as unclassified (EC Directive 83/467/EEC, 1983); hence further studies were not conducted with higher doses.
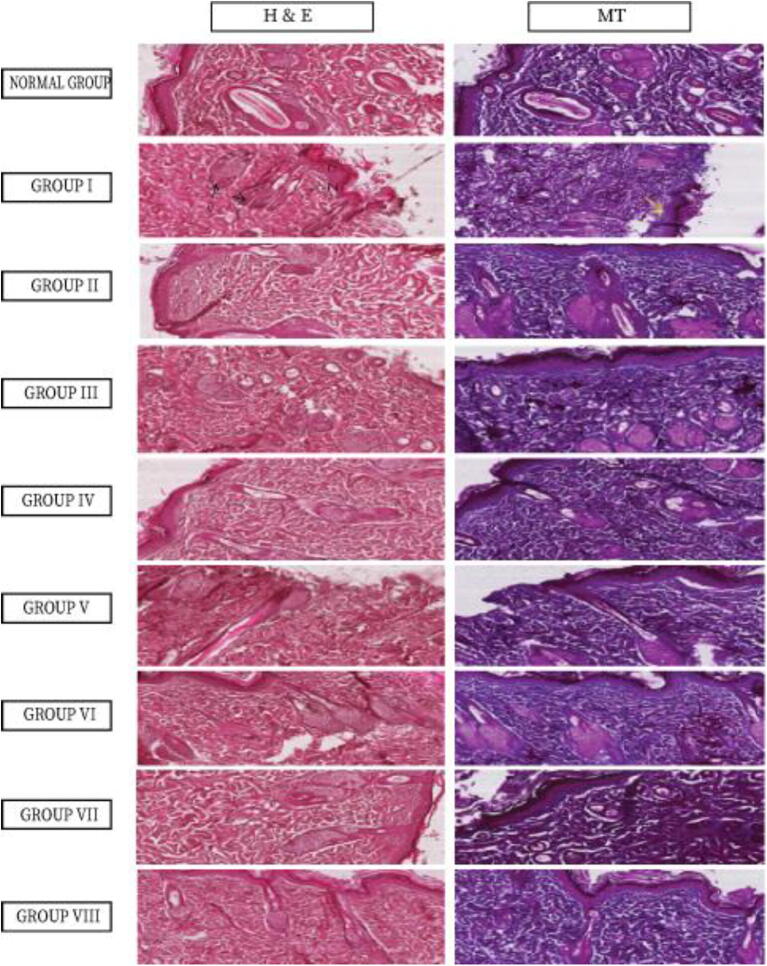
Histopathological results showed that for healthy animal and group II, the skin appeared normal in both general stain (H&E) and special stain (MT). Group III-VIII showed variable improvement but still, skin tissue suffers from moderate to low tissue damage depend on plants extracts used. The most improvement in the animals treated with 10% of L. acacia gel (Group VI), and animals treated with 10% C. obtusifolia gel (Group VIII). See variables table for score information (Table 2 and Fig. 5).
Table 2.
Wound healing variables score information.
| Animal groups | Degeneration | Necrosis | Occlusion | Loss of epidermis | Loss of collagen | Muscle weakness | Total |
|---|---|---|---|---|---|---|---|
| Normal Group | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Group I | 4 | 4 | 4 | 4 | 4 | 4 | 24 |
| Group II | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Group III | 3 | 3 | 4 | 2 | 2 | 1 | 15 |
| Group IV | 2 | 2 | 2 | 2 | 1 | 0 | 9 |
| Group V | 3 | 3 | 1 | 4 | 2 | 2 | 15 |
| Group VI | 2 | 1 | 1 | 0 | 1 | 0 | 5 |
| Group VII | 3 | 2 | 1 | 1 | 3 | 1 | 11 |
| Group VIII | 1 | 1 | 1 | 0 | 1 | 0 | 4 |
Note: Histopathological result of all animal groups to see wound healing variables score information.
Fig. 5.
Histopathological study using H&E method and Masson trichrome method for animal groups’ I-VIII. In Group I with H&E showed severe damage to skin layers and area of complete loss of epidermis (L). Also shows remarkable necrosis (N) and degeneration (D) as well as occlusion (O) of capillaries that irrigate hair follicles. Special stain (MT) shows severe loss of collagen fibres in the dermis area (yellow arrow) magnification is 200X.
4. Discussion
Medicinal plants can offer valuable phytochemicals constituents which can play a significant role in fighting infection. Also, the natural product has become the current idea of pharmacological research and drug discovery. In this study, for the first time, investigated the wound healing activities of three Sudanese medicinal plants using in vivo animal model along with the screening of antibacterial activities. Plants tested counter to Gram-positive and Gram-negative bacteria. There are slightly different in activities between them, and this could be attributed to its chemical constituents. Generally, the biological activities of Sudanese herbal medicines connected with their ability to treat or prevent ailments, the most bioactive compounds recognized in these plants are phenolics, alkaloids, tannins, flavonoids, saponins, and steroids (Karar and Kuhnert, 2017).
In this study, L. acacia showed the best antimicrobial result for both Gram-negative and Gram-positive bacteria, and it gave an excellent result for wound healing activities in the two concentrations (5 and 10%) comparing with standard silver sulphadiazine (1% w/w) ointment. Furthermore, there is no evidence of data cited about wound healing activities of L. acacia aerial parts. In contrast, several studies have reported the antimicrobial activities of L. acacia against a wide range of bacteria, and our finding ultimately agreed with their finding (Elegami et al., 2001, El-Shafei et al., 2018). These antibacterial activities may be due to presence of flavanocoumarin, loranthin, catechin, quercetin, rutin, gallic acid and methyl gallate which were previously isolated from L. acacia and showed significant activity towards bacteria (Badr et al., 2013).
For C. proximus our result likely agree with the previous study stated that this herb has antimicrobial properties after investigation of antimicrobial activity crude extract against Gram-positive and antifungal (Al-Taweel et al., 2013, Selim, 2011). While, C. obtusifolia contains anthraquinones, benzyl-β-resorcylate glycosides, flavonoids, triterpenoids, anthrones which has Antioxidant (DPPH), Neuroprotective effect, α Amylase activity, Lipase activity, Protease activity and Antitumor (Huang et al., 2012, Ju et al., 2010, Cong et al., 2014, Vadivel et al., 2012, Wu et al., 2012).
5. Conclusions
This study investigated the antimicrobial activities and in vivo wound healing activities for three medicinal plants. Histological studies were performed on animals’ skin. Our study demonstrated that all tested plants have various antimicrobial and wound healing activities. But L. acacia exhibited the best antimicrobial and wound healing activities results in comparing with the reference drug. Thus, it is essential to consider L. acacia as a prospective source in progress in the synthesis of a new antimicrobial medicine in curing infectious diseases.
Funding
This project is not funded by any institutional or orgnization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This publication was supported by the Deanship of Scientific Research at Prince Sattam bin Abdulaziz University, Alkharj, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akkol E.K., Koca U., Peşin I., Yilmazer D., Toker G., Yeşilada E. Exploring the wound healing activity of Arnebia densiflora (Nordm.) Ledeb. by in vivo models. J. Ethnopharmacol. 2009;124:137–141. doi: 10.1016/j.jep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Al-Taweel A.M., Fawzy G.A., Perveen S., el Tahir K.E.H. Gas chromatographic mass analysis and further pharmacological actions of Cymbopogon proximus essential oil. Drug Res. 2013;63:484–488. doi: 10.1055/s-0033-1347239. [DOI] [PubMed] [Google Scholar]
- Badr J.M., Shaala L.A., Youssef D.T.A. Loranthin: A new polyhydroxylated flavanocoumarin from Plicosepalus acacia with significant free radical scavenging and antimicrobial activity. Phytochem. Lett. 2013;6:113–117. [Google Scholar]
- Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Q., Shang M., Dong Q., Liao W., Xiao F., Ding K. Structure and activities of a novel heteroxylan from Cassia obtusifolia seeds and its sulfated derivative. Carbohydr. Res. 2014;393:43–50. doi: 10.1016/j.carres.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Dkhil M.A., Zreiq R., Hafiz T.A., Mubaraki M.A., Sulaiman S., Algahtani F., Abdel-Gaber R., Al-Shaebi E.M., Al-Quraishy S. Anthelmintic and antimicrobial activity of Indigofera oblongifolia leaf extracts. Saudi J. Biol. Sci. 2020;27:594–598. doi: 10.1016/j.sjbs.2019.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Askary H.I., Meselhy M.R., Galal A.M. Sesquiterpenes from Cymbopogon proximus. Mol.: J. Synthetic Chem. Nat. Product Chem. 2003;8:670–677. [Google Scholar]
- El-Shafei G., Al-Hazmi B., Marghelani A., Al-Moalem D., Badr J., Moneib N. Antimicrobial activity of different extracts of Plicosepalus acacia. Records Pharm. Biomed. Sci. 2018;1:47–51. [Google Scholar]
- Elegami A.A., Elnima E.I., Muddathir A.K., Omer M.E. Antimicrobial activity of Plicosepalus acaciae. Fitoterapia. 2001;72:431–434. doi: 10.1016/s0367-326x(01)00268-4. [DOI] [PubMed] [Google Scholar]
- Elkhalifa K.F., Ibrahim M.A., Elghazali G. A survey of medicinal uses of Gash Delta vegetation, Eastern Sudan. Saudi J. Biol. Sci. 2006;13:1–6. [Google Scholar]
- Hamad, A., Ahmed, H., 2016. Association of connective tissue fibers with estrogen expression in breast lesions among sudanese females. Int. Clin. Pathol. J., 2.
- Hamad A.M., Ahmed H.G. Association of some carbohydrates with estrogen expression in breast lesions among Sudanese females. J. Histotechnol. 2018;41:2–9. [Google Scholar]
- Huang Y.-L., Chow C.-J., Tsai Y.-H. Composition, characteristics, and in-vitro physiological effects of the water-soluble polysaccharides from Cassia seed. Food Chem. 2012;134:1967–1972. doi: 10.1016/j.foodchem.2012.03.127. [DOI] [PubMed] [Google Scholar]
- Ju M.S., Kim H.G., Choi J.G., Ryu J.H., Hur J., Kim Y.J., Oh M.S. Cassiae semen, a seed of Cassia obtusifolia, has neuroprotective effects in Parkinson's disease models. Food Chem. Toxicol.: Int. J. Published Brit. Ind. Biol. Res. Assoc. 2010;48:2037–2044. doi: 10.1016/j.fct.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Karar M.G.E., Kuhnert N. Herbal drugs from sudan: traditional uses and phytoconstituents. Pharmacogn. Rev. 2017;11:83–103. doi: 10.4103/phrev.phrev_15_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid H., Abdalla W.E., Abdelgadir H., Opatz T., Efferth T. Gems from traditional north-African medicine: medicinal and aromatic plants from Sudan. Nat. Prod. Bioprospecting. 2012;2:92–103. [Google Scholar]
- Kim, S. Suvarna, Christopher, Layton, Bancroft, J. D. 2018. Bancroft's Theory and Practice of Histological Techniques. 8th edition. Elsevier. Page: 131.
- Magaldi S., Mata-Essayag S., Hartung De Capriles C., Perez C., Colella M.T., Olaizola C., Ontiveros Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004;8:39–45. doi: 10.1016/j.ijid.2003.03.002. [DOI] [PubMed] [Google Scholar]
- Maroyi A. Traditional use of medicinal plants in south-central Zimbabwe: review and perspectives. J. Ethnobiol. Ethnomed. 2013;9:31. doi: 10.1186/1746-4269-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moglad, E.H., Alhassan, M.S., Abdalkareem, E.A., Abdalla, A.N., Kuse, M., 2019. Ethyl acetate fraction of Solanum nigrum L.: Cytotoxicity, induction of apoptosis, cell cycle in breast cancer cells, and gas chromatography-mass spectrometry analysis. Asian J. Pharm. 13(3), 246-251.
- Moglad E.H., Ska Boon, Hto Ali. Various medicinal plants: a promising treatment for multidrug-resistant bacteria isolated from wound infection. Int. J. Pharm. Sci. Res. 2020;11(2):839–843. doi: 10.13040/IJPSR.0975-8232.11(2).839-43. [DOI] [Google Scholar]
- Mohammed I.N., Babikir H.E. Traditional and spiritual medicine among Sudanese children with epilepsy. Sudanese J. Paediatrics. 2013;13:31–37. [PMC free article] [PubMed] [Google Scholar]
- Moreno-Salazar S.F., Robles-Zepeda R.E., Johnson D.E. Plant folk medicines for gastrointestinal disorders among the main tribes of Sonora, Mexico. Fitoterapia. 2008;79:132–141. doi: 10.1016/j.fitote.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Niyogi P., Raju N.J., Reddy P.G., Rao B.G. Formulation and evaluation of antiinflammatory activity of solanum pubescens wild extracts Gel on albino wister rats. Int. J. Pharm. 2012;2:484–490. [Google Scholar]
- Noman O.M., Mothana R.A., Al-Rehaily A.J., Al Qahtani A.S., Nasr F.A., Khaled J.M., Alajmi M.F., Al-Said M.S. Phytochemical analysis and anti-diabetic, anti-inflammatory and antioxidant activities of Loranthus acaciae Zucc. Grown in Saudi Arabia. Saudi Pharm. J. 2019;27:724–730. doi: 10.1016/j.jsps.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisation for Economic Co-Operation and Development (OECD) Guidelines for 382 testing of chemicals 423, 2001. Acute oral toxicity–acute toxic class method. #423, Paris, 383 France.
- SELIM, S. 2011. Chemical composition, antioxidant and antimicrobial activity of the essential oiland methanol extract of the Egyptian lemongrass Cymbopogon proximus Stapf. Grasas y Aceites, 62.
- Vadivel V., Kunyanga C.N., Biesalski H.K. Antioxidant potential and type II diabetes-related enzyme inhibition of Cassia obtusifolia L.: effect of indigenous processing methods. Food Bioprocess Technol. 2012;5:2687–2696. [Google Scholar]
- Verma D., Bharat M., Nayak D., Shanbhag T., Shanbhag V., Rajput R. Areca catechu: Effect of topical ethanolic extract on burn wound healing in albino rats. IJPCS. 2012:1. [Google Scholar]
- Wu X., Ruan J., Yang V.C., Wu Z., Lou J., Duan H., Zhang J., Zhang Y., Guo D. Three new acetylated benzyl-beta-resorcylate glycosides from Cassia obtusifolia. Fitoterapia. 2012;83:166–169. doi: 10.1016/j.fitote.2011.10.009. [DOI] [PubMed] [Google Scholar]