Abstract
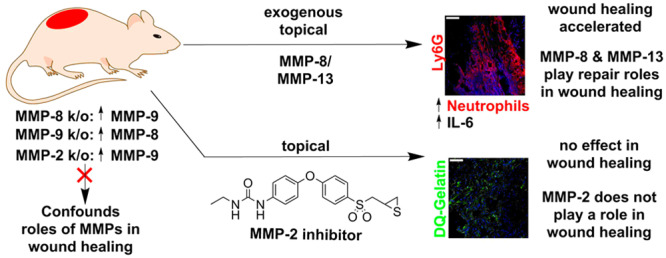
Matrix metalloproteinases (MMPs) play important roles in wound healing, but attribution of their functions in repair of wounds has been challenging. Commonly used tools such as MMP-knockout mice and zymography often confound analysis, which is complicated further as these enzymes exist in three distinct forms with only one being catalytically competent. With the use of topical exogenously administered recombinant MMP-8 and MMP-13 to diabetic and nondiabetic mouse wounds, we show that these proteinases facilitate wound repair by upregulating IL-6 and increasing neutrophil trafficking with an early onset of inflammation. Furthermore, by spatiotemporal control in the use of a selective MMP-2 inhibitor, along with immunoprecipitation and Western blotting, we provide definitive demonstration that MMP-2 does not affect wound healing, contrary to reports. MMP-2 is found in wounds complexed with TIMPs, which is catalytically incompetent.
Keywords: MMP-2, MMP-8, MMP-13, wound healing, diabetic mice, MMP-knockout
Cutaneous wound healing is highly complex and involves inflammation, angiogenesis, and tissue remodeling.1 Wounds that fail to successfully complete the stages of wound healing become chronic, which occur frequently in patients with diabetes. Diabetic foot ulcers (DFUs) affect 26.1 million individuals in the world every year,2 and 25% of diabetic patients suffer DFUs.2 The standard of care for DFUs is debridement, off-loading, and treatment of infected wounds with antibiotics. These therapies are not very effective, resulting in 108,000 amputations annually in the United States.3 Amputation results in poor prognosis, with 44% mortality within 1 year.4 Investigation of the mechanisms involved in the healing recalcitrance of chronic wounds and what can accelerate wound repair is needed to improve treatment options.
As both normal wound healing and the pathology of DFUs require remodeling of the extracellular matrix, matrix metalloproteinases (MMPs) are involved.1 There are 24 human MMPs, and each exists in three forms (latent, activated regulated by complexation with tissue inhibitor of metalloproteinases or TIMPs, and activated unregulated). Of the three forms, only the activated unregulated MMP is catalytically competent. The majority of the current methods for profiling of MMPs are unable to differentiate the active form from the other two. We developed an affinity resin that fishes out only the active form of MMPs from diseased tissues, which can then be identified and quantified by mass spectrometry.5 Using this approach, we identified two MMPs in their active forms (MMP-8 and MMP-9) in wounds of diabetic mice.5 Using selective inhibitors, MMP-9-knockout mice, and exogenous recombinant MMP-8, we demonstrated that MMP-9 is detrimental to diabetic wound healing, whereas MMP-8 plays a beneficial role in promoting repair of the diabetic wound.5,6
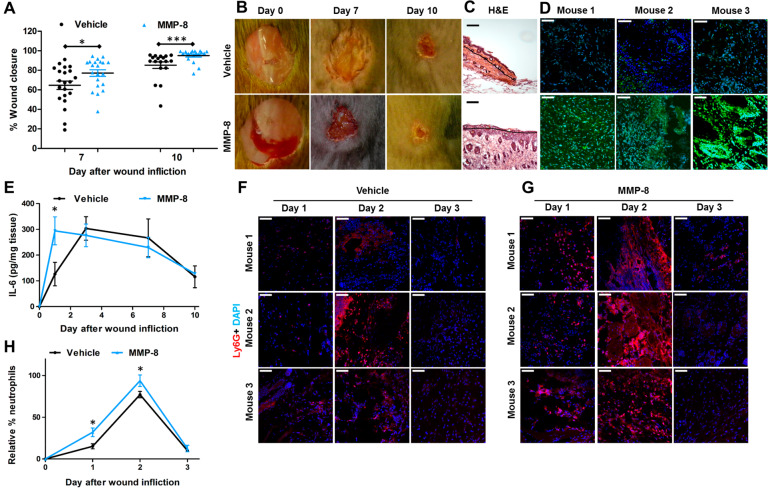
The question that becomes pertinent is whether MMP-8 also promotes nondiabetic wound repair. Gutierrez-Fernandez et al. had found that MMP-8 is involved in wound repair using MMP-8-knockout mice, which resulted in delayed wound healing due to increased inflammation.7 However, gene ablation of MMP-8 results in a compensatory increase of MMP-9.7 The results are confounding as we had shown that MMP-9 plays a detrimental role in diabetic wound healing.5,6,8 Tools and approaches other than the use of transgenic mice should be employed to address this question. We cloned the gene for mouse MMP-8, expressed it, and purified the recombinant protein to homogeneity. When applied topically to wounds of nondiabetic mice at 10× the protein levels found in these mice, the exogenously added MMP-8 accelerated nondiabetic wound healing more significantly than did vehicle (Figure 1A,B), leading to complete re-epithelialization (Figure 1C) in contrast to the control group, which exhibited partial re-epithelialization. We confirmed increased MMP-8 activity in the MMP-8-treated wounds by in situ zymography (ISZ, Figure 1D). Interleukin (IL)-6 is a marker of inflammation. IL-6-deficient mice have delayed wound healing resulting from decreased leukocyte infiltration, inflammation, angiogenesis, and re-epithelialization.9 We measured IL-6 levels in wounds by ELISA and found that they were significantly increased on day 1 (Figure 1E). IL-6 is also known to regulate neutrophils that are drawn to the wound site to defend against infection during the inflammation stage of wound healing.10 Lymphocyte antigen 6 complex locus G6D (Ly6G) is a protein that is expressed primarily in neutrophils,11 making Ly6G an excellent marker for inflammation. We stained the wounds with Ly6G antibody (Figure 1F,G) and found that MMP-8 treatment increased neutrophil trafficking on days 1 and 2 compared to vehicle-treated ones (Figure 1H). Thus, topical application of exogenous MMP-8 accelerates wound healing by upregulating IL-6 and increasing neutrophil trafficking that results in an earlier onset of inflammation that facilitates wound healing.
Figure 1.
MMP-8 accelerates wound healing in nondiabetic mice. (A) Wound healing; n = 21 mice/group on day 7 and n = 18 mice/group on day 10; *p < 0.05, ***p < 0.001. (B) Representative wound images and (C) H&E staining on day 10; 10× lens. Re-epithelialization is shown by the black lines (scale bars, 50 μm). (D) ISZ of the wounds with DQ-collagen (green) merged with DAPI (blue); n = 3 mice/group, 40× lens (scale bars, 50 μm). (E) Levels of IL-6 are significantly upregulated on day 1 in wounds treated with MMP-8. Staining with Ly6G for infiltrating neutrophils (red) merged with DAPI (blue) of (F) vehicle-treated wounds and (G) MMP-8-treated wounds. (H) Ly6G neutrophil quantification of (F) and (G) reveals upregulation of infiltrating neutrophils on days 1 and 2 in MMP-8-treated mice relative to vehicle-treated ones. For (E) and (H), n = 3 mice/group/time point; *p < 0.05.
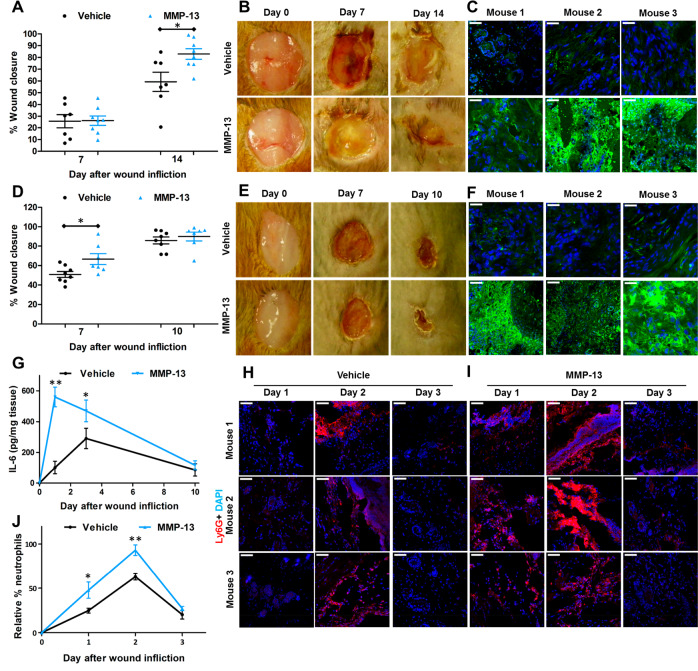
Mice have two collagenases, MMP-8 and MMP-13. As topical application of MMP-8 accelerates wound healing in nondiabetic mice, could MMP-13 (not found in the wound) play a similar role in wound healing? Underscoring the complications in the use of knockouts, MMP-13-knockout mice show no difference in wound healing (re-epithelialization, inflammation, angiogenesis, remodeling) compared to wild-type mice.12 This observation was attributed to the documented compensatory increase in MMP-8.12 Hence, the knockout strain could not provide clarity on the role of the enzyme. Notwithstanding, as both MMP-8 and MMP-13 are collagenases, could their functions in wound healing, in principle, be redundant? To investigate this, we treated diabetic and nondiabetic mice with exogenously added recombinant MMP-13 at 10× the level of MMP-8 found in the wounds. We found that indeed topical application of MMP-13 accelerated wound healing in both diabetic (Figure 2A,B) and nondiabetic mice (Figure 2D,E). We documented the increased collagenase activity (due to exogenous MMP-13) in the wounds by ISZ (Figure 2C,F). Similar to the effect of MMP-8, exogenous MMP-13 application to wounds of nondiabetic mice upregulated IL-6 levels (Figure 2G) and increased neutrophil trafficking that facilitates wound repair by an earlier onset of inflammation (Figure 2H–J). Collagenase activity—be it from MMP-8 or MMP-13—facilitates wound healing.
Figure 2.
MMP-13 accelerates wound healing. (A) Wound closure and (B) representative wound images of diabetic mice. (C) ISZ of diabetic wounds with DQ collagen (green) merged with DAPI (blue). (D) Wound closure and (E) representative wound images of nondiabetic mice. (F) ISZ of nondiabetic wounds with DQ-collagen merged with DAPI. (G) Levels of IL-6 are significantly upregulated on day 1 and day 2 in nondiabetic wounds treated with MMP-13. Staining with Ly6G for infiltrating neutrophils (red) merged with DAPI (blue) of (H) vehicle-treated wounds and (I) MMP-13-treated wounds. (J) Neutrophil quantification with Ly6G of (H) and (I) reveals upregulation of infiltrating neutrophils on days 1 and 2 in nondiabetic wounds of MMP-13-treated mice relative to vehicle-treated ones. For (A) and (D), mean ± SEM; n = 8 mice/group on day 7; n = 8 in MMP-13-treated on day 14 or 10, respectively; n = 7 in vehicle-treated on day 14 or 10, respectively; *p < 0.05. For (C) and (F), n = 3 mice/group, 40× lens, scale bars 50 μm. For (G–J), n = 3 mice/group/time point; *p < 0.05 and **p < 0.01.
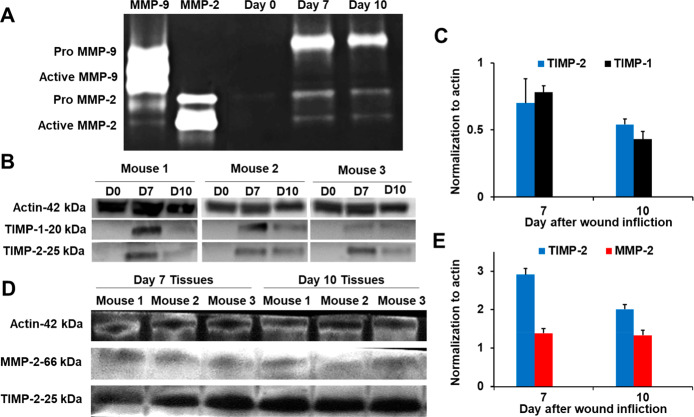
MMP-2, a gelatinase (turns over denatured collagen), has been reported to play a role in both human diabetic and nondiabetic wounds13 as well as in mouse diabetic wounds.14 MMP-2 in those studies was identified by gelatin zymography, a method that does not by itself distinguish between MMP-2-TIMP complexed and active MMP-2. This is due to denaturation/renaturation of the samples in gelatin zymography, which results in dissociation of the MMP-2-TIMP complex, giving a band for active MMP-2 undifferentiated whether it is in complex with TIMP or not. The MMP-2-TIMP complex obviously is catalytically incompetent, so the differentiation of the two forms is critical in attributing a role for MMP-2. We analyzed wound tissues of diabetic mice by our affinity resin/proteomics and did not detect the presence of active MMP-2.5 This documented that unregulated (uncomplexed by TIMP) active MMP-2 did not exist in the wound tissue. Nonetheless, to complement the earlier study, we also analyzed the nondiabetic mouse wounds by gelatin zymography and indeed observed proMMP-9, proMMP-2, and active MMP-2 (Figure 3A). We reasoned that the active MMP-2 band observed by gelatin zymography is in fact MMP-2 complexed to TIMPs, which would not be detected by the affinity resin/proteomics analysis. We analyzed the wound tissue by Western blot and confirmed the presence of TIMP-1 and TIMP-2 in the wounds (Figure 3B). Furthermore, immunoprecipitation of wound homogenates with TIMP-2 antibody and Western blot analysis showed the presence of MMP-2 together with TIMP-2, indicating that MMP-2 exists in its complexed form with TIMP-2 in the wound environment (Figure 3C). Hence, the activity of MMP-2 in the wound is kept in check. The MMP-2-TIMP complex is catalytically incompetent and cannot contribute to the wound processes by turnover of protein substrates.
Figure 3.
MMP-2 observed in nondiabetic mouse wounds is in complex with TIMPs. (A) Gelatin zymography indicates the presence of proMMP-9, proMMP-2, and active MMP-2. (B) Analyses (n = 3 mice per time point) by Western blot show the presence of TIMP-1 and TIMP-2. Actin was used as a loading control. (C) Quantification (n = 3 mice per time point) of TIMP-1 and TIMP-2 in (B). (D) Immunoprecipitation of wound homogenates (n = 3 mice per time point) with TIMP-2 antibody, followed by Western blot analysis, indicates that MMP-2 is in complex with TIMP-2. (E) Quantification (n = 3 mice per time point) of TIMP-2 and MMP-2 in (D).
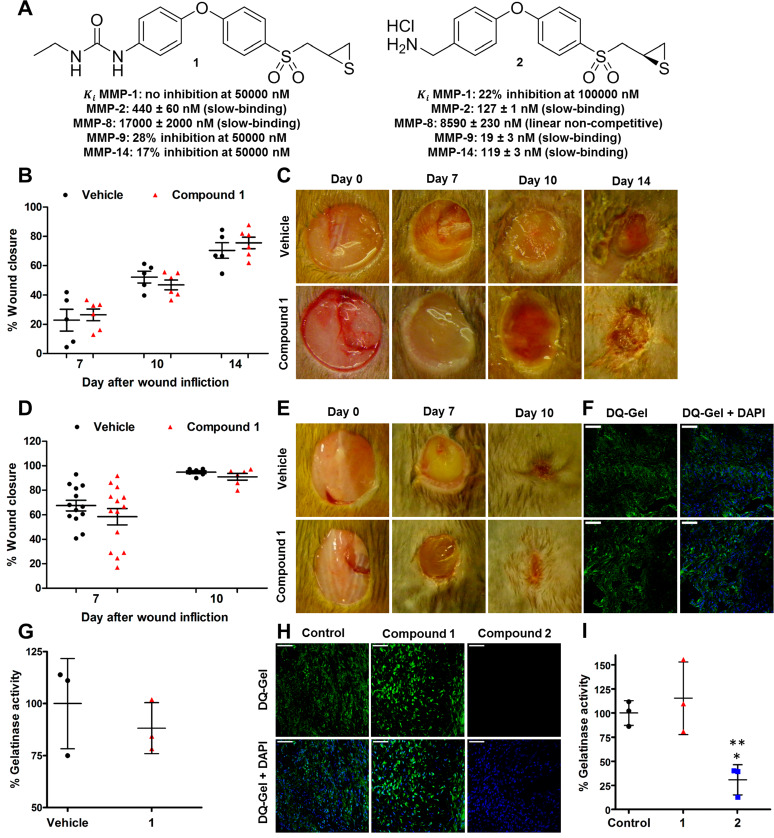
To complicate the picture, gene ablation of MMP-2 results in compensatory upregulation of MMP-9, both of which are gelatinases.15 Because of the compensatory upregulation of MMP-9, which we have shown to be detrimental to wound healing, MMP-2-knockout mice are of diminished utility in studying the role of the enzyme in wound healing. Thus, we resorted to a highly selective MMP-2 inhibitor from our laboratory (compound 1, Figure 4A) to ascertain the role of MMP-2. By so doing, we had complete spatiotemporal control of intervening in the activity of MMP-2 if it indeed were to play any role in wound healing. This is in contrast to the use of knockout strains. Compound 1 inhibits MMP-2 as a slow-binding inhibitor with a Ki value of 440 ± 60 nM and poorly inhibits or does not inhibit other MMPs.16 The residence time of compound 1 bound to MMP-2 is 24.5 min.16 This value is longer than those for TIMP-1 and TIMP-2 bound to MMP-2 (6.9 and 10.4, min, respectively),17 also slow-binding protein inhibitors. In essence, compound 1 is more effective as an MMP-2 inhibitor than are TIMPs. It is a useful tool in assessing the MMP-2 activity. Wounds of diabetic (Figure 4B,C) and nondiabetic (Figure 4D,E) mice treated with compound 1 showed no effect on wound healing (neither acceleration nor delay), compared to vehicle-treated mice. No difference in gelatinase activity was observed in MMP-2 inhibitor-treated wounds by ISZ, as the gelatinase activity that is observed is due to MMP-9 and compound 1 does not inhibit MMP-9 (Figure 4F,G). Levels of compound 1 in the wounds were 51 ± 12 μM at 1 h after dose administration, a concentration well above the Ki for MMP-2 that should provide effective inhibition of MMP-2 and no inhibition of MMP-9 (28% inhibition at 50 μM). However, as there is no active MMP-2 in the wounds, compound 1 has no effect on wound healing. To further demonstrate that active MMP-2 does not affect wound healing, we investigated the effect of ex vivo inhibition of MMP-2 (by using compound 1) and that of both gelatinases (by using compound 2) by ISZ of nondiabetic wounds. Compound 2 inhibits MMP-2, MMP-9, and MMP-14 as a slow-binding inhibitor with a Ki values of 127 ± 1, 19 ± 3, and 119 ± 3 nM, respectively (Figure 4A).8 ISZ analysis showed that compound 1 did not inhibit gelatinase activity, whereas compound 2 did (Figure 4H,I). These studies collectively indicate that MMP-9 is the only active gelatinase in the wound environment, whereas MMP-2 exists in complex with TIMP-2, a catalytically incompetent form.
Figure 4.
Selective MMP-2 inhibition has no effect on wound healing. (A) Chemical structures of compounds 1 and 2 ((R)-ND-336) and their inhibition profiles. (B) Wound closure and (C) representative wound images in diabetic mice after topical administration of compound 1. Mean ± SEM; n = 5 mice/time point for vehicle-treated; n = 6 mice/time point for MMP-2 inhibitor-treated. (D) Wound closure and (E) representative wound images in nondiabetic mice after topical application of compound 1. Mean ± SEM; n = 13 mice/group on day 7; n = 6 mice/group on day 10. (F) ISZ of nondiabetic wounds with DQ-gelatin (green) and merged DAPI (blue). (G) Quantification of gelatinase activity of (F) in wounds of nondiabetic mice; n = 3 mice/group. (H) ISZ of nondiabetic wounds with DQ-gelatin (green) treated ex vivo with 10× the Ki values of compounds 1 and 2. (I) Quantification of gelatinase activity of (H); n = 3 mice/group; *p < 0.05 between compound 2 and control, **p < 0.01 between compound 2 and compound 1. For (F) and (H), 40× lens (scale bars, 50 μm).
MMP-knockout mice are frequently used to ascertain the role of a particular MMP in disease or repair. However, gene ablation frequently results in compensatory increases of other MMPs, providing confounding results, which could mislead. The present report underscores the use of other methods in lieu of knockout strains to ascertain the role of MMPs in remodeling of wounds. Furthermore, we used exogenous topically applied recombinant MMP-8 and MMP-13 to wounds of diabetic and nondiabetic mice and found that these proteinases accelerate wound healing by upregulating IL-6 and increasing neutrophil trafficking, which result in an earlier onset of inflammation that facilitates wound healing. MMP-8 is seen in the wound environment, but MMP-13 is not. Nonetheless, their overlapping activities as collagenases indicated that MMP-13 could perform the function of MMP-8, as we document here this is indeed the case. On the other hand, and in contrast to literature assertions, we show that MMP-2 has no effect on wound healing. MMP-2 is merely found in wounds in a catalytically incompetent form in complex with TIMPs. It does not play a role in wound healing. The roles of MMPs as key enzymes in wound remodeling as a prerequisite for healing is undisputed. The present work brings clarity to the roles of the subset of MMPs implicated in wound healing in the literature.
Methods
Compounds
Compounds 1(16) and 2(8) were synthesized
as described,16 dissolved in 20% DMSO/80%
propylene glycol or water, respectively, filter-sterilized (Acrodisc),
and stored at 4 °C. The solutions were freshly prepared every
2 days and warmed to room temperature before dosing. The internal
standard (compound 3) was synthesized as described.18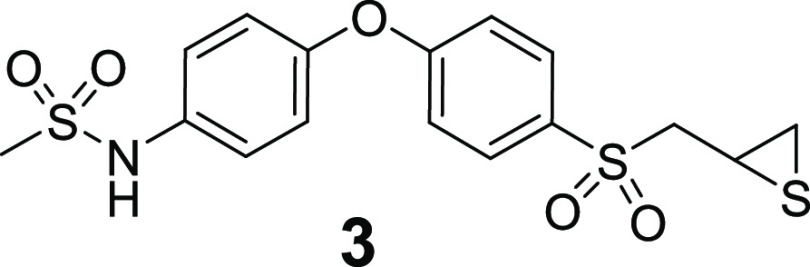
Collagenases
MMP-8 was cloned, expressed, and purified as described.6 MMP-13 was purchased from Mybiosource. The enzymatic activity was verified using the fluorogenic substrate Mca-KPLGL-Dpa-AR-NH2 (R&D Systems) in reaction buffer (50 mM Tris, 200 mM NaCl, 5 mM CaCl2, 20 μM ZnSO4, 0.05% Brij-35, pH 7.6) and monitored on a Cary Eclipse fluorescence spectrophotometer (Varian). Km, kcat, and Vmax values of MMP-13 were 5.4 ± 0.9 μM, 0.45 ± 0.03 s–1, and 8.8 ± 0.6 × 10–4 μM s–1, respectively. MMP-8 and MMP-13 solutions were prepared freshly every 2 days and stored at 4 °C.
Animals
Female db/db mice (BKS.Cg-Dock7m +/+ Leprdb/J, 8 weeks old, 38 ± 3 g) and female wild-type mice (C57BLKS/6J, 8 weeks old, 19 ± 2 g) were purchased from Jackson Laboratory. Animals were fed 5001 Laboratory Rodent Diet (LabDiet) and water ad libitum. Mice were maintained in polycarbonate shoebox cages with hardwood bedding (The Andersons, Inc.) at 72 ± 2 °F and exposed to 12 h light/12 h dark. All animal studies were conducted with approval and oversight by the Institutional Animal Care and Use Committee at the University of Notre Dame.
Full-Thickness Excision Animal Model
A single 8 mm diameter full-thickness excisional wound was created on the shaved dorsal thorax using a biopsy punch (Miltex) while under isoflurane anesthesia. The wound was covered with Tegaderm dressing (3M Company). Treatments were started the following day (day 1). Animals (n = 3 mice/group/time time) were sacrificed and wounds were excised and immediately frozen in liquid nitrogen. On the last day, the wounds were excised and embedded in OCT compound and cryosectioned for analysis by H& E staining and ISZ.
Wound Measurements
The wounds were photographed and measured as described.8 Wound closure was calculated as percent change in wound area relative to day 0. Wound closure measurements were done on days 0, 7, and 14 for the diabetic mice and on days 0, 7, and 10 for the wild-type animals.
Histological Evaluation, ISZ, and Ex Vivo Inhibition
Wounds were harvested and processed as described.8 Re-epithelialization was determined19 on a Nikon Eclipse 90i fluorescence microscope (Nikon Instruments Inc.). ISZ was performed using DQ-gelatin or DQ-collagen (Molecular Probes, Inc.) as described.6 Ly6G immunofluorescence staining was performed and quantified based on a published protocol20 with minor modifications. Embedded tissues were blocked with 5% BSA/0.1% Tween-20 in PBS, followed by 1 h incubation with anti-Ly6G antibody (1A8; BD Biosciences) at 5 μg/mL final concentration. Sections were washed and counterstained with DAPI, imaged by confocal microscopy, and quantified with ImageJ. The ex vivo inhibition experiment was performed on cryosectioned wound tissues with 1 h incubation at 10× the Ki values of MMP-2 for compound 1 and of MMP-9 for compound 2, followed by ISZ analysis. The control was treated with PBS. H&E, Ly6G immunofluorescence staining, ex vivo inhibition, and ISZ were assessed on n = 3 mice/group/time point.
MMP-8 Study
Wild-type mice (n = 42) were divided into two groups; the wounds were topically treated with MMP-8 (1 μg/wound/day in reaction buffer) or vehicle (50 μL reaction buffer, 50 mM Tris (pH 7.5), 10 mM CaCl2, 150 mM NaCl, 0.05% (w/v) Brij-35) once a day for 10 days.
Measurement of IL-6 by ELISA
Wound tissues (n = 3 mice/group) were homogenized in a Bullet blender (NextAdvance) in cold lysis buffer containing free-EDTA protease inhibitor cocktail (Pierce). The extracts were centrifuged (15000 rpm, 20 min, 4 °C); the supernatants were collected and quantified for protein content by the Bradford protein assay (Bio-Rad). All samples were normalized and stored at −80 °C until analysis. The levels of IL-6 were quantified by ELISA using the manufacturer’s procedure (Abcam).
MMP-13 Studies
Two studies were carried out. The first study used 16 db/db mice treated topically with vehicle (50 μL of reaction buffer) or MMP-13 (1 μg/wound/day) once a day for 14 days. The second study was similar to the first study, except for the use of 16 wild-type mice and topical administration of MMP-13 for 10 days.
Selective MMP-2 Inhibitor Study
Two studies were carried out: (a) 24 db/db mice treated with vehicle (50 μL of 20% DMSO/80% propylene glycol) or inhibitor (250 μg/wound/day) for 14 days and (b) 28 wild-type mice were treated with vehicle (same as in the first study) or with inhibitor (50 μg/wound/day) for 10 days.
Gelatin Zymography and Western Blot Analysis
Wound tissues (n = 3 mice per time point) were homogenized in lysis buffer containing protease inhibitor cocktail without EDTA. The protein content of samples was determined by the BCA method; 0.5 mg of total protein extract was analyzed by gelatin zymography to determine activity of MMP-2 and MMP-9, as previously described.21 Wound homogenates were analyzed for TIMP-1 and TIMP-2 by Western blot, as per a published protocol22 with a few modifications. Samples (50 μg of total protein) were loaded onto 8–16% SDS-polyacrylamide gels (GenScript), and electrophoresis was used to separate the proteins. The proteins were transferred to a nitrocellulose membrane (BioRad) and blocked overnight with 5% nonfat dry milk in TBST (10 mM Tris (pH 7.6), 150 mM NaCl, 0.05% Tween-20). The next day, the membranes were probed for 2 h with primary antibodies against TIMP-1 (Thermo Fisher) at 1:250, TIMP-2 (EMD Millipore) at 1:250, or MMP-2 (Thermo Fisher) at 1:500, and α-actin (Sigma) at 1:2000 in TBST. After the wash, the blots were probed for 1.5 h with secondary goat anti-mouse IgG antibody conjugated to horseradish peroxidase at 1:1000 in TBST. The blots were incubated with ECL Plus (Fisher) for 3–5 min, followed by imaging by StormScanner (GE Healthcare) and exposed to film. Relative quantities of TIMP-1, TIMP-2, and MMP-2 were determined by densitometry using ImageJ (version 1.52) and normalized to total amount of actin.
Immunoprecipitation with TIMP-2 Antibody
Immunoprecipitation was performed following the manufacturer’s instructions (Pierce). Wound homogenates (n = 3 mice per time point) were incubated with control agarose resin of the immunoprecipitation kit to obtain precleared homogenate and then incubated with TIMP-2 antibody to capture the complex of TIMP-2 and MMP-2, followed by Western blot analysis and densitometry analysis by ImageJ; levels of TIMP-2 and MMP-2 were normalized to the total amount of actin.
Quantification of Compound 1 in Wounds
Wound tissues (n = 3) were harvested on day 10 at 1 h after topical treatment and stored at −80 °C until analysis. The wound tissues were weighed and homogenized at 4 °C for 30 min with one volume equivalent of acetonitrile containing internal standard 3 at 2.5 μM final concentration. The homogenate was centrifuged (20000g, 20 min), and the supernatant was analyzed by UPLC on a Kinetex C18 column (2.6 μm, 2.1 mm × 100 mm, Phenomenex) with a Waters TQD triple quadrupole detector monitored with MassLynx MS software using electrospray ionization in the negative ion mode. The mass spectrometry parameters, MRM transitions, and chromatographic conditions were the same as described.16 Peak-area ratios relative to internal standard and linear regression parameters derived from the calibration curves in blank mouse tissue were used to quantify the levels of compound 1 in the wounds.
Statistical Analysis
Wound closure was analyzed for statistical significance using a two-tail Mann–Whitney U test (GraphPad Prism 5). Cytokine data were analyzed by the Student t-test (Excel) using a two-tail distribution and unequal variance; p < 0.05 was considered statistically significant.
Acknowledgments
We thank Sarah Chapman for the preparation of wound tissue sections and H&E staining. T.T.N. is a Ruth L. Kirschtein National Research Service Award Fellow of the Chemistry–Biochemistry–Biology Interface Program at the University of Notre Dame, supported by Training Grant T32 GM075762 from the National Institutes of Health. This work was supported by the American Diabetes Association Pathway to Stop Diabetes (Grant 1-15-ACN-06).
Author Present Address
§ (M.A.S.) Biomedical Engineering Department, University of Kentucky, Lexington, KY 40506.
The authors declare no competing financial interest.
References
- Nguyen T. T., Mobashery S., and Chang M. (2016) Roles of matrix metalloproteinases in cutaneous wound healing, in Wound Healing: New Insights into Ancient Challenges (Alexandrescu V. A., Ed.), pp 37–71, InTech. [Google Scholar]
- Armstrong D. G.; Boulton A. J. M.; Bus S. A. (2017) Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 376, 2367–2375. 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2017) National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States.
- Fortington L. V.; Geertzen J. H.; van Netten J. J.; Postema K.; Rommers G. M.; Dijkstra P. U. (2013) Short and long term mortality rates after a lower limb amputation. Eur. J. Vasc. Endovasc. Surg. 46, 124–131. 10.1016/j.ejvs.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Gooyit M.; Peng Z.; Wolter W. R.; Pi H.; Ding D.; Hesek D.; Lee M.; Boggess B.; Champion M. M.; Suckow M. A.; Mobashery S.; Chang M. (2014) A chemical biological strategy to facilitate diabetic wound healing. ACS Chem. Biol. 9, 105–110. 10.1021/cb4005468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M.; Nguyen T. T.; Suckow M. A.; Wolter W. R.; Gooyit M.; Mobashery S.; Chang M. (2015) Acceleration of diabetic wound healing using a novel protease-anti-protease combination therapy. Proc. Natl. Acad. Sci. U. S. A. 112, 15226–15231. 10.1073/pnas.1517847112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Fernandez A.; Inada M.; Balbin M.; Fueyo A.; Pitiot A. S.; Astudillo A.; Hirose K.; Hirata M.; Shapiro S. D.; Noel A.; Werb Z.; Krane S. M.; Lopez-Otin C.; Puente X. S. (2007) Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). FASEB J. 21, 2580–2591. 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. T.; Ding D. R.; Wolter W. R.; Perez R. L.; Champion M. M.; Mahasenan K. V.; Hesek D.; Lee M.; Schroeder V. A.; Jones J. I.; Lastochkin E.; Rose M. K.; Peterson C. E.; Suckow M. A.; Mobashery S.; Chang M. (2018) Validation of Matrix Metalloproteinase-9 (MMP-9) as a Novel Target for Treatment of Diabetic Foot Ulcers in Humans and Discovery of a Potent and Selective Small-Molecule MMP-9 Inhibitor That Accelerates Healing. J. Med. Chem. 61, 8825–8837. 10.1021/acs.jmedchem.8b01005. [DOI] [PubMed] [Google Scholar]
- Lin Z. Q.; Kondo T.; Ishida Y.; Takayasu T.; Mukaida N. (2003) Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J. Leukocyte Biol. 73, 713–721. 10.1189/jlb.0802397. [DOI] [PubMed] [Google Scholar]
- Fielding C. A.; McLoughlin R. M.; McLeod L.; Colmont C. S.; Najdovska M.; Grail D.; Ernst M.; Jones S. A.; Topley N.; Jenkins B. J. (2008) IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J. Immunol. 181, 2189–2195. 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- Daley J. M.; Thomay A. A.; Connolly M. D.; Reichner J. S.; Albina J. E. (2008) Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukocyte Biol. 83, 64–70. 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Hartenstein B.; Dittrich B. T.; Stickens D.; Heyer B.; Vu T. H.; Teurich S.; Schorpp-Kistner M.; Werb Z.; Angel P. (2006) Epidermal development and wound healing in matrix metalloproteinase 13-deficient mice. J. Invest. Dermatol. 126, 486–496. 10.1038/sj.jid.5700084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmann R.; Ambrosch A.; Schultz G.; Waldmann K.; Schiweck S.; Lehnert H. (2002) Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 45, 1011–1016. 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- Wall S. J.; Bevan D.; Thomas D. W.; Harding K. G.; Edwards D. R.; Murphy G. (2002) Differential expression of matrix metalloproteinases during impaired wound healing of the diabetes mouse. J. Invest. Dermatol. 119, 91–98. 10.1046/j.1523-1747.2002.01779.x. [DOI] [PubMed] [Google Scholar]
- Hsu J. Y.; McKeon R.; Goussev S.; Werb Z.; Lee J. U.; Trivedi A.; Noble-Haeusslein L. J. (2006) Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J. Neurosci. 26, 9841–9850. 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooyit M.; Song W.; Mahasenan K. V.; Lichtenwalter K.; Suckow M. A.; Schroeder V. A.; Wolter W. R.; Mobashery S.; Chang M. (2013) O-Phenyl Carbamate and Phenyl Urea Thiiranes as Selective Matrix Metalloproteinase-2 Inhibitors that Cross the Blood-Brain Barrier. J. Med. Chem. 56, 8139–8150. 10.1021/jm401217d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. W.; Gervasi D. C.; Mobashery S.; Fridman R. (1997) Kinetic analysis of the binding of human matrix metalloproteinase-2 and −9 to tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2. J. Biol. Chem. 272, 29975–29983. 10.1074/jbc.272.47.29975. [DOI] [PubMed] [Google Scholar]
- Gooyit M.; Song W.; Mahasenan K. V.; Lichtenwalter K.; Suckow M. A.; Schroeder V. A.; Wolter W. R.; Mobashery S.; Chang M. (2013) O-phenyl carbamate and phenyl urea thiiranes as selective matrix metalloproteinase-2 inhibitors that cross the blood-brain barrier. J. Med. Chem. 56, 8139–8150. 10.1021/jm401217d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkalcevic V. I.; Cuzic S.; Parnham M. J.; Pasalic I.; Brajsa K. (2009) Differential evaluation of excisional non-occluded wound healing in db/db mice. Toxicol. Pathol. 37, 183–192. 10.1177/0192623308329280. [DOI] [PubMed] [Google Scholar]
- Kowanetz M.; Wu X. M.; Lee J.; Tan M.; Hagenbeek T.; Qu X. P.; Yu L. L.; Ross J.; Korsisaari N.; Cao T.; Bou-Reslan H.; Kallop D.; Weimer R.; Ludlam M. J. C.; Kaminker J. S.; Modrusan Z.; van Bruggen N.; Peale F. V.; Carano R.; Meng Y. G.; Ferrara N. (2010) Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl. Acad. Sci. U. S. A. 107, 21248–21255. 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M.; Sohail A.; Fridman R. (2012) Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol. Biol. 878, 121–135. 10.1007/978-1-61779-854-2_8. [DOI] [PubMed] [Google Scholar]
- Delfin D. A.; Zang K. E.; Schill K. E.; Patel N. T.; Janssen P. M.; Raman S. V.; Rafael-Fortney J. A. (2012) Cardiomyopathy in the dystrophin/utrophin-deficient mouse model of severe muscular dystrophy is characterized by dysregulation of matrix metalloproteinases. Neuromuscul. Disord. 22, 1006–1014. 10.1016/j.nmd.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]