Abstract
Kidney disease is a worldwide public health problem that affects millions of people worldwide. Globally, many risk factors for kidney disease progression have been identified. The global prevalence of acute and chronic forms of kidney disease is rising continuously. Nephrotoxicity is defined as rapid dysfunction of kidney due to toxic influence of medications and chemicals. Nephroprotective agents are material that has potential to minimize the effects of nephrotoxic agents. Plants have been shown to be potential therapeutic agents to protect against nephrotoxicity. The purpose of the present study was to evaluate the nephroprotective effect of basil leaves extract against thioacetamide (TAA) in male rats. Experimental male rats were divided into four groups. Rats of the first group were served as controls. Rats of the second group were exposed to TAA. Rats of the third group were treated with basil leaves extract and TAA. Rats of the fourth group were treated with basil leaves extract. After the end of experimental duration (6 Weeks), rats of the second group showed significantly increases of serum creatinine, blood urea nitrogen and uric acid levels, while the levels of serum superoxide dismutase and glutathione were significantly decreased. Histopathologically, renal sections from rats treated with only TAA showed several alterations in the structure of most renal corpuscles including a degeneration of glomeruli and Bowman's capsules. Treatment with basil leaves extract improved the observed biochemical and histopathological changes induced by TAA intoxication. These new findings indicate that the extract of basil leaves represent protective roles on biochemical and histopathological changes induced by TAA toxicity due to its antioxidant activities.
Keywords: Nephrotoxicity, Thioacetamide, Ocimum basilicum, Antioxidant, Rats
1. Introduction
The kidney is an essential organ required by the body to perform several important functions (Ferguson et al., 2008). The kidney is the main organ required by the human body to achieve and perform different important functions including detoxification, regulation of extracellular fluids, homeostasis, and excretion of toxic metabolites (Stevens et al., 2006). The kidney is concerned with many homeostatic mechanisms. It maintains the overall chemical composition of the intracellular environment by regulating the quantity of water, sodium chloride, potassium, phosphate and numerous other substances in the body (Hoenig and Zeidel, 2014). Toxic nephropathies are an important and relatively common category of kidney damage. Although they generally are reversible when detected early, they may be permanent, leading to chronic kidney disease (CKD). Toxic nephropathies are defined primarily as kidney injury caused by any number of medications, diagnostic agents, alternative products, herbal adulterants, or other toxin exposures, which includes environmental agents and chemicals (Perazella, 2010). Nephrotoxicity occurs when kidney-specific detoxification and excretion do not function optimally due to the damage or destruction of kidney function by exogenous or endogenous toxicants (Kim and Moon, 2012).
Thioacetamide (TAA) is an organosulfur compound and also known as thioacetimidic acid, or acetothioamide (C2H5NS). TAA is one of the several agents that produce centrilobular necrosis of the liver and has been so employed. Chronic TAA intoxication was established as a reliable and reproducible experimental model of fibrosis and cirrhosis in rodents by either the oral or intraperitoneal routes (Müller et al., 1988, Muñoz Torres et al., 1991, Salguero et al., 2008). Experimentally, prolonged administration of TAA leads to hyperplastic liver nodules, liver cell adenomas and hepatocarcinomas (Yeh et al., 2004). Moreover, it has been reported that TAA can also injure different organ systems besides liver, including lungs, intestine, kidneys, spleen, thymus and pancreas (Barker and Smuckler, 1972, Barker and Smuckler, 1974, Ortega et al., 1997, Al-Bader et al., 1999, Al-Bader et al., 2000, Latha et al., 2003, Al-Attar et al., 2017).
Traditional herbal medicine and their preparations have been widely used for the thousands of years in developing and developed countries owing to its natural origin and lesser side effects (Kamboj, 2012). The therapeutic use of plants and their extracts may be a promising approach for the treatment of different diseases (Sakr and Nooh, 2013). Among the plants known for medicinal value, the plants of genus Ocimum belonging to family Lamiaceae are very important for their therapeutic potentials. Basil (Ocimum basilicum) possesses high power against antioxidation (Marinava and Ynishlieva, 1997). Basil genus is widely used in folk medicine to treat a wide range of diseases and many studies have established that basil leaves extracts has numerous pharmacological activities (Chiang et al., 2005, Bozin et al., 2006, de Almeida et al., 2007, Samson et al., 2007). The antioxidative effect of basil is mainly due to its content of phenolic components, such as flavonoids, phenolic acids, rosmarinic acid and aromatic compounds (Gülçin et al., 2007). Alomar and Al-Attar (2019) investigated the effect of basil leaves extract on liver fibrosis induced by TAA in male rats. Administration of basil leaves extract to rats exposed to TAA led to inhibition of biochemical and liver histopathological alterations. They concluded that the protective role of basil leaves extract attributed to its antioxidant effects. Therefore, the objective of the present study was to evaluate the possible protective effects of basil leaves extract against TAA-induced nephrotoxicity in male rats.
2. Material and methods
2.1. Basil leaves extraction
The extraction of basil leaves was prepared according to the method of Alomar and Al-Attar (2019). Basil leaves were purchased from a local market with a highly degree of quality assurance. The leaves were botanically authenticated and its identification was confirmed by a specialist of plant taxonomy. Two hundred grams of dried basil leaves were powdered and added to 7 L of hot water. After 3 h, the mixture was slowly boiled for 30 min. and the mixture was cooled at room temperature. The obtained mixture was gently subjected to an electric mixer for 20 min. and filtered using Whitman filter paper. Thereafter, the filtrates were evaporated in an oven at 40 °C to produce dried residues (active principles). With references to the powdered samples, the yield means of leaves extract were 17.6%.
2.2. Experimental animals
Forty male Wistar rats (236–284 g) were utilized in the current study. Animals were allocated 10 per cage. Mean daily animal room temperature ranged from 19 to 21 °C and mean daily relative humidity equal 65% during the study. Light timers were set to provide a 12 h light/12 h dark photoperiod. Animals were fed ad libitum on normal commercial chow and had free access to water. The experimental treatments were conducted in accordance with ethical guidelines of the Animal Care and Use Committee of King Abdulaziz University.
2.3. Experimental protocol
Male rats were randomly distributed into four experimental groups. Rats of the first group were served as controls and intraperitoneally injected with saline solution (0.9% NaCl), twice weekly. Rats of the second group were given 300 mg/kg body weight of TAA (Sigma-Aldrich Corp., St. Louis, MO, USA) by intraperitoneal injection, twice weekly. Rats of the third group were orally supplemented with basil leaves extract at a dose of 300 mg/ kg body weight/ day and they were intraperitoneally injected with TAA at the same dose given to the second group. Rats of the fourth group were intraperitoneally injected with saline solution (0.9% NaCl), twice weekly and were orally exposed to basil leaves extract at the same dose given to the third group. After the end of experimental duration (6 weeks), animals were anaesthetized with diethyl ether. Blood samples were collected from orbital venous plexus in non-heparinized tubes, centrifuged at 2500 rpm for 15 min. and blood sera were then collected and stored at −80 °C prior immediate determination of selected biochemical parameters. Serum creatinine level was estimated according to the method of Larsen (1971). Blood urea nitrogen (BUN) level was measured according to the method of Patton and Crouch (1977). The method of Young (1990) was used to determine the level of serum uric acid. The methods of Nishikimi et al., 1972, Beutler et al., 1963 were used to evaluate the levels of serum superoxide dismutase (SOD) and glutathione (GSH) respectively. For histopathological examination, kidney tissues were quickly removed, immersed in 10% formalin, dehydrated and embedded in paraffin, sectioned at 4 µm, stained with hematoxylin and eosin (H&E) and evaluated by light microscopy. Images representative of typical histological profile in control and all treated groups were captured with the aid of light microscope (Olympus BX61- USA) connected to motorized controller unit (Olympus bx-ucb- USA) and photographed by a camera (Olympus DP72- USA).
2.4. Statistical analysis
Numerical data were represented as mean ± standard deviation (S.D). Statistical Package for Social Sciences (SPSS) for Windows version 22.0 software was used for statistical analysis. Data were analyzed using one-way analysis of variance (ANOVA). Results were considered statistically significant at P < 0.05.
3. Results
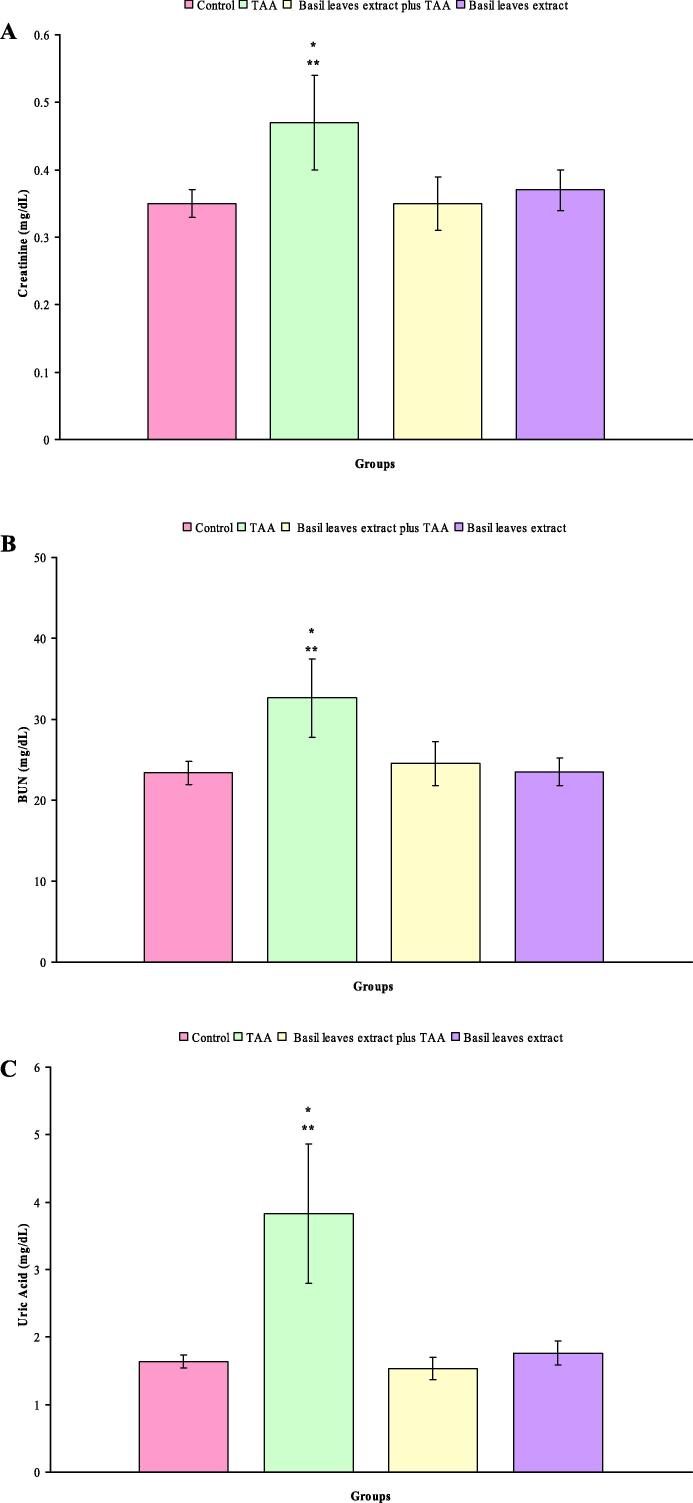
The levels of serum creatinine, BUN and uric acid in control, TAA, basil leaves extract plus TAA and basil leaves extract treated rats are shown in Fig. 1A–C. Statistically increase in the level of serum creatinine was noted in rats treated with TAA (+34.3%, P < 0.002). Additionally, there were no significant differences in the level of serum creatinine in rats exposed to basil leaves extract plus TAA and basil leaves extract compared with control rats (Fig. 1A). TAA intoxicated rats showed a significant elevation in the level of serum BUN (+39.7%, P < 0.001) compared with control rats. This parameter was insignificantly altered in basil leaves extract plus TAA and basil leaves extract treated rats as compared with control rats (Fig. 1B). In comparison with control rats, the levels of serum uric acid were statistically increased in rats treated with TAA (+133.5%, P < 0.001). Insignificant changes were observed in the level of serum uric acid in rats treated with basil leaves extract plus TAA and basil leaves extract (Fig. 1C).
Fig. 1.
(A–C) Levels of serum creatinine (A), BUN (B) and uric acid (C) in control, TAA, basil leaves extract plus TAA and basil leaves extract treated rats after six weeks. * Indicates a significant difference between control and treated groups. ** Indicates a significant difference between rats treated with TAA and basil leaves extract plus TAA and basil leaves extract.
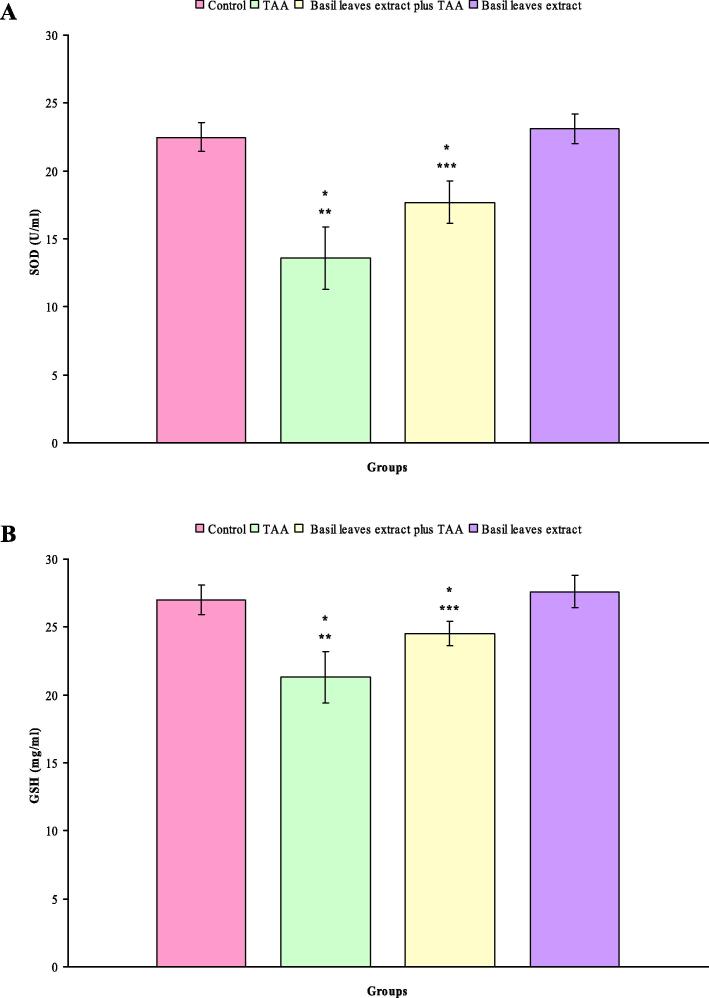
Fig. 2A and B showed the levels of serum SOD and GSH in all experimental groups. Relative to the control rats, the experimental rats treated with TAA exhibited significantly decline in the level of serum SOD (- 39.6%, P < 0.000). The level of serum SOD was statistically decreased in basil leaves extract plus TAA treated rats (−21.7%, P < 0.001). In addition, no statistically significant difference was noted in the level of serum SOD in basil leaves extract treated rats compared with control rats (Fig. 2A). Significant decreases in the level of serum GSH were observed in rats treated with TAA (−21.3%, P < 0.000) and basil leaves extract plus TAA (−9.5%, P < 0.05) compared with control rats. Moreover, no statistically significant difference was noted in the level of serum GSH in rats supplemented with basil leaves extract compared with control rats(Fig. 2B).
Fig. 2.
(A–B) Levels of serum SOD (A) and GSH (B) in control, TAA, basil leaves extract plus TAA and basil leaves extract treated rats after six weeks. * Indicates a significant difference between control and treated groups. ** Indicates a significant difference between rats treated with TAA and basil leaves extract plus TAA and basil leaves extract. ***indicates a significant difference between rats treated with basil leaves extract plus TAA and basil leaves extract.
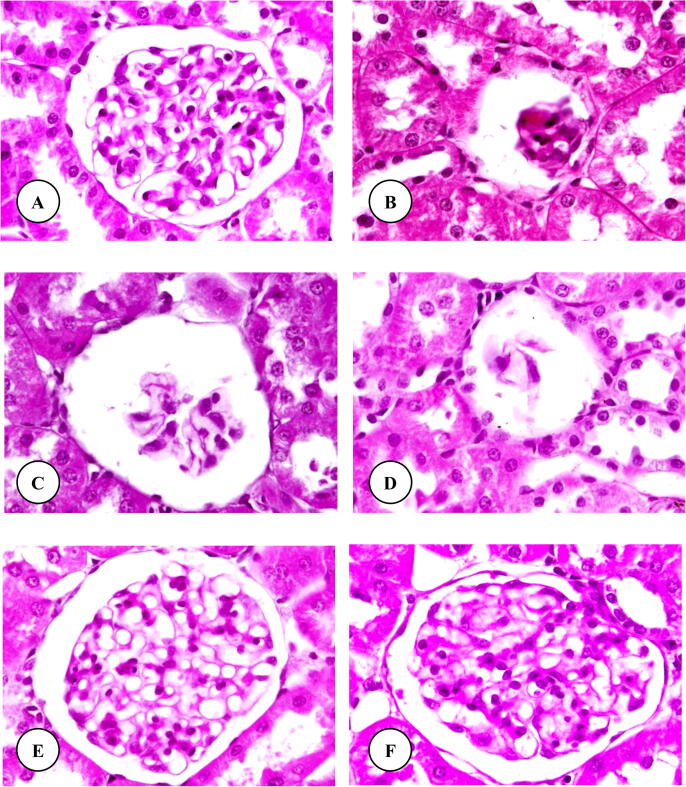
Histopathological examination of kidney sections of control, TAA, basil leaves extract plus TAA and basil leaves extract treated rats are represented in Fig. 3A-F. As shown in Fig. 3A, 3E and 3F of the control (group 1) and basil leaves extract plus TAA (group 3) and basil leaves extract (group 4) treated rats, normal kidney or renal sections were observed. Fig. 3A showed the normal structure of renal (Malpighian) corpuscle. The normal renal corpuscle consists of a tuft of capillaries, the glomerulus, surrounded by a double-walled epithelial capsule called Bowman’s capsule. Between the two layers of the capsule is the urinary or Bowman’s space. Histopathological examination of renal sections from rats treated with only TAA (group 2) showed several alterations in the structure of most renal corpuscles including a degeneration of glomeruli and Bowman's capsules (Fig. 3B–D).
Fig. 3.
(A–F) Photomicrographs of renal corpuscle of control (A), TAA (B–D), basil leaves extract plus TAA (E) and basil leaves extract (F) treated rats. Original magnification X1000.
4. Discussion
The present study showed that rats exposed to TAA display a pronounced impairment in kidney (renal) function which is confirmed by the enhancement of serum creatinine, BUN and uric acid levels, and histopathological changes. Creatinine, BUN and uric acid are waste products of protein metabolism that need to be excreted by the kidney, therefore a marked increase of these parameters, as observed in this study, confirms an indication of functional damage to the kidney (Panda, 1999). Serum levels of creatinine, BUN and uric acid are useful tools in diagnosis as the pick any disturbances to the renal system early enough to allow for projection and possible remedies. TAA is the most potent nephrotoxic substance because of its rapid elimination and cumulative injury when it is given intermittently, presumably by free radical mediated lipid (Dashti et al., 1997, Begum et al., 2011). However, the present increases of serum creatinine, BUN and uric acid levels are generally in accordance with previous studies on experimental animals exposed to TAA (Bakhtiary et al., 2012, Kadir et al., 2013, Omnia et al., 2014, Zargar et al., 2019). Moreover, Al-Attar et al., 2017, Ahmad et al., 2018 investigated the nephrotoxicity induced by TAA exposure in male mice and female rats. They reported that the levels of serum creatinine, BUN and uric acid were statistically increased accompanied with renal histological damage.
The present study showed that TAA induced oxidative stress which confirmed by the decreases of serum SOD and GSH levels. These findings clearly showed that TAA induced oxidative stress in experimental rats. Both enzymatic and non-enzymatic antioxidant system are essential for cellular response in order to deal with oxidative stress under physiological condition. Therefore, SOD and GSH were used as indexes to evaluate the level of oxidative stress (Medina and Moreno-Otero, 2005, Karabulut et al., 2010, Mallikarjuna et al., 2010, Dey and Lakshmanan, 2013).
Oxidative stress plays a fundamental role in the pathogenesis of nephrotoxicity. Reactive oxygen species (ROS) are one of the main causes leading to the progression of pathophysiological changes of kidney diseases. ROS have many physiological roles in cells; however, they can exert also deleterious effects such as lipid peroxidation, cellular protein oxidation and DNA damage. These effects may lead to altered integrity of plasma membrane and mitochondrial membrane. Also, the oxidative damage can result in impaired function of the protein and suppression of both cell repair and proliferation (El-Desouky et al., 2019). SODs belong to a family of antioxidant enzymes that catalyze the dismutation of superoxide to yield H2O2 and oxygen (Johnson et al., 2005). SOD is essentially a protective enzyme which scavenges the superoxide ions produced as cellular by products during oxidative stress (Pushpakiran et al., 2004). Its decreased activity can lead to adverse effects because superoxide anions are extremely toxic and may accumulate in the cells. GSH, a tripeptide present in the majority of cells, is responsible for hydrophilic xenobiotics conjugation. GSH serves many vital physiological functions including protection of cells from ROS, detoxification of exogenous compounds, and amino acid transport (Kojima-Yuasa et al., 2005, Mendoza-Cózatl et al., 2005). Sulphydryl group of GSH is essential for its antioxidant activity against some forms of ROS in cells (Cnubben et al., 2001). Much of the pathology is associated with the decrease in intracellular GSH concentration (Aruoma et al., 1989).
Histopathologically, the present study showed that TAA caused several alterations in the structure of kidney, including an abnormal structure of renal corpuscles, which appearing a highly degeneration of glomeruli and Bowman's capsules. Moreover, these results showed that rats exposed to TAA only display a pronounced impairment in kidney function which confirmed by the enhancement of serum creatinine, BUN and uric acid levels, and histopathological changes. Moreover, these results demonstrated that the cortex was more affected than medulla due to long-term treatment with TAA. This could be partly due to uneven distribution of metabolites of TAA in the tissue of the kidney where about 90% of the total renal blood flow enters the cortex via the bloodstream. Accordingly, a relative high concentration of metabolites of TAA might reach the cortex via the bloodstream than that would enter the medulla. Metabolic studies of TAA induced tissue damage suggest that TAA is metabolized by the mixed function oxidase system to its toxic metabolites sulfine (sulfoxide) and sulfene (sulfone) which are then distributed among several organs, including plasma, liver, kidney, bone marrow, adrenals and other tissues (Edward and Baker, 1974). Later, TAA undergoes an extensive metabolism to acetate and it is excreted through the urine within 24 h (Spira and Raw, 2000, Kadir et al., 2011). TAA is toxic to selected populations of cells (hepatocytes, proximal convoluted tubular cells in kidney and cortical thymocytes) and several animals studies have shown that toxin-induced renal tubular damage plays a crucial role in the reduction of glomerular filtration rate either through obstruction and back leak of renal filtrate or secondary to ROS (Edward and Baker, 1974, Leena and Balaraman, 2005). Moreover, the toxic effects of TAA on kidney of experimental animals were investigated by several studies. These studies showed that the light microscopic examinations of renal tissues revealed severe histopathological changes including congestion of the glomeruli and focal mesengial cell proliferation, increased deposition of the collagen in the renal medulla and fibrin in the cortex, disrupted and swollen cells of convoluted tubules and lobulated atrophied glomeruli, tubular epithelial cell necrosis associated with diffuse tubular swelling, and inflammatory cell infiltration (Al-Bader et al., 1999, Mahmoud, 2006, Kadir et al., 2013, Ahmad et al., 2018).
The current results indicate the treatment with basil leaves extract significantly attenuated the physiological and histopathological changes induced by TAA in male rats. The protective action of basil leaves extract possibly via its antioxidant effect in reversing physiological and histopathological alterations of kidney induced by TAA. Therefore, the protective effect of this extract can be credited to rich antioxidant contents. The accumulations of free radicals in organs or tissues are strongly associated with oxidative damages in biomolecules and cell membranes. This can lead to many chronic diseases, such as inflammatory, cancer, diabetes, aging, cardiac dysfunction, and other degenerative diseases (Wang et al., 2004). The medicinal value of any plant depends on bioactive phytochemical constituents that produce definite physiological action in the human body. Bioactive phytochemical constituents like alkaloids, phenolics, flavonoids, essential oils, tannins and saponins are usually responsible for medicinal importance of herbal plants (Kähkönen et al., 1999, Krishnaiah et al., 2009). Nephroprotective agents are material that has potential to minimize the effects of nephrotoxic agents (Chinnappan et al., 2019). Basil is mainly used for culinary or medicinal purposes because of its high concentration of antioxidant phenolic compounds, such as rosmarinic acid and other caffeic acid derivatives (Makri and Kintzios, 2008, Kiferle et al., 2011). Gülçin et al. (2007) showed that the antioxidative effect of basil is mainly due to its content of phenolic components, such as flavonoids, phenolic acids, rosmarinic acid and aromatic compounds. The antioxidant activity of phenolic compounds is mainly caused by their redox properties, which permit them to act as reducing agents, hydrogen donors and singlet oxygen quenchers (Hakkim et al., 2007). Additionally, several experimental investigations showed that the protective effects of basil against experimental nephrotoxicity, myocardial infarction, colon tumors and liver fibrosis were attributed to its antioxidants and free radicals scavenging properties (Gajula et al., 2010, Sakr and Al-Amoudi, 2012, Ahmed and Masoud, 2014, Alomar and Al-Attar, 2019). From the results of the current study, it can be concluded that the extract of basil leaves could be used as safe potential natural products in the treatment of TAA toxicity due to its antioxidant properties. Moreover, further experimental investigations will be required to study the influence of different concentrations and doses of basil leaves extract against the toxicity of TAA.
Acknowledgments
Acknowledgments
The author gratefully acknowledges with sincere thanks to King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia, for their financial and technical support of this study (No.1-18-01-009-0192).
Declaration of Competing Interest
The author has declared that there are no conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad A., Al-Abbasi F.A., Sadath S., Ali S.S., Abuzinadah M.F., Alhadrami H.A., Mohammad Alghamdi A.A., Aseeri A.H., Khan S.A., Husain A. Ameliorative effect of camel's milk and Nigella Sativa oil against thioacetamide-induced hepatorenal damage in rats. Pharmacogn. Mag. 2018;14:27–35. doi: 10.4103/pm.pm_132_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A.A.M., Masoud R.A. Cardioprotective potential of basil oil and vitamin E against oxidative stress in experimental myocardial infarction induced by epinephrine in rats. AAMJ. 2014;12:204–237. [Google Scholar]
- Al-Attar A.M., Alrobai A.A., Almalki D.A. Protective effect of olive and juniper leaves extracts on nephrotoxicity induced by thioacetamide in male mice. Saudi J. Biol. Sci. 2017;24:15–22. doi: 10.1016/j.sjbs.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bader A., Mathew T.C., Khoursheed M., Asfar S., al-Sayer H., Dashti H.M. Thioacetamide toxicity and the spleen: histological and biochemical analysis. Anat. Histol. Embryol. 2000;29:3–8. doi: 10.1046/j.1439-0264.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- Al-Bader A.A., Mathew T.C., Abul H., Al-Mosawi M., Dashti H.M., Kumar D., Singal P.K. Thioacetamide induced changes in trace elements and kidney damage. J. Trace Elem. Exp. Med. 1999;12:1–14. [Google Scholar]
- Alomar M.Y., Al-Attar A.M. Effect of basil leaves extract on liver fibrosis induced by thioacetamide in male rats. Int. J. Pharmacol. 2019;15:478–485. [Google Scholar]
- Aruoma O.I., Halliwell B., Hoey B.M., Butler J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Bakhtiary S.A., Iqbal M.M., Ibrahim Md. Hepatoprotective and nephroprotective activity of Phyllanthus amarus Schum & Thonn. seed extract. Ann. Phytomed. 2012;1:97–104. [Google Scholar]
- Barker E.A., Smuckler E.A. Altered microsome function during acute thioacetamide poisoning. Mol. Pharmacol. 1972;8:318–326. [PubMed] [Google Scholar]
- Barker E.A., Smuckler E.A. Nonhepatic thioacetamide injury. II. The morphologic features of proximal renal tubular injury. Am. J. Pathol. 1974;74:575–590. [PMC free article] [PubMed] [Google Scholar]
- Begum Q., Noori S., Mahboob T. Antioxidant effect of sodium selenite on thioacetamide-induced renal toxicity. Pak. J. Biochem. Mol. Biol. 2011;44:21–26. [Google Scholar]
- Beutler E., Duron O., Kelly M.B. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Bozin B., Mimica-Dukic N., Simin N., Anackov G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006;54:1822–1828. doi: 10.1021/jf051922u. [DOI] [PubMed] [Google Scholar]
- Chiang L.C., Ng L.T., Cheng P.W., Chiang W., Lin C.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005;32:811–816. doi: 10.1111/j.1440-1681.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- Chinnappan S.M., George A., Krishnamurthy P., Choudhary Y., Choudhary V.K., Ramani Y., Dewangan R. Nephroprotective effect of herbal extract Eurycoma longifolia on paracetamol-induced nephrotoxicity in rats. Evid. Based Complement. Alternat. Med. 2019;2019:4916519. doi: 10.1155/2019/4916519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnubben N.H.P., Rietjens I.M.C.M., Wortelboer H., van Zanden J., van Bladeren P.J. The interplay of glutathione-related processes in antioxidant defense. Environ. Toxicol. Pharmacol. 2001;10:141–152. doi: 10.1016/s1382-6689(01)00077-1. [DOI] [PubMed] [Google Scholar]
- Dashti H.M., Mathew T.C., Jadaon M.M., Ashkanani E. Zinc and liver cirrhosis: Biochemical and histopathologic assessment. Nutrition. 1997;13:206–212. doi: 10.1016/s0899-9007(96)00403-0. [DOI] [PubMed] [Google Scholar]
- de Almeida I., Alviano D.S., Vieira D.P., Alves P.B., Blank A.F., Lopes A.H., Alviano C.S., Rosa Mdo S. Antigiardial activity of Ocimum basilicum essential oil. Parasitol. Res. 2007;101:443–452. doi: 10.1007/s00436-007-0502-2. [DOI] [PubMed] [Google Scholar]
- Dey A., Lakshmanan J. The role of antioxidants and other agents in alleviating hyperglycemia mediated oxidative stress and injury in liver. Food Funct. 2013;4:1148–1184. doi: 10.1039/c3fo30317a. [DOI] [PubMed] [Google Scholar]
- Edward A., Baker E.A.S. Nonhepatic thioacetamide injury. The morphologic features of proximal renal tubular injury. Am. J. Pathol. 1974;74:576–590. [PMC free article] [PubMed] [Google Scholar]
- El-Desouky M.A., Mahmoud M.H., Riad B.Y., Taha Y.M. Nephroprotective effect of green tea, rosmarinic acid and rosemary on N-diethylnitrosamine initiated and ferric nitrilotriacetate promoted acute renal toxicity in Wistar rats. Interdiscip. Toxicol. 2019;12:98–110. doi: 10.2478/intox-2019-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M.A., Vaidya V.S., Bonventre J.V. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008;245:182–193. doi: 10.1016/j.tox.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajula D., Verghese M., Boateng J., Shackelford L., Mentreddy S.R., Sims C., Asiamah D., Walker L.T. Basil (Ocimum basilicum and Ocimum tenuiflorum) reduces azoxymethane induced colon tumors in fisher 344 male rats. Res. J. Phytochem. 2010;4:136–145. [Google Scholar]
- Gülçin I., Elmastş M.Y., Aboul Enein H. Determination of antioxidant and radical scavenging activity of basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother. Res. 2007;21:354–361. doi: 10.1002/ptr.2069. [DOI] [PubMed] [Google Scholar]
- Hakkim F.L., Shankar C.G., Girija S. Chemical composition and antioxidant property of holly basil (Ocimum sanctum L.) leaves, stems, and inflorescence and their in vitro callus cultures. J. Agric. Food Chem. 2007;55:9109–9117. doi: 10.1021/jf071509h. [DOI] [PubMed] [Google Scholar]
- Hoenig M.P., Zeidel M.L. Homeostasis, the milieu int́erieur, and the wisdom of the nephron. Clin. J. Am. Soc. Nephrol. 2014;9:1272–1281. doi: 10.2215/CJN.08860813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W.T., Johnson L.A.K., Henry C., Lukaski H.C. Serum superoxide dismutase 3 (extracellular superoxide dismutase) activity is a sensitive indicator of Cu status in rats. J. Nutr. Biochem. 2005;16:682–692. doi: 10.1016/j.jnutbio.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Kadir F.A., Othman F., Abdulla M.A., Hussan F., Hassandarvish P. Effect of Tinospora crispa on thioacetamide-induced liver cirrhosis in rats. Indian J. Pharmacol. 2011;43:64–68. doi: 10.4103/0253-7613.75673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadir F.A., Kassim N.M., Abdulla M.A., Yehye W.A. Effect of oral administration of ethanolic extract of Vitex negundo on thioacetamide-induced nephrotoxicity in rats. BMC Complement. Altern. Med. 2013;13:294. doi: 10.1186/1472-6882-13-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähkönen M.P., Hopia A.I., Vuorela H.J., Rauha J.P., Pihlaja K., Kujala T.S., Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Kamboj A. 1st ed. In Tech Publication; Rijeka, Croatia: 2012. Analytical evaluation of herbal drugs. Drug discovery research in Pharmacognosy. [Google Scholar]
- Karabulut A.B., Gui M., Karabulut E., Kiran T.R., Ocak S.G., Otlu O. Oxidant and antioxidant activity in rabbit livers treated with zoledronic acid. Transplant. Proc. 2010;42:3820–3822. doi: 10.1016/j.transproceed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Kiferle C., Lucchesini M., Mensuali-Sodi A., Maggini R., Raffaelli A., Pardossi A. Rosmarinic acid content in basil plants grown in vitro and in hydroponics. Cent. Eur. J. Biol. 2011;6:946–957. [Google Scholar]
- Kim S.Y., Moon A. Drug-induced nephrotoxicity and its biomarkers. Biomol. Ther. (Seoul) 2012;20:268–272. doi: 10.4062/biomolther.2012.20.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima-Yuasa A., Umeda K., Ohkita T., Opare Kennedy D., Nishiguchi S., Matsui-Yuasa I. Role of reactive oxygen species in zinc deficiency-induced hepatic stellate cell activation. Free Radic. Biol. Med. 2005;39:631–640. doi: 10.1016/j.freeradbiomed.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Krishnaiah D., Devi T., Bono A., Sarbatly A. Studies on phytochemical constituents of six Malaysian medicinal plants. J. Med. Plants Res. 2009;3:67–72. [Google Scholar]
- Larsen K. Creatinine assay by a reaction-kinetic principle. Clin. Chim. Acta. 1971;41:209–217. doi: 10.1016/0009-8981(72)90513-x. [DOI] [PubMed] [Google Scholar]
- Latha S.M., Pai M.R., Pai P.K. Thioacetamide toxicity and the lung: Histological analysis. Indian J. Physiol. Pharmacol. 2003;47:476–478. [PubMed] [Google Scholar]
- Leena P., Balaraman R. Effect of green tea extract on cisplatin induced oxidative damage on kidney and testes of rats. Ars. Pharm. 2005;46:5–18. [Google Scholar]
- Mahmoud N.H. Protective effect Of Panax ginseng against thioacetamide cytotoxicity in liver and kidney of albino rat. J. Egypt. Soc. Toxicol. 2006;34:43–54. [Google Scholar]
- Makri O., Kintzios S. Ocimum sp. (basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants. 2008;13:123–150. [Google Scholar]
- Mallikarjuna K., Shanmugam K.R., Nishanth K., Wu M.C., Hou C.W., Kuo C.H., Reddy K.S. Alcohol-induced deterioration in primary antioxidant and glutathione family enzymes reversed by exercise training in the liver of old rats. Alcohol. 2010;44:523–529. doi: 10.1016/j.alcohol.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Marinava E.M., Ynishlieva N.V. Antioxidative activity of extracts from selected species of the family lamiaceae in sunflower oil. Food Chem. 1997;58:245–248. [Google Scholar]
- Medina J., Moreno-Otero R. Pathophysiological basis for antioxidant therapy in chronic liver disease. Drugs. 2005;65:2445–2461. doi: 10.2165/00003495-200565170-00003. [DOI] [PubMed] [Google Scholar]
- Mendoza-Cózatl D., Loza-Tavera H., Hernández-Navarro A., Moreno-Sánchez R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol. Rev. 2005;29:653–671. doi: 10.1016/j.femsre.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Müller A., Machnik F., Zimmermann T., Schubert H. Thioacetamide-induced cirrhosis-like liver lesions in rats – usefulness and reliability of this animal model. Exp. Pathol. 1988;34:229–236. doi: 10.1016/s0232-1513(88)80155-5. [DOI] [PubMed] [Google Scholar]
- Muñoz Torres E., Paz Bouza J.I., López Bravo A., Abad Hernández M.M., Carrascal Marino E. Experimental thioacetamide-induced cirrhosis of the liver. Histol. Histopathol. 1991;6:95–100. [PubMed] [Google Scholar]
- Nishikimi M., Roa N.A., Yogi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Bioph. Res. Common. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Omnia M.A., Nabila M.A., Nadia R.R. Biochemical effects of propolis and bee pollen in experimentally-induced hyperammonemia in rats. Benha Vet. Med. J. 2014;27:8–24. [Google Scholar]
- Ortega M.A., Torres M.I., Fernandez M.I., Rios A., Sánchez-Pozo A., Gil A. Hepatotoxic agent thioacetamide induces biochemical and histological alterations in rat small intestine. Dig. Dis. Sci. 1997;42:1715–1723. doi: 10.1023/a:1018817600238. [DOI] [PubMed] [Google Scholar]
- Panda N.C. Textbook of biochemistry and human biology. Prentise Hall; India: 1999. Kidney; pp. 290–296. [Google Scholar]
- Patton G.J., Crouch S.R. Determination of urea (urease modified Berthelot reaction) Anal. Chem. 1977;49:464–469. [Google Scholar]
- Perazella M.A. Toxic Nephropathies: Core Curriculum 2010. Am J Kidney Dis. 2010;55:399–409. doi: 10.1053/j.ajkd.2009.10.046. [DOI] [PubMed] [Google Scholar]
- Pushpakiran G., Mahalakshmi K., Anuradha C.V. Taurine restores ethanol-induced depletion of antioxidants and attenuates oxidative stress in rat tissues. Amino Acids. 2004;27:91–96. doi: 10.1007/s00726-004-0066-8. [DOI] [PubMed] [Google Scholar]
- Sakr S.A., Al-Amoudi W.A. Effect of leave extract of Ocimum basilicum on deltamethrin induced nephrotoxicity and oxidative stress in albino rats. J. Appl. Pharm. Sci. 2012;02:22–27. [Google Scholar]
- Sakr S.A., Nooh H.Z. Effect of Ocimum basilicum extract on cadmium-induced testicular histomorphometric and immunohistochemical alterations in albino rats. Anat. Cell Biol. 2013;46:122–130. doi: 10.5115/acb.2013.46.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salguero P.R., Roderfeld M., Hemmann S., Rath T., Atanasova S., Tschuschner A., Gressner O.A., Weiskirchen R., Graf J., Roeb E. Activation of hepatic stellate cells is associated with cytokine expression in thioacetamide-induced hepatic fibrosis in mice. Lab. Invest. 2008;88:1192–1203. doi: 10.1038/labinvest.2008.91. [DOI] [PubMed] [Google Scholar]
- Samson J., Sheeladevi R., Ravindran R. Oxidative stress in brain and antioxidant activity of Ocimum sanctum in noise exposure. Neurotoxicology. 2007;28:679–685. doi: 10.1016/j.neuro.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Spira B., Raw I. The effect of thioacetamide on the activity and expression of cytosolic rat liver glutathione-S-transferase. Mol. Cell. Biochem. 2000;211:103–110. doi: 10.1023/a:1007114801362. [DOI] [PubMed] [Google Scholar]
- Stevens L.A., Coresh J., Greene T., Levey A.S. Assessing kidney function – Measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- Wang S., Konorev E.A., Kotamraju S., Joseph J., Kalivendi S., Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms intermediacy of H2O2-and p53- dependent pathways. J. Biol. Chem. 2004;279:25535–25543. doi: 10.1074/jbc.M400944200. [DOI] [PubMed] [Google Scholar]
- Yeh C.N., Maitra A., Lee K.F., Jan Y.Y., Chen M.F. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: An animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis. 2004;25:631–636. doi: 10.1093/carcin/bgh037. [DOI] [PubMed] [Google Scholar]
- Young, D.S., 1990. Effects of drugs on clinical laboratory test. 3rd Edn. 3, 19-25.
- Zargar S., Alonazi M., Rizwana H., Wani T.A. Resveratrol reverses thioacetamide-induced renal assault with respect to oxidative stress, renal function, DNA damage, and cytokine release in Wistar rats. Oxid. Med. Cell. Longev. 2019;2019:1702959. doi: 10.1155/2019/1702959. [DOI] [PMC free article] [PubMed] [Google Scholar]