Abstract
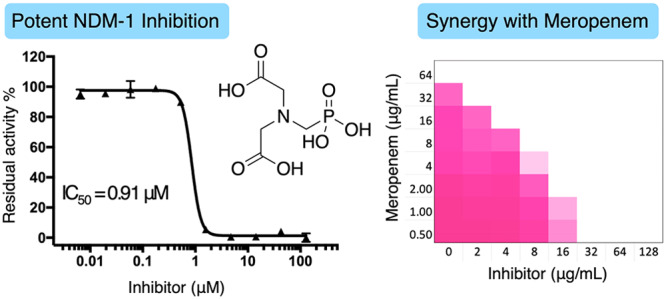
In the search for new inhibitors of bacterial metallo-β-lactamases (MBLs), a series of commonly used small molecule carboxylic acid derivatives were evaluated for their ability to inhibit New Delhi metallo-β-lactamase (NDM)-, Verona integron-encoded metallo-β-lactamase (VIM)-, and imipenemase (IMP)-type enzymes. Nitrilotriacetic acid (3) and N-(phosphonomethyl)iminodiacetic acid (5) showed promising activity especially against NDM-1 and VIM-2 with IC50 values in the low-to-sub μM range. Binding assays using isothermal titration calorimetry reveal that 3 and 5 bind zinc with high affinity with dissociation constant (Kd) values of 121 and 56 nM, respectively. The in vitro biological activity of 3 and 5 against E. coli expressing NDM-1 was evaluated in checkerboard format, demonstrating a strong synergistic relationship for both compounds when combined with Meropenem. Compounds 3 and 5 were then tested against 35 pathogenic strains expressing MBLs of the NDM, VIM, or IMP classes. Notably, when combined with Meropenem, compounds 3 and 5 were found to lower the minimum inhibitory concentration (MIC) of Meropenem up to 128-fold against strains producing NDM- and VIM-type enzymes.
Keywords: NDM-1, MBL inhibitors, zinc binding, isothermal titration calorimetry, synergy
Antibiotic resistance threatens to reduce the efficacy of currently available antibiotics and places a substantial burden on global health and the world economy.1,2 Resistance to β-lactam antibiotics can be caused by a diverse group of enzymes known as β-lactamases. While based on sequence homology, these enzymes are categorized into classes A–D (known as Ambler classification),3 mechanistically they are classified as serine-β-lactamases (SBLs, Ambler classes A, C, and D) or metallo-β-lactamases (MBLs, Ambler class B).4 SBLs inactivate β-lactams via the hydrolytic action of a nucleophilic serine in their active site. First-generation SBL inhibitors, including clavulanic acid, sulbactam, and tazobactam as well as the more recently approved avibactam and vaborbactam, are available to rescue β-lactams in the presence of SBL-producing bacteria.5,6 MBLs on the other hand are metallo-enzymes that hydrolyze β-lactams by the action of a zinc-activated nucleophilic water molecule that is formed in the active site. To date, there are no FDA-approved MBL inhibitors available. Of particular concern are the clinically important MBLs including the New Delhi metallo-β-lactamase (NDM), VIM, and IMP families that possess carbapenemase activity,7 adding further urgency to the development of MBL inhibitors to combat MBL-producing bacterial infections.
Small molecules with the ability to inhibit MBLs have been the topic of a number of comprehensive reviews.8−11 The majority of the known MBL inhibitors contain functional groups that can bind zinc. In this regard, the most common small molecules possessing anti-MBL activity are thiol-containing compounds,12−15 sulfonylhydrazones,16 bis-carboxylic acids,17,18 picolinic acids,19,20 and commonly used chelating agents,21,22 including their bacteria-targeting analogs.23,24 As an example, the natural product aspergillomarasmine A (AMA) was recently identified by Wright and co-workers who screened fungal extracts for anti-MBL activity. AMA was shown to be a potent inhibitor of both NDM- and VIM-type enzymes and importantly displays in vivo efficacy.25 Also of interest are the recently developed cyclic boronate SBL- and MBL-inhibitors, which mimic the tetrahedral intermediate formed upon the nucleophilic attack of a serine-hydroxyl group (SBLs) or zinc-bound water molecule (MBLs) at the β-lactam unit.26−30 In addition, recently, reports have also described compounds with alternative modes of MBL inhibition including covalent inhibitors31−33 and DNA aptamers proposed to operate via allosteric mechanisms of inhibition.34
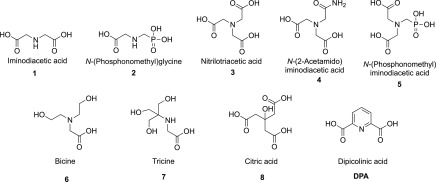
In reviewing the literature, we noted that sulfonic acid buffer components such as MES and PIPES have previously been reported to be weak MBL inhibitors.35 This prompted us to investigate the possibility of identifying new MBL inhibitor candidates among other commonly used small molecule buffer components containing multiple carboxylic acid and/or phosphonate functionalities. Given that zinc binding is a key aspect of the mechanism of action for a majority of MBL inhibitors, we specifically focused our attention on common buffer reagents and structurally related small molecules reported to interact with metals (Figure 1).
Figure 1.
Small molecule carboxylic acids as potential MBL inhibitors.
The panel of small molecules shown in Figure 1 were first screened for their inhibitory activity against purified MBLs including NDM-1, VIM-2, and IMP-28. The substrate used for the enzyme inhibition assay was a fluorescent cephalosporin derivative developed by Schofield and co-workers for assessing MBL activity.36 As shown in Table 1, nitrilotriacetic acid (NTA, 3) and its bioisosteres (4; 5, N-(phosphonomethyl)iminodiacetic acid) showed promising activity against NDM-1 and VIM-2, superior to that of dipicolinic acid (DPA), a well-studied MBL inhibitor.19,20 Notably, the much weaker inhibitory activity of the disubstituted analogs 1 and 2 point to the necessity of three carboxyl(phosphoryl) substituents in order to achieve potent inhibition of NDM-1 and VIM-2, most probably by tightly chelating zinc ions. Interestingly, compounds 1–8 all exhibited little-to-no activity against IMP-28. This observation is in line with previous investigations that have found the IMP class of MBLs to be less sensitive to inhibition by zinc-binding agents.25,37 To establish whether the inhibition measured was time dependent, the IC50 values of compounds 3, 5, and DPA for NDM-1 were also determined after preincubating the inhibitor and enzyme for various times including 0, 10, 20, 40, and 60 min, as previously described for a different class of NDM-1 inhibitors.38 As shown in Figure S3, preincubation time does not significantly affect the potency of the tested compounds under the assay conditions used.
Table 1. IC50 Values Determined against NDM-1, VIM-2, and IMP-28.
| IC50 (μM)a |
|||
|---|---|---|---|
| compound | NDM-1 | VIM-2 | IMP-28 |
| 1 | >200 | >200 | >200 |
| 2 | 75 ± 2 | 41 ± 6 | >200 |
| 3 | 1.3 ± 0.07 | 2.4b | 112 ± 3 |
| 4 | 2.3 ± 0.05 | 25b | >200 |
| 5 | 0.91 ± 0.05 | 0.68 ± 0.02 | 39 ± 7 |
| 6 | >200 | >200 | >200 |
| 7 | >200 | >200 | >200 |
| 8 | 132 ± 15 | 102 ± 7 | >200 |
| DPA | 3.8 ± 0.04 | 2.9 ± 0.5 | 17 ± 1 |
Values reported as mean ± SD of at least 3 independent experiments.
Due to the complex shape of the log[concentration] – activity plot, accurate fitting was not possible; the reported values are therefore an estimation (see the Supporting Information).
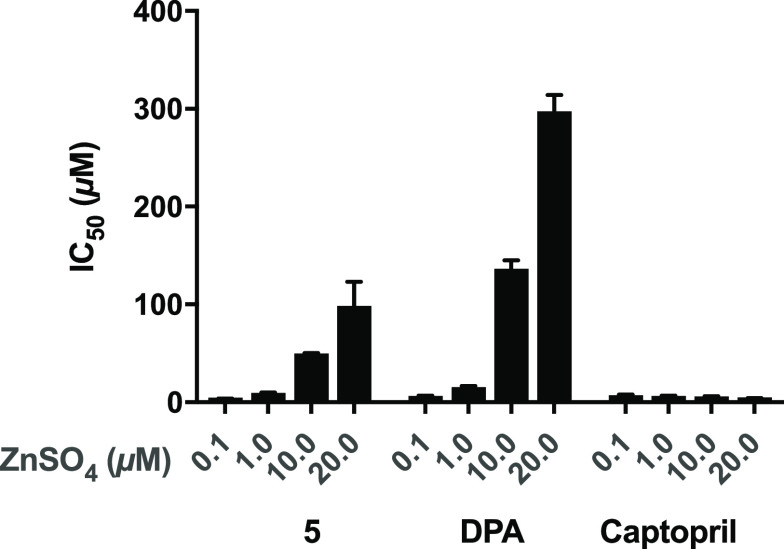
The majority of MBL inhibitors fall into one of two groups: those that interact with zinc as part of their binding in the MBL active site forming a ternary complex or those that actively strip zinc from the MBL active site driven by their strong chelating ability.39,40 Captopril is an example for the former, while known chelating agents such as EDTA and AMA represent the latter.19,41 In determining the IC50 value of N-(phosphonomethyl)iminodiacetic acid 5 against NDM-1, it was noted that, in the presence of different concentrations of zinc sulfate (ranging from 0.1 μM to 20 μM), the IC50 values measured also changed, revealing a zinc-dependent effect similar to that for DPA. In comparison, and as expected, the inhibitory activity of captopril is not influenced by varying the concentration of exogenous zinc added to the assay media (Figure 2). These findings support a zinc-sequestration based mechanism of NDM-1 inhibition for compound 5.
Figure 2.
Effect of zinc on the inhibitory activity of compound 5, DPA, and captopril against NDM-1.
Further evidence for high affinity zinc binding by compound 5 was obtained by the use of isothermal titration calorimetry (ITC). As we have previously demonstrated for thiol based small molecule zinc-binding MBL inhibitors,13 ITC allows for the direct determination of the dissociation constant (Kd) as well as the thermodynamic parameters ΔG, ΔH, and ΔS. Among the small molecules tested as part of the current study, compounds 3–5 were found to be strong zinc binders with Kd values of 121, 231, and 56 nM, respectively (Table 2). Interestingly, the affinity of compounds 3–5 for other biologically relevant divalent cations like Ca2+ and Mg2+ was negligible by ITC with binding interactions too weak to allow for an accurate determination of thermodynamic parameters. Previous reports have also described potentiometric titration42−44 and ITC based methods for studying the metal binding properties of related compounds.45−47 It should be noted that, in these earlier studies, the associated Kd values measured for the binding of Ca2+ and Zn2+ by DPA were somewhat lower than the values obtained in our investigations, an effect we ascribe to differences in the buffers used. Specifically, given the buffering capacity of the test compounds evaluated in our study, we chose to employ 100 mM Tris buffers to avoid any pH mismatch. Notably, our ITC data reveal a strong correlation between these compounds’ capacity to inhibit MBL activity and their zinc binding ability (see Table S1 for the full ITC data).
Table 2. ITC Based Thermodynamic Parameters for the Binding of Zinc by Compounds 3–5.
| compound | Kd (nM) | ΔH (kcal/mol) | –TΔS (kcal/mol) | ΔG (kcal/mol) |
|---|---|---|---|---|
| 3 | 121 ± 7 | –4.890 ± 0.219 | –4.546 ± 0.248 | –9.400 ± 0.042 |
| 4 | 231 ± 10 | –2.957 ± 0.069 | –6.100 ± 0.079 | –9.057 ± 0.026 |
| 5 | 56 ± 15 | –3.077 ± 0.113 | –6.837 ± 0.274 | –9.910 ± 0.155 |
The results of our investigations, as well as other recently published studies, indicate that the incorporation of the phosphonic acid moiety is a promising approach in designing potent MBL inhibitors.48−50 In line with our findings related to the enhanced potency of compound 5 relative to compound 3 are recent studies showing that phosphonic acid analogues of picolinic acid demonstrate increased potency against NDM-1.20,49 In addition, phosphonate analogues of the well-known mercapto-carboxylic acid MBL inhibitors (represented by thiomandelic acid) demonstrate enhanced inhibitory activity.48 In light of our findings and the studies mentioned above, the incorporation of a phosphonic acid moiety into the structures of other MBL inhibitors such as cyclic boronates (exemplified by VNRX-5133)30 may also provide access to new classes of hybrid MBL inhibitors.
The ability of compounds 1–8 to restore the activity of Meropenem, a last resort carbapenem, against a representative MBL-expressing strain was evaluated using a clinical NDM-1 positive isolate (coded E. coli RC0089). Using a checkerboard assay, multiple concentration combinations of MBL inhibitor + Meropenem were tested, allowing for the calculation of the fractional inhibitory concentration (FIC) index according to the following expression (where an FIC index (FICI) of <0.5 indicates a synergistic relationship):
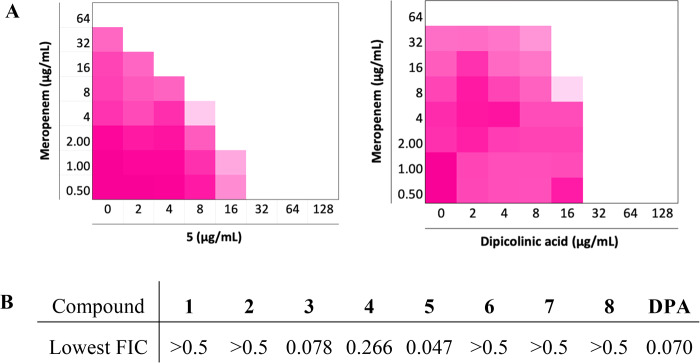
Among the compounds tested, 3–5 showed a synergistic relationship with Meropenem with compound 5 demonstrating the highest potency with the lowest FIC index of 0.047 (Figure 3). Compounds 3 and 5 were both very effective in restoring the activity of Meropenem against the NDM-1 producing E. coli strain used in the initial screen and were therefore also tested in combination with Meropenem against a larger panel of 38 Gram-negative clinical isolates displaying carbapenem resistance (Table S2). While compounds 3 and 5 exhibited no antibacterial activity at the highest tested concentration of 256 μg/mL, both were found to effectively enhance the activity of Meropenem against strains expressing NDM- and VIM-type enzymes. When administered at a concentration of 32 μg/mL, both 3 and 5 reduced the minimum inhibitory concentration (MIC) of Meropenem by up to 128-fold against these strains, a synergism equivalent to or better than that observed for DPA. Overall, compound 5 reduced the MIC of Meropenem to its clinically susceptible concentration (≤1 μg/mL) for 67% of the NDM- and VIM-type producing isolates tested, while for compound 3 and DPA, this ratio was 37% and 53%, respectively. In comparison, when tested against strains expressing IMP-type enzymes, the synergistic activity of 3 and 5 was modest, leading to no more than a 4-fold reduction of MIC in most cases, a trend also mirrored for DPA. In addition, the complete lack of synergy observed against strains expressing serine-carbapenemases such as KPC-2 and OXA-48 further demonstrates the inhibitory activities of compounds 3 and 5 to be MBL-specific. Also, among the bacterial species screened, P. aeruginosa proved to be more resistant to the synergistic combinations tested. This is apparent when comparing the antibacterial activities of the combinations against NDM-1 and VIM-2 producing P. aeruginosa isolates versus the corresponding E. coli and K. pneumoniae counterparts (see Table S2).
Figure 3.
(A) Checkerboard plots for compound 5 and DPA in combination with Meropenem tested against an NDM-1 producing strain of E. coli. The optical density of the bacteria at 600 nm (OD600) has been shown as a color gradient between white (no bacterial growth) and magenta (maximum growth). (B) The lowest FIC values calculated for compounds 1–8.
Conclusion
The clinically most relevant MBLs continue to be the NDM, VIM, and IMP classes and present a significant challenge to the efficacy of virtually all classes of β-lactam antibiotics including “last-line-of-defense” carbapenems such as Meropenem. Despite this, no inhibitors are clinically available to combat resistant infections caused by Gram-negative pathogens that express MBLs. The current study expands our understanding of the diversity of small molecule carboxylic acids that inhibit MBLs and synergize with carbapenems. By screening a series of available and commonly used small molecule carboxylates, we found that nitrilotriacetic acid (3) and its phosphoric acid analogue N-(phosphonomethyl)iminodiacetic acid (5) are both potent inhibitors of NDM- and VIM-type enzymes with sub- to low-μM IC50 values. Using ITC, both 3 and 5 were shown to bind zinc with nanomolar affinity. When further tested against a broad panel of MBL-producing Gram-negative pathogens, compounds 3 and 5 effectively reduced the MIC of Meropenem against NDM- and VIM-type enzymes. As for the well-characterized MBL inhibitors DPA and AMA, the mechanism of inhibition for 3 and 5 appears to be largely driven by zinc-sequestration. While AMA was reported to have in vivo efficacy,25 it is unlikely that strong zinc-binding small molecule carboxylates like 3 and 5 are suitable as clinical candidates. Rather, such compounds represent readily available inhibitors for biochemical studies of MBLs. Furthermore, given their small size and structural simplicity, such compounds may serve as leads for further optimization. One approach may be to administer such compounds as prodrugs that are activated only upon entry to the bacterial cell. In the absence of clinically approved MBL-inhibitors and with increasing rates of MBL-driven carbapenem resistance, it is important that many approaches, including unconventional avenues, be explored in the pursuit of an effective therapeutic response.
Methods
Enzyme Production and Purification
The plasmid constructs of NDM-1 and VIM-2 were a kind gift from Prof. Christopher J. Schofield (Oxford University). The IMP-28 construct was designed in the pET28b vector with a C-terminal His-tag. The corresponding enzymes were expressed and purified as described in the Supporting Information.
IC50 and Zinc Dependency Assay
All the test compounds were dissolved and serially diluted in 50 mM HEPES, pH 7.2, supplemented with 0.01% triton X-100 and 1 μM ZnSO4. The MBL enzymes (60 pM NDM-1, 100 pM VIM-2, and 60 pM IMP-28) were then added to the wells and incubated at 25 °C for 15 min. Next, the fluorescent cephalosporin substrate FC536 (0.5 μM for NDM-1 and VIM-2, 16 μM for IMP-28) was added to the wells, and the fluorescence was monitored immediately over 30–40 scanning cycles (λex 380 nm, λem 460 nm) on a Tecan Spark plate reader. Using the initial velocity data plotted against inhibitor concentration, the half-maximal inhibitory concentrations were calculated by an IC50 curve-fitting model in GraphPad Prism 7 software. 2,6-Dipicolinic acid was used as the positive control. The IC50 of captopril, dipicolinic acid, and compound 5 was also evaluated in the presence of different concentrations of zinc sulfate (0.1, 1, 10, and 20 μM) against NDM-1, following the procedure described above.
Isothermal Titration Calorimetry (ITC)
The test compounds were evaluated for their ability to bind zinc using an automated PEAQ-ITC calorimeter (Malvern). Zinc sulfate dissolved in 100 mM Tris (pH 7.0) was titrated into the test compounds dissolved in the same buffer over 19 × 2 μL aliquots (except for the first aliquot, which was 0.4 μL). The titrations were performed at 25 °C, and reference power was set at 10 μcal/s. Peak integration and curve-fitting was done using the PEAQ-ITC data analysis software provided by the manufacturer. The blank titrations included buffer titrated in the test compounds and zinc sulfate titrated in buffer, both of which showed negligible signals attributed to the heat of dilution (see the Supporting Information for the thermograms).
Antibacterial Assays
All antibacterial assays were carried out following the guidelines published by the Clinical and Laboratory Standards Institute (CLSI). Bacterial strains and clinical isolates were cultured on blood agar and incubated overnight at 37 °C. Fresh colonies were suspended in tryptic soy broth (TSB) and incubated at 37 °C with shaking. Following growth to the exponential phase (OD600 = 0.5), the bacterial suspension was diluted to 106 CFU/mL in Mueller-Hinton broth (MHB) and added to the test compounds prepared as described for each assay.
Single Concentration Synergy Assay
On a polypropylene microplate, Meropenem was dissolved and serially diluted in MHB (25 μL/well). Compounds 3, 5, and DPA with a final concentration of 32 μg/mL (25 μL/well) were then added to the wells. Following the addition of the diluted bacterial suspensions prepared as described above (50 μL/well), the microplates were incubated at 37 °C with shaking, and after 16–20 h, the plates were inspected for the bacterial growth. Minimum inhibitory concentration (MIC) values were reported as the lowest concentration of the antibiotic/test compounds that prevents the visible growth of bacteria. All the assays were performed in triplicate, and the median values were used to report MICs.
OD600 Checkerboard Assay
Meropenem was dissolved and serially diluted on the polypropylene microplates in MHB (25 μL/well). The test compounds dissolved and serially diluted to the final concentration ranging from 128 to 1 μg/mL were then added to Meropenem (25 μL/well). E. coli RC0089, a clinical isolate producing NDM-1, grown to the exponential phase and diluted in MHB was added to the microplate (50 μL/well), which was then incubated at 37 °C with shaking. After 16–20 h, the optical density of the wells was scanned at 600 nm on a Tecan Spark plate reader.
Acknowledgments
Financial support was provided by The European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 725523).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.9b00459.
Procedure for enzyme production and purification, IC50 curves, time-course of NDM-1 inhibition by 3, 5, and DPA, thermodynamic data and ITC thermograms, checkerboard synergy graphs, and MIC data against clinical isolates (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ferri M.; Ranucci E.; Romagnoli P.; Giaccone V. (2017) Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 57, 2857–2876. 10.1080/10408398.2015.1077192. [DOI] [PubMed] [Google Scholar]
- Aslam B.; Wang W.; Arshad M. I.; Khurshid M.; Muzammil S.; Rasool M. H.; Nisar M. A.; Alvi R. F.; Aslam M. A.; Qamar M. U.; Salamat M. K. F.; Baloch Z. (2018) Antibiotic resistance: a rundown of a global crisis. Infect. Drug Resist. 2018, 1645–1658. 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P. (1980) The structure of beta-lactamases. Philos. Trans. R. Soc. London B. Biol. Sci. 289, 321–331. [DOI] [PubMed] [Google Scholar]
- Drawz S. M.; Bonomo R. A. (2010) Three decades of beta-lactamase inhibitors. Clin. Microbiol. Rev. 23, 160–201. 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani K. H. M. E.; Martin N. I. (2018) β-lactam/β-lactamase inhibitor combinations: an update. MedChemComm 9, 1439–1456. 10.1039/C8MD00342D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. (2018) Game Changers: New β-Lactamase Inhibitor Combinations Targeting Antibiotic Resistance in Gram-Negative Bacteria. ACS Infect. Dis. 4, 84–87. 10.1021/acsinfecdis.7b00243. [DOI] [PubMed] [Google Scholar]
- Mojica M. F.; Bonomo R. A.; Fast W. (2016) B1-Metallo-β-Lactamases: Where Do We Stand?. Curr. Drug Targets 17, 1029–1050. 10.2174/1389450116666151001105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast W.; Sutton L. D. (2013) Metallo-β-lactamase: Inhibitors and reporter substrates. Biochim. Biophys. Acta, Proteins Proteomics 1834, 1648–1659. 10.1016/j.bbapap.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Groundwater P. W.; Xu S.; Lai F.; Váradi L.; Tan J.; Perry J. D.; Hibbs D. E. (2016) New Delhi metallo-β-lactamase-1: Structure, inhibitors and detection of producers. Future Med. Chem. 8, 993–1012. 10.4155/fmc-2016-0015. [DOI] [PubMed] [Google Scholar]
- McGeary R. P.; Tan D. T.; Schenk G. (2017) Progress toward inhibitors of metallo-β-lactamases. Future Med. Chem. 9, 673–691. 10.4155/fmc-2017-0007. [DOI] [PubMed] [Google Scholar]
- Linciano P.; Cendron L.; Gianquinto E.; Spyrakis F.; Tondi D. (2019) Ten Years with New Delhi Metallo-β-lactamase-1 (NDM-1): From Structural Insights to Inhibitor Design. ACS Infect. Dis. 5, 9–34. 10.1021/acsinfecdis.8b00247. [DOI] [PubMed] [Google Scholar]
- Mollard C.; Moali C.; Papamicael C.; Damblon C.; Vessilier S.; Amicosante G.; Schofield C. J.; Galleni M.; Frère J. M.; Roberts G. C. K. (2001) Thiomandelic acid, a broad spectrum inhibitor of zinc β-lactamases. Kinetic and spectroscopic studies. J. Biol. Chem. 276, 45015–45023. 10.1074/jbc.M107054200. [DOI] [PubMed] [Google Scholar]
- Tehrani K. H. M. E.; Martin N. I. (2017) Thiol-Containing Metallo-β-Lactamase Inhibitors Resensitize Resistant Gram-Negative Bacteria to Meropenem. ACS Infect. Dis. 3, 711–717. 10.1021/acsinfecdis.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner D.; Kramer J. S.; Klingler F.-M.; Wittmann S. K.; Hartmann M. R.; Kurz C. G.; Kohnhäuser D.; Weizel L.; Brüggerhoff A.; Frank D.; Steinhilber D.; Wichelhaus T. A.; Pogoryelov D.; Proschak E. (2018) Challenges in the Development of a Thiol-Based Broad-Spectrum Inhibitor for Metallo-β-Lactamases. ACS Infect. Dis. 4, 360–372. 10.1021/acsinfecdis.7b00129. [DOI] [PubMed] [Google Scholar]
- Meng Z.; Tang M.-L.; Yu L.; Liang Y.; Han J.; Zhang C.; Hu F.; Yu J.-M.; Sun X. (2019) Novel Mercapto Propionamide Derivatives with Potent New Delhi Metallo-β-Lactamase-1 Inhibitory Activity and Low Toxicity. ACS Infect. Dis. 5, 903–916. 10.1021/acsinfecdis.8b00366. [DOI] [PubMed] [Google Scholar]
- Siemann S.; Evanoff D. P.; Marrone L.; Clarke A. J.; Viswanatha T.; Dmitrienko G. I. (2002) N-Arylsulfonyl Hydrazones as Inhibitors of IMP-1 Metallo-β-Lactamase. Antimicrob. Agents Chemother. 46, 2450–2457. 10.1128/AAC.46.8.2450-2457.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L.; Yang K.-W.; Zhou L.-S.; Xiao J.-M.; Yang X.; Zhai L.; Zhang Y.-L.; Crowder M. W. (2012) N-Heterocyclic dicarboxylic acids: Broad-spectrum inhibitors of metallo-β-lactamases with co-antibacterial effect against antibiotic-resistant bacteria. Bioorg. Med. Chem. Lett. 22, 5185–5189. 10.1016/j.bmcl.2012.06.074. [DOI] [PubMed] [Google Scholar]
- Hiraiwa Y.; Saito J.; Watanabe T.; Yamada M.; Morinaka A.; Fukushima T.; Kudo T. (2014) X-ray crystallographic analysis of IMP-1 metallo-β-lactamase complexed with a 3-aminophthalic acid derivative, structure-based drug design, and synthesis of 3,6-disubstituted phthalic acid derivative inhibitors. Bioorg. Med. Chem. Lett. 24, 4891–4894. 10.1016/j.bmcl.2014.08.039. [DOI] [PubMed] [Google Scholar]
- Chen A. Y.; Thomas P. W.; Stewart A. C.; Bergstrom A.; Cheng Z.; Miller C.; Bethel C. R.; Marshall S. H.; Credille C. V.; Riley C. L.; Page R. C.; Bonomo R. A.; Crowder M. W.; Tierney D. L.; Fast W.; Cohen S. M. (2017) Dipicolinic Acid Derivatives as Inhibitors of New Delhi Metallo-β-lactamase-1. J. Med. Chem. 60, 7267–7283. 10.1021/acs.jmedchem.7b00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. Y.; Thomas P. W.; Cheng Z.; Xu N. Y.; Tierney D. L.; Crowder M. W.; Fast W.; Cohen S. M. (2019) Investigation of Dipicolinic Acid Isosteres for the Inhibition of Metallo-β-Lactamases. ChemMedChem 14, 1271–1282. 10.1002/cmdc.201900172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somboro A. M.; Tiwari D.; Bester L. A.; Parboosing R.; Chonco L.; Kruger H. G.; Arvidsson P. I.; Govender T.; Naicker T.; Essack S. Y. (2015) NOTA: A potent metallo-β-lactamase inhibitor. J. Antimicrob. Chemother. 70, 1594–1596. 10.1093/jac/dku538. [DOI] [PubMed] [Google Scholar]
- Azumah R.; Dutta J.; Somboro A. M.; Ramtahal M.; Chonco L.; Parboosing R.; Bester L. A.; Kruger H. G.; Naicker T.; Essack S. Y.; Govender T. (2016) In vitro evaluation of metal chelators as potential metallo- β -lactamase inhibitors. J. Appl. Microbiol. 120, 860–867. 10.1111/jam.13085. [DOI] [PubMed] [Google Scholar]
- Yarlagadda V.; Sarkar P.; Samaddar S.; Manjunath G. B.; Mitra S. D.; Paramanandham K.; Shome B. R.; Haldar J. (2018) Vancomycin Analogue Restores Meropenem Activity against NDM-1 Gram-Negative Pathogens. ACS Infect. Dis. 4, 1093–1101. 10.1021/acsinfecdis.8b00011. [DOI] [PubMed] [Google Scholar]
- Schnaars C.; Kildahl-Andersen G.; Prandina A.; Popal R.; Radix S.; Le Borgne M.; Gjøen T.; Andresen A. M. S.; Heikal A.; Økstad O. A.; Fröhlich C.; Samuelsen Ø.; Lauksund S.; Jordheim L. P.; Rongved P.; Åstrand O. A. H. (2018) Synthesis and Preclinical Evaluation of TPA-Based Zinc Chelators as Metallo-β-lactamase Inhibitors. ACS Infect. Dis. 4, 1407–1422. 10.1021/acsinfecdis.8b00137. [DOI] [PubMed] [Google Scholar]
- King A. M.; Reid-Yu S. A.; Wang W.; King D. T.; De Pascale G.; Strynadka N. C.; Walsh T. R.; Coombes B. K.; Wright G. D. (2014) Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature 510, 503–506. 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnc A.; Lang P. A.; Panduwawala T. D.; Brem J.; Schofield C. J. (2019) Will morphing boron-based inhibitors beat the β-lactamases?. Curr. Opin. Chem. Biol. 50, 101–110. 10.1016/j.cbpa.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill S. T.; Tyrrell J. M.; Navratilova I. H.; Calvopiña K.; Robinson S. W.; Lohans C. T.; McDonough M. A.; Cain R.; Fishwick C. W. G.; Avison M. B.; Walsh T. R.; Schofield C. J.; Brem J. (2019) Studies on the inhibition of AmpC and other β-lactamases by cyclic boronates. Biochim. Biophys. Acta, Gen. Subj. 1863, 742–748. 10.1016/j.bbagen.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Langley G. W.; Cain R.; Tyrrell J. M.; Hinchliffe P.; Calvopiña K.; Tooke C. L.; Widlake E.; Dowson C. G.; Spencer J.; Walsh T. R.; Schofield C. J.; Brem J. (2019) Profiling interactions of vaborbactam with metallo-β-lactamases. Bioorg. Med. Chem. Lett. 29, 1981–1984. 10.1016/j.bmcl.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnc A.; Brem J.; Hinchliffe P.; Calvopiña K.; Panduwawala T. D.; Lang P. A.; Kamps J. J. A. G.; Tyrrell J. M.; Widlake E.; Saward B. G.; Walsh T. R.; Spencer J.; Schofield C. J. (2019) Bicyclic Boronate VNRX-5133 Inhibits Metallo- and Serine-β-Lactamases. J. Med. Chem. 62, 8544–8556. 10.1021/acs.jmedchem.9b00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.; Trout R. E. L.; Chu G.-H.; McGarry D.; Jackson R. W.; Hamrick J. C.; Daigle D. M.; Cusick S. M.; Pozzi C.; De Luca F.; Benvenuti M.; Mangani S.; Docquier J.-D.; Weiss W. J.; Pevear D. C.; Xerri L.; Burns C. J. (2020) Discovery of Taniborbactam (VNRX-5133): A Broad-Spectrum Serine- and Metallo-β-lactamase Inhibitor for Carbapenem-Resistant Bacterial Infections. J. Med. Chem. 63, 2789. 10.1021/acs.jmedchem.9b01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. W.; Cammarata M.; Brodbelt J. S.; Fast W. (2014) Covalent Inhibition of New Delhi Metallo-β-Lactamase-1 (NDM-1) by Cefaclor. ChemBioChem 15, 2541–2548. 10.1002/cbic.201402268. [DOI] [PubMed] [Google Scholar]
- Chiou J.; Wan S.; Chan K.-F.; So P.-K.; He D.; Chan E. W.; Chan T.; Wong K.; Tao J.; Chen S. (2015) Ebselen as a potent covalent inhibitor of New Delhi metallo-β-lactamase (NDM-1). Chem. Commun. 51, 9543–9546. 10.1039/C5CC02594J. [DOI] [PubMed] [Google Scholar]
- Christopeit T.; Albert A.; Leiros H.-K. S. (2016) Discovery of a novel covalent non-β-lactam inhibitor of the metallo-β-lactamase NDM-1. Bioorg. Med. Chem. 24, 2947–2953. 10.1016/j.bmc.2016.04.064. [DOI] [PubMed] [Google Scholar]
- Khan N. H.; Bui A. A.; Xiao Y.; Sutton R. B.; Shaw R. W.; Wylie B. J.; Latham M. P. (2019) A DNA aptamer reveals an allosteric site for inhibition in metallo-β-lactamases. PLoS One 14, e0214440 10.1371/journal.pone.0214440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. M. D.; Wu J. K.; Toney J. H. (1998) Unanticipated Inhibition of the Metallo-β-lactamase from Bacteroides fragilis by 4-Morpholineethanesulfonic Acid (MES): A Crystallographic Study at 1.85-Å Resolution. Biochemistry 37, 6791–6800. 10.1021/bi9730339. [DOI] [PubMed] [Google Scholar]
- van Berkel S. S.; Brem J.; Rydzik A. M.; Salimraj R.; Cain R.; Verma A.; Owens R. J.; Fishwick C. W. G.; Spencer J.; Schofield C. J. (2013) Assay Platform for Clinically Relevant Metallo-β-lactamases. J. Med. Chem. 56, 6945–6953. 10.1021/jm400769b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proschak A.; Kramer J.; Proschak E.; Wichelhaus T. A. (2018) Bacterial zincophore [S,S]-ethylenediamine-N,N′-disuccinic acid is an effective inhibitor of MBLs. J. Antimicrob. Chemother. 73, 425–430. 10.1093/jac/dkx403. [DOI] [PubMed] [Google Scholar]
- Cahill S. T.; Cain R.; Wang D. Y.; Lohans C. T.; Wareham D. W.; Oswin H. P.; Mohammed J.; Spencer J.; Fishwick C. W. G.; McDonough M. A.; Schofield C. J.; Brem J. (2017) Cyclic Boronates Inhibit All Classes of β-Lactamases. Antimicrob. Agents Chemother. 61, e02260-16. 10.1128/AAC.02260-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju L. C.; Cheng Z.; Fast W.; Bonomo R. A.; Crowder M. W. (2018) The Continuing Challenge of Metallo-β-Lactamase Inhibition: Mechanism Matters. Trends Pharmacol. Sci. 39, 635–647. 10.1016/j.tips.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotondo C. M.; Wright G. D. (2017) Inhibitors of metallo-β-lactamases. Curr. Opin. Microbiol. 39, 96–105. 10.1016/j.mib.2017.10.026. [DOI] [PubMed] [Google Scholar]
- Bergstrom A.; Katko A.; Adkins Z.; Hill J.; Cheng Z.; Burnett M.; Yang H.; Aitha M.; Mehaffey M. R.; Brodbelt J. S.; Tehrani K. H. M. E.; Martin N. I.; Bonomo R. A.; Page R. C.; Tierney D. L.; Fast W.; Wright G. D.; Crowder M. W. (2018) Probing the Interaction of Aspergillomarasmine A with Metallo-β-lactamases NDM-1, VIM-2, and IMP-7. ACS Infect. Dis. 4, 135–145. 10.1021/acsinfecdis.7b00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung L.; Rajan K. S.; Merdinger E.; Grecz N. (1971) Coordinative binding of divalent cations with ligands related to bacterial spores. Equilibrium studies. Biophys. J. 11, 469–482. 10.1016/S0006-3495(71)86229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab H. A.; Anwar Z. M.; Sokar M. (2004) Metal Ion Complexes Containing Nucleobases and Some Zwitterionic Buffers. J. Chem. Eng. Data 49, 62–72. 10.1021/je0301702. [DOI] [Google Scholar]
- Krishnamoorthy C. R.; Nakon R. (1991) Free Metal Ion Depletion by Good’s Buffers. IV. Bicine 1:1 and 2:1 Complexes with Mg(II), Ca(II), Mn(II), Co(II), Ni(II), Cu(II) and Zn(II). J. Coord. Chem. 23, 233–243. 10.1080/00958979109408254. [DOI] [Google Scholar]
- Wyrzykowski D.; Pilarski B.; Jacewicz D.; Chmurzyński L. (2013) Investigation of metal-buffer interactions using isothermal titration calorimetry. J. Therm. Anal. Calorim. 111, 1829–1836. 10.1007/s10973-012-2593-y. [DOI] [Google Scholar]
- Wyrzykowski D.; Tesmar A.; Jacewicz D.; Pranczk J.; Chmurzyński L. (2014) Zinc(II) complexation by some biologically relevant pH buffers. J. Mol. Recognit. 27, 722–726. 10.1002/jmr.2398. [DOI] [PubMed] [Google Scholar]
- Tesmar A.; Wyrzykowski D.; Muñoz E.; Pilarski B.; Pranczk J.; Jacewicz D.; Chmurzyński L. (2017) Simultaneous determination of thermodynamic and kinetic parameters of aminopolycarbonate complexes of cobalt(II) and nickel(II) based on isothermal titration calorimetry data. J. Mol. Recognit. 30, e2589 10.1002/jmr.2589. [DOI] [PubMed] [Google Scholar]
- Lassaux P.; Hamel M.; Gulea M.; Delbrück H.; Mercuri P. S.; Horsfall L.; Dehareng D.; Kupper M.; Frère J.-M.; Hoffmann K.; Galleni M.; Bebrone C. (2010) Mercaptophosphonate Compounds as Broad-Spectrum Inhibitors of the Metallo-β-lactamases. J. Med. Chem. 53, 4862–4876. 10.1021/jm100213c. [DOI] [PubMed] [Google Scholar]
- Hinchliffe P.; Tanner C. A.; Krismanich A. P.; Labbé G.; Goodfellow V. J.; Marrone L.; Desoky A. Y.; Calvopiña K.; Whittle E. E.; Zeng F.; Avison M. B.; Bols N. C.; Siemann S.; Spencer J.; Dmitrienko G. I. (2018) Structural and Kinetic Studies of the Potent Inhibition of Metallo-β-lactamases by 6-Phosphonomethylpyridine-2-carboxylates. Biochemistry 57, 1880–1892. 10.1021/acs.biochem.7b01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton O. A.; Jaishankar P.; Akhtar A.; Adams J. L.; Shaw L. N.; Renslo A. R.; Chen Y. (2019) Heteroaryl Phosphonates as Noncovalent Inhibitors of Both Serine- and Metallocarbapenemases. J. Med. Chem. 62, 8480–8496. 10.1021/acs.jmedchem.9b00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.