Abstract
The aim of this study is to determine the effect of salinization and wastewater stresses on the growth, some cellular contents (total soluble proteins, total soluble carbohydrates, nucleic acids, and amino acids composition) and ultrastructure using TEM of unicellular green alga Scenedesmus bijugatus. Treatment of S. bijugatus by NaCl at 10 and 50 mg L−1 significantly increased the growth of this alga and its cellular macro-molecules. While, treatment above this concentration with NaCl significantly inhibited the growth and cellular macro-molecules. On the other hand, treatment by NaCl at the pre-lethal concentration (300 mg L−1) had different effects on its detected amino acids. Whereas, Asp. Acid, Pro, Cys, Val, Iso-leu, leu, Phe.ala and Lys were slightly stimulated with salinization treatment. On contrast the levels of amino acids: Thr, Ser, Glu.acid, Gly, Ala, Mth, His and Arg were markedly inhibited. Ultrastructure examination of treated S. bijugatus by 300 mg L−1 of NaCl for 8 days showed increase of starch granules, shrinkage of cell contents and thickening of cell wall. The recorded data indicated also that treatment by wastewater with all concentrations led to stimulatory effects on their growth and cellular macro-molecules except at 100% wastewater which had inhibitory effects on Asp., Gly., Thr., Ser., Pro., Glu., Ala., Meth., and Cyst., of S. bijugatus. Also, wastewater induced a slight change in the treated S. bijugatus as elevation in starch granules and presence of thylakoid membranes although not clear as in the control.
Keywords: Salinity, Wastewater, Scenedesmus bijugatus
1. Introduction
Increasing of salinity in the freshwater bodies during natural and human activities alters the planktonic communities to a considerable range. It is one of the processes that alter the growth curve and biochemical constituents of aquatic algae. In the last decade, the research indicates that different levels of salinity are correlating with changes in biochemical constituents of microalgae (Tayar et al., 2020). The degree of tolerance of a microalga to salinization stress is dependent on the constituents of the growth culture and growth conditions such as light intensity and a favorable temperature (Rai et al., 2015). In this respect, Kuo et al., 2016, Collotta et al., 2018 observed that the response of these algae varied from tolerance to full strength medium to very high sensitivity even to low concentrations of sea water medium.
One of the most important factors that qualitatively and quantitatively influence algal populations in aquatic habitats, is the number of soluble salts in such habitats. Many microalgal genera have been adapted to grow efficiently in wastewater (Kuo et al., 2016, Collotta et al., 2018). In this respect, Chan et al. (1979) mentioned that cells of Chlorella salina grow well in domestic sewage having high salt concentrations which can be used for biodiesel production. Czernas (1978) also, obtained variable results while studying the effect of salinity on three-selected algal species, namely Microcystis aeruginosa, Nitzschia gracilis and Selenastrum capricornutum. Meanwhile, Frank and Wegmann (1974) explained that the osmotic regulation of the metabolism of the salt-tolerant alga, Dunaliella, is a compartmentation phenomenon, at high NaCl concentrations, the reaction occurring in the cytoplasm are inhibited, while those in the chloroplast remain operating. On the other hand, working on the diatom Skeletonema costatum by Rijstenbil et al. (1989a) salinity suppresses the photosynthetic process, dark respiration, and cell growth. However, the cellular pools of glucose appeared in lower form on the contrary to the carbohydrate content which remained constant, while the protein content slightly increased. On the other hand, a total descends of the overcapacity of ammonium uptake were distinguished. Increase in salinity produced an increase in general cellular activity, especially in enzyme activity like glutamate dehydrogenase, glutamine synthetase and glutamate synthase and hence an increase in the ammonium uptake. The same work has been applied to the diatom Ditylum brightwellii by Rijstenbil et al. (1989b) and similar findings were reported.
Wastewater in the broadest sense is water contaminated by prior use, requiring either treatment or disposal (Abdel-Raouf et al., 2003). The term wastewater is limited to water containing organic wastes and nutrients compatible with algae growth (Whitton et al., 2015). It includes municipal sewage, the discharge from food processing plants and various chemical industries, anaerobic digester effluent and agricultural wastewaters (Abdel-Raouf et al., 2012). Approximately 25 elements are known to be essential for most forms of life including the microalgae (Reddy et al., 2014, Han et al., 2016, Li et al., 2019). All but a few of these elements are so widely distributed in nature that they are unlikely to be limiting to growth. In the case of algae, the nutrients most likely to be limiting are carbon, nitrogen, and phosphorus in that order. The algae in wastewater utilize both inorganic and organic carbon unlike clean algal culture systems (Boboescu et al., 2014, Wirth et al., 2018, Amenorfenyo et al., 2019, Shetty et al., 2019a).
Although some species of algae are very fast growing which are simply able to out-compete most contaminants. Not all wastewater is suitable for algae. If the wastewater has higher concentrations of ammonia then this will affect the performance of the algae and the wastewater must be either diluted or the feed rate reduced. These attempts were made to clarify the effects of salinization and wastewater treatments on the growth of Scenedesmus bijugatus and its some cellular macromolecules.
2. Materials and methods
2.1. The biological materials
In the present investigation, axenic cultures of the unicellular green alga Scenedesmus bijugatus was used. S. bijugatus was previously isolated from Wadi-Hanifa, a wadi in the Najd region, Riyadh Province, in central Saudi Arabia.
2.1.1. Media used for culturing algal taxa
2.1.1.1. Z-medium
Staub (1961) has described this medium which prepared from premixed stock solutions. The stock solutions should be stored at 4 °C in refrigerator.
2.1.2. Maintenance of the studied algal cultures
Stock cultures were storage at 5 °C on agar slants (El-Nawawy et al., 1958). Subculturing should be conducted each month and keep purified.
2.1.3. Algal growth conditions
Preliminary tests were conducted by using a wide range of temperature (24–30 °C), light duration (12–24 h), light intensities (1000–6000 Lux) and pH values (6–8) to obtain the optimum growth conditions for the studied alga. As a result of these experiments Scenedesmus bijugatus incubated at 28 °C and continuous light at 4000 Lux. The algal cultures were harvested on the 8th day then used in the experimental study.
2.2. Uptake test of investigated environmental stresses from culture media by S. bijugatus
According to Wong and Pak (1992), a preliminary experiment using a wide range of different NaCl concentrations and different domestic wastewater percentages, was carried out to determine the suitable concentrations of these materials which could be tolerated by the studied alga. Selection of these concentrations was based on the response of the studied alga to it, which had a slightly or marked effects on their growth, and also to avoid the non-effective and directly lethal concentrations on the alga experimented with.
The actual experiment was then carried out by placing the appropriate volumes of selected concentrations of the studied NaCl or domestic wastewater percentages (after filtration through bacterial filter) into the culture media making up to 100 mls with particle-free deionized water and algal cells with a known density (initial inoculum): 2 × 103 cells/ml of Scenedesmus bijugatus using 250 mls measuring conical flasks as culture vessels. The pH of the media was adjusted by 1 M HCl/NaOH prior to autoclaving. The culture media were aerated (to provide CO2) through the cotton plugs. Three replicates for each concentration of the studied stresses in addition to the control were prepared. Then the culture vessels were incubated under conditions required for the growth of the studied alga for a required period of growth. At the final growth period, the algal mats were harvested, washed three times with distilled water and subjected for determination of the investigated cellular macromolecules according to the described plan of this study.
2.3. Chemical analysis
2.3.1. Optical density
The optical density of the homogenized green algal suspension was measured at 760 nm (Adhikary, 1983).
2.3.2. Chlorophyll a content
Chlorophyll a content of S. bijugatus was determined according to the method described by Strickland and Persons (1972). The concentration of chlorophyll a (ugL-1) was calculated using the equation of Strickland and Persons (1968).
2.3.3. Determination of total soluble proteins
This was conducted according to the method of Lowery et al., (1951), using bovine serum albumin as a standard protein.
2.3.4. Extraction and determination of total soluble carbohydrates
2.3.4.1. Extraction
The algal growth suspension after being dried at room temperature, were ground to a fine powder and extracted with mixture of 5 mls of 2% phenol water and 10 mls of 30% trichloroacetic acid (Said and Ramzy, 1964).
2.3.4.2. Determination
Total water-soluble carbohydrates were determined by anthrone technique according to Umbreit et al. (1969).
2.3.5. Quantitative estimation of nucleic acids (RNA-DNA)
The method applied for total RNA and DNA determination (Schmidt and Thannhauser, 1945) with some little modifications as described by Morse and Carter (1949) was applied.
2.3.5.1. Ribonucleic acid (RNA) content
It was estimated calorimetrically by the orcinol reaction as described by Dische (1953). To 0.5 ml RNA extract 3 ml of an acid reagent (0.5 ml of a 10% FeCl3·6H2O was mixed with 100 ml of concentrated HCl) were added. This was followed by adding 0.2 ml of a freshly prepared 6% solution of orcinol in 96% ethanol. The mixture was heated in a boiling water bath for 20 min and its optical density was measured at 660 mu.
2.3.5.2. Deoxyribonucleic acid (DNA) content
It was estimated by DPA (diphenylamine) colour reaction described by Burton (1956). Samples of 1.0 ml DNA extract were mixed with 2.0 ml of DPA reagent (1.5% g of steam distilled diphenylamine were dissolved in 100 ml of redistilled glacial acetic acid then 1.5 ml of concentrated H2SO4 were added and mixed well) let stand at 30 °C for 16–20 h. Their optical densities were measured at 540 mu. with pure RNA and DNA, the method gives a linear relation between the concentration of RNA and DNA and the optical density.
2.3.6. Amino acid determination
Amino acid determination was performed according to the method of Winder and Eggum (1966).
2.3.7. Transmission electron microscope
After 8 days of treatment for Scenedesmus bijugatus by NaCl (300 mg L−1) and wastewater (100%), the cells become to make ready for harvesting by centrifugation at 2500 r.p.m. for 10 min at 4 °C, immediately fixed in fresh 3% glutaraldehyde-formaldehyde at 4 °C for 18–24 h. The specimens were then washed in phosphate buffer (pH 7.4) and then postfixes in isotonic 1% osmium tetroxide for 1 h at 4 °C (Mercer and Birbeck, 1966). Ultrathin sections were then prepared using the ultramicrotome glass knives, stained with uranyl acetate and lead citrate (Reynolds, 1963) and examined by Philips 400 T electron microscope at 60–80 KV.
2.4. Statistical analysis
Data obtained in the present investigation were statistically analyzed using the Least Significant Difference test (L.S.D) at 1% and 5% levels of probability (Snedecor and Cochran, 1967).
Doubling time:
where te = doubling time; ti = treatment time; to = zero time; b = cell no. of sample at treatment time; a = cell no. of sample at zero time.
3. Results
Preliminary tests using wide range of salinization treatments (mgL-1 of NaCl) were conducted on the growth of the studied alga Scenedesmus bijugatus (as mentioned in materials and methods) to avoid the non-effective and directly lethal concentrations of NaCl. The obtained results indicated that the most suitable concentrations of NaCl for this study were: 10, 50, 100, 200, 300 and 400 mg L−1. The recorded results in the present study were mean values of three replicates of determinations.
3.1. Salinization treatments
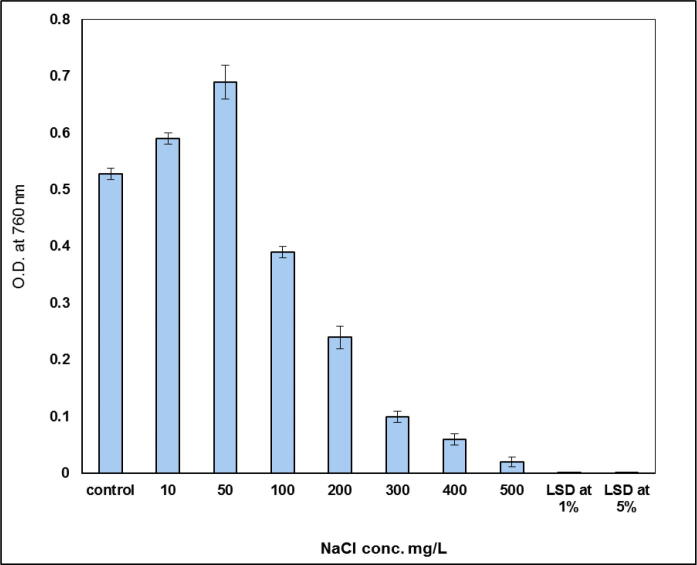
Salinization treatments were generally performed to monitor the tolerance of the green alga Scenedesmus bijugatus to salt stress. Fig. 1 revealed that Scenedesmus bijugatus was more sensitive to salinization treatments. Generally, growth of the alga was inhibited with increasing salinization levels. The obtained results indicated that growth of S. bijugatus was significantly stimulated by 8 and 10% at low NaCl concentration (10 and 50 mg L−1, respectively). Increasing salt level led to a dramatically inhibition in the growth of the studied alga.
Fig. 1.
Effect of salinity concentrations on the growth rate (O.D. at 760 nm) of S. bijugatus.
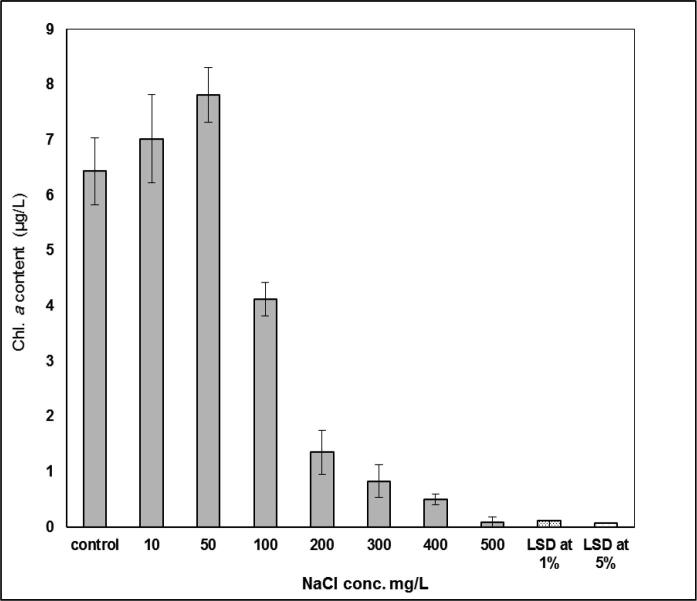
Accordingly, chl. a contents (Fig. 2) of S. bijugatus decreased with rise in the corresponding salinisation levels. It must be pointed out that at low concentration of NaCl (10 and 50 mg L−1) a marked stimulation in chl. a content took place by 7 and 10%, respectively. In general, chl. a content was gradually inhibited with increasing the levels of salinization above 50–500 mg L−1, respectively.
Fig. 2.
Effect of salinity concentrations on chlorophyll a content (µg L-1) of S. bijugatus.
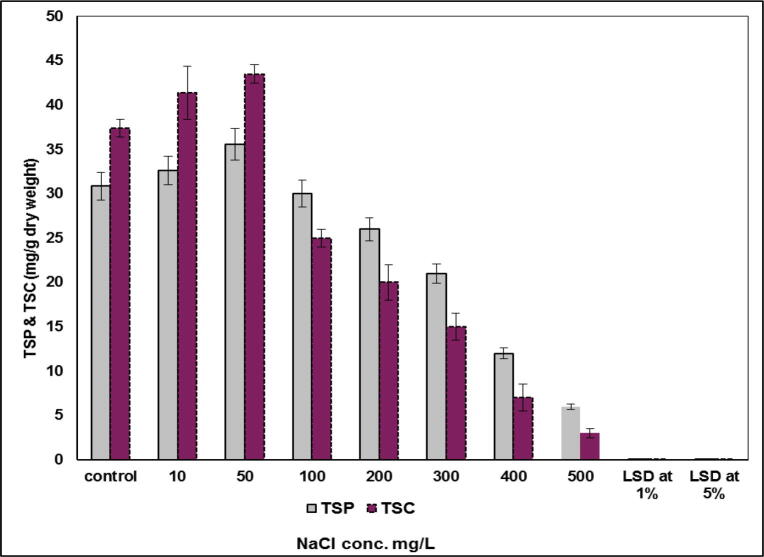
Fig. 3 showed the effect of different concentrations of NaCl on the total soluble proteins which showmen slightly stimulation (7 and 10%) at low levels of NaCl (10 and 50 mg L−1, respectively). Increasing NaCl level to 100 and 200 mg L−1 led to an inhibition in total soluble proteins to 4 and 12%, respectively). Sharp declines in total soluble proteins 30, 50 and 80% were observed at high concentrations of NaCl at 300, 400 and 500 mg L−1 respectively. Also, there is an increasing in the total soluble carbohydrates at low salinization level of 10 and 50 mg L−1 by 12 and 18%. However, increasing salinization levels led to gradual inhibition in total soluble carbohydrates reached to severely reduced total soluble carbohydrates by 93 and 96% at 400 and 500 mg L−1, respectively.
Fig. 3.
Effect of salinity (mg L-1) on total soluble proteins and total soluble carbohydrates (mg L-1 dry weight) of S. bijugatus.
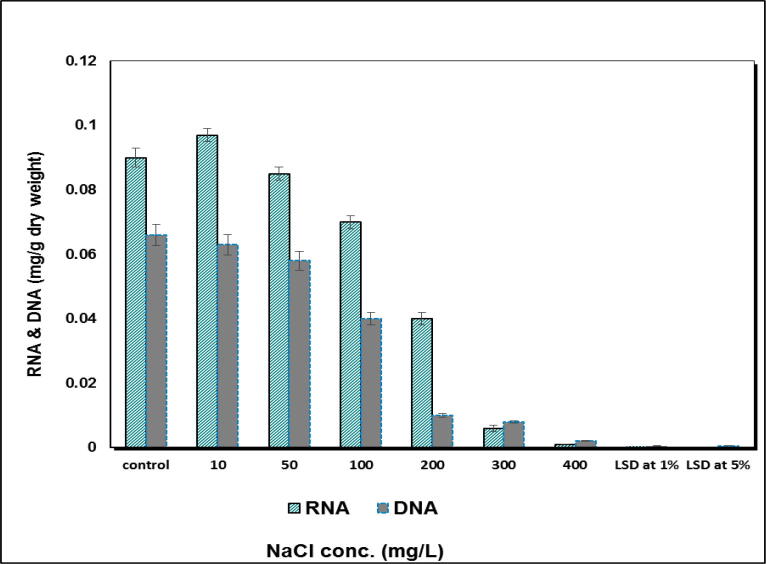
Regarding nucleic acids a significant decrease in their contents was recorded with the increase of salinization level (Fig. 4). RNA was slightly stimulated (5%) at low salinization level (10 mg L−1) followed by a dramatically inhibition at all studied NaCl concentrations. Similarly, DNA was highly inhibited at treatment by 10 and 50 mg L−1 NaCl treatment. However, a marked reduction in DNA values was noticed at 100, 200, 300 and 400 mg L−1 of NaCl concentrations.
Fig. 4.
Effect of salinity (mg L-1) on RNA & DNA (mg L-1 dry weight) of S. bijugatus.
Apparently, analysis showed a wide variation in amino acids content of the treated cultures at the pre-lethal NaCl concentration (300 mg L−1) throughout 8 days (Fig. 5). Analysis also, revealed that some of the detected amino acids were markedly inhibited while the others were slightly stimulated by salinization treatment. Data in Fig. 5 revealed that Asp. Acid, Pro, Cys, Val, Iso-leu, leu, Phe.ala and Lys were slightly stimulated with salinization treatment. On contrast the levels of amino acids: Thr, Ser, Glu.acid, Gly, Ala, Mth, His and Arg were markedly inhibited. It was noticeable that the amino acid Tyrosine was detected among the recorded amino acids upon treatment with 300 mg L−1 of NaCl, although, its none detected in untreated cultures alga (control).
Fig. 5.
Percentages of amino acids contents in S. bijugatus cells grown on nutrient medium treated by (300 mg L-1) of NaCL through 8 days.
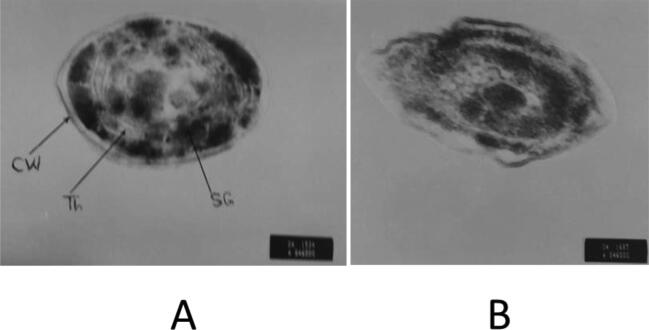
Regarding the transmission electron microscopy of S. bijugatus treated by 300 mg L−1 of NaCl throughout 8 days, data showing irregular cell wall shape, mixing of plastids and cytoplasmic contents, thylakoid break down and crystalline inclusions were found (see Plate 1).
Plate 1.
Electron micrograph of S. bijugatus cells, (A): untreated control and (B): cells after salinization treatment by 300 mg L−1 of NaCl (8 days) showing irregular cell wall shape, mixing of plastids and cytoplasmic contents, thylakoid break down and crystalline inclusions were found. X = 60000.
3.2. Effect of domestic wastewater
In the present investigation primary treatment carried out through the preliminary sieving step at station to get-rid of the large suspended solids, then subjected to sterilization through a Bacterial Filter in laboratory.
-
(a)
Wastewater quality
Physicochemical characteristics of some parameters of the domestic wastewater samples, under the experimental investigation were represented in Table 1. During the study period, the measured pH-value for wastewater samples ranged between 8 and 9.5, which lies mainly in the alkaline side. In addition, the electrical conductivity (E.C.) was estimated in the range between 1.09 and 1.8 mohs cm−1. The total soluble salts (T.S.S) of wastewater fluctuated within a relatively narrow range all over the period of study and the average estimated value ranged between 590 and 600 mg L−1. The bicarbonate level was measured in the range of 5.5–6.3 mg L−1 during the investigation period. The average reached 6 mg L−1. Apparently, the wastewater samples were completely depleted of any detectable carbonate. The chloride content of the water samples at the investigated site was relatively low and recorded an average of 2.5 mg L−1. The data presented in Table 1 showed that the sulphate content of wastewater was very low and estimated as 0.06 mg L−1 during the investigation period. The data further revealed that the detected cations were limited to Ca++ (2.03 mg L−1), Mg++ (2.41 mg L−1), Na+ (4.00 mg L−1) and K+ (0.65 mg L−1).
-
(b)
Wastewater affecting S. bijugatus
Table 1.
Average values in mg L−1 of some physicochemical characteristics of used wastewater sample.
| Parameter | Values | |
|---|---|---|
| Physical values | Water Color | Yellowish |
| Water Odor | Unacceptable | |
| Chemical values | pH | 9.5 ± 0.5 |
| E.C | 1.8 ± 0.5 m mohs cm−1 | |
| T. S. S | 590 ± 2.1 | |
| Cl¯ | 2.5 ± 0.02 mg L−1 | |
| SO4 | 0.05 ± 0.001 mg L−1 | |
| HCO3− | 5.8 ± 0.02 mg L−1 | |
| CO3− | – | |
| Ca++ | 2.03 ± 0.2 mg L−1 | |
| Mg++ | 2.41 ± 0.3 mg L−1 | |
| Na+ | 4.00 ± 0.4 mg L−1 | |
| K+ | 0.65 ± 0.3 mg L−1 | |
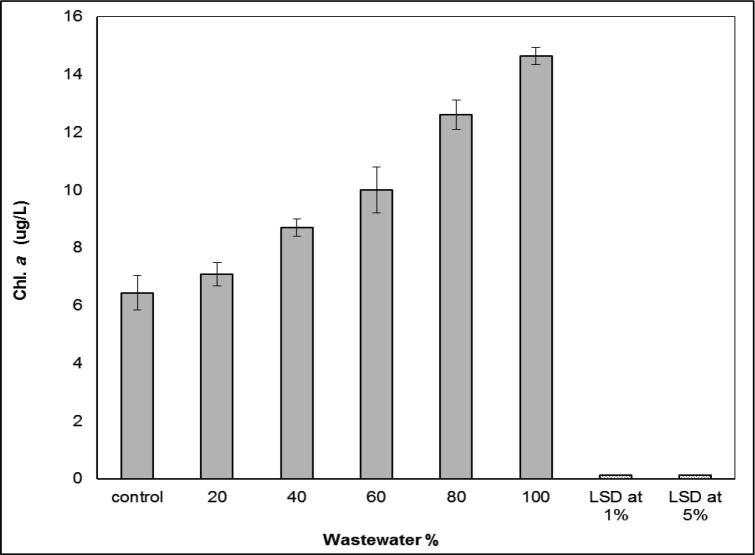
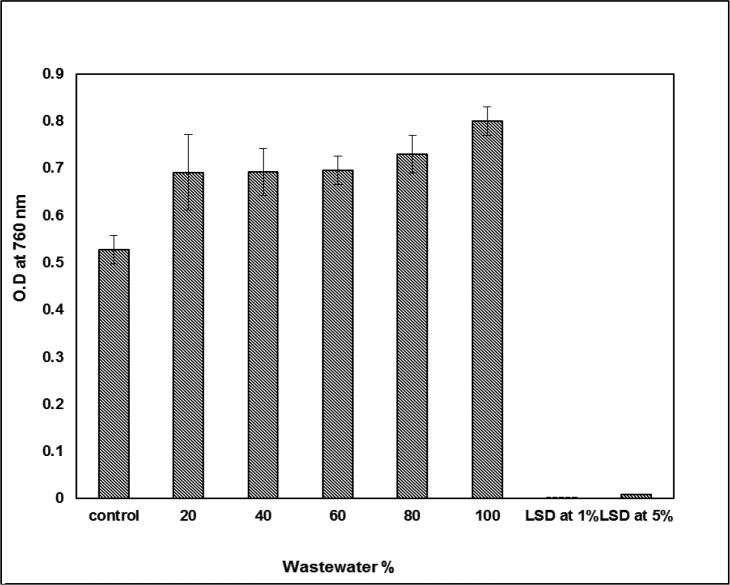
The treatment of the experimental alga S. bijugatus with wastewater showed a steady increase in the rate of growth which appeared by a clear optical density in Fig. 6. It was showed the stimulating effect of growth. On the other hand, the recorded results of the chlorophyll a contents also showed a high and steady elevation, which suggests that wastewater, is a stimulant supporting the growth of algae as shown in Fig. 7.
Fig. 6.
Effect of wastewater percentage on the growth of S. bijugatus (O.D. at 760 nm).
Fig. 7.
Effect of wastewater percentage on chlorophyll a content (µg L-1) of S. bijugatus.
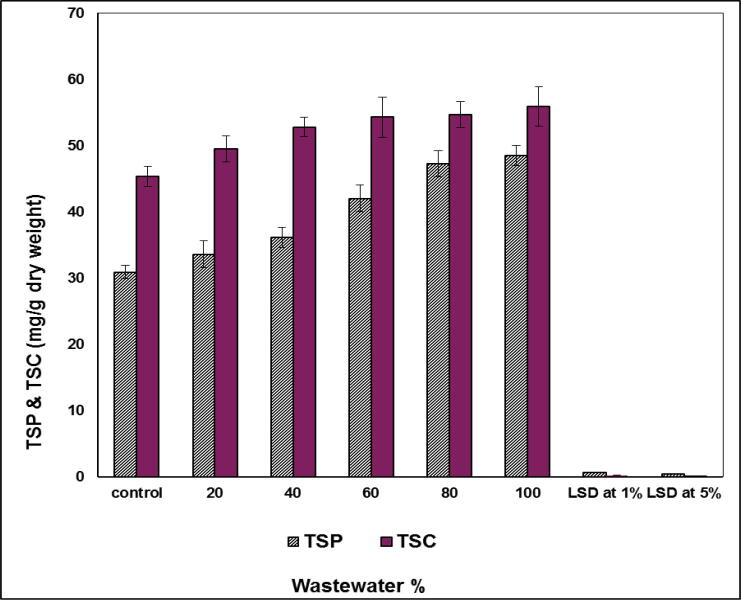
Data recorded in Fig. 8 revealed that total soluble proteins of S. bijugatus grown on nutrient media containing 20, 40 and 60% wastewater recorded an elevations in protein values reached 5, 10 and 18%, respectively. Further increasing in wastewater conc. to 80 and 100% exhibited high stimulation percentages of total soluble proteins to 35 and 45%, respectively. Similarly, the estimated total soluble carbohydrates increased gradually with the increase in wastewater percentage. At low doses of wastewater (20%) increasing of total soluble carbohydrates recorded 7%, while the highest content of total soluble carbohydrates 25% was exhibited at application of 100% wastewater treatment (see Fig. 8).
Fig. 8.
Effect of wastewater (mg L-1) on total soluble proteins and total soluble carbohydrates (mg L-1 dry weight) S. bijugatus.
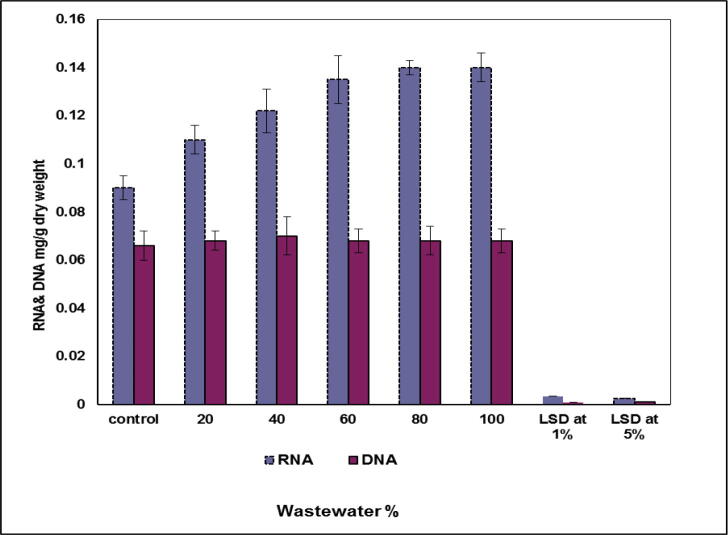
Regarding the studied DNA and RNA (Fig. 9), data revealed a significant stimulation in RNA values upon increasing wastewater conc. Whereas, RNA recorded a significant stimulation of 14, 26 and 35% in Scenedesmus cells grown on nutrient media containing 20, 40 and 60% of wastewater, respectively. Moreover, the high increase in the level of RNA value (37%) was exhibited in the studied algal cells growing in media contains 80 and 100% wastewater. On contrast, DNA exhibited a suppression effects (see Fig. 9).
Fig. 9.
Effect of wastewater concentration on RNA and DNA (mg g−1 dry weight) of S. bijugatus.
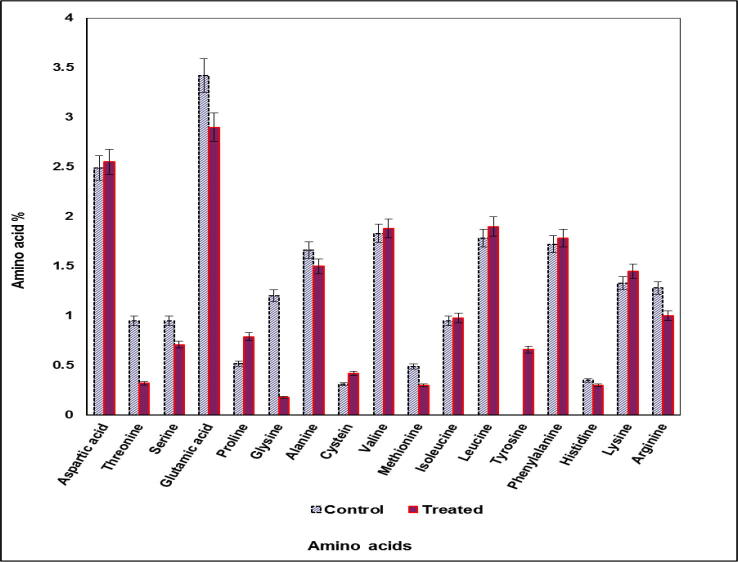
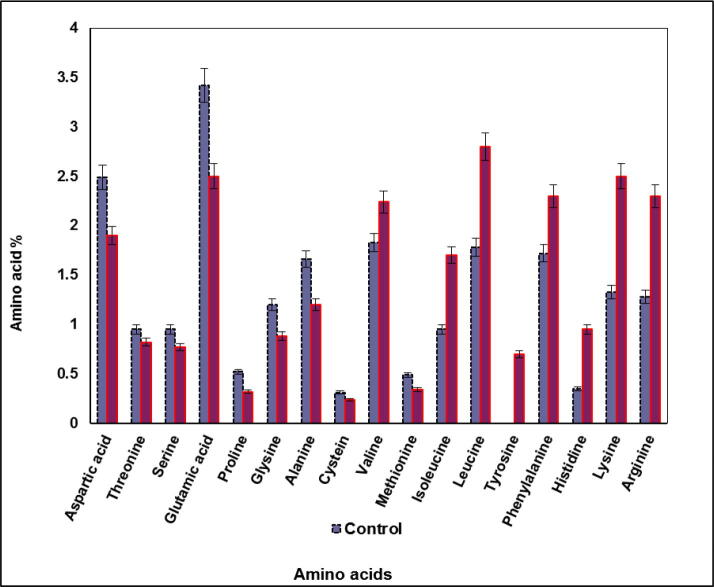
It is noteworthy that there were major striking differences in amino acid contents of S. bijugatus treated by wastewater comparable with untreated alga grown on synthetic nutrient medium (Fig. 10). Although data revealed that wastewater reduced the level of certain amino acids it also, stimulated synthesis other amino acids. Generally, stimulation biosynthesis of amino acids was higher than the inhibition ratio recorded in wastewater treated algal cultures. Whereas, Asp.acid, Thr, Ser, Glu.acid, Pro, Gly, Ala, Cys, Mth were hardly affected by wastewater. In contrary accumulation of Val, Iso-leu, Leu, Phe.ala, His, Lys and Arg were significant encouraged by wastewater treatment. Tyrosine was detected among the recorded amino acids upon treatment with 100% wastewater, although, it is none detected in untreated culture.
Fig. 10.
Percentages of amino acids content of S. bijugatus grown on 100% wastewater for 8 days.
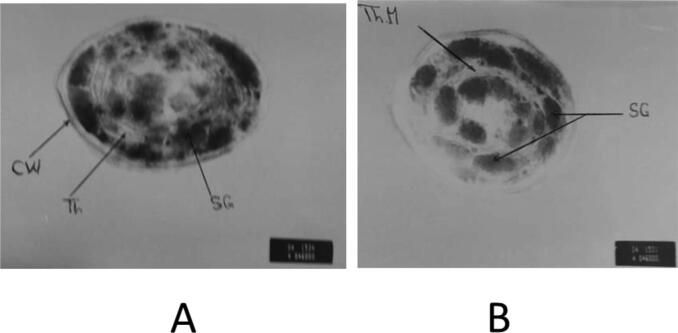
Regarding the transmission electron microscopy of S. bijugatus cell; grown on wastewater medium for 8 days (Plate 2) revealed that wastewater induced a slight change in the treated S. bijugatus cell such as increasing in starch granules and presence of thylakoid membranes, although not as clear as in the control.
Plate 2.
Electron micrograph of S. bijugatus cells, (A) untreated control and (B): cells treated with 100% wastewater for 8 days, showing increase in starch granules and presence of thylakoid membranes although not as clear as in the control. X = 60000.
4. Discussion
Salinity imposes significant stresses in various living organisms including microalgae. Salinity represents one of the most important factors, which exert stress, or even injury of the metabolism of algal cell. The obtained data (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5) indicated that S. bijugatus was sensitive to salinization treatments. Whereas, the stimulation effect of salinization appeared on the total soluble carbohydrates and the total soluble proteins of the investigated alga. Although many species of micro-algae are tolerant to great variations in salinity, their chemical composition can be affected (Lanping et al., 2013). The increase in total protein content in algae subjected to salinity stress conditions have reported by many authors (Ashraf, 1989, Cowan and Rose, 1991). This may be due to suggestions that salinity stresses enhance protein synthesis (Langdale et al., 1973, Mishra et al., 2008, El.Din, 2015). According to Rafiqul et al., 2003, Ghezelbash et al., 2008 suggested that the algae adapted to salinity stress by increasing carbohydrates in the cells.
The collected data further reveal that non-halotolerant organisms like S. bijugatus is unable to retain its metabolic activities. It is evident that most freshwater algae show low growth and survival in high salinity concentrations and are generally unable to survive beyond a low threshold of salinity (Shetty et al., 2019b). Many photoautotrophs show a considerable decrease in metabolic activity under rising NaCl effort. This lowering can be attributed to a shortage in different cations, production of (ROS) and osmotic pressure, which interrupts with various metabolic activities (Shetty et al., 2019b). Hasaneen et al. (2008) reported that compatible osmolytes such as glycine betaine, choline and proline are synthesized in response to salt stress. These osmotic adjustments protect subcellular structure and reduce oxidative damage caused by free radicals produced in response to stress of high salinity (Hong et al., 2000).
The stimulation behavior at NaCl concentration justify the findings of Hagemann et al., (1990), who reported that microalgal cells have adopted to synthesis and accumulate osmo-protective compounds to achieve an equilibrium of osmotic potential, so increasing carbohydrates proteins, RNA at certain concentrations may be attributed to the above previous reasons.
Increasing of RNA contents which displayed at 10 mg L−1 of NaCl for S. bijugatus, a trend that was reversed with increasing salinity concentrations may be considered as a tool for defense mechanism against salinization while the inhibition of DNA accumulation of the studied alga may be attributed to direct or indirect interfering of salinization effect on metabolic conversion. On the other hand, the detected individual amino acids of S. bijugatus that were treated by (300 mg L−1) of NaCl revealed that salinity may be interfering with synthesis and/or degradation of the detected amino acids, because salinity stimulated certain amino acids and inhibited to others.
In this investigation the effect of 300 mg L−1 of NaCl for 8 days on the unicellular green alga S. bijugatus was studied by employing transmission electron microscopy. Examination of S. bijugatus showed an increase in starch granules, shrinkage of cell contents which led to clear separation between the cell components and cell wall, absence of thylakoid membranes and complete disorganization of the cell contents (see Plate 1). It is agreed with Kirst (1989) how report that, under conditions of extreme hypo- or hyperosmotic stresses the photosynthesis and respiration are inhibited in all algae investigated which leads to loosing of cell contents.
Although wastewater was studied as an environmental stress, the obtained results revealed that wastewater appeared as a stimulatory agent for growth and the studied macro-molecules of the investigated alga such as increasing of total soluble proteins and total soluble carbohydrates. Data revealed that undiluted wastewater (100%) achieved the highest levels of growth and the studied cellular macro-molecules of Scenedesmus bijugatus. These results are in agreement with (Shelef et al., 1978, Goldman, 1979, Gross et al., 1986, Mohammed, 1994, Hammouda et al., 1995), who found that wastewater is very rich in phosphorus, nitrogen and other compounds which are necessary for algal growth. Accordingly, in the presence of excess nutrients, the algae are capable of rapid growth and multiplication so, the photosynthetic activity increased which led to increasing the total soluble carbohydrates (El-Nabarawy and Welter, 1984).
Ultrastructure examination of S. bijugatus which grown on undiluted wastewater for 8 days, respectively showed a general increase in starch granules inside the cells (see Plate 2). Such observation could be explained by the increase of total soluble carbohydrates in the studied alga.
5. Conclusion
Pollution in water resources, from permitting agricultural drainage, factory drainage, and sewage, increases the risk of these water resources to normal life and natural flora in these sources. The current study demonstrated that increasing the salinity resulting from polluted drainage of waterways leads to a fatal effect on algae, a group of organisms that are used as evidence of pollution. The study also confirmed the seriousness of different types of water drainage, which is exclusive to water sources without treatment, which leads to pollution of waterways, thus increasing pollution, which is characteristic of the risk to water flora. Therefore, I recommend preserving water sources by preventing the disposal of water wastes, whether agricultural or industrial, in waterways without emphasized treatment.
6. Consent for publication
Not applicable.
7. Availability of data and materials
All data generated or analyzed during this study are included in this published article.
8. Authors' contributions
Not applicable.
Funding
Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This publication was supported by the Deanship of Scientific Research at Prince Sattam Bin Abdulaziz University, Alkharj, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Raouf, N., Ibraheem, I.B.M., Hammouda, O., 2003. Eutrophication of River Nile as indicator of pollution. In: Al-Azhar Bull. of Sci., Proceeding of 5th Int. Sci. Conf., 25–27.
- Abdel-Raouf N., Al-Homaidan A.A., Ibraheem I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012;19(3):257–275. doi: 10.1016/j.sjbs.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikary S.P. Growth measurements by monitoring light scattering of a filamentous blue-green alga which does not give uniform and stable suspension in culture vessels. Zeitschrift für Allg. Mikrobiologie. 1983;23:475–483. [Google Scholar]
- Amenorfenyo D.K., Huang X., Zhang Y., Zeng Q., Zhang N., Ren J., Huang Q. Microalgae brewery wastewater treatment: potentials, benefits and the challenges. Int. J. Environ. Res. Public Health. 2019;16(11):1910. doi: 10.3390/ijerph16111910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M. The effect of NaCl on water relations, chlorophyll protein and proline contents of two cultivars of Black gram. Plant Soil. 1989;119:205–210. [Google Scholar]
- Boboescu I.Z., Llie M., Gherman V.D., Mirel I., Pap B., Negrea A., Kondorosi E., Biro T., Maroti G. Revealing the factors influencing a fermentative biohydrogen production process using industrial wastewater as fermentation substrate. Biotechnol. Biofuels. 2014;7:139. doi: 10.1186/s13068-014-0139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem., J. 1956;62:315. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.Y., Wonk K.H., Wong P.K. Nitrogen and phosphorus removal from sewage effluent with high salinity by Chlorella salina. Environ. Pollut. 1979;18:39. [Google Scholar]
- Collotta M.P., Champagne W.M., Tomasoni G. Wastewater and waste CO2 for sustainable biofuels from microalgae. Algal Res. 2018;29:12–21. doi: 10.1016/j.algal.2017.11.013. [DOI] [Google Scholar]
- Cowan K.A., Rose D.P. Abscisic acid metabolism in salt-stressed cells of Dunaliella salina. Plant Physiol. 1991;97:798–803. doi: 10.1104/pp.97.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernas K. The use of selected algal tests in determining the influence of potassium and sodium on the eutrophication of waters. Acta Hydro. 1978;20:323. [Google Scholar]
- Dische, E.L., 1953. J. Am. Chem. Soc., 22, 3014 (cited in physiological studies on the herbicide cotoran, S.S.; Roushdy, 1983 , M.Sc. Thesis , Fac. of Sci., Ain-Shams Univ., 131).
- El.Din S.M. Effect of seawater salinity concentrations on growth rate, pigment contents and lipid concentration in Anabaena fertilissma. Egyptian Soc. Environ. Sci. 2015;11(1):59–65. [Google Scholar]
- El-Nabarawy, M.T., Welter, A.N., 1984. Utilization of algal cultures and assays by industry. (Algae as ecological indicators edited by L.Elliot Shubert) Academic Press. Inc. London, 32. pp.
- El-Nawawy A.S., Lotfi M., Fahmy M. Studies on the ability of some blue-green algae to fix atmospheric nitrogen and their effect on growth and yield of paddy. Argic. Res. Rev. 1958;36(2):308–320. [Google Scholar]
- Frank G., Wegmann K. Physiology and biochemistry of glycerol biosynthesis in Dunaliella. Biol. Zbl. 1974;93:707. [Google Scholar]
- Ghezelbash F., Farboodnia T., Hedari R., Agh N. Biochemical effect of different salinities and luminance on green microalgae. J. Biolog. Sci. 2008;2:217–221. [Google Scholar]
- Goldman J.C. Outdoor algal mass cultures II, Photosynthetic yield limitations. Water Res. 1979;13:119–136. [Google Scholar]
- Gross R., Schoeneberger H., Gross U. The nutritional quality of Scenedesmus acutus in a semi-industrial plant in Peru. J. Environ. Pathol-Toxicol. Oncol. 1986;6(5–6):47–57. [PubMed] [Google Scholar]
- Hagemann M., Wölfel L., Krüger B. Alterations of protein synthesis in the cyanobacterium synechocystis sp. PCC 4803 after a salt shock. J. Gen. Microbiol. 1990;136:1393–1399. [Google Scholar]
- Hammouda O., Gaber A., Abdel-Raouf N. Microalgae and wastewater treatment. Ecotoxicol. Environ. Saf. 1995;31:205–210. doi: 10.1006/eesa.1995.1064. [DOI] [PubMed] [Google Scholar]
- Han W., Huang J., Zhao H., Li Y. Continuous biohydrogen production from waste bread by anaerobic sludge. Bioresour. Technol. 2016;212:1–5. doi: 10.1016/j.biortech.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Hasaneen M.N.A., Youns M.E., El-Bialy D.M. Plant growth metabolism and adaptation in relation to stress conditions. Plant Sci. 2008;17:320–328. [Google Scholar]
- Hong K., Zhang Z., Verma D. Removal of feedback inhibition of pyrroline- 5- carboxylate synthetase results in increased proline accumulation and protection of plants form osmotic stress. Plant Physiol. 2000;122:1129–1136. doi: 10.1104/pp.122.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirst G.O. Salinity tolerance of eukaryotic marine algae. Annu. Rev. Plant Physiol. Plant Mol. Bioi. 1989;40:21–53. [Google Scholar]
- Kuo C.M., Jian J.F., Lin T.H., Chang Y.B., Wan X.H., Lai J.T. Simultaneous microalgal biomass production and CO2 fixation by cultivating Chlorella sp. GD with aquaculture wastewater and boiler flue gas. Bioresour. Technol. 2016;221:241–250. doi: 10.1016/j.biortech.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Langdale G.W., Thomas J., Littlelon T.G. Nitrogen metabolism of star grass as affected by nitrogen and soil salinity. Agron. J. 1973;65:468–480. [Google Scholar]
- Lanping D., Yuanyuan M., Bingxin H., Shanwen C. Effect of salinity and temperature on growth and pigment contents in Hypnea Cervicornis. Bio. Res. Inter. 2013;10:1–8. doi: 10.1155/2013/594308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Pan J., Yan M., Tang J., Qin W., Liu Y. Treatment of fracturing wastewater using microalgae-bacteria consortium. Can. J. Chem. Eng. 2019;98:484–490. doi: 10.1002/cjce.23631. [DOI] [Google Scholar]
- Lowery O.H., Rosebrought N.J., Furr A., Randall R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mercer E.N., Birbeck M.S. 2nd Ed. Black Well Scientific Publications; Oxford: 1966. “Electron Microscopy”. A. Hand Book for Biologists. [Google Scholar]
- Mishra A., Mandoli A., Jha B. Physiological characterization and stress-induced metabolic responses of Dunaliella salina isolated from salt pan. J. Ind. Microbiol. Biotechnol. 2008;35:1093–1101. doi: 10.1007/s10295-008-0387-9. [DOI] [PubMed] [Google Scholar]
- Mohammed, N.A., 1994. Application of algal ponds for wastewater treatment and algal production. M.Sc. Thesis. Fac. of Sci. Cairo Univ. (Beni-Suef branch).
- Morse J.L., Carter C.F. The synthesis of nucleic acid in cultures of Escherchia coli, strains band B / R. J. Bacterial. 1949;58:317. doi: 10.1128/jb.58.3.317-326.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiqul I.M., Hassan A., Sulebele G., Orosco C.A., Jalal K.C. Salt stress culture of blue green algae. Pakistan J. Sci. 2003;6:648–650. [Google Scholar]
- Rai M.P., Gautom T., Sharma N. Effect of Salinity, pH, Light Intensity on Growth and Lipid Production of Microalgae for Bioenergy Application. On Line J. Biol. Sci. 2015;15(4):260–267. [Google Scholar]
- Reddy V., Amulya M., Rohit K., Sarma P.M., Venkata-Mohan S. Valorization of fatty acid waste for bioplastics production using Bacillus tequilensis: Integration with dark-fermentative hydrogen production process. Int. J. Hydrogen Energy. 2014;39:7616–7626. doi: 10.1016/j.ijhydene.2013.09.157. [DOI] [Google Scholar]
- Reynolds E.S. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell Biol. 1963;17:208. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijstenbil J.W., Mur L.R., Wijnholds J.A., Sinke J.J. Impact of a temporal salinity decrease on growth and nitrogen metabolism of the marine diatom Skeletonema costatum in continuous cultures. Mar. Biol. 1989;101:121–129. [Google Scholar]
- Rijstenbil J.W., Wijnholds J.A., Sinke J.J. Implications of salinity fluctuations for growth and nitrogen metabolism of the marine diatom Ditylum brightwillii in comparison with Skeletonema costatum. Mar. Biol. 1989;101:131–141. [Google Scholar]
- Said A., Ramzy M.A. Sucrose determination as a mean of estimations of the “Draw Bak Tax” on exported halawa tehinia. Bull. Fac. of Sci. Cairo Univ. 1964;39:209. [Google Scholar]
- Schmidt C., Thannhauser S.J. A method for the determination of deoxyribonucleic acid, ribonucleic acid and phosphorproteins in animal tissues. J. Biol. Chem. 1945;161:83. [PubMed] [Google Scholar]
- Shelef G., Moraine R., Oron G. Photosynthetic biomass production from sewage. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1978;11:3–14. [Google Scholar]
- Shetty, P., Boboesuc, I.Z., Pap, B., Wirth, R., Kovacs, K.L., Biro, T., Futo, Z., White, R.A., Maroti, G., 2019a. Exploitation of Algal-Bacterial Consortia in Combined Biohydrogen Generation and Wastewater Treatment. Front. Energy Res., doi: 10.3389/fenrg.2019.00052.
- Shetty P., Gitau M.M., Maróti G. Salinity Stress Responses and Adaptation Mechanisms in Eukaryotic Green Microalgae. Cells. 2019;8(2):1657. doi: 10.3390/cells8121657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor, G.W., Cochran, W.G., 1967. Statistical methods. 6th Ed. Iowa State Univ. Press. Ames. Iowa, USA, P. 275.
- Staub R. Ernähr unzsphysiologish autökologische unter suchungen an der planktischen blau-alg Osillatoria rubescens D.C. Schweiz. Zeitschr. Fur Hydrologie. 1961;23:82–198. [Google Scholar]
- Strickland J.D.H., Persons T.R. A practical handbook of seawater analysis. Bull. Fisheries Res. Board Canada. 1968;167:1–311. [Google Scholar]
- Strickland, J.D.H., Persons, T.R., 1972. A practical handbook of seawater analysis - 2nd Ed. Bull. Fish. Res. Bd. Canada, 167-311.
- Tayar S., Abed R., Abed A. Investigation on physicochemical properties of wastewater grown microalgae methyl ester and its effects on CI engine. Environ. Climate Technol. 2020;24(1):72–87. [Google Scholar]
- Umbreit, W.W., Burris, R.H., Stauffer, J.F., Cohen, P.P., Johnsen, W.J., Leepage, G.A., Patter, V.R., Schneider, W.C., 1969. Manometric techniques: A manual describing methods applicable to the study of tissue metabolism. Published by Minneapolis, Burgess Publishing Company. Antiquariat Dr. Götzhaber (Luckenbach, Germany).
- Whitton R., Ometto F., Pidou M., Jarvis P., Villa R., Jefferson B. Microalgae for municipal wastewater nutrient remediation: mechanisms, reactors and outlook for tertiary treatment. Environ. Technol. Rev. 2015;4(1):133–148. [Google Scholar]
- Winder K., Eggum O.B. Protein hydrolysis. A description of the method used at the department of animal Physiology in Copenhagen. Acta Agric. Scondinavia. 1966;16:115. [Google Scholar]
- Wirth R., Lakatos G., Bojti T., Maroti G., Bagi Z., Rakhely G., Kovacs K.L. Anaerobic gaseous biofuel production using microalgal biomass – a review. Anaerobe. 2018;52:1–8. doi: 10.1016/j.anaerobe.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Wong M.H., Pak D.C.H. Removal of Cu2+ and Ni2+ by free and immobilized microalgae. Biomed. Environ. Sci. 1992;5:99–108. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.