Abstract
We studied 1859 subjects with confirmed COVID-19 from seven centers in Wuhan 1651 of whom recovered and 208 died. We interrogated diverse covariates for correlations with risk of death from COVID-19. In multi-variable Cox regression analyses increased hazards of in-hospital death were associated with several admission covariates: (1) older age (HR = 1.04; 95% Confidence Interval [CI], 1.03, 1.06 per year increase; P < 0.001); (2) smoking (HR = 1.84 [1.17, 2.92]; P = 0.009); (3) admission temperature per °C increase (HR = 1.32 [1.07, 1.64]; P = 0.009); (4) Log10 neutrophil-to-lymphocyte ratio (NLR; HR = 3.30 [2.10, 5.19]; P < 0.001); (5) platelets per 10 E + 9/L decrease (HR = 0.996 [0.994, 0.998]; P = 0.001); (6) activated partial thromboplastin (aPTT) per second increase (HR = 1.04 [1.02, 1.05]; P < 0.001); (7) Log10 D-dimer per mg/l increase (HR = 3.00 [2.17, 4.16]; P < 0.001); and (8) Log10 serum creatinine per μmol/L increase (HR = 4.55 [2.72, 7.62]; P < 0.001). In piecewise linear regression analyses Log10NLR the interval from ≥0.4 to ≤1.0 was significantly associated with an increased risk of death. Our data identify covariates associated with risk of in hospital death in persons with COVID-19.
Subject terms: Infectious diseases, Risk factors
Introduction
The SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2) pandemic has caused many deaths from coronavirus disease 2019 (COVID-19) [1–8]. The outbreak began in December 2019 in Wuhan city, Hubei province, China [9–16]. There are several studies or risk factors for death from COVID-19 but most have relatively few subjects and come from 1 or 2 centers [17–26]. We analyzed prognostic covariates for death in 1859 subjects with confirmed COVID-19 from 7 centers in Wuhan city from January 20 to April 4, 2020. Curiously, we found COVID-19 progressed similarly until day 10 after admission but progressed more slowly in the cohort of subjects who died compared with those recovering, possibly because of therapy interventions [27, 28]. Subjects who died had a greater frequency of comorbidities before COVID-19 and complications after developing COVID-19. We were able to show a correlation between likelihood of death and several hematological and other laboratory covariates at diagnosis.
Methods
Subjects
From 20 January to 4 April 2020, all consecutive patients ≥18 years were enrolled from Union Hospital (main part, Union West Hospital and Union Tumor Hospital), Wuhan Central Hospital, General Hospital of Central Theater Command, PLA, Wuhan Third Hospital and Wuhan Jin-Yin-Tan Hospital. These hospitals were reconstructed and designated as COVID-19 treatment centers. Between February 4 and February 18, 2020 persons with clinical symptoms and a lung computed tomography (CT) scan consistent with COVID-19 were diagnosed as having COVID-19 without confirmation of SARS-CoV-2-infection by quantitative reverse transcript polymerase chain reaction (qRT-PCR). After hospitalization subjects were tested by qRT-PCR to confirm the diagnosis and monitor their course. Beginning 4 March, 2020, anti-SARS-CoV-2 IgM and/or IgG antibodies were assayed at Union Hospital and Wuhan Central Hospital by the centers to confirm the diagnosis and to evaluate suspected cases of COVID-19 which were qRT-PCR-negative [29]. Subjects in whom we could not confirm SARS-CoV-2-infection by a qRT-PCR, IgM/IgG assay, or either were excluded from the study. Subjects recovering from COVID-19 were discharged and transferred to designated hotels, Fangcang shelter hospitals [30] or Leishenshan Hospital for 2–4 weeks of isolation or further care if needed. The study was approved by the Ethics Committees of Union Hospital (2020-0095) and of Wuhan Central Hospital (2020-007). Written and orally informed consent from subjects was waived by the Ethics Committees.
Data collection
We obtained epidemiological, demographic, clinical, laboratory, radiological, therapy, and outcomes data from electronic medical records using a standardized data collection form. Therapies included antibiotics, anti-viral therapy, corticosteroids, and supportive care including supplemental oxygen, mechanical ventilation (with and without intubation), and extracorporeal membrane oxygenation (ECMO). Data were independently entered and cross validated by two physicians (WH and JY). A third researcher (QL) adjudicated discordances. Missing data were retrieved from the relevant hospital.
SARS-CoV-2 testing and laboratory covariants
Methods for diagnosis of SARS-CoV-2-infection by qRT-PCR are described [24]. Before January 11, 2020 testing was done by a few institutions such as the Chinese Center for Disease Control and Prevention. Beginning January 11, 2020, qRT-PCR testing was done at local Centres for Disease Control and Prevention and from January 27, 2020 in the study hospitals. Beginning March 4, 2020 IgM/IgG antibodies to SARS-CoV-2 were tested at Union Hospital and after March 5, 2020 in Wuhan Central Hospital. Nasopharyngeal swab specimens were obtained every other day if there was clinical improvement judged by clinical signs and symptoms and lung CT scan. Subjects recovering were discharged after ≥2 negative qRT-PCR tests >24 h apart. Studies on admission included a CBC and differential, biochemistry panel, coagulation profile, and tests of inflammation including C-reactive protein (CRP), procalcitonin, lactate dehydrogenase (LDH), and ferritin. All subjects had a lung CT scan.
Definitions
Exposure history was defined as exposure to persons with confirmed SARS-CoV-2-infection or visiting the Huanan Wholesale Seafood Market, possible origin site of the SARS-CoV-2 epidemic in Wuhan city. Smoking history was defined as current or former smoker (stopping >5 years ago) with exposure of ≥20 cigarettes per day for ≥1 year (1 pack year). Fever was defined as temperature ≥37.3 °C. Diagnosis of bacterial infection required ≥1 positive culture or a positive antigen detection test. Acute kidney injury, acute respiratory distress syndrome (ARDS), and acute cardiac injury were diagnosed according to guidelines or as reported [24, 31, 32]. Liver damage was defined as more than 2x upper limit of normal. Severity of COVID-19 was classified as; (1) mild; (2) moderate; (3) severe; or (4) critical according to the Chinese management guideline for COVID-19 (version 7) [29, 33]. Recovery was defined as complete resolution of clinical signs and symptoms, normalization of the lung CT scan (if abnormal) and ≥2 negative qRT-PCR tests for SARS-CoV-2. Subjects dying of unrelated causes were excluded from analyses of COVID-19-related deaths. Invasive and noninvasive mechanical ventilations were defined as mechanical ventilation with and without intubation.
Statistical analysis
Demographics and clinical covariates were presented using descriptive statistics with frequencies (percentage) for discrete variables and median (IQR) and range for continuous variables. Medians were compared using independent group t test and Mann–Whitney test for normally and abnormally distributed data. Proportions for categorical variables were compared by χ2 test. Missing data were ignored without multiple imputations. Covariates considered for correlations with death included age, sex, occupation, signs and symptoms, laboratory and radiological findings, smoking history, comorbidities including arterio-sclerotic cardio-vascular disease (ASCVD), arterial hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), cancer, ARDS, infection, septic shock, acute renal failure, myocardial infarction, liver injury, gastro-intestinal bleeding, disseminated vascular coagulation, and multiple organ failure. Neutrophil-to-lymphocyte ratio (NLR), D-dimer and serum creatine (Scr) were log10 transformed before the analyses because of non-normal distributions. Uni- and multi-variable Cox regression models were used to evaluate associations of covariants with risk of death. R version 3.5.2 was used for statistical analyses. For unadjusted comparisons, a two-sided alpha of <0.05 was considered significant. Analyses were not adjusted for multiple comparisons.
Results
Admission clinical covariates
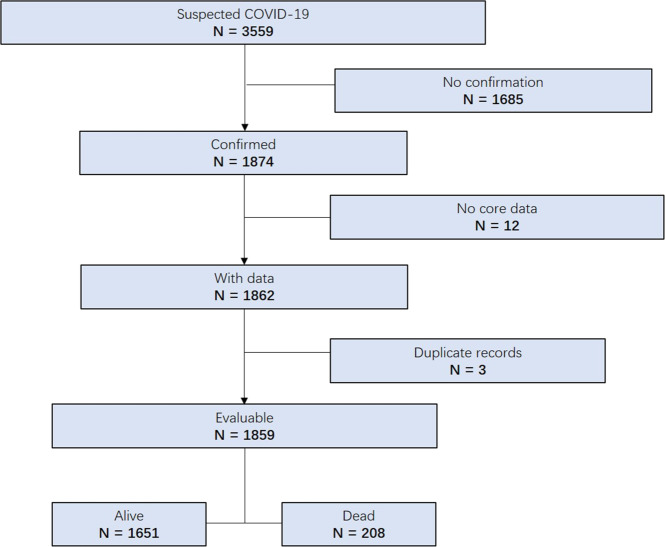
From January 20 to April 4, 2020, 3559 subjects who died or had been discharged with clinical- or qRT-PCR-confirmed COVID-19 [34, 35] were enrolled. As shown in Fig. 1, 1859 subjects were included for analysis, with SARS-CoV-2-infection confirmed in 1790 subjects by qRT-PCR and by antibody testing in 69. Of the 1859 subjects, 208 died (11%), 157 (8%) recovered and discharged but remained hospitalized in other units for 2–4 weeks of recovery-related care, and 1494 (80%) discharged and transferred to COVID-19 designated hotels or Fangcang shelter hospitals [30] for 2–4 weeks of isolation.
Fig. 1.
Study flow diagram.
Median age was 59 years (Interquartile Range [IQR] 45–68 years; Table 1). 806 (43%) were 60–79 years and 122 (7%) >80 years. 934 subjects were male (50%). 111 (6%) were current or former smokers, 71 (5%), health care provider, and 14 (1%), with pregnancy or puerperium. In total, 4 subjects were exposed at Huanan Seafood Wholesale Market and 78 (4%) had close contact with persons with confirmed SARS-CoV-2 infection. 579 (31%) had hypertension, 268 (14%), ASCVD, 262 (14%), diabetes, 98 (5%), gastro-intestinal disease, 69 (4%), cancer and 61 (3%), COPD. Most common signs and symptoms included fever (n = 1448, 78%), shortness of breath (n = 716, 39%), dry (n = 619, 43%) or wet cough (n = 715, 39%), fatigue (n = 695, 37%), chills (n = 281, 19%) and myalgia (n = 315, 17%). Bilateral pneumonia (n = 1570, 88%) and Ground-glass opacity (n = 1331, 75%) were two most findings in lung CT scan. 34 (2%) subjects had mild, 1170 (63%), moderate, 453 (24%), severe and 202 (11%) critical COVID-19.
Table 1.
Demographic and clinical covariates.
| Total n = 1859 | Alive n = 1651 (89) | Died n = 208 (11) | P value | |
|---|---|---|---|---|
| Age, median (IQR), years | 59 (45, 68) | 57 (43, 66) | 70 (63, 78) | <0.001 |
| Age distribution | <0.001 | |||
| <40 years | 342 (18) | 337 (20) | 5 (2) | |
| 40–59 years | 589 (32) | 556 (34) | 33 (16) | |
| 60–79 years | 806 (43) | 681 (41) | 125 (60) | |
| ≥80 years | 122 (7) | 77 (5) | 45 (22) | |
| Female sex | 925 (50) | 870 (53) | 55 (26) | <0.001 |
| Smoking history | 111 (6) | 86 (5) | 25 (13) | <0.001 |
| Former smoker | 66 (4) | 54 (3) | 12 (6) | |
| Current smoker | 45 (2) | 32 (2) | 13 (7) | |
| Health care provider | 71 (5) | 69 (6) | 2 (1) | 0.008 |
| Pregnancy/Puerperium | 14 (1) | 14 (1) | 0 (0) | 0.394 |
| Exposure history | 0.005 | |||
| Huanan Seafood Market | 4 (0.2) | 1 (0.1) | 3 (1) | |
| Close contact with patients | 78 (4) | 72 (4) | 6 (3) | |
| Comorbidity | ||||
| ASCVD | 268 (14) | 205 (12) | 63 (30) | <0.001 |
| Hypertension | 579 (31) | 475 (29) | 104 (50) | <0.001 |
| Diabetes | 262 (14) | 203 (12) | 59 (28) | <0.001 |
| COPD | 61 (3) | 49 (3) | 12 (6) | 0.039 |
| Cancer | 69 (4) | 52 (3) | 17 (8) | <0.001 |
| Chronic kidney disease | 45 (2) | 25 (2) | 20 (10) | <0.001 |
| Gastro-intestinal disease | 98 (5) | 82 (5) | 16 (8) | 0.097 |
| Auto-immune disease | 10 (1) | 9 (1) | 1 (1) | 0.999 |
| Psychiatric disorders | 7 (0.5) | 6 (0.5) | 1 (1) | 0.586 |
| Signs and symptoms | ||||
| Fevera | 1448 (78) | 1274 (77) | 174 (84) | 0.025 |
| Temperature (°C)b | 36.6 (36.4, 37.0) | 36.6 (36.4, 37.0) | 36.8 (36.5, 37.5) | <0.001 |
| Shortness of breath | 716 (39) | 572 (35) | 144 (70) | <0.001 |
| Dry cough | 619 (43) | 554 (43) | 65 (38) | 0.205 |
| Wet cough | 715 (39) | 609 (37) | 106 (51) | <0.001 |
| Fatigue | 695 (37) | 595 (36) | 100 (48) | <0.001 |
| Nausea or vomiting | 124 (9) | 114 (9) | 10 (6) | 0.186 |
| Diarrhea | 243 (13) | 213 (13) | 30 (14) | 0.522 |
| Chills | 281 (19) | 236 (18) | 45 (26) | 0.015 |
| Rhinorrhea | 33 (2) | 30 (2) | 3 (1) | 0.999 |
| Myalgia | 315(17) | 282 (17) | 33 (16) | 0.681 |
| Headache | 107 (6) | 101 (6) | 6 (3) | 0.061 |
| Radiological features | ||||
| Bilateral pneumonia | 1570 (88) | 1402 (87) | 168 (96) | <0.001 |
| Consolidation | 326 (18) | 266 (17) | 60 (35) | <0.001 |
| Ground-glass opacity | 1331 (75) | 1213 (76) | 118 (69) | 0.042 |
| Patchy shadows | 736 (41) | 664 (41) | 72 (42) | 0.81 |
| COVID-19 stage | <0.001 | |||
| Mild | 34 (2) | 34 (2) | 0 (0) | |
| Moderate | 1170 (63) | 1162 (70) | 8 (4) | |
| Severe | 453 (24) | 427 (26) | 26 (13) | |
| Critical | 202 (11) | 28 (2) | 174 (84) | |
Data are median (IQR) or n (%).
ASCVD atherosclerotic cardio- and cerebro-vascular disease, COPD chronic obstructive pulmonary disease.
a≥1 temperature ≥37.3 °C from onset of symptoms to admission.
bAdmission temperature.
Comparison of survivors and nonsurvivors by clinical covariates
Subjects who died were older (medians 70 versus 57 years; P < 0.001, Table 1), more likely male (74% versus 47%; P < 0.001), more likely smokers (13% versus 5%; P < 0.001) and less likely health care providers (1% versus 6%, P = 0.008). More subjects who died were exposure at the Huanan Seafood Wholesale Market (1% versus 0.1%, P = 0.005). Nonsurvivors more likely to have comorbidities of hypertension (50% versus 29%; P < 0.001), ASCVD (30% versus 12%; P < 0.001), diabetes (28% versus 12%; P < 0.001), COPD (6% versus 3%; P = 0.039), cancer (8% versus 3%; P < 0.001) and kidney failure (10% versus 2%; P < 0.001). Fever from illness onset to admission (84% versus 77%; P = 0.025), shortness of breath (70% versus 35%; P < 0.001), wet cough (51% versus 37%; P < 0.001), fatigue (48% versus 36%; P < 0.001), chills (26% versus 18%; P = 0.015), bilateral pneumonia (96% versus 87%; P < 0.001) and lung consolidation on CT scan (35% versus 17%; P < 0.001) were more common in subjects who died. Paradoxically, survivors were more likely to have ground-glass lung opacity on lung CT scan (76% versus 69%; P = 0.042). Subjects who died were more likely to have critical COVID-19 on admission (84% versus 2%; P < 0.001) and less likely to have moderate severity (4% versus 70%; P < 0.001).
Comparison of survivors and nonsurvivors by laboratory covariates
There were significant differences in admission laboratory covariates between survivors and nonsurvivors (Table 2). Subjects who died had higher median neutrophils (7 × 10 E + 9/L [IQR 4–10 × 10 E + 9/L] versus 3 × 10 E + 9/L [IQR 2–4 × 10 E + 9/L]; P < 0.001), lower median lymphocytes (0.6 × 10 E + 9/L [IQR 0.4–0.9 × 10 E + 9/L] versus median 1.2 × 10 E + 9/L [IQR 0.9–1.6 × 10 E + 9/L]; P < 0.001), lower median platelets (163 × 10 E + 9/L [IQR 113–223 × 10 E + 9/L] versus 207 10 E + 9/L [IQR 161–268 × 10 E + 9/L]; P < 0.001) and higher median neutrophil-to-lymphocyte ratios (NLR; 11 [IQR 6–20] versus 3 [IQR 2–4]; P < 0.001). Nonsurvivors had lower median proportions of CD3-positive cells (60% [IQR 52–70%] versus 72% [IQR 63–79%]; P < 0.001), median proportions of CD8-positive cells (16% [IQR 11–20%] versus 24% [IQR 18–30%]; P < 0.001), median proportions of NK-cells (8% [IQR 3–12%] versus 10%, [IQR 6–17%]; P = 0.011), and higher proportions of B-lymphocyte (15% [IQR 9–28%] versus 12% [IQR 9–17%]; P = 0.033) and higher median CD4/CD8 ratios (3 [IQR 2–4] versus 2 [IQR 1–3]; P < 0.001) compared with survivors.
Table 2.
Laboratory covariates on admission.
| Covariates (normal range) | N | Total | Alive | Died | P value |
|---|---|---|---|---|---|
| CBC | |||||
| Neutrophils ×10E + 9/L (1.8–6.3) | 1816 | 3 (2, 5) | 3 (2, 4) | 7 (4, 10) | <0.001 |
| Lymphocytes ×10 E + 9/L (1.1–3.2) | 1847 | 1.0 (0.8, 1.6) | 1.2 (0.9, 1.6) | 0.6 (0.4, 0.9) | <0.001 |
| Monocytes ×10 E + 9/L (0.1–0.6) | 1805 | 0.4 (0.3, 0.5) | 0.4 (0.3, 0.5) | 0.3 (0.2, 0.5) | <0.001 |
| Hemoglobin, g/L (115–150) | 1433 | 128 (117, 139) | 128 (117, 139) | 129 (117, 140) | 0.539 |
| Platelets ×10 E + 9/L (125–350) | 1814 | 203 (155, 264) | 207 (161, 268) | 163 (113, 223) | <0.001 |
| NLR | 1814 | 3 (2, 5) | 3 (2, 4) | 11 (6, 20) | <0.001 |
| Inflammation covariates | |||||
| hCRP, mg/L (<4) | 995 | 4 (1, 10) | 3 (1, 10) | 10 (10, 80) | <0.001 |
| Procalcitonin, ng/ml (< 0.5) | 1643 | 0.06 (0.05, 0.1) | 0.05 (0.04, 0.1) | 0.3 (0.1, 0.6) | <0.001 |
| LDH, U/L (109–245) | 1729 | 212 (170, 292) | 201 (165, 261) | 412 (306, 561) | <0.001 |
| Ferritin, ng/ml (4.6–204) | 308 | 567 (246, 1218) | 470 (197, 940) | 1579 (1206, 2000) | <0.001 |
| Coagulation covariates | |||||
| aPTT, s (28–43.5) | 1356 | 34 (30, 38) | 34 (30, 38) | 37 (31, 42) | <0.001 |
| Fibrinogen, g/L (2–4) | 1323 | 3.7 (2.9, 4.6) | 3.7 (2.9, 4.6) | 4.3 (3.2, 5.1) | <0.001 |
| D-dimer, mg/L (<0.5) | 1602 | 0.4 (0.2, 1.1) | 0.4 (0.2, 0.8) | 2.5 (0.7, 8) | <0.001 |
| Biochemical covariates | |||||
| ALT, U/L (5–35) | 1832 | 38 (22, 67) | 36 (21, 63) | 58 (30, 139) | <0.001 |
| AST, U/L (8–40) | 1830 | 32 (22, 49) | 30 (22, 44) | 64 (40, 140) | <0.001 |
| Total bilirubin, μmol/L (5.1–19) | 1586 | 14 (10, 19) | 13 (10, 18) | 24 (15, 36) | <0.001 |
| Creatine kinase, U/L (26–140) | 1493 | 88 (54, 165) | 81 (52, 135) | 262 (135, 636) | <0.001 |
| BNP, pg/ml (< 100) | 838 | 61 (18, 242) | 47 (15, 140) | 467 (121, 1467) | <0.001 |
| Myoglobin, ng/ml (<140) | 972 | 35 (21, 73) | 30 (21, 51) | 576 (175, 1013) | <0.001 |
| Troponin I, ng/L (<26.2) | 1083 | 4 (1, 14) | 3 (1, 8) | 161 (46, 712) | <0.001 |
| BUN, mmol/L (2.9–8.2) | 1815 | 5 (4, 7) | 5 (4, 6) | 15 (9, 27) | <0.001 |
| Scr, μmol/L (44–106) | 1813 | 71 (59, 85) | 69 (58, 81) | 108 (76, 256) | <0.001 |
| Lymphocyte subsets | |||||
| CD3+, (58–84%) | 759 | 71 (62, 78) | 72 (63, 79) | 60 (52, 70) | <0.001 |
| CD4+, (25–51%) | 759 | 41 (32, 48) | 41 (33, 48) | 37 (28, 46) | 0.094 |
| CD8+, (14–39%) | 759 | 23 (17, 30) | 24 (18, 30) | 16 (11, 20) | <0.001 |
| NK cell, (3–30%) | 561 | 10 (6, 16) | 10 (6, 17) | 8 (3, 12) | 0.011 |
| B lymphocyte, (4–18%) | 561 | 13 (9, 18) | 12 (9, 17) | 15 (9, 28) | 0.033 |
| CD4 + /CD8 + Ratio (0.41–2.72) | 755 | 2 (1, 3) | 2 (1, 3) | 3 (2, 4) | <0.001 |
| Cytokines | |||||
| IL-4, pg/ml (0.1–3.2) | 505 | 3 (2, 4) | 3 (2, 4) | 2 (2, 3) | 0.21 |
| IL-6, pg/ml (0.1–2.9) | 857 | 10 (4, 42) | 9 (4, 32) | 79 (23, 525) | <0.001 |
| IL-10, pg/ml (0.1–5) | 505 | 4 (3, 6) | 4 (3, 5) | 10 (5, 22) | <0.001 |
| TNF-α, pg/ml (0.1–23) | 505 | 3.3 (2.2, 5.4) | 3.5 (2.3, 5.6) | 2.7 (1.9, 3.6) | 0.009 |
| IFN-γ, pg/ml (0.1–18) | 505 | 3.1 (2.0, 4.1) | 3.1 (2.0, 4.1) | 2.7 (1.8, 4.2) | 0.313 |
Data are median (IQR).
NLR neutrophil-to-lymphocyte ratio, hCRP high-sensitivity c-reactive protein, LDH lactate dehydrogenase, aPTT activated partial thromboplastin time, ALT alanine aminotransferase, AST aspartate aminotransferase, BNP B-type natriuretic peptide, BUN blood urea nitrogen, Scr serum creatinine, IL interleukin, TNF tumor necrosis factor, IFN interferon.
Subjects who died had longer median aPTT (37 s [IQR 31–42 s] versus 34 s [IQR 30–38 s]; P < 0.001) and higher median concentrations of fibrinogen (4.3 g/L [IQR 3.2–5.1 g/L] versus 3.7 g/L [IQR 2.9–4.6 g/L]; P < 0.001) and median D-dimer concentrations (2.5 mg/L [IQR 0.7–8 mg/L] versus 0.4 mg/L [IQR 0.2–0.8 mg/L]; P < 0.001).
Subjects who died also had higher median hCRP concentrations (10 mg/L [IQR 10–80 mg/L] versus 3 mg/L [IQR 1–10 mg/L]; P < 0.001), median procalcitonin concentrations (0.3 ng/ml [IQR 0.1–0.6 ng/mL] versus 0.05 ng/ml [IQR 0.04–0.1 ng/mL]; P < 0.001), median LDH activities (412 U/L [IQR 306–561 U/L] versus 201 U/L [IQR 165–261 U/L]; P < 0.001), median ferritin concentrations (1579 ng/mL [IQR 1206-2000 ng/mL] versus 470 ng/mL [IQR 197–940 ng/mL]; P < 0.001), median IL-6 concentrations (79 pg/mL [IQR 23–525 pg/mL] versus 9 pg/mL [IQR 4–32 pg/mL]; P < 0.001), median IL-10 concentrations (10 pg/mL [IQR 5–22 pg/mL] versus 4 pg/mL[IQR 3–5 pg/mL]; P < 0.001) and lower median TNF-α concentrations (2.7 pg/mL [IQR 1.9–3.6 pg/mL] versus 3.5 pg/mL [IQR 2.3–5.6 pg/mL]; P = 0.009).
Subjects who died also had higher median activities of alanine aminotransferase (ALT; 58 U/L [IQR 30–139 U/L] versus 36 U/L [IQR 21–63 U/L]; P < 0.001), aspartate aminotransferase (AST; 64 U/L [IQR 40–140 U/L] versus 30 U/L [IQR 22–44 U/L]; P < 0.001), creatine kinase (262 U/L [IQR 135–636 U/L] versus 81 U/L [IQR 52–135 U/L]; P < 0.001) and median concentrations of total bilirubin (24 μmol/L [IQR 15–36 μmol/L] versus 13 μmol/L [IQR 10–18 μmol/L]; P < 0.001), b-type natriuretic peptide (BNP; 467 pg/ml [IQR 121–1467 pg/ml] versus 47 pg/ml, [IQR 15–140 pg/ml]; P < 0.001), myoglobin (576 ng/ml [IQR 175–1013 ng/ml] versus 30 ng/ml [IQR 21–51 ng/ml]; P < 0.001) and troponin I (161 ng/L [IQR 46–712 ng/L] versus 3 ng/L [IQR 1–8 ng/L]; P < 0.001), blood urea nitrogen (BUN; 15 mmol/L [IQR 9–27 mmol/L] versus 5 mmol/L [IQR 4–6 mmol/L]; P < 0.001) and Scr (108 μmol/L [IQR 76–256 μmol/L] versus 69 μmol/L [IQR 58–81 μmol/L]; P < 0.001).
Complications and treatments for survivors and non-survivors
Subjects who died were more likely to have complications (207 [99.5%] versus 1039 [63%]; P < 0.001) including ARDS (174 [84%] versus 53 [3%]; P < 0.001), bacterial infection (180 [87%] versus 383 [23%]; P < 0.001) and liver damage (102 [50%] versus 354 [22%]; P < 0.001; Table 3). Nonsurvivors also had higher incidence of heart injury (130 [63%] versus 99 [6%]; P < 0.001), multiple organ failure (126 [61%] versus 3 [0.2%]; P < 0.001), acute kidney injury (82 [39%] versus 17 [1%]; P < 0.001), septic shock (63 [37%] versus 1 [0.1%]; P < 0.001), abnormal coagulation parameters (47 [23%] versus 2 [0.1%]; P < 0.001) and gastro-intestinal bleeding (29 [14%] versus 5 [0.3%]; P < 0.001).
Table 3.
Complications and therapy.
| Total n = 1859 | Alive n = 1651 (89) | Died n = 208 (11) | P value | |
|---|---|---|---|---|
| Complications | ||||
| ARDS | 227 (12) | 53 (3) | 174 (84) | <0.001 |
| Bacterial infections | 563 (30) | 383 (23) | 180 (87) | <0.001 |
| Septic shock | 64 (4) | 1 (0.1) | 63 (37) | <0.001 |
| Acute kidney injury | 99 (5) | 17 (1) | 82 (39) | <0.001 |
| Cardiac injury | 229 (12) | 99 (6) | 130 (63) | <0.001 |
| Abnormal LFT | 456 (25) | 354 (22) | 102 (50) | <0.001 |
| Gastro-intestinal bleeding | 34 (2) | 5 (0.3) | 29 (14) | <0.001 |
| Coagulopathy | 49 (3) | 2 (0.1) | 47 (23) | <0.001 |
| Multiple organ failure | 129 (7) | 3 (0.2) | 126 (61) | <0.001 |
| Therapy | ||||
| Antibiotics | 1559 (85) | 1356 (84) | 203 (98) | <0.001 |
| Antifungal drugs | 71 (4) | 32 (2) | 39 (20) | <0.001 |
| Oseltamivir | 757 (41) | 688 (42) | 69 (33) | 0.021 |
| Umifenovir | 1386 (75) | 1226 (74) | 160 (77) | 0.351 |
| Lopinavir/Ritonavir | 339 (23) | 280 (22) | 59 (35) | <0.001 |
| Interferon | 387 (21) | 344 (21) | 43 (21) | 0.957 |
| Corticosteroids | 753 (41) | 588 (36) | 165 (80) | <0.001 |
| IVIG | 506 (29) | 401 (26) | 105 (52) | <0.001 |
| High-flow nasal cannula oxygen therapy | 233 (16) | 81 (6) | 152 (89) | <0.001 |
| Noninvasive mechanical ventilation | 145 (8) | 27 (2) | 118 (57) | <0.001 |
| Invasive mechanical ventilation | 85 (5) | 12 (1) | 73 (35) | <0.001 |
| ECMO | 4 (0.2) | 1 (0.1) | 3 (1) | 0.005 |
| CRRT | 23 (2) | 4 (0.3) | 19 (11) | <0.001 |
| Outcomes | ||||
| ICU admission | 106 (6) | 36 (2) | 70 (34) | <0.001 |
| Time from illness onset to ICU admission, median (IQR), days | 14 (10, 20) | 14 (10, 21) | 14 (10, 20) | 0.962 |
| ICU length of stay, median (IQR), days | 10 (4, 17) | 10 (5, 18) | 10 (4, 16) | 0.676 |
| Time from illness onset to repeated negative SARS-CoV-2 tests, median (IQR), days | 22 (17, 28) | 22 (17, 28) | 21 (15, 27) | 0.284 |
| Time from illness onset to admission, median (IQR), days | 10 (7, 15) | 10 (7, 16) | 9 (6, 12) | <0.001 |
| Time from illness onset to progression, median (IQR), days | 10 (7, 15) | 10 (6, 14) | 12 (9, 18) | <0.001 |
| Time from illness onset to outcome, median (IQR), days | 30 (23, 37) | 31 (24, 38) | 21 (14, 28) | <0.001 |
| Time from diagnosis to outcome, median (IQR), days | 19 (13, 27) | 20 (14, 28) | 11 (5, 17) | <0.001 |
| Time from admission to outcome, median (IQR), days | 18 (12, 23) | 18 (14, 23) | 10 (6, 19) | <0.001 |
LFT liver function test, ARDS acute respiratory distress syndrome, IVIG intravenous immunoglobin, ECMO extra-corporeal membrane oxygenation, CRRT continuous renal replacement therapy, ICU intensive care unit.
Also, subjects who died were more likely to receive antibiotics (203 [98%] versus 1356 [84%]; P < 0.001), antifungal drugs (39 [20%] versus 32 [2%]; P < 0.001), lopinavir and ritonavir (59 [35%] versus 280 [22%]; P < 0.001), corticosteroids (165 [80%] versus 588 [36%]; P < 0.001), intravenous immunoglobin (IVIG; 105 [52%] versus 401 [26%]; P < 0.001), high-flow nasal cannula oxygen therapy (152 [89%] versus 81 [6%]; P < 0.001), noninvasive mechanical ventilation (118 [57%] versus 27 [2%]; P < 0.001), invasive mechanical ventilation (73 [35%] versus 12 [1%]; P < 0.001), ECMO (3 [1%] versus 1 [0.1%]; P = 0.005) and continuous renal replacement therapy (CRRT) (19 [11%] versus 4 [0.3%]; P < 0.001).
Nonsurvivors had briefer median intervals from onset of symptoms to admission (9 d [IQR 6–12 d] versus 10 d [IQR 7–16 d]; P < 0.001) and median intervals from onset of symptoms to death or discharge (21 d [IQR 14–28] versus 31 d [IQR 24–38 d]; P < 0.001), and from admission to death or discharge (10 d [IQR] 6–19 d versus 18 d [IQR 14–23 d]; P < 0.001) but longer median intervals from onset of symptoms to progression (median 12 d [IQR 9–18 d] versus 10 d [QR 6–14 d]; P < 0.001). There were no differences between survivors and nonsurvivors in median intervals from symptoms onset to ICU admission (14 d [IQR 10–20 d] versus 14 d [IQR 10–21 d]; P = 0.962) or median intervals to negative SARS-CoV-2 testing (21 d [IQR 15–27 d] versus 22 d [IQR 17–28 d]; P = 0.284). In total, 178 of the 208 subjects who died (86%) had a positive qRT-PCR test until death.
Risk factors for death
In total, 33 covariants had significant associations with risk of death in uni-variable analyses, 8 of which remained significant in multi-variable analyses including age (HR = 1.04 [1.03, 1.06]; P < 0.001), smoking history (HR = 1.84 [1.17, 2.92]; P = 0.009), temperature value (°C) at admission (HR = 1.32 [1.07–1.64]; P = 0.009), log10 NLR (HR = 3.30 [2.10, 5.19]; P < 0.001), admission platelet concentration (HR = 0.996; [0.994–0.998]; P = 0.001), aPTT on admission (HR = 1.04 [1.02, 1.05]; P < 0.001), Log10 D-dimer (HR = 3.00 [2.17, 4.16]; P < 0.001), and Log10 Cr (HR = 4.55 [2.72, 7.62]; P < 0.001; Table 4).
Table 4.
Risk factors for death.
| Uni-variable HR (95% CI) | P value | Multivariable HR (95% CI) | P value | |
|---|---|---|---|---|
| Clinical covariates | ||||
| Age, years | 1.07 (1.06–1.08) | <0.001 | 1.04 (1.03–1.06) | <0.001 |
| Female sex (vs male) | 0.35 (0.26–0.48) | <0.001 | .. | .. |
| Smoking history (vs nonsmoking) | 2.43 (1.59–3.73) | <0.001 | 1.84 (1.17–2.92) | 0.009 |
| Health care provider (vs non health care provider) | 0.24 (0.06–0.96) | 0.044 | .. | .. |
| Comorbidity (Yes/No) | ||||
| ASCVD | 2.56 (1.90–3.45) | <0.001 | .. | .. |
| Diabetes | 2.47 (1.82–3.34) | <0.001 | .. | .. |
| Hypertension | 2.21 (1.68–2.90) | <0.001 | .. | .. |
| Cancer | 2.59 (1.58–4.26) | <0.001 | .. | .. |
| Symptoms and complications (Y/N) | ||||
| Dyspnea | 6.26 (4.76–8.24) | <0.001 | .. | .. |
| Wet cough | 1.63 (1.24–2.14) | 0.001 | .. | .. |
| ARDS | 54.21 (37.13–79.14) | <0.001 | .. | .. |
| Bacterial infections | 18.36 (12.16–27.72) | <0.001 | .. | .. |
| Temperature at admission (°C) | 1.50 (1.28–1.75) | <0.001 | 1.32 (1.07–1.64) | 0.009 |
| Laboratory covariates | ||||
| Neutrophils ×10E + 9/L | 1.23 (1.20–1.26) | <0.001 | .. | .. |
| Lymphocytes x10E + 9/L | 0.07 (0.05–0.10) | <0.001 | .. | .. |
| Log10 NLR | 1.06 (1.06–1.07) | <0.001 | 3.30 (2.10–5.19) | <0.001 |
| Platelets x10E + 9/L | 0.99 (0.99–1.00) | <0.001 | 0.996 (0.994–0.998) | 0.001 |
| hCRP, mg/L | 1.02 (1.02–1.02) | <0.001 | .. | .. |
| Procalcitonin, ng/ml | 1.23 (1.18–1.28) | <0.001 | .. | .. |
| LDH, U/L | 1.00 (1.00–1.00) | <0.001 | .. | .. |
| Ferritin, ng/ml | 1.00 (1.00–1.00) | <0.001 | .. | .. |
| aPTT, s | 1.06 (1.04–1.08) | <0.001 | 1.04 (1.02–1.05) | <0.001 |
| Log10 D-dimer, mg/L | 1.09 (1.08–1.11) | <0.001 | 3.00 (2.17–4.16) | <0.001 |
| Total bilirubin, μmol/L | 1.03 (1.03–1.04) | <0.001 | .. | .. |
| Creatine kinase, U/L | 1.00 (1.00–1.00) | <0.001 | .. | .. |
| Troponin I, ng/L | 1.00 (1.00–1.00) | <0.001 | .. | .. |
| BUN, mmol/L | 1.06 (1.05–1.07) | <0.001 | .. | .. |
| Log10 Scr, μmol/L | 1.00 (1.00–1.00) | <0.001 | 4.55 (2.72–7.62) | <0.001 |
| IL-6 | 1.00 (1.00–1.00) | <0.001 | .. | .. |
| IL-10 | 1.00 (1.00–1.00) | 0.004 | .. | .. |
| CD3-positive, % | 0.95 (0.93–0.97) | <0.001 | .. | .. |
| CD8-positive, % | 0.91 (0.88–0.94) | <0.001 | .. | .. |
| CD4/CD8 ratio | 1.27 (1.18–1.37) | <0.001 | .. | .. |
CI confidence interval, ASCVD atherosclerotic cardio- and cerebro-vascular disease, ARDS acute respiratory distress syndrome, NLR neutrophil-to-lymphocyte ratio, hCRP high-sensitive c-reactive protein, LDH lactate dehydrogenase, aPTT activated partial thromboplastin time, BUN blood urea nitrogen, Scr serum creatinine.
We further showed the linear relationship between these covariants except age, smoking history, and temperature at admission and risk of death (Fig. 2). Based on the steep curve of Log10 NLR we conducted a further piecewise linear regression analysis of NLR and death. The results indicate a Log10 NLR value of ≥0.4 to ≤1.0 is significantly associated with risk of death (Table 5).
Fig. 2. The linear relationship between admission covariants and risk of death.
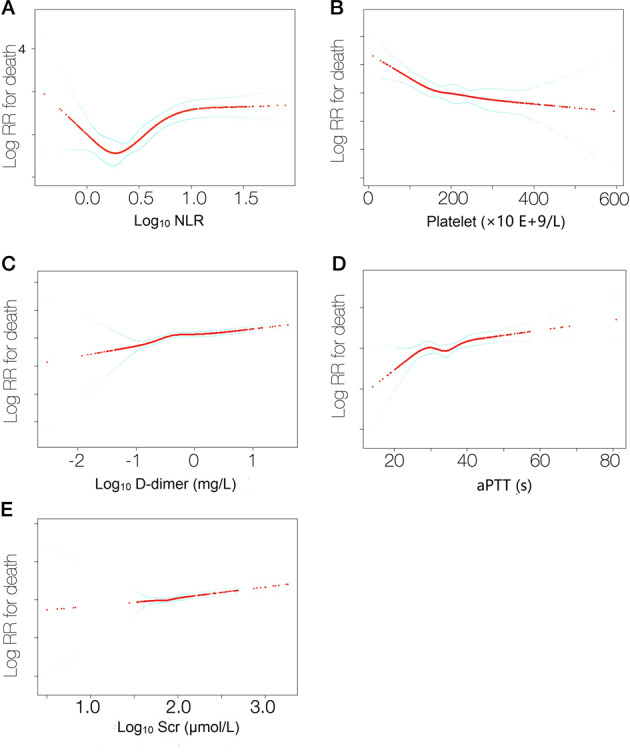
(a) log10NLR, (b) platelet (x 10 E+9/L), (c) log10D-dimer (mg/L), (d) aPTT (s) and (e) log10Scr (μmol/L).
Table 5.
Piecewise linear regression analysis of the effect of Neutrophil-to-lymphocyte ratio (NLR) on risk of death.
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| Log10 NLR | 3.30 (2.10–5.19) | <0.001 |
| Log10 NLR < 0.4 | 0.44 (0.02–10.09) | 0.608 |
| Log10 NLR ≥ 0.4, ≤ 1.0 | 14.06 (3.23–61.21) | <0.001 |
| Log10 NLR > 1.0 | 0.49 (0.17–1.44) | 0.195 |
Discussion
We identified eight hospital admission covariates which are independent risk factors for death in almost 2000 persons with COVID-19 including older age, smoking history, higher body temperature (°C), and levels of D-dimer, aPTT, Scr, platelet, and NLR on admission. Several are reported by others; however, we were unable to confirm other risk factors reported in smaller datasets [15, 18–21, 23, 24, 26, 36].
We identified Log10 NLR as an independent risk factor for death with an HR = 14.1 (3.2, 61.2) with Log10 values ≥0.4 to ≤1.0 but not otherwise (Table 5). Although higher neutrophil and lower lymphocyte concentrations and higher NLR were previously reported [23, 26, 36–38], Log10NLR has not.
There are important limitations to our study. First, not all covariates were available in all subjects including body mass index and SOFA score (a factor for death identified by logistic regression analysis) [24]. Also, BNP and TNI were reported in different units and were therefore not included for multi-variable Cox regression analyses. Second, at the data lock 65 (1.8%) subjects remained hospitalized and are excluded from our analyses. Third, some covariates such as bacterial coinfection and BUN could not be accurately analysed and interpreted together with other covariants for interactions. Our conclusions although based on a large dataset require confirmation. Nevertheless, they may be useful in predicting outcomes in persons with COVID-19.
Acknowledgements
We thank participating patients, families, and health care providers. Funded by the National Natural Science Foundation of China (NSFC; 81974009 to QL and 81974221 to ZC) and the Fundamental Research Funds for the Central Universities (2020kfyXGYJ086 to QL). RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme.
Author contributions
YH and QL designed the study. LeC, JY, WH, LCh, GY, FD, WC, YC, JY, LC, DW, QR, LL, QL, WR, FG, HW, and ZC collected the data. All authors had full access to the data, were involved in data interpretation and vouch for the accuracy of the analyses. QL, LeC, and RPG prepared the typescript which all authors approved final approval and supported the decision to submit for publication.
Data availability
All data generated or analyzed are included in this typescript including supplement.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lei Chen, Jianming Yu, Wenjuan He, Li Chen, Guolin Yuan, Fang Dong, Wenlan Chen, Yulin Cao, Jingyan Yang, Liling Cai
Contributor Information
Qiubai Li, Email: qiubaili@hust.edu.cn.
Yu Hu, Email: dr_huyu@126.com.
References
- 1.WHO. Coronavirus disease (COVID-2019) situation report. 2020 2020/02/27: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200517-covid-20200519-sitrep-20200118.pdf?sfvrsn=20200521c20200510dafe_20200518. Accessed 18 May 2020.
- 2.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed]
- 3.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–6. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–6. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 6.MacLaren G, Fisher D, Brodie D. Preparing for the most critically Ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020;323:1245–6. doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 7.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically Ill patients in the Seattle Region — Case Series. N Engl J Med. 2020: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed]
- 8.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–3. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel Coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel Coronavirus–infected Pneumonia. N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus–infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Wang D, Guo J, Yuan G, Yang Z, Gale RP, et al. COVID-19 in persons with chronic myeloid leukaemia. Leukemia. 2020: 10.1038/s41375-020-0853-6. [DOI] [PMC free article] [PubMed]
- 17.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020: 10.1164/rccm.202003-200543OC. [DOI] [PMC free article] [PubMed]
- 18.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 20.Du R-H, Liang L-R, Yang C-Q, Wang W, Cao T-Z, Li M, et al. Predictors of mortality for patients with COVID-19 Pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020:10.1183/13993003.13900524-13992020. [DOI] [PMC free article] [PubMed]
- 21.Liang W-H, Guan W-J, Li C-C, Li Y-M, Liang H-R, Zhao Y, et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicenter) and outside Hubei (non-epicenter): a Nationwide Analysis of China. Eur Respir J. 2020: 10.1183/13993003.13900562-13992020. [DOI] [PMC free article] [PubMed]
- 22.Tu W-J, Cao J, Yu L, Hu X, Liu Q. Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med. 2020: 10.1007/s00134-00020-06023-00134. [DOI] [PMC free article] [PubMed]
- 23.Wu C, Chen X, Cai Y, Xia JA, Zhou X, Xu S, et al. risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 24.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Resp Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21:1–10. doi: 10.1186/s12931-019-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Chen C, Hu F, Wang J, Zhao Q, Gale RP. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia. 2020;34:1503–11. doi: 10.1038/s41375-020-0848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gale RP Perspective: SARS-CoV-2, COVID-19 and haematologists. Acta Haematol. 2020: 10.1159/000508021. [DOI] [PMC free article] [PubMed]
- 29.National Health Commission of China. Chinese Clinical Guidance for COVID-19 pneumonia Diagnosis and Treatment (7th version). 2020: http://www.nhc.gov.cn/yzygj/s7653p/202003/202046c209294a202007dfe202004cef202080dc202007f205912eb201989.shtml. Accessed 10 March 2020.
- 30.Chen S, Zhang Z, Yang J, Wang J, Zhai X, Bärnighausen T, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395:1305–14. doi: 10.1016/S0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–9. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 32.Force* TADT. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 33.He W, Chen L, Chen L, Yuan G, Fang Y, Chen W, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020: 10.1038/s41375-41020-40836-41377. [DOI] [PMC free article] [PubMed]
- 34.National Health Commission of China. The novel coronavirus pneumonia diagnosis and treatment program, 5th version. 2020: http://www.nhc.gov.cn/yzygj/s7653p/202002/202003b202009b202894ac202009b204204a202079db202005b208912d204440.shtml. Accessed 05 April 2020.
- 35.WHO. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. 2020: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed 5 April.
- 36.Zhao X, Zhang B, Li P, Ma C, Gu J, Hou P, et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. medRxiv 2020: 10.1101/2020.1103.1117.20037572.
- 37.Liang W, Liang H, Ou L, Chen B, Chen A, Li C, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020: 10.1007/s00134-00020-06023-00134. [DOI] [PMC free article] [PubMed]
- 38.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed are included in this typescript including supplement.