Abstract
Red palm weevil (Rhynchophorus ferrugineus) is a voracious pest of date palm worldwide. Pakistan ranks sixth in date palm production globally. Losses to date palm plantations in Pakistan sometimes surpass 10%-20%. Most of the traditional management strategies used by farmers have been found insignificant to combat this voracious pest. The entomopathogenic fungi, Beauveria bassiana [QA-3(L) and QA-3(H)] and insecticides, Nitenpyram (Active 10% SL) [NIT (L) and NIT (H)] were applied to larval (2nd, 4th, and 6th), pupal and adult stages of R. ferrugienus. Integration or alone application of fungi with insecticides at different concentration under laboratory conditions. Combined application was depicted additive and synergistic interactions. Contrarily, highest cumulative mortality (100%) was recorded in 2nd instar larvae as compared to later instar larvae at combined application. The maximum pupal and adult mortality remained 89% and 66% respectively after treatment with [QA-3 (H) + NIT (L)]. The combination of B. bassiana at higher concentration whereas Nitenpyram at lower dose was found more lethal to larvae, pupae and adults of R. ferrugineus. This signifies the need of combining B. bassiana and bio-rational insecticides that can reduce the cost of management with least harm to environment and natural enemies.
Keywords: Rhynchophorus ferrugineus, Date palm, Entomopathogenic fungi, Bio-rational insecticides
1. Introduction
Date palm (Phoenix dactylifera L.) is an economically important fruit crop worldwide owning to high nutritional values and economic benefits (Shabani et al., 2016). Among different dates producing countries, Pakistan ranks at sixth position (FAO, 2014). A country with the facet problems of low nutrition availability per capita can use cultivation of date palm to meet the required intake level (Hassan et al., 2006). In Pakistan, around 10–20% of annual production is compromised due to several contributing factors (Baloch et al., 1992) including insect pests palm (Wakil et al., 2015). Among notable insects damaging date palm, Red Palm Weevil, Rhynchophorus ferrugienus (Coleoptera: Curculionidae) appear to be one of cryptic insect rather difficult to manage by traditional approaches (El-Sufty et al., 2007; Arab and El-Deeb, 2012). R. ferrugineus is voracious feeder of date palm, coconut palm and oil palms in the world (Saleem et al., 2019). R. ferrugineus damages greater than 29 different palm species in economic trade zones of date palm particularly in Africa, South East Asia and Middle East (Wakil et al., 2015). For 30 years, this invasive pest is causing economic losses in date palms (Güerri-Agulló et al., 2010). Its infestation was recorded in 50% of date producing countries (Yasin et al., 2017), causing yield losses up to 0.7–10 tons/ha (Singh and Rethinam, 2005). In Pakistan, date palm producing provinces like Sindh and Balochistan have several report of RPW in orchids as well newly developed residential colonies where date palm is extensively grown as ornamentals (Wakil et al., 2015). However, on the basis of personal visual observation in Punjab, RPW infestation upto Multan are on the board, but in upper Punjab i.e. Faisalabad, Lahore and Islamabad are yet free of infestation.
Owing to concealed feeding behavior, R. ferrugineus is very difficult to monitor and manage (Arab and El-Deeb, 2012, Dembilio and Jaques, 2015, Faleiro et al., 2016). Different approaches for the control of this pest practiced in the past such as: proper phyto-sanitation, pheromone trapping (Abdel-Moety et al., 2012), bio-control and microbial agents (Paoli et al., 2014), and application of fumigating, injecting, and spraying techniques are being used for suppress the RPW populations (Al-Rajhy et al., 2005). With the concealed feeding habit, traditional strategies are not enough to manage this pest whereas insecticides are highly criticized for resistance development, environmental hazards and health concerns (Abraham et al., 1998).
Neonicotinoid insecticides are economically most important at worldwide to reduce the infestations of insect pests (Jeschke et al., 2011), and are better option in integrated pest management programs. Beauveria bassiana sensu lato (Balsamo) Vuillemin (Hypocreales: Clavicipitaceae) is an effective alternate to curtail R. ferrugineus. It is low cost, environment friendly, and safe for non-target insects (Hussain et al., 2013). With the ability to readily capture the host defence and degrade cuticle (Hussain et al. 2010), high adoption rate (Hussain et al., 2010), hydrophobic ability (Sevim et al., 2012), entompathgenic fungi is a priceworthy choice.
Present study focuses on the integration of insecticide with B. bassiana to lower the cost of production as well as to target the insect pest effectively. Conventional management practices of using insecticides are simply escaped by concealed feeding habit of RPW. Previous studies on managing RPW mainly focus on use of insecticides or entopathogens separately. B. bassiana has the ability to synergise the action of synthetic insecticides and offer an integrated control of RPW (Anderson et al., 1989, Malik et al., 2016). B. bassiana can challenge the voracious and widespread damage of RPW and its integration with bio-rational insecticides can enhance the spectrum of management with no harm to environment and natural enemies.
2. Material and methods
2.1. Isolation, cultivation and preparation of fungal isolates
In order to sort out local strains of entomopathogenic fungus (EPF), the soils around Multan, Pakistan was sampled extensively. The damp soil that remains uncultivated/undisturbed was found inhabiting the entomopathogens particularly B. bassiana. After removing the top layer of soil (10 cm) containing debris and rocks, about half kg of soil was taken with sampling auger in plastic zipper bags, transferred into laboratory and stored at 4 °C. Shade dried soil was ground into fine powder and sieved out. About 10 g of soils was taken from each sample and placed in plastic petri plates (9 cm dia). Galleria mellonella 2nd instar larvae were buried in each petri plate. Fungus appearing on susceptible larvae after few days of incubation was isolated using method described by Zimmermann, 1986, Zimmerman, 1998). From the infected insect, spores were collected and shifted to nutrient media plates (PDA) for mass culturing. Single spore method was used to purify the fungal culture. Morphological characters of spores, cottony growth and colony color was used to identify the fungal isolates (Barnett and Hunter, 1999). Extensive preliminary studies were conducted to isolate, purify and mass-culture of the test strain of B. bassiana. Other opportunistic and less virulent fungal strains found during the study are not mentioned in this manuscript.
2.2. Collection and maintenance of R. Ferrugineus
Larvae and adult R. ferrugineus were collected from infected date palm plantation in Bahawalpur (29.3544° N, 71.6911° E) and Multan (30.1575° N, 71.5249° E). Infected plants were sensed with the visual observation and feeding sound of R. ferrugineus by ear touching the stem. R. ferrugineus culture was established from larvae and adults reared in plastic cages (30 × 60 × 60 cm) in the Insect Rearing Laboratory, Institute of Plant Protection, MNS University of Agriculture, Multan. Shredded sugarcane pieces (6-8″) were offered to weevils for feeding, oviposition and hatching (Saleem et al., 2019). Batch of five pairs of adult weevils were set to mate and oviposit on sugarcane sets (24 cm). Clean, fresh and infestation free sugarcane stem pieces were offered as diet to the growing larvae of R. ferrugineus. For egg harvesting, the adults of both sexes were kept on sugarcane sawdust and egg collection recorded every 2 days. Laboratory condition was maintained at 25 ± 2 °C and relative humidity 65 ± 5%, 12:12 D:L photoperiod (Kaakeh, 2006).
2.3. Compatibility of B. Bassiana and Nitenpyram
Nitenpyram (Active) 10% AS provided by Agro Mart of Warble Pvt Ltd. Pakistan) was used to integrate with EPF (@ 250 and 500 μL l−1). Nitenpyram is a fast acting, orally administered systemic insecticide readily available for uptake after feeding. Fungal growth media (PDA) after autoclaving at 121 °C for 20 min was cooled down and two concentrations of insecticide (250 and 500 μL l−1) were added to the media, shaken for 2 min to homogenize the contents. About 10 ml of the nutrient media was filled in each petri plate and 1 ml of fungal suspension (1 × 107 conidia ml−1) was uniformly spread with the help of sterile spatula to find out their compatibility. For each concentration of Nitenpyram (or control), five petri dishes were used to consider the germination whereas a pure nutrient media served the purpose of control. Biological index (BI) scale was planned by Alves et al. (2007) taken as standard for categorizing the compatibility or lethality of different chemicals on in vitro growing EPF. The Biological Index-values were determined on the basis of formula: BI = [47×(VG) + 43×(SP) + 10×(GR)]/100. The BI > 66 was regarded as compatible when 42 ≤ BI ≤ 66 as moderately toxic and BI < 42 as toxic (Alves et al., 2007).
2.4. Impact of B. Bassiana and Nitenpyram on development and survival of R. Ferrugineus
Second, fourth and sixth instar larvae, pupae and adults of R. ferrugineus were evaluated for insecticidal nature. Laboratory bioassays was carried out using B. bassiana (QA-3) (1 × 107, 1 × 108 conidia ml−1), and Nitenpyram (@ 250 and 500 μL l−1) in individual and combined form. Both larvae and were treated in a batch (n = 12). Larvae of each instar were directly exposed in fungal solution for 60 s and adults for 90 s (Dembilio et al., 2010), air dried in sterilized Petri plate for 10 min (2.5 cm diameter) lined with moist filter paper (Ma et al., 2008). Untreated larvae and adults were dipped in distilled water having 0.01% Tween 80 and placed individually in screw cups (150 ml) with a central hole closed with a fire-glued metallic fine-knit mesh and left to feed on sugarcane pieces. Larvae kept in the dark in a controlled room at 25 ± 2 °C and 65 ± 5% R.H. Mortality was observed after 5, 10, 15 and 20 days of application, and pupal and adult mortality were recorded after seven days of treatments. Experiment was repeated thrice with four replications. From surviving insects, pupation, adult emergence was recorded.
2.5. Statistical analysis
Data recorded in percentages were arcsine transformed to meet the normality assumption and means were put to analysis of variance in Minitab software (Minitab, 2007). Treatment mean significance was determined in Tukey’s HSD test (0.05%) (Sokal and Rohlf, 1995).
3. Results
3.1. Fungal isolations
B. bassiana [4 isolates; QA-4, QA-3, QA-2 and QA-1] identified on the basis of morphological characteristics was used for assay. Fungal conidia growing in clusters in a specific snowball shape and flask shaped basal section (zig-zag geniculate rachis) were marked as B. bassiana (Elllis et al., 2001). All isolates of Bb were exposed to 2nd instar of R. ferrugineus for screening and only one of these was found compatible and virulent showing mortality > 80%. The median lethal concentrations were determined to find their virulence. LC50 values for isolate QA-3 [1.80 (1.36–2.45)], LC90 [5.54 (3.53–12.31)] were found quite lower than the other isolates.
These isolates were tested for compatibility with EPF (B. bassiana) using biological index scale by spreading fungal suspension on PDA media plates containing Nitenpyram mixed during media preparation. The B. bassiana isolate QA-3 was found compatible as compared to other isolates were moderately toxic (QA-1 and QA-2), while QA-4 was found non-compatible/toxic. Further study for mortality was conducted with this selected isolate (Table 1).
Table 1.
Median lethal concentrations (μL L-1) and biological index of four different isolates of Beauveria bassiana.
| EPF isolate | LC50 | LC90 | Chi sq | Slope | Biological Index | Compatibility to EPF |
|---|---|---|---|---|---|---|
| QA-1 | 5.79 (3.08–4.92) | 12.72 (6.54–36.15) | 3.4 | 2.15 ± 0.61 | 60.17 | Moderate |
| QA-2 | 3.01 (1.67–2.52) | 6.82 (4.55–16.30) | 2.27 | 3.76 ± 0.73 | 64.53 | Moderate |
| QA-3 | 1.80 (1.36–2.45) | 5.54 (3.53–12.31) | 3.83 | 4.28 ± 0.62 | 83.61 | Compatible |
| QA-4 | 4.53 (2.82–34.94) | 16.32 (6.24–226.77) | 0.22 | 3.39 ± 1.29 | 40.12 | Toxic |
EPF = entomopathogenic fungus; LC50 = lethal concentration to kill the 50% of tested population; LC90 = lethal concentration to kill the 90% of tested population.
3.2. Virulence of B. Bassiana and Nitenpyram to R. Ferrugineus
Bioassays for larvae were conducted on 2nd, 4th and 6th instar, pupa and adult of R. ferrugineus using B. bassiana and Nitenpyram individual and their possible combinations. Significant differences were observed among treatments and larval instars. The insect mortality was increased in concentration dependent manner whereas decrease in pupation and adult emergence was recorded vice versa (Table 2). Second instar larvae were found more susceptible than fourth and sixth instar larvae as mortality was found linearly related to developmental stage. Larvae were exposed to QA-3 (1 × 108 conidia ml−1) high concentration and low concentration of Nitenpyram (250 μL l−1) were provided synergistic effect, compatible for integrated management of R. ferrugineus (Tables 2, 3, 4). Maximum mortality (100%) was observed for 2nd instar larvae as compared to 4th and 6th instar. Similar observations were recorded at same concentrations against the pupa and adults of R. ferrugineus (Table 5). Maximum pupal and adult mortality was 89% and 66% was recorded.
Table 2.
Mean mortality (%±SE) of 2nd instar larvae of Rhynchophorus ferrugineus treated with Beauveria bassiana (QA-3), and Nitenpyram (NIT). B. bassiana was used each @ 1 × 107 and 1 × 108 conidia/mL and Nitenpyram was applied @ 250 μL L−1 and 500 μL L−1.
| Treatment | Interval | Observed mortality | Expected mortality | Type of interaction |
|---|---|---|---|---|
| QA-3 (L) | 5d | 6.25 | – | – |
| 10d | 8.52 | – | – | |
| 15d | 17.43 | – | – | |
| 20d | 25.76 | – | – | |
| QA-3 (H) | 5d | 10.42 | – | – |
| 10d | 15.34 | – | – | |
| 15d | 21.40 | – | – | |
| 20d | 34.47 | – | – | |
| NIT (L) | 5d | 16.67 | – | – |
| 10d | 21.78 | – | – | |
| 15d | 34.66 | – | – | |
| 20d | 47.92 | – | – | |
| NIT (H) | 5d | 21.02 | – | – |
| 10d | 26.33 | – | – | |
| 15d | 40.91 | – | – | |
| 20d | 51.89 | – | – | |
| QA-3 (L) + NIT (L) | 5d | 25.76 | 22.92 | Additive |
| 10d | 30.87 | 30.30 | Additive | |
| 15d | 47.73 | 52.08 | Additive | |
| 20d | 69.32 | 73.67 | Additive | |
| QA-3 (L) + NIT (H) | 5d | 29.74 | 27.27 | Additive |
| 10d | 34.47 | 34.47 | Additive | |
| 15d | 62.88 | 58.33 | Additive | |
| 20d | 76.33 | 77.65 | Additive | |
| QA-3 (H) + NIT(L) | 5d | 33.90 | 27.08 | Synergistic |
| 10d | 45.64 | 37.12 | Synergistic | |
| 15d | 78.41 | 56.06 | Synergistic | |
| 20d | 100.00 | 82.39 | Synergistic | |
| QA-3 (H) + NIT (H) | 5d | 31.82 | 31.44 | Additive |
| 10d | 43.94 | 41.67 | Additive | |
| 15d | 67.42 | 62.31 | Additive | |
| 20d | 85.04 | 86.36 | Additive |
Table 3.
Mean mortality (%±SE) of 4th instar larvae of Rhynchophorus ferrugineus treated with Beauveria bassiana (QA-3), and Nitenpyram (NIT). B. bassiana was used each @ 1 × 107 and 1 × 108 conidia/mL and Nitenpyram was applied @ 250 μL L−1 and 500 μL L−1.
| Treatment | Interval | Observed mortality | Expected mortality | Type of interaction |
|---|---|---|---|---|
| QA-3 (L) | 5d | 2.08 | – | – |
| 10d | 6.44 | – | – | |
| 15d | 15.15 | – | – | |
| 20d | 23.86 | – | – | |
| QA-3 (H) | 5d | 6.25 | – | – |
| 10d | 10.80 | – | – | |
| 15d | 19.32 | – | – | |
| 20d | 30.30 | – | – | |
| NIT (L) | 5d | 10.61 | – | – |
| 10d | 15.15 | – | – | |
| 15d | 28.22 | – | – | |
| 20d | 39.02 | – | – | |
| NIT (H) | 5d | 14.77 | – | – |
| 10d | 21.78 | – | – | |
| 15d | 30.11 | – | – | |
| 20d | 47.92 | – | – | |
| QA-3 (L) + NIT (L) | 5d | 18.94 | 12.69 | Synergistic |
| 10d | 25.76 | 21.59 | Synergistic | |
| 15d | 36.55 | 43.37 | Additive | |
| 20d | 56.25 | 62.88 | Additive | |
| QA-3 (L) + NIT (H) | 5d | 21.21 | 16.86 | Synergistic |
| 10d | 28.03 | 28.22 | Additive | |
| 15d | 43.37 | 45.27 | Additive | |
| 20d | 61.17 | 71.78 | Additive | |
| QA-3 (H) + NIT(L) | 5d | 27.46 | 16.86 | Synergistic |
| 10d | 36.55 | 25.95 | Synergistic | |
| 15d | 51.89 | 47.54 | Additive | |
| 20d | 78.03 | 69.32 | Additive | |
| QA-3 (H) + NIT (H) | 5d | 25.38 | 21.02 | Synergistic |
| 10d | 32.39 | 32.58 | Additive | |
| 15d | 47.73 | 49.43 | Additive | |
| 20d | 69.32 | 78.22 | Additive |
Table 4.
Mean mortality (%±SE) of 6th instar larvae of Rhynchophorus ferrugineus treated with Beauveria bassiana (QA-3), and Nitenpyram (NIT). B. bassiana was used each @ 1 × 107 and 1 × 108 conidia/mL and Nitenpyram was applied @ 250 μL L−1 and 500 μL L−1.
| Treatment | Interval | Observed mortality | Expected mortality | Type of interaction |
|---|---|---|---|---|
| QA-3 (L) | 5d | 2.08 | – | – |
| 10d | 8.33 | – | – | |
| 15d | 14.96 | – | – | |
| QA-3 (H) | 5d | 4.17 | – | – |
| 10d | 10.61 | – | – | |
| 15d | 17.05 | – | – | |
| NIT (L) | 5d | 6.25 | – | – |
| 10d | 14.77 | – | – | |
| 15d | 23.86 | – | – | |
| NIT (H) | 5d | 10.61 | – | – |
| 10d | 19.13 | – | – | |
| 15d | 26.14 | – | – | |
| QA-3 (L) + NIT (L) | 5d | 14.96 | 8.33 | Synergistic |
| 10d | 23.30 | 19.13 | Additive | |
| 15d | 32.58 | 38.83 | Additive | |
| QA-3 (L) + NIT (H) | 5d | 17.05 | 12.69 | Synergistic |
| 10d | 27.65 | 23.48 | Additive | |
| 15d | 39.21 | 41.10 | Additive | |
| QA-3 (H) + NIT(L) | 5d | 23.30 | 10.42 | Synergistic |
| 10d | 33.90 | 21.40 | Synergistic | |
| 15d | 45.64 | 40.91 | Additive | |
| QA-3 (H) + NIT (H) | 5d | 21.21 | 14.77 | Synergistic |
| 10d | 29.74 | 25.76 | Additive | |
| 15d | 41.10 | 43.18 | Additive |
Table 5.
Mean mortality (%±SE) of Pupa and Adult of R. ferrugineus treated with B. bassiana (QA-3), and Nitenpyram (NIT). B. bassiana was used each @ 1 × 107 and 1 × 108 conidia/ml and Nitenpyram was applied @ 250 μL l−1 and 500 μL l−1.
| Treatment | Pupa |
Adult |
|||||
|---|---|---|---|---|---|---|---|
| Interval | Observed Mortality | Expected Mortality | Type of interaction | Observed Mortality | Expected Mortality | Type of interaction | |
| QA-3 (L) | 7d | 25.95 | – | – | 21.21 | – | – |
| QA-3 (H) | 7d | 34.66 | – | – | 27.65 | – | – |
| NIT (L) | 7d | 36.93 | – | – | 25.37 | – | – |
| NIT (H) | 7d | 43.18 | – | – | 29.73 | ‘ | – |
| QA-3 (L) + NIT (L) | 7d | 56.25 | 62.88 | Additive | 38.25 | 46.59 | Additive |
| QA-3 (L) + NIT (H) | 7d | 62.88 | 69.13 | Additive | 44.69 | 50.95 | Additive |
| QA-3 (H) + NIT(L) | 7d | 89.02 | 71.59 | Synergistic | 66.09 | 53.03 | Synergistic |
| QA-3 (H) + NIT (H) | 7d | 69.51 | 77.84 | Additive | 48.86 | 57.39 | Additive |
3.3. Growth and development of R. Ferrugineus
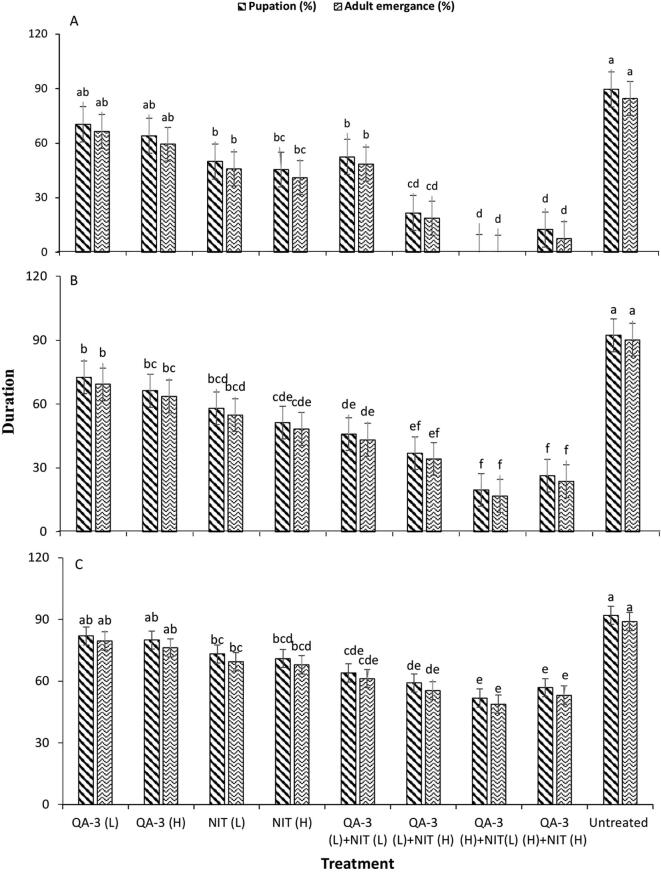
Growth and development of R. ferrugineus (larval to adult) was affected by the application of different treatments. B. bassiana (QA-3) and Nitenpyram when applied onto larvae, significant differences were observed. Percent pupation and adult emergence has inverse relation to toxicity of B. bassiana in second, fourth, and sixth instar larvae. Harmful effect on development was more in integration of B. bassiana and Nitenpyram followed by B. bassiana and Nitenpyram used singly (Fig. 1a–c).
Fig. 1.
Pupation and adult emergence (% ± SE) of red palm weevil, Rhynchophorous ferrugineus when second (a), fourth (b) and sixth (c) instar larvae were exposed to individual and combined application of Beauveria bassiana (1 × 107, 1 × 108 conidia mL−1) and Nitenpyram (250 and 500 µL L−1).
4. Discussion
Mortality data recorded showed that simultaneous use of Nitenpyram boost up the pathogenicity of B. bassiana against R. ferrugineus. Insects when exposed to synthetic insecticides face physiological or chemical mediated disorders and this condition is favored for fungal penetration into cuticle and to grab the nutrition leading to death of target insect (Hassan et al., 1989, Shahina et al., 2009). Compatibility of more than two agents to be used simultaneously is a pivotal feature, Malik et al. (2016) reported that there were no inhibitory effect of insecticides on the growth of B. bassiana. Integrating microbial agents and insecticides has proved to reduce induced resistance in insects and additionally it can augment their efficacy to synergize each other thus offering to be used in lower concentrations (Boman, 1980). Synergistic effects was firstly described Easwaramoorthy et al. (1979), where two insecticides boosted up the effect of Verticillium lecanii against coffee green scale. Integration of insecticides (abamectin, triflumuron and carbaryl) with B. bassiana provided highest mortality of Leptinotarsa decemlineata (Anderson et al., 1989). In our study, integration also provided synergistic interaction at higher B. bassiana (QA-3) and lower Nitenpyram concentrations. Cumulative mortality of R. ferrugineus was maximum with combination of higher concentration of B. bassiana with low concentration of Nitenpyram. However, the second instar of R. ferrugineus was more susceptible stage against [(QA-3) (1 × 108 conidia ml−1) + Nitenpyram (250 μL l−1)]. These combinations provided very promising results against the pupa and adults of R. ferrugineus.
EPF is a natural enemy of insects that has great potential to be developed as biological control agents. Lin et al. (2017) recovered Indigenous EPF from the soil and proved to be lethal against R. ferrugineus. Similar observation was recorded by El Kichaoui et al. (2017) who collected EPF from the Ghaza strip and proved to be effective for the control of R. ferrugineus. EPF isolated from same species are often proved more lethal to target insect. Ricaño et al. (2013) described that isolates of B. bassiana which was collected from R. ferrugineus have more virulence than from other isolates. Dembilio et al. (2010) reported that B. bassiana strains recovered from pupa have potential against all stages of R. ferrugineus. Sun et al. (2016) reported that Metarhizium sp. collected from soils in China have a great potential against R. ferrugineus, which showed 100% mortality. Yasin et al. (2017) reported 88% larval mortality with B. bassiana at high concentration. Gindin et al. (2006) recorded higher number of R. ferrugineus adult mortality when treated with M. anisopliae and B. bassiana dry spores, two weeks after treatment. Under the laboratory, field and semi field trials B. bassiana is widely used against R. ferrugineus (Ricaño et al., 2013). Many features of insects as poor nutrition, physical stress, weak immune system, competition render these as susceptible individual for pathogenic action (Inglis et al., 2001). EPF are promising microbial agents for this pest and are environmental safely, harmless to non-target species (Rossetti et al., 2015). EPF easily survive in darker and moist area where R. ferrugineus generally thrives.
Growth and development is very important phenomenon for completion of life span of the insects and reproduction. Any disturbance in insect life may vulnerable to biotic and abiotic factors (predators, parasitoids or environmental factors) that ultimately disrupt and reduced the growth and development. Therefore, larval period is susceptible towards such phenomena (Marzban et al., 1997). However, in our study treatments caused decreased growth period of R. ferrugineus pupal and adult stages, and even affected fecundity and survival rate of the insects into the next generation.
The experiment depicted that fungal isolates in integration with Nitenpyram provided significant mortality of RPW. Hence, integration of Nitenpyram in sequential manners with B. bassiana might be effectively used for this weevil management. This integration promises to provide greater effectiveness than alone treatments but more work is needed under field conditions.
5. Conclusion
The present study showed that EPF, B. bassiana can help to delay the onset of resistance and is most virulent against the R. ferrugineus. Furthermore, the environmental hazards of pesticides demand some eco-friendly and bio-rational alternatives. Application of date palms with EPF would be a better choice instead of insecticides while dealing with a hidden insect pest like R. ferrugineus. Based on the assumptions to be tested, EPF seem to be the best choice and successful alternatives for alleviation of the pesticide resistance problem and hence achieving the goal of increased productivity. It can be concluded that integration of entomopathogenic fungi and insecticides had an advantage for pest management of R. ferrugineus. Further research may test the usefulness and effectiveness of EPF in the field in date palm orchids.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgement
This study was supported by projects (SRGP No. 1181) from the Higher Education Commission (HEC), Islamabad, Pakistan. Khalid Ali Khan as an author of the manuscript extends his appreciation to the Deanship of Scientific Research at King Khalid University for supporting a part of this work through Research Group (R.G.P1)-108/40.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Moety E.M., Lotfy H., Rostom Y. Trace determination of red palm weevil, Rhynchophorus ferrugineus, pheromone at trapping locations under Egyptian climate. Int. J. Agric. Food Res. 2012;2:13–20. [Google Scholar]
- Abraham V.A., Al Shuaibi M.A., Faleiro J.R., Abozuhairah R.A., Vidyasagar P.S.P.V. An integrated management approach for red palm weevil, Rhynchophorus ferrugineus Oliv., a key pest of date palm in the Middle East. J. Agric. Sci. 1998;3:77–83. [Google Scholar]
- Al-Rajhy D.H., Hussein H.I., Al-Shawaf A.M.A. Insecticidal activity of carbaryl and its mixture with piperonylbutoxide against red palm weevil Rhynchophorus ferrugineus (Olivier) (Curculionidae: Coleoptera) and their effects on acetylcholinesterase activity. Pakistan J. Biol. Sci. 2005;8:679–682. [Google Scholar]
- Alves, S.B., Haddad, M.L., Faion, M., de Baptista, G.C., Rossi-Zalaf, L.S., 2007. Novo ındice biologico para classificac ¸ao da toxicidade de ~ agrotoxicos para fungos entomopatog enicos. In: ^ X SICONBIOL, 2007, Brasılia Anai. Siconbiol.
- Anderson T.E., Hajek A.E., Roberts D.W., Priesler H.K., Robertson J.L. Colorado potato beetle (Coleoptera: Chrysomelidae) effects of combinations of Beauveria bassiana with insecticides. J. Eco. Entomol. 1989;82:83–89. [Google Scholar]
- Arab Y.A., El-Deeb H.M. The use of endophyte beauveria bassiana for bioprotection of date palm seedlings against red palm weevil and Rhizoctonia root-rot disease. Sci. J. King Faisal Univ. (Basic Appl. Sci.) 2012;13:1433. [Google Scholar]
- Baloch H.B., Rustamani M.A., Khuro R.D., Talpur M.A., Hussai T. Incidence and abundance of date palm weevil in different cultivars of date palm. Zool. Soc. Pakistan, Lahore, Pakistan. 1992;12:445–447. [Google Scholar]
- Barnett H.L., Hunter B.B. 4th ed. APS Press; St. Paul: 1999. Illustrated Genera of Imperfect Fungi, The American Phytopathological Society; p. 218. [Google Scholar]
- Boman H.G. Insect responses to microbial infections. In: Burges H.D., editor. Microbial Control of Pest and Plant Diseases 1970–1980. Academic Press; New York: 1980. pp. 769–784. [Google Scholar]
- Dembilio Ó., Quesada-Moraga E., Santiago-Álvarez C., Jacas J.A. Potential of an indigenous strain of the entomopathogenic fungus Beauveria bassiana as a biological control agent against the Red Palm Weevil Rhynchophorus ferrugineus. J. Invertebr. Pathol. 2010;104(3):214–221. doi: 10.1016/j.jip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Dembilio Ó., Jaques J.A. Biology and management of red palm weevil. In: Wakil W., Faleiro J.R., Miller T.A., editors. Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges, Sustainability in Plant and Crop Protection. Springer International Publishing Switzerland; 2015. pp. 13–36. [Google Scholar]
- Easwaramoorthy S., Regupathy A., Santharam G., Jayaraj S. The effect of sub normal concentration of insecticides in combination with the fungal pathogen, Cephalosporium lecanii Zimm.in the control of coffee green scale. Coccus viridis Green Zeitschrift für Angewandte Entomol. 1979;86:161–166. [Google Scholar]
- El Kichaoui, A., Bara’a, Y.A., El-Hindi, M.W., 2017. Isolation, Molecular Identification and under Lab Evaluation of the Entomopathogenic Fungi M. anisopliae and B. bassiana against the Red Palm Weevil R. ferrugineus in Gaza Strip. Adv. Microbiol. 7 (1), 109.
- El-Sufty R, Al-Awash SA, Al-Amiri AM, Shahdad AS, Al-BathraAH, Musa SA (2007). Biological control of red palm weevil, Rhynchophorus ferrugineus (Col.: Curculionidae) by the entomopathogenic fungus Beauveria bassiana in United Arab Emirates. Proceeding of the 3rd International Conference on Date Palm. Acta Horticulturae 736: 399–404.
- Elllis R., Basturkmen H., Loewen S. Learner uptake in communicataive ESL lessons. Language Learn. 2001;51(2):281–318. [Google Scholar]
- Faleiro, JR., Jaques, J.A., Carrillo, D., 2016. Integrated pest management (IPM) of palm pests. In: Abrol DP (ed) Integrated Pest Management in the Tropics, 439–497. New India Publishing Agency, New Delhi, India.
- FAO, Food and Agriculture Organization of the United Nations, 2014. Food and agricultural commodities production for Pakistan for 2012. www. faostat.fao.org/DesktopDefault.aspx?PageID=339&lang=en&country=16 5. Accessed 24 July 2014.
- Gindin G., Levski S., Glazer I., Soroker V. Evaluation of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana against the red palm weevil Rhynchophorus ferrugineus. Phytoparasitica. 2006;34:370–379. [Google Scholar]
- Güerri-Agulló B., Gómez-Vidal S., Asensio L., Barranco P., Lopez-Llorca L.V. Infection of the red palm weevil (Rhynchophorus ferrugineus) by the Entomopathogenic Fungus Beauveria bassiana: a SEM study. Mic. Res. Tech. 2010;73:714–725. doi: 10.1002/jemt.20812. [DOI] [PubMed] [Google Scholar]
- Hassan A.E.M., Dillon R.J., Charnely A.K. Influence of accelerated germination of conidia on the pathogenicity of Metarhizium anisopliae for Manduca sexta. J. Invertebr. Pathol. 1989;54:277–279. [Google Scholar]
- Hassan S., Bakhsh K., Gill Z.A., Maqbool A., Ahmad W. Economics of growing date palm in Punjab. Pakistan. Int. J. Agric. Biol. 2006;8(6):788–792. [Google Scholar]
- Hussain A., Rizwan-ul-Haq M., Al-Jabr A.M., Al-Ayied H.Y. Managing invasive populations of red palm weevil: a worldwide perspective. J. Food Agric. Environ. 2013;11:456–463. [Google Scholar]
- Hussain A., Tian M.Y., He Y.R., Lin R. In vitro and in vivo culturing impacts on the virulence characteristics of serially passed entomopathogenic fungi. J. Food Agric. Environ. 2010;8:481–487. [Google Scholar]
- Inglis G.D., Goettel M.S., Butt T.M., Strasser A. Use of hyphomycetous fungi for managing insect pests. In: Butt T.M., Jackson C., Magan N., editors. Fungi as Biocontrol Agents. CAB International; Wallingford, UK: 2001. pp. 24–69. [Google Scholar]
- Jeschke P., Nauen R., Schindler M., Elbert A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011;59:2897–3290. doi: 10.1021/jf101303g. [DOI] [PubMed] [Google Scholar]
- Kaakeh W. Toxicity of imidacloprid to developmental stages of Rhynchophorus ferrugineus (Curculionidae: Coleoptera): laboratory and field tests. Crop Prot. 2006;25(5):432–439. [Google Scholar]
- Lin, G.L.E., Salim, J.M., Halim, M.F.A., AZMI, W.A., 2017. Entomopathogenic fungi isolated from the soil of Terengganu, Malaysia as potential bio-pesticides against the red palm weevil, Rhynchophorus ferrugineus. J. Sustain. Sci. Manag. 12 (2), 71–79.
- Ma X.M., Liu X.X., Ning X., Zhang B., Han F., Guan X.M., Zhang Q.W. Effects of Bacillus thuringiensis toxin Cry1Ac and Beauveria bassiana on Asiatic corn borer (Lepidoptera: Crambidae) J. Inverteb. Pathol. 2008;99(2):123–128. doi: 10.1016/j.jip.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Malik, M.A., Manzoor, M., Ali, H., Muhammad, A., ul Islam, S., Qasim, M., Ahmad, N., Idrees, A., Muhammad A., Saqib, H.S.A., 2016. Evaluation of imidacloprid and entomopathogenic fungi, Beauveria bassiana against the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae). J. Entomol. Zool. Stud. 4, 262–268.
- Marzban R., He Q., Liu X., Zhang Q. Effects of Bacillus thuringiensis toxin Cry1Ac and cytoplasmic polyhedrosis virus of Helicoverpa armigera (Hübner) (HaCPV) on cotton bollworm (Lepidoptera: Noctuidae) J. Invertebr. Pathol. 1997;101:71–76. doi: 10.1016/j.jip.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Minitab Inc., 2007. Meet Minitab 15, State College, PA: Minitab.
- Paoli F., Dallai R., Cristofaro M., Arnone S., Francardi V., Roversi P.F. Morphology of the male reproductive system, sperm ultrastructure and γ-irradiation of the red palm weevil Rhynchophorus ferrugineus Oliv. (Coleoptera: Dryophthoridae) Tissue Cell. 2014;46(4):274–285. doi: 10.1016/j.tice.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Ricaño J., Güerri-Agulló B., Serna-Sarriás M.J. Evaluation of the pathogenicity of multiple isolates of Beauveria bassiana (Hypocreales: Clavicipitaceae) on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) for the assessment of a solid formulation under simulated field conditions. Fla. Entomol. 2013;96:1311–1324. [Google Scholar]
- Rossetti S.B., Mastore M., Protasonim M., Brivio M.F. Effects of an entomopathogen nematode on the immune response of the insect pest red palm weevil: focus on the host antimicrobial response. J. Invertebr. Pathol. 2015;133:110–119. doi: 10.1016/j.jip.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Saleem M.A., Qayyum M.A., Ali M., Amin M., Tayyab M., Maqsood S. Effect of sub-lethal doses of Beauveria bassiana and nitenpyram on the development of red palm weevil, Rhynchophorus ferrugineus (Olivier) Pak. J. Zool. 2019;51(2):559–565. [Google Scholar]
- Sevim A., Donzelli B.G.G., Wu D.L., Demirbag Z., Gibson D.M., Turgeon B.G. Hydrophobin genes of the entomopathogenic fungus, Metarhizium brunneum, are differentially expressed and corresponding mutants are decreased in virulence. Curr. Genet. 2012;58:79–92. doi: 10.1007/s00294-012-0366-6. [DOI] [PubMed] [Google Scholar]
- Shabani F., Kumar L., Nojoumian A.M., Esmaeili A., Toghyani M. Projected future distribution of date palm and its potential use in alleviating micronutrient deficiency. J. Sci. Food Agric. 2016;96:1132–1140. doi: 10.1002/jsfa.7195. [DOI] [PubMed] [Google Scholar]
- Shahina F., Salma J., Mehreen G., Bhatti M.I., Tabassum K.A. Rearing of Rhynchophorus ferrugineus in laboratory and field conditions for carrying out various efficacy studies using EPNs. Pak. J. Nematol. 2009;27:219–228. [Google Scholar]
- Singh S.P., Rethinam P. Trapping a major tactic of BEPM strategy of palm weevils. Cord. 2005;21:57–79. [Google Scholar]
- Sokal, R.R., Rohlf, F.J., 1995. Biometry: The Principles and Practice of Statistics in Biological Research. Third edition. W. H. Freeman and Company, New York, New York, USA.
- Sun X., Yan W., Qin W., Zhang J., Niu X., Ma G., Li F. Screening of tropical isolates of Metarhizium anisopliae. SpringerPlus. 2016;5(1):1–5. doi: 10.1186/s40064-016-2780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakil W., Faleiro J.R., Miller T.A. Springer International Publishing; Switzerland: 2015. Sustainable pest management in date palm: current status and emerging challenges; p. 429. [Google Scholar]
- Yasin, M., Wakil, W., Ghazanfar, M.U., Qayyum, M.A., Tahir, M., Bedford, G.O., 2017. Virulence of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against red palm weevil, Rhynchophorus ferrugineus (Olivier). Entomol. Res. https://doi.org/10.1111/1748-5967.12260.
- Zimmerman, G., 1998. Suggestions for a standardised method for reisolation of entomopathogenic fungi from soil using the bait method (G. Zimmermann, J. Appl. Entomol. 102, 213-215, 1986). IOBC/WPRS Bulletin, Insect pathogens and insect parasitic nematodes, 21, 289.
- Zimmermann G. The Galleria bait method for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 1986;102:213–215. [Google Scholar]