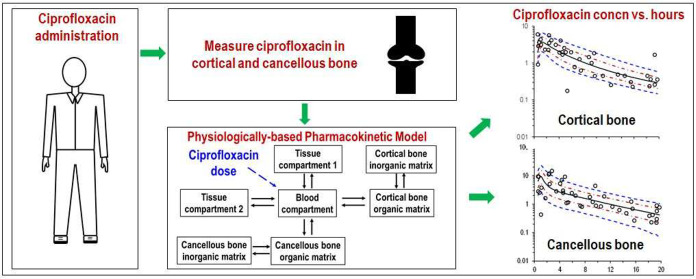
Abstract
Ciprofloxacin is highly active against bacteria that commonly cause bone infections. However, the time-course of ciprofloxacin in bone has not been characterized using population pharmacokinetic modeling. Thirty-nine patients received a 1-h infusion of 400 mg of ciprofloxacin before orthopedic surgery. Blood and bone samples were collected at 0.5 to 20 h following the start of the infusion. Bone samples were separated into cortical and cancellous bone and pulverized under liquid nitrogen using a cryogenic mill. Ciprofloxacin in plasma, and cortical and cancellous bone was quantified by liquid chromatography–tandem mass spectrometry. A physiologically based pharmacokinetic modeling approach was utilized to describe the concentration–time profiles in plasma and bone. Ciprofloxacin concentrations ranged from 0.176 to 5.98 mg/L (median, 1.67; density, 1.99 g/cm3) in cortical, and 0.224 to 14.6 mg/L (median, 1.22; 1.92 g/cm3) in cancellous bone. The average observed cortical bone/plasma concentration ratio was 0.67 at 0.5 to 2 h (n = 7) and 5.1 at 13 to 20 h (n = 9). For cancellous bone the respective average ratios were 0.77 and 4.4. The population PK model included a central (blood) compartment, two peripheral tissue compartments, and compartments for the organic and inorganic (hydroxyapatite) matrix in cortical and cancellous bone. The population mean ciprofloxacin clearance was 20.7 L/h. The estimated partition coefficients of the organic bone matrix were 3.39 for cortical and 5.11 for cancellous bone. Ciprofloxacin achieved higher concentrations in bone than plasma. Slow redistribution from bone to plasma may have been due to binding to the inorganic bone matrix. The developed model presents a step toward optimized antibiotic dosing in osteomyelitis.
Keywords: pharmacokinetic modeling, bone penetration, physiologically based pharmacokinetic model, fluoroquinolones
Bone infections, which are most commonly caused by Staphylococcus aureus, remain challenging to treat for a number of reasons, including a paucity of information on the extent and time-course of penetration of antibiotics into bone.1−5 The incidence of bone infections is expected to increase, largely due to the rising number of orthopedic device-related infections associated with increasing demand for prosthetic joint surgeries.6,7 The global antimicrobial resistance crisis is exacerbating the problem.8 Osteomyelitis typically requires prolonged antibiotic treatment, has a high risk of recurrence and may irreversibly damage the bone.1−3 Successful surgical prophylaxis and postsurgical antibiotic therapy are critical for the respective prevention and treatment of osteomyelitis.2,9 Both require the attainment of effective concentrations at the infection site in bone.9 Thus, studying the extent and time-course of antibiotic bone penetration is important, especially as bone is less vascularized than many other regions , for example, the lungs or skin.10 Such studies are challenging to perform and analyze, and several limitations apply to the vast majority of bone penetration studies.11
While microdialysis studies conducted over several hours can be very informative in understanding penetration of drugs in many soft tissues, this technique has substantial limitations when applied to bone.12,13 Therefore, bone penetration studies are typically undertaken by simultaneous collection of a sample of bone and a sample of plasma at one time (e.g., during orthopedic surgery) following an antibiotic dose in each subject.11,14 The drug concentration in each of these biological matrices enables determination of a bone to plasma concentration ratio for the time at which the sample was collected. However, it is essential to recognize that the time-course of concentration in bone often lags behind that in plasma. As a result of this system hysteresis the bone to plasma drug concentration ratio depends on the sampling time, unless equilibrium has been reached. Clearly, such time-dependent changes in the ratio hamper interpretation of data within a given study and comparison of ratios across different studies.11 Furthermore, the pharmacokinetic/pharmacodynamic (PK/PD) indices that best predict activity for most antibiotics used against bone infections, including fluoroquinolones and β-lactams,5,15,16 are respectively the area under the unbound plasma concentration–time curve divided by the minimum inhibitory concentration (fAUC/MIC) and the percentage of a dosing interval during which the unbound plasma concentration exceeds the MIC (fT>MIC).17,18 It is axiomatic that these indices cannot be reliably determined based on one or even a small number of time points. Clearly, a collection of multiple bone samples from a patient over a wide range of different times after drug administration raises practical and ethical considerations. The need for sufficient samples over a sufficient time can be addressed by an informative study design in which bone and plasma samples are collected from a number of different patients with the times of sample collection being distributed across a sufficient time after a single dose or across a dosing interval in a multiple dosing regimen. Such a sparse sampling protocol for each of the included patients together with population PK analysis makes possible a description of the whole time-course of concentrations in both bone and plasma.10 Subsequent Monte Carlo simulations can predict the expected probability of PK/PD target attainment for clinically relevant dosage regimens, as we have reported using traditional compartmental modeling in which the physiological context is not considered.19,20
The development of a minimal or full physiologically based pharmacokinetic (PBPK) model,21,22 with the addition of compartments that describe measured concentrations in bone, offers a promising approach as a step toward an improved understanding of antibiotic PK in bone. Such an approach incorporates physiological information; in the case of bone penetration studies this can include consideration of the different compartments and blood flows within bone. Despite their potential, it appears that physiologically based modeling approaches have not been applied to describe the time-course of measured concentrations of an antibiotic in bone.
Ciprofloxacin has high activity against pathogens causing bone infections, including those growing in biofilms.9 Generally high bone to plasma concentration ratios (∼0.3 to 1.2) have been reported for ciprofloxacin from samples collected at different distinct time points postdose.23−25 However, the complete time-course of ciprofloxacin concentrations in bone has not been evaluated by population PK modeling. We aimed to elucidate the complete time-course of ciprofloxacin in plasma, cortical bone, and cancellous bone via measuring the concentrations of ciprofloxacin in bone and plasma from a cohort of patients who had received the drug prior to orthopedic surgery. We sought to develop a population PK model by utilizing a physiologically based approach to describe all ciprofloxacin concentrations in plasma and bone simultaneously. Subsequently, we aimed to calculate probabilities of PK/PD target attainment based on Monte Carlo simulations.
Results and Discussion
Despite several decades of use of antibiotics for the treatment of osteomyelitis there remain major gaps in understanding the extent and time-course of concentrations in bone. We sought to close some of these gaps by exploring the use of a physiologically based population PK modeling approach to describe the disposition in bone of ciprofloxacin, a representative of an important class of antibiotics used in the treatment of osteomyelitis. A key decision at the outset related to the way in which events in bone would be monitored. We chose to collect bone samples at the time of surgical intervention for the management of pre-existing orthopedic conditions in the noninfected patients included in the study. Microdialysis studies aiming to determine antibiotic concentrations in interstitial bone fluid have been reported.12,13 However, for insertion of the microdialysis catheter a hole needs to be drilled into the bone, resulting in a dead space that may fill with blood clots and extracellular fluid exudations.12 Measured concentrations are assumed to represent those in the dead space and the interstitial fluid of the adjacent bone tissue,12 but it is unknown how drilling a hole into bone impacts on these concentrations,13 and how concentrations in blood clots and exudations accumulating in the drill hole relate to the concentrations in intact bone. In addition, the microdialysis technique presents practical and ethical issues for opportunistic studies in which sampling of bone tissue occurs during a surgical procedure, as was the case in the noninfected patients in the present study (Table 1).
Table 1. Characteristics of Patients Included in the Studya.
| average ± standard deviation or percentage | |
|---|---|
| body weight (kg) | 74 ± 20 |
| height (cm) | 165 ± 8 |
| age (years) | 66 ± 9 |
| females/males (%) | 68/32 |
| creatinine clearance (mL/min) | 68 ± 20 |
| white blood cells (103/μL) | 7.8 ± 2.1 |
| hemoglobin (g/dL) | 12 ± 1.1 |
| hematocrit (%) | 37.9 ± 3.4 |
| platelets (103/μL) | 256 ± 70 |
| international normalized ratio (INR) | 0.96 ± 0.11 |
Demographic and clinical chemistry details were available from 37 of the 39 patients.
Observed Ciprofloxacin Concentrations
Figure 1 presents the ciprofloxacin concentrations in plasma, cortical bone, and cancellous bone for each patient on both the semilogarithmic scale over the 20-h sampling period and the linear scale over the first 6 h. Individual plasma ciprofloxacin concentrations ranged from 7.86 mg/L at 0.67 h to <0.05 mg/L at 19.3 h. One extremely high plasma concentration was ≥3.5-fold greater than all other plasma concentrations; it was considered as an outlier, the only such occurrence in the study, and commented out of the modeling data set.26 At 0.58 to 19.75 h, concentrations of ciprofloxacin were between 5.98 and 0.176 mg/L (overall median concentration 1.67 mg/L) in cortical bone and between 14.6 and 0.224 mg/L (median 1.22 mg/L) in cancellous bone.
Figure 1.
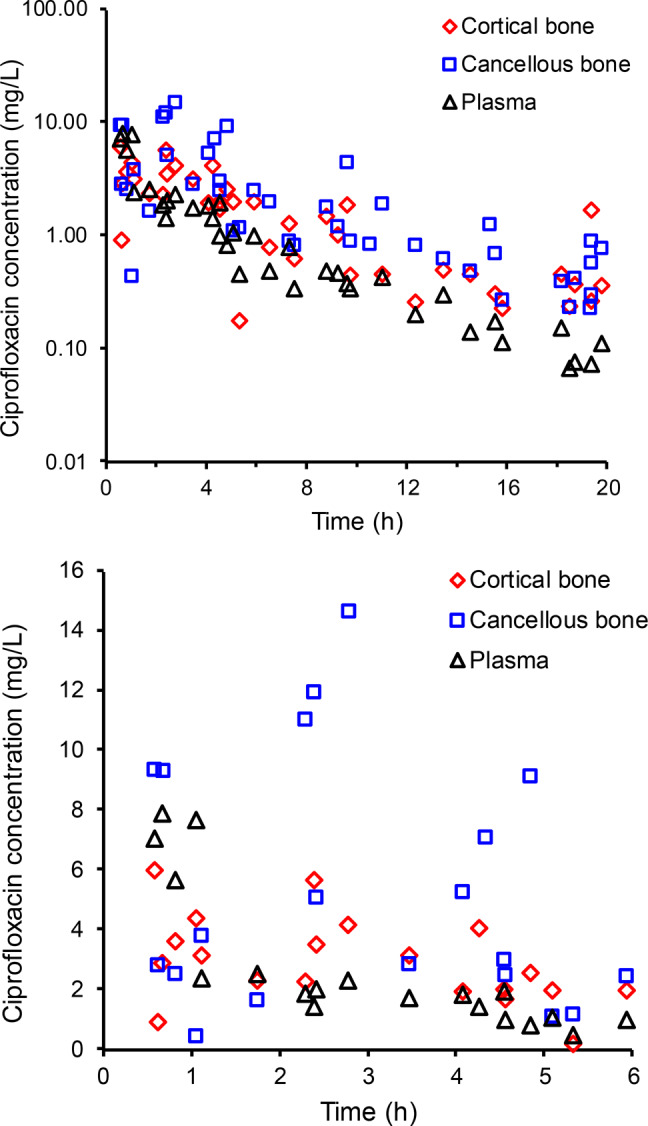
Ciprofloxacin concentrations in plasma, cortical bone and cancellous bone on log-scale (upper panel) and over the first 6 h on linear scale (lower panel). Ciprofloxacin concentrations in bone are expressed as mg/L, using a density of 1.99 g/cm3 for cortical and 1.92 g/cm3 for cancellous bone.
The collection of simultaneous samples of plasma and bone from 39 patients with collection times ranging from approximately 0.5 to 20 h afforded the opportunity to assess the relative concentration time-courses of ciprofloxacin in the respective physiological compartments. Initially, ciprofloxacin concentrations in plasma were similar to or exceeded those in bone, whereas toward the end of the observation period concentrations were higher in bone than in plasma (Figure 1). The average cortical bone to plasma concentration ratio increased from 0.67 between 0.5 and 2.0 h (n = 7) to 5.1 between 13 and 20 h (n = 9, the plasma sample from one patient was below the limit of quantification). For cancellous bone the average concentration ratios increased from 0.77 between 0.5 and 2.0 h (n = 7) to 4.4 between 13 and 20 h (n = 9). This increase in the bone-to-plasma concentration ratios over time reflects initially the time required for distribution down a concentration gradient from plasma into bone, and later the slower decline of ciprofloxacin concentrations in bone compared to plasma as the drug redistributed out of bone. While these data provide evidence for system hysteresis, it is clear that the maximum concentration of ciprofloxacin in cortical and cancellous bone is reached within the first few hours after intravenous administration (Figure 1). Rapid uptake into bone is advantageous, because early attainment of sufficiently high antibiotic concentrations at an infection site is considered an essential element of antimicrobial therapy.27,28
A small number of studies have investigated ciprofloxacin penetration into bone.23−25,29,30 In three of these studies, a bone density of 1.0 was assumed,23−25 most studies did not report ciprofloxacin concentrations in both cortical and cancellous bone,23−25,29 and in two studies ciprofloxacin was administered orally.23,24 Massias et al. investigated cortical bone uptake of ciprofloxacin at steady state in 21 patients after oral doses of ciprofloxacin 500 mg twice daily.23 The average concentration ratios were between 0.27 and 1.2 at 1 to 13 h postdose. These investigators used naïve averaging and the trapezoidal rule based on data from five time points to calculate an area under the curve (AUC) ratio of 0.63 between cortical bone of the mastoid process and serum.23 Fong et al. reported on bone concentrations of ciprofloxacin following a single oral dose in noninfected patients undergoing hip or knee replacement surgery or osteotomy and in patients with osteomyelitis.24 Samples of bone and serum were collected 1.5 to 4.75 h after dosing in noninfected patients and 2 to 4.5 h after ciprofloxacin administration in the patients with osteomyelitis. After 750 mg of ciprofloxacin was administered to seven noninfected patients, the average ± standard deviation (SD) concentration of ciprofloxacin in cortical bone (cancellous bone was not examined) was 0.7 ± 0.4 mg/kg and in serum 2.6 ± 1.1 mg/L; the corresponding values for the same dose administered to four patients with osteomyelitis were 1.4 ± 1.0 mg/kg and 2.9 ± 2.2 mg/L.24 Bone-to-serum concentration ratios for individual patients were not reported and it is not possible to determine them from the data presented. Care is needed when interpreting the data of Fong et al. because of the small number of patients7,4 in each group and the lack of information on the actual sample times post-administration in each patient. In a study by Wacha et al., cortical bone concentrations of 0.11 to 0.94 mg/kg were observed 0.5 to 5 h after intravenous administration of 200 mg of ciprofloxacin to 20 elderly patients undergoing above-knee amputation, total hip replacement, or osteosynthesis.29 Caution is required with interpretation of this study as a microbiological assay was used to quantify ciprofloxacin. Leone et al. determined skull bone concentrations of ciprofloxacin in 14 patients undergoing brain tumor excision.25 Ciprofloxacin (200 mg) was administered intravenously 0.5 h before skin incision and the average (±SD) bone-to-serum concentration ratios were 0.44 (±0.29) at opening and 0.97 (±1.57) at closure. Meissner et al. reported bone and serum concentrations of ciprofloxacin following intravenous administration of a 200 mg dose to 20 patients prior to implantation of a total hip-joint endoprosthesis.30 The average concentrations in cancellous and cortical bone after ciprofloxacin administration were, respectively, 2.16 mg/L and 1.42 mg/L after 1 h; 1.16 mg/L and 0.42 mg/L after 4–5 h; and 0.27 mg/L and 0.15 mg/L after 13–19 h. For the reasons indicated above, it is not possible to directly compare the results of these previous studies with those of the current study.
Population PK Analysis
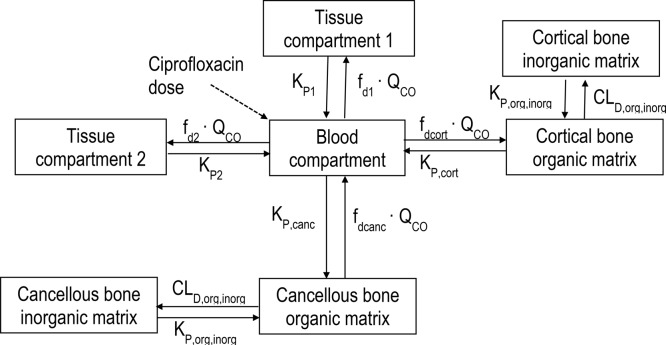
The granularity of the concentration versus time data (Figure 1) rendered them suitable for physiologically based population PK modeling. The developed model (Figure 2) demonstrated highly satisfactory predictive performance for the concentrations in plasma, cortical bone, and cancellous bone (Figure 3). Table 2 presents the population parameter estimates, their interindividual variabilities and standard errors; the latter indicate that the model parameters were estimated with generally good precision. The estimated partition coefficients (relative to plasma or blood) were 1.49 (KP1) and 1.18 (KP2) for the two (nonbone) peripheral compartments, and 3.39 (KPcort) and 5.11 (KPcanc) for the organic matrix of cortical and cancellous bone. Any attempts to understand the PK of ciprofloxacin in bone, including the bone to plasma partition coefficients, requires an appreciation of the composition and structure of bone.
Figure 2.
Final structural PK model.
Figure 3.
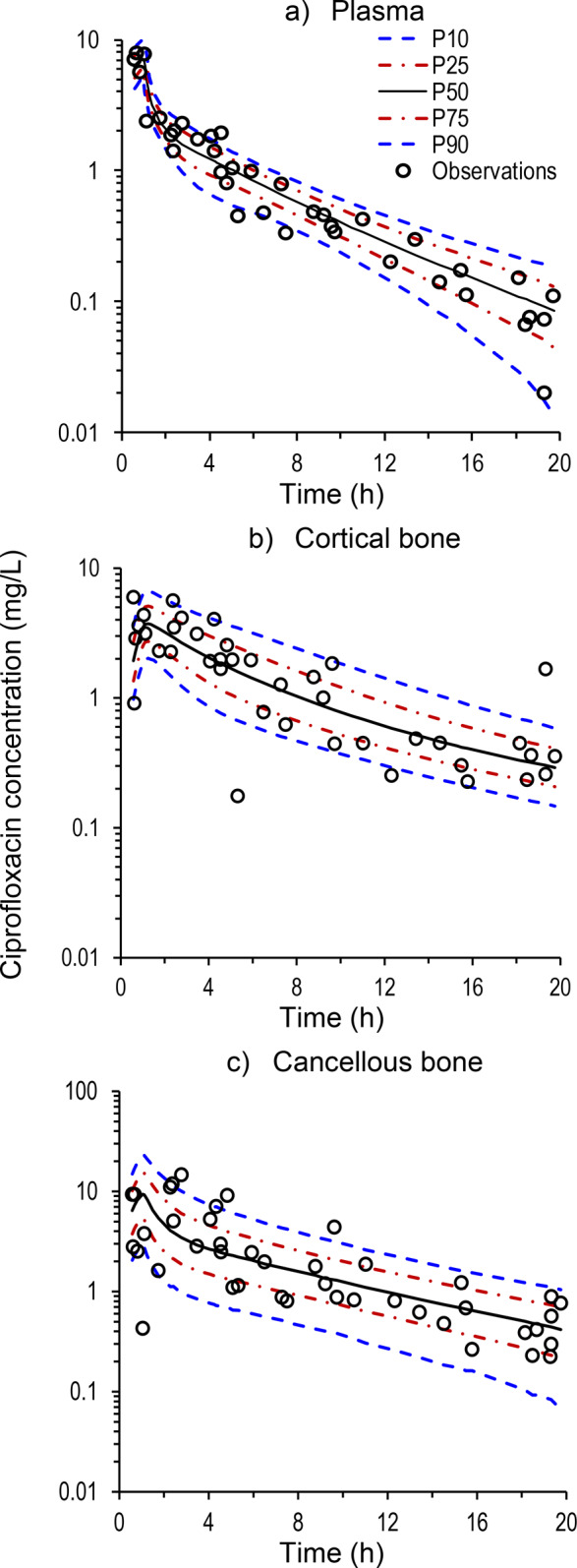
Visual predictive checks for ciprofloxacin in (a) plasma, (b) cortical bone, and (c) cancellous bone. The plots present the observed concentrations, the 90% prediction interval (10th percentile [P10] to 90th percentile [P90]), and the predicted interquartile range (25th percentile [P25] to 75th percentile [P75]).
Table 2. Population Parameter Estimates, Interindividual Variability (IIV) and Standard Errors (SE) for Ciprofloxacin in Plasma, Cortical Bone, and Cancellous Bone.
| parameter | symbol | unit | estimate (IIV %CV) | SE %CV |
|---|---|---|---|---|
| Central and Estimated Tissue Compartments | ||||
| total body clearance | CL | L/h | 20.7 (12%) | 4.0% |
| blood volume | VB | L | 5.85a (22%) | |
| volume of tissue compartment 1 | V1 | L | 5.07 (11%) | 8.2% |
| cardiac output | QCO | L/h | 312a (27%) | |
| fd1 | fd1 | 0.111 (35%) | 42% | |
| fd2 | fd2 | 0.115 (80%) | 19% | |
| partition coefficient for shallow peripheral compartment | KP1 | 1.49 (13%) | 13% | |
| partition coefficient for deep peripheral compartment | KP2 | 1.18 (13%) | 6.8% | |
| proportional residual variability | CVCP | % | 0.06% | |
| additive residual variability | SDCP | mg/L | 0.035 | |
| Cortical and Cancellous Bone | ||||
| fraction of organic bone matrix | forg | 0.350a (41%) | ||
| partition coefficient between organic and inorganic bone matrix | KP,org,inorg | 0.203 (24%) | 30% | |
| distribution clearance between organic and inorganic bone matrix | CLD,org,inorg | L/h | 0.0074 (25%) | 41% |
| Cortical Bone | ||||
| cortical bone volume | Vcort | L | 2.12a (23%) | |
| partition coefficient for the organic matrix of cortical bone | KPcort | 3.39 (25%) | 7.8% | |
| distribution clearance for cortical bone (CLD,cort = fdcort·QCO) | CLD,cort | L/h | 2.00a (41%) | |
| proportional residual variability | CVcort | % | 2.8% | |
| additive residual variability | SDcort | mg/L | 0.053 | |
| Cancellous Bone | ||||
| cancellous bone volume | Vcanc | L | 0.54a (30%) | |
| partition coefficient for the organic matrix of cancellous bone | KPcanc | 5.11 (36%) | 12% | |
| distribution clearance for cancellous bone (CLD,canc = fdcanc·QCO) | CLD,canc | L/h | 3.19a (46%) | |
| proportional residual variability | CVcanc | % | 47% | |
| additive residual variability | SDcanc | mg/L | 0.053 | |
Population mean parameter fixed to physiological value based on Brown et al.39
Bone is composed of an organic and an inorganic matrix.5,19,31 The organic bone matrix represents ∼35% of total bone mass and consists mainly of collagen fibrils (∼90%), in addition to glycoproteins, proteoglycans, and extracellular fluid. Blood vessels in bone are located in Haversian and Volkmann’s canals that transverse the matrix. Bone cells represent only 1–2% of total bone mass and in their most mature form as osteocytes are trapped inside the matrix.31,32 The inorganic matrix accounts for ∼65% of total bone mass and consists of calcium phosphate crystals (hydroxyapatite) deposited inside the organic bone matrix. Currently no bioanalytical methods are available to reliably quantify drug concentrations separately within those different components of bone.
Our population PK model suggests that the bone matrix was in relatively rapid equilibrium with plasma, although system hysteresis was evident, and the increase in bone to plasma concentration ratios over time was due to ciprofloxacin binding to the matrix, thus creating a reservoir. The binding of ciprofloxacin to inorganic bone material and penetration into most or all parts of the organic bone components is the most likely contributor to the high bone-to-plasma partition coefficients. The estimated KP,org,inorg of 0.203 may suggest that overall a large fraction of the ciprofloxacin resides in the organic bone matrix (including bone fluid and blood vessels) or is in relatively rapid equilibration with the organic matrix. On this basis, a smaller fraction would be more tightly bound to the hydroxyapatite crystals embedded inside the organic matrix and act as a depot compartment, with comparatively slow redistribution of ciprofloxacin (CLD,org,inorg). For most fluoroquinolones for which cortical and cancellous bone have been measured separately, the penetration was higher in cancellous bone,11 as was observed in the current study for ciprofloxacin. Comparison of the estimated partition coefficients for the two (nonbone) peripheral compartments (KP1 and KP2) with those for the organic bone matrix (KPcort and KPcanc) indicates that, on average, ciprofloxacin had higher affinity for bone than other tissues.
To the best of our knowledge, our physiologically based population PK model is the first to use measured concentrations of ciprofloxacin in cortical and cancellous bone, together with those in plasma, to describe the time-course of ciprofloxacin in bone. A whole body PBPK model was recently published for ciprofloxacin; however, that model was developed based on plasma concentrations only and did not include any compartment for bone. In addition, the model was based upon PK data from intensive care patients.33 Indeed, there are very few PBPK models for other antibiotics. A PBPK model in PK-Sim was previously used to predict clindamycin exposure in bone; however, no measurements of clindamycin in bone were included, and profiles of predicted bone concentrations over time were not reported.34 A PBPK model for the antituberculosis drug capreomycin included a bone compartment, but there were no measurements of the drug in bone to inform the model nor predictions of capreomycin concentrations in bone.35 The model developed for ciprofloxacin in the current study to describe its uptake and disposition in bone enabled Monte Carlo simulations to forecast the probabilities of target attainment (PTA) for clinically used dosing regimens of ciprofloxacin.
Probabilities of Target Attainment
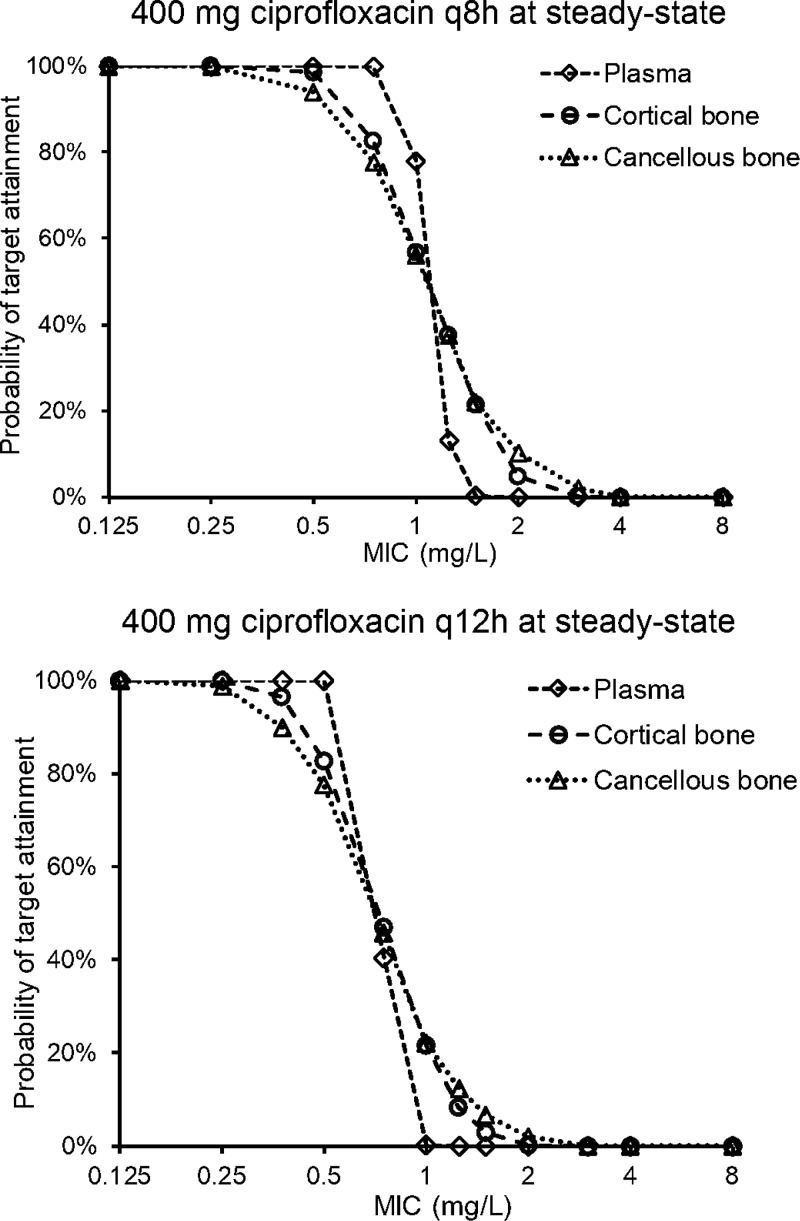
From the Monte Carlo simulations, the median ciprofloxacin penetration into cortical bone, calculated based on the ratio of AUCcort/AUCplasma for 1000 virtual patients at steady state was 1.62 with an interquartile range of 1.26 to 2.09 and 10th to 90th percentile of 1.02 to 2.58. For cancellous bone the median AUCcanc/AUCplasma was 2.53 with an interquartile range of 1.87 to 3.37 and 10th to 90th percentile of 1.43 to 4.45. These AUCbone/AUCplasma ratios are conservative and reflect the total bone to plasma AUC ratios. Therefore, they are lower than the estimates for KPcort and KPcanc that represent the partition coefficients between the organic bone matrix and plasma. On the basis of the fAUCplasma/MIC target of ≥4020,36,37 for successful clinical and microbiological outcome (see Methods), a plasma protein binding of 25%38 and the median bone/plasma AUC ratios above, the targets for bone were AUCcort/MIC of ≥86 and AUCcanc/MIC of ≥135. The targets for bone are based on the total AUC expressed in mg·h/L with respective bone densities of 1.92 and 1.99 g/cm3,39 as opposed to mg·h/kg. The PTA plots shown in Figure 4 are based on the patients in the current study, that is, reflecting the characteristics of patients who are likely to undergo joint replacement surgery, receiving two clinically used dosing regimens. The FDA approved dose of intravenous ciprofloxacin for treatment of bone and joint infections is 400 mg every 8 to 12 h.40 With a dosage regimen of 400 mg of ciprofloxacin every 8 h (q8h) at steady state, the PK/PD breakpoints were 0.75 mg/L for plasma, and 0.5 mg/L for cortical and cancellous bone. For 400 mg of ciprofloxacin administered q12h at steady state, the breakpoints were 0.5 mg/L for plasma, and 0.375 mg/L for cortical and cancellous bone. The breakpoints in bone are slightly lower than those in plasma due to the higher interindividual variability of the ciprofloxacin concentrations in bone compared to plasma.
Figure 4.
Probabilities of target attainment for plasma, cortical bone, and cancellous bone after 1-h infusions of 400 mg of ciprofloxacin q8h (top) and q12h (bottom) at steady state. The fAUC/MIC target for plasma was 40,20 and the AUC/MIC targets for cortical and cancellous bone were 86 and 135, respectively.
The breakpoints above from the PTA analysis need to be considered relative to the breakpoint of the Clinical and Laboratory Standards Institute (CLSI). The CLSI susceptible MIC breakpoint for ciprofloxacin against S. aureus is ≤1 mg/L, a breakpoint that is based on the plasma exposure-response (i.e., PK/PD) relationship across a range of different infections, for example, pneumonia and bloodstream infections.41 The PK/PD breakpoints for plasma (0.75 and 0.5 mg/L for the 400 mg q8h and q12h regimens, respectively) and bone (0.5 and 0.375 mg/L for the 400 mg q8h and q12h regimens, respectively) from the PTA analysis in this study suggest a susceptible breakpoint, even at the maximum approved FDA daily dose, that might be lower than that of CLSI (1 mg/L).
The above breakpoints require careful consideration for several reasons. The PTA analysis was based on the total bone-to-plasma AUC ratios of ciprofloxacin, that is, an even distribution of the antibiotic throughout the bone tissue. However, it is most likely that neither bacteria nor antibiotics distribute evenly throughout the bone tissue;5,11 therefore, the actual breakpoints in bone for clinical and microbiological cure might be higher.19 The precise location of bacteria in bone infection is not well-known. Based on their size (e.g., ∼1 μm for S. aureus), bacteria are expected to distribute through the Haversian and Volkmann canals (∼70 μm diameter) in bone, but not into hydroxyapatite crystals. S. aureus can enter into and survive in bone cells, which may explain relapses of bone infections.42−44 In addition, this bacterial pathogen adheres to components of the organic bone matrix such as collagen.43 Ciprofloxacin and other fluoroquinolones have been reported to bind to hydroxyapatite and, importantly, to be biologically active after dissociation.45 It is also important to recognize that the ability of ciprofloxacin to penetrate into various types of mammalian cells and exert antibacterial activity against intracellular S. aureus may be advantageous for the treatment of chronic osteomyelitis.46,47 While the reverse engineered PK/PD targets for S. aureus used in the PTA analysis are based on clinical and microbiological cure,20,36,37 higher targets might be required for suppression of emergence of resistance, but such targets are not available. Finally, it must be recognized that the susceptible MIC breakpoints of CLSI and from the current PTA analysis were derived based on the use of ciprofloxacin alone. However, combination antibiotic therapy is recommended for the treatment of osteomyelitis.5,15,16
Strengths and Limitations of the Study
Strengths of this study include the following: with 39 patients included it is the largest study of the PK of ciprofloxacin in bone and plasma; samples of both cortical and cancellous bone, in addition to plasma, were collected over 20 h such that the time-course of ciprofloxacin in each of these biological matrices could be described; the physiologically based population PK model developed is the first for any antibiotic to be informed by actual measurements of concentrations in bone compartments; the application of a physiologically based population PK modeling approach enabled maximum yield of information from the sparse sample collection design that was imposed by practical and ethical constraints around the elective surgical procedures; and, the use of Monte Carlo simulations and PTA analyses enabled assessment of the likely adequacy of common dosing regimens, including the maximum FDA approved dose, to achieve PK/PD targets. The major limitation of the study is that even though concentrations were measured in two types of bone, each of those in turn is a heterogeneous matrix. Unfortunately, it is not possible analytically to quantify drug concentration in the various component parts of cortical and cancellous bone. Added to this problem is that bacterial cells may not be distributed uniformly in bone. Like most studies, the current investigation measured antibiotic concentrations in noninfected bone. This enables studying a more homogeneous patient group; however, the presence of an infection might impact on antibiotic PK in bone.
Conclusion
The PK and PK/PD of antibiotics in relation to bone infections is a neglected area of research due to multiple challenges. However, the incidence of bone infections, which frequently require prolonged intravenous antibiotics and hospital stays, has been projected to increase. In the current study we have characterized the time-course of disposition of ciprofloxacin in major bone compartments and plasma. Through the use of physiologically based population PK modeling we showed that ciprofloxacin rapidly penetrates bone and subsequently achieves concentrations that are substantially higher than those observed concurrently in plasma. On the basis of the developed PK model and through the use of Monte Carlo simulations and PTA analyses it appears that the currently approved maximum daily dose of ciprofloxacin would not be sufficient for all wild-type S. aureus isolates with MICs up to the CLSI susceptible breakpoint. This reinforces the general recommendation for the use of combination antibiotic therapy for osteomyelitis. Finally, we have exemplified the power of the mathematical modeling approaches employed in this study to inform and assist optimization of the administration of antibiotics for difficult to treat infections.
Methods
Study Participants and Ethics
The study included 39 patients scheduled for orthopedic surgery. Patient characteristics are presented in Table 1. The vast majority of patients underwent hip replacement, generally due to coxarthrosis. In some patients, surgery was required due to fracture of the femoral neck, humerus, femur, tibia, or pelvic column. The study was approved by the ethics committee of the Landesärztekammer Hessen, Frankfurt am Main, Germany, and performed according to the revised version of the Declaration of Helsinki. All patients gave their written informed consent prior to enrollment and commencement of the study.
Ciprofloxacin Administration and Sample Collection
Each patient received a single dose of 400 mg of ciprofloxacin (Ciprobay 400, Bayer AG, Leverkusen, Germany) as a 1-h intravenous infusion at 0.5 to 20 h before surgery. Blood and bone samples were collected at the time of bone resection: ≥0.5 to <1 h (n = 4 patients), ≥1 to <2 h (n = 3), ≥2 to <3 h (n = 4), ≥3 to <5 h (n = 6), ≥5 to <7 h (n = 4), ≥7 to <9 h (n = 3), ≥9 to <13 h (n = 5), ≥13 to <16 h (n = 4) and ≥18 to ≤20 h (n = 6) after the start of the ciprofloxacin infusion. The exact times of ciprofloxacin administration and collection of blood and bone samples were recorded. The vast majority of bone samples were from the femoral head or neck; three were from the humerus, and one each from the femur, tibia, and pelvic column. Blood samples were centrifuged immediately at 4 °C and the plasma was harvested. Bone samples were immediately frozen on dry ice. All samples were stored at −80 °C until analysis.
Quantification of Ciprofloxacin Concentrations in Plasma and Bone
Plasma samples (100 μL) were prepared by adding 25 μL of a mixture of 80% acetonitrile and 20% perchloric acid containing the internal standard (pipemidic acid). After mixing for 30 s, the samples were centrifuged (5 min, 11 000 rpm). An aliquot (10 μL) of the supernatant was injected into the liquid chromatography (LC) system. Bone samples were separated into cortical and cancellous tissue, each of which was pulverized under liquid nitrogen by a cryogenic mill. Approximately 0.050 g of the bone powder was accurately weighed and placed into a polypropylene tube. Six times the amount (300 μL) of extraction buffer [65% Milli-Q-water, 10% 100 mM sodium dihydrogen phosphate buffer (pH 6.5), 5% methanol, 20% acetonitrile] was added, and in the next step the mixture was placed in a rotating shaker for 20 min at room temperature. After centrifugation for 5 min (3,600 rpm at 4 °C), 100 μL of the supernatant was prepared in the same way as the plasma samples and 10 μL of the final supernatant was injected into the LC system. For analysis of the bone samples, calibration standards and spiked quality control samples were prepared by adding appropriate amounts of standard solutions to ciprofloxacin-free bone tissue and prepared as described above. Chromatographic separation was performed on a Spherisorb ODS II, 5 μm, (250 mm × 4.6 mm i.d.) Grom HPLC and Analytik GmbH column, (Herrenberg-Kayh, Germany). The LC system consisted of a HPLC-Pump Merck Hitachi L-6000 A (Darmstadt, Germany), a Merck Hitachi LaChrom L-7250 autosampler (Darmstadt, Germany), and a Jasco fluorescence detector FP-920 (Groß-Umstadt, Germany). The mobile phase was 0.1 M citric acid monohydrate, 0.031 M ammonium perchlorate, 5 mM tetrabutylammonium hydrogensulfate (87%), and acetonitrile (13%) with a flow rate of 1.3 mL/min. Ciprofloxacin and the internal standard were monitored by fluorescence detection (excitation, 278 nm; emission, 460 nm). For evaluation of the calibration standards, a weighted linear regression (1/x) was performed with theoretical concentrations of calibration standards and measured peak height ratios (peak height ciprofloxacin/peak height internal standard).
No interferences were observed in plasma and bone for ciprofloxacin and the internal standard. Linearity of the ciprofloxacin calibration curves was demonstrated from 0.00231 to 10.2 mg/L in plasma and from 0.00428 to 8.87 mg/L in bone homogenate. The interday precision and accuracy of the spiked quality control standards of ciprofloxacin in human plasma (0.0100 mg/L, 0.0641 mg/L, 0.801 mg/L, and 8.01 mg/L) ranged from 1.9 to 4.8% and 99.2 to 102.7%. The interday precision and accuracy of the spiked quality control standards of ciprofloxacin in bone homogenate (0.0480 mg/L and 4.92 mg/L) ranged from 0.8 to 3.4% and 92.7 to 99.9%.
Population PK Analysis
For the population PK analysis, ciprofloxacin concentrations in bone were expressed as mg/L, using a density of 1.99 g/cm3 for cortical and 1.92 g/cm3 for cancellous bone.39 Nonlinear mixed-effects modeling was performed utilizing the S-ADAPT platform (version 1.57) with the Monte Carlo parametric expectation maximization algorithm,48 and SADAPT-TRAN for pre- and postprocessing.49,50 For model evaluation, plots of observed versus individual-fitted and observed versus population-fitted ciprofloxacin concentrations, visual predictive checks, the normalized prediction distribution error, and the objective function in S-ADAPT (−1·log-likelihood) were utilized.
A modeling approach as described by Cao and Jusko21 was employed to describe the concentration–time profiles of ciprofloxacin in plasma. Physiologically based compartments were added to the model to represent cortical and cancellous bone. The concentrations in plasma and bone from all patients were modeled simultaneously. The blood to plasma concentration ratio of ciprofloxacin in humans closely approximates unity.51,52 Thus, the concentration of ciprofloxacin measured in plasma was used as a surrogate of blood concentration. The ciprofloxacin concentrations in the blood compartment (CB) were modeled as
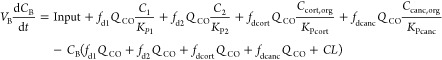 |
1 |
where VB is blood volume, QCO is cardiac output blood flow, C1 and C2 are the ciprofloxacin concentrations in (nonbone) tissue compartments 1 and 2, fd1 and fd2 are the fractions of QCO going to tissue compartments 1 and 2, and Kp1 and Kp2 are the tissue partition coefficients for these compartments. Ccort,org and Ccanc,org are the ciprofloxacin concentrations in the organic matrix of cortical and cancellous bone, fdcort and fdcanc are the fractions of QCO going to cortical and cancellous bone, Kpcort and Kpcanc are the tissue partition coefficients for the compartments representing the organic matrix in cortical and cancellous bone, and CL is the systemic clearance. The VB, QCO, fdcort, and fdcanc were fixed to physiological values (Table 2).39 The distribution clearance for cortical bone CLD,cort was defined as fdcort·QCO, and the distribution clearance for cancellous bone CLD,canc was defined as fdcanc·QCO (Table 2).
The ciprofloxacin concentrations in tissue compartment 1 (C1) were modeled as
| 2 |
where V1 is the volume of tissue compartment 1.
The ciprofloxacin concentrations in tissue compartment 2 (C2) were modeled as
| 3 |
where V2, the volume of tissue compartment 2, was a secondary parameter due to physiological restrictions, and was determined by VB + V1 + V2 + Vcort + Vcanc = TBW, with Vcort representing the cortical bone volume, Vcanc the cancellous bone volume, and TBW total body weight. This relationship was adapted from Cao and Jusko21 by including the bone volumes. Similarly, due to physiological restrictions, fd1 + fd2 + fdcort + fdcanc was ≤1, such that total blood flow was ≤ QCO.21 The Vcort and Vcanc were fixed to physiological values (Table 2).39
Ciprofloxacin in the organic cortical bone compartment (organic matrix) was modeled as
| 4 |
where forg was the fraction of organic bone matrix (fixed to the physiological value39), CLD,org,inorg described the distribution of ciprofloxacin between organic and inorganic bone matrix, Ccort,inorg represented the ciprofloxacin in the inorganic cortical bone compartment, and Kp,org,inorg was the partition coefficient between organic and inorganic bone matrix.
Ciprofloxacin in the inorganic cortical bone compartment was modeled as
| 5 |
The overall concentration of ciprofloxacin in cortical bone was described by
| 6 |
Ciprofloxacin in the organic cancellous bone compartment was modeled as
| 7 |
where Ccanc,inorg represented ciprofloxacin in the inorganic cancellous bone compartment.
The amount of ciprofloxacin in the inorganic cancellous bone compartment was modeled as
| 8 |
The overall concentration of ciprofloxacin in cancellous bone was described by
| 9 |
All the processes included in the model were necessary to describe the data and this was confirmed by leaving out one process at a time. The interindividual variability was assumed to be log-normally distributed for all parameters. The residual variability for blood, cortical, and cancellous bone was described by combined proportional and additive error models.
PK/PD Targets
Reverse-engineered PK/PD targets, which we reported previously, were used.20 These targets were based on data from published clinical studies with ciprofloxacin in osteomyelitis patients, a plasma protein binding of 25%, and susceptibility data for S. aureus which is a very common cause of bone infection.20 The targets for successful clinical or microbiological outcome were fAUCplasma/MIC of ≥15,53 ≥36,36 ≥43,37 and ≥66.54 As the targets calculated based on two of the studies investigating clinical and microbiological outcome were very similar,36,37 the average from these two studies, that is, fAUCplasma/MIC ≥ 40,20 was used for the Monte Carlo simulations. The AUC/MIC targets for bone were calculated based on the fAUCplasma/MIC targets mentioned above, the ciprofloxacin plasma protein binding of 25% and the median bone/plasma AUC ratios from the current study.
Monte Carlo Simulations
The ciprofloxacin profiles in plasma and bone were simulated for two FDA approved regimens, 400 mg administered as a 1-h intravenous infusion every 8 h or every 12 h,40 at steady state in 1000 virtual patients, and the AUCplasma, fAUCplasma, AUCcort, AUCcanc, AUCcort/AUCplasma, and AUCcanc/AUCplasma were determined from the population PK modeling. The probabilities of target attainment (PTA) were derived by calculating the fraction of subjects who attained the PK/PD target at each MIC. The PK/PD breakpoint was defined as the highest MIC for which the PTA was ≥90%.
Acknowledgments
This work was supported by an Australian National Health and Medical Research Council Ideas grant (APP1184428) to C.B.L. and R.L.N.
The authors declare no competing financial interest.
References
- Kim B. N.; Kim E. S.; Oh M. D. (2014) Oral antibiotic treatment of staphylococcal bone and joint infections in adults. J. Antimicrob. Chemother. 69, 309–22. 10.1093/jac/dkt374. [DOI] [PubMed] [Google Scholar]
- Lew D. P.; Waldvogel F. A. (2004) Osteomyelitis. Lancet 364, 369–79. 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- Masters E. A.; Trombetta R. P.; de Mesy Bentley K. L.; Boyce B. F.; Gill A. L.; Gill S. R.; Nishitani K.; Ishikawa M.; Morita Y.; Ito H.; Bello-Irizarry S. N.; Ninomiya M.; Brodell J. D. Jr.; Lee C. C.; Hao S. P.; Oh I.; Xie C.; Awad H. A.; Daiss J. L.; Owen J. R.; Kates S. L.; Schwarz E. M.; Muthukrishnan G. (2019) Evolving concepts in bone infection: redefining ″biofilm″, ″acute vs. chronic osteomyelitis″, ″the immune proteome″ and ″local antibiotic therapy″. Bone Res. 7, 20. 10.1038/s41413-019-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffulli N.; Papalia R.; Zampogna B.; Torre G.; Albo E.; Denaro V. (2016) The management of osteomyelitis in the adult. Surgeon 14, 345–360. 10.1016/j.surge.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Kavanagh N.; Ryan E. J.; Widaa A.; Sexton G.; Fennell J.; O’Rourke S.; Cahill K. C.; Kearney C. J.; O’Brien F. J.; Kerrigan S. W. (2018) Staphylococcal Osteomyelitis: Disease Progression, Treatment Challenges, and Future Directions. Clin. Microbiol. Rev. 31, e00084–17. 10.1128/CMR.00084-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S. M.; Lau E. C.; Son M. S.; Chang E. T.; Zimmerli W.; Parvizi J. (2018) Are We Winning or Losing the Battle With Periprosthetic Joint Infection: Trends in Periprosthetic Joint Infection and Mortality Risk for the Medicare Population. J. Arthroplasty 33, 3238–3245. 10.1016/j.arth.2018.05.042. [DOI] [PubMed] [Google Scholar]
- Sendi P.; Zimmerli W. (2012) Antimicrobial treatment concepts for orthopaedic device-related infection. Clin. Microbiol. Infect. 18, 1176–84. 10.1111/1469-0691.12003. [DOI] [PubMed] [Google Scholar]
- Marston H. D.; Dixon D. M.; Knisely J. M.; Palmore T. N.; Fauci A. S. (2016) Antimicrobial Resistance. JAMA 316, 1193–1204. 10.1001/jama.2016.11764. [DOI] [PubMed] [Google Scholar]
- Zimmerli W.; Sendi P. (2017) Orthopaedic biofilm infections. APMIS 125, 353–364. 10.1111/apm.12687. [DOI] [PubMed] [Google Scholar]
- Landersdorfer C. B., Bulitta J. B., and Sorgel F. (2015) Pharmacokinetics and Pharmacodynamics of Antibiotics in Bone, in Bone and Joint Infections: From Microbiology to Diagnostics and Treatment (Zimmerli W, Ed.) p 21–37, John Wiley & Sons, Chichester UK. [Google Scholar]
- Landersdorfer C. B.; Bulitta J. B.; Kinzig M.; Holzgrabe U.; Sorgel F. (2009) Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin. Pharmacokinet. 48, 89–124. 10.2165/00003088-200948020-00002. [DOI] [PubMed] [Google Scholar]
- Bogehoj M.; Emmeluth C.; Overgaard S. (2007) Blood flow and microdialysis in the human femoral head. Acta Orthop 78, 56–62. 10.1080/17453670610013420. [DOI] [PubMed] [Google Scholar]
- Traunmuller F.; Schintler M. V.; Metzler J.; Spendel S.; Mauric O.; Popovic M.; Konz K. H.; Scharnagl E.; Joukhadar C. (2010) Soft tissue and bone penetration abilities of daptomycin in diabetic patients with bacterial foot infections. J. Antimicrob. Chemother. 65, 1252–7. 10.1093/jac/dkq109. [DOI] [PubMed] [Google Scholar]
- Thabit A. K.; Fatani D. F.; Bamakhrama M. S.; Barnawi O. A.; Basudan L. O.; Alhejaili S. F. (2019) Antibiotic penetration into bone and joints: An updated review. Int. J. Infect. Dis. 81, 128–136. 10.1016/j.ijid.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Berbari E. F.; Kanj S. S.; Kowalski T. J.; Darouiche R. O.; Widmer A. F.; Schmitt S. K.; Hendershot E. F.; Holtom P. D.; Huddleston P. M. 3rd; Petermann G. W.; Osmon D. R. (2015) Infectious Diseases Society of A. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin. Infect. Dis. 61, e26–46. 10.1093/cid/civ482. [DOI] [PubMed] [Google Scholar]
- Spellberg B.; Lipsky B. A. (2012) Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin. Infect. Dis. 54, 393–407. 10.1093/cid/cir842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. A. (1998) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26, 1–10. quiz 11–2 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- Drusano G. L. (2004) Antimicrobial pharmacodynamics: critical interactions of ’bug and drug’. Nat. Rev. Microbiol. 2, 289–300. 10.1038/nrmicro862. [DOI] [PubMed] [Google Scholar]
- Landersdorfer C. B.; Kinzig M.; Bulitta J. B.; Hennig F. F.; Holzgrabe U.; Sorgel F.; Gusinde J. (2009) Bone penetration of amoxicillin and clavulanic acid evaluated by population pharmacokinetics and Monte Carlo simulation. Antimicrob. Agents Chemother. 53, 2569–78. 10.1128/AAC.01119-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landersdorfer C. B.; Kinzig M.; Hennig F. F.; Bulitta J. B.; Holzgrabe U.; Drusano G. L.; Sorgel F.; Gusinde J. (2009) Penetration of moxifloxacin into bone evaluated by Monte Carlo simulation. Antimicrob. Agents Chemother. 53, 2074–81. 10.1128/AAC.01056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.; Jusko W. J. (2012) Applications of minimal physiologically-based pharmacokinetic models. J. Pharmacokinet. Pharmacodyn. 39, 711–23. 10.1007/s10928-012-9280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M.; Peck C.; Tucker G. (2011) Physiologically-based pharmacokinetics in drug development and regulatory science. Annu. Rev. Pharmacol. Toxicol. 51, 45–73. 10.1146/annurev-pharmtox-010510-100540. [DOI] [PubMed] [Google Scholar]
- Massias L.; Buffe P.; Cohen B.; Cudennec Y.; Gehanno P.; Sterkers O.; Farinotti R. (1994) Study of the distribution of oral ciprofloxacin into the mucosa of the middle ear and the cortical bone of the mastoid process. Chemotherapy 40 (Suppl 1), 3–7. 10.1159/000239309. [DOI] [PubMed] [Google Scholar]
- Fong I. W.; Ledbetter W. H.; Vandenbroucke A. C.; Simbul M.; Rahm V. (1986) Ciprofloxacin concentrations in bone and muscle after oral dosing. Antimicrob. Agents Chemother. 29, 405–8. 10.1128/AAC.29.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone M.; Sampol-Manos E.; Santelli D.; Grabowski S.; Alliez B.; Durand A.; Lacarelle B.; Martin C. (2002) Brain tissue penetration of ciprofloxacin following a single intravenous dose. J. Antimicrob. Chemother. 50, 607–9. 10.1093/jac/dkf179. [DOI] [PubMed] [Google Scholar]
- Mould D. R.; Upton R. N. (2013) Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT: Pharmacometrics Syst. Pharmacol. 2, e38. 10.1038/psp.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. (2010) Early antimicrobial therapy in severe sepsis and septic shock. Curr. Infect Dis Rep 12, 336–44. 10.1007/s11908-010-0128-x. [DOI] [PubMed] [Google Scholar]
- Martinez M. N.; Papich M. G.; Drusano G. L. (2012) Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob. Agents Chemother. 56, 2795–805. 10.1128/AAC.05360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacha H.; Wagner D.; Schafer V.; Knothe H. (1990) Concentration of ciprofloxacin in bone tissue after single parenteral administration to patients older than 70 years. Infection 18, 173–6. 10.1007/BF01642108. [DOI] [PubMed] [Google Scholar]
- Meissner A.; Borner K. (1993) Concentration of ciprofloxacin in bone tissue. Aktuelle Traumatol 23, 80–4. [PubMed] [Google Scholar]
- Cotran R. S., Kumar V, Collins T, Robbins S. L., and Saunders W. B. (1994). Robbins pathological basis of disease, W. B. Saunders Co., Philadelphia, PA. [Google Scholar]
- Landersdorfer C. B., Bulitta J. B., and Sorgel F. (2015) Pharmacokinetics and Pharmacodynamics of Antibiotics in Bone, in Bone and Joint Infections: From Microbiology to Diagnostics and Treatment (Zimmerli W, Ed.) p 21–37, John Wiley & Sons, Chichester UK. [Google Scholar]
- Sadiq M. W.; Nielsen E. I.; Khachman D.; Conil J. M.; Georges B.; Houin G.; Laffont C. M.; Karlsson M. O.; Friberg L. E. (2017) A whole-body physiologically based pharmacokinetic (WB-PBPK) model of ciprofloxacin: a step towards predicting bacterial killing at sites of infection. J. Pharmacokinet. Pharmacodyn. 44, 69–79. 10.1007/s10928-016-9486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornik C. P.; Wu H.; Edginton A. N.; Watt K.; Cohen-Wolkowiez M.; Gonzalez D. (2017) Development of a Pediatric Physiologically-Based Pharmacokinetic Model of Clindamycin Using Opportunistic Pharmacokinetic Data. Clin. Pharmacokinet. 56, 1343–1353. 10.1007/s40262-017-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfeld B.; Metzler C. P.; Lyons M. A.; Mayeno A. N.; Brooks E. J.; Degroote M. A. (2012) A physiologically based pharmacokinetic model for capreomycin. Antimicrob. Agents Chemother. 56, 926–34. 10.1128/AAC.05180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix D. E.; Cumbo T. J.; Kuritzky P.; DeVito J. M.; Schentag J. J. (1987) Oral ciprofloxacin in the treatment of serious soft tissue and bone infections. Efficacy, safety, and pharmacokinetics. Am. J. Med. 82, 146–53. [PubMed] [Google Scholar]
- Hoogkamp-Korstanje J. A. (1987) Treatment of chronic postsurgical osteomyelitis with ciprofloxacin. Pharm. Weekbl., Sci. Ed. 9 (Suppl), S90–2. 10.1007/BF02075271. [DOI] [PubMed] [Google Scholar]
- Begg E. J.; Robson R. A.; Saunders D. A.; Graham G. G.; Buttimore R. C.; Neill A. M.; Town G. I. (2000) The pharmacokinetics of oral fleroxacin and ciprofloxacin in plasma and sputum during acute and chronic dosing. Br. J. Clin. Pharmacol. 49, 32–8. 10.1046/j.1365-2125.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. P.; Delp M. D.; Lindstedt S. L.; Rhomberg L. R.; Beliles R. P. (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 13, 407–84. 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- Anonymous . CIPRO IV(ciprofloxacin) injection, for intravenous use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019857s066lbl.pdf (202/05/13).
- CLSI. Clinical and Laboratory Standards Institute (2020) Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.: M100 Ed30th., Wayne, PA, USA. [Google Scholar]
- Hudson M. C.; Ramp W. K.; Nicholson N. C.; Williams A. S.; Nousiainen M. T. (1995) Internalization of Staphylococcus aureus by cultured osteoblasts. Microb. Pathog. 19, 409–19. 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- Jevon M.; Guo C.; Ma B.; Mordan N.; Nair S. P.; Harris M.; Henderson B.; Bentley G.; Meghji S. (1999) Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect. Immun. 67, 2677–81. 10.1128/IAI.67.5.2677-2681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A.; von Eiff C.; Kahl B. C.; Becker K.; McNamara P.; Herrmann M.; Peters G. (2006) Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4, 295–305. 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- Wittmann D. H.; Kotthaus E. (1986) Further methodological improvement in antibiotic bone concentration measurements: penetration of ofloxacin into bone and cartilage. Infection 14 (Suppl 4), S270–3. 10.1007/BF01661291. [DOI] [PubMed] [Google Scholar]
- Seral C.; Van Bambeke F.; Tulkens P. M. (2003) Quantitative analysis of gentamicin, azithromycin, telithromycin, ciprofloxacin, moxifloxacin, and oritavancin (LY333328) activities against intracellular Staphylococcus aureus in mouse J774 macrophages. Antimicrob. Agents Chemother. 47, 2283–92. 10.1128/AAC.47.7.2283-2292.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisto F.; Bonomi A.; Cavicchini L.; Cocce V.; Scaltrito M. M.; Bondiolotti G.; Alessandri G.; Parati E.; Pessina A. (2014) Human mesenchymal stromal cells can uptake and release ciprofloxacin, acquiring in vitro anti-bacterial activity. Cytotherapy 16, 181–90. 10.1016/j.jcyt.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Bauer R. J. (2011) S-ADAPT/MCPEM user’s guide (version 1.57), software for pharmacokinetic, pharmacodynamic MCPEM and population data analysis, Berkeley, CA. [Google Scholar]
- Bulitta J. B.; Bingolbali A.; Shin B. S.; Landersdorfer C. B. (2011) Development of a new pre- and post-processing tool (SADAPT-TRAN) for nonlinear mixed-effects modeling in S-ADAPT. AAPS J. 13, 201–11. 10.1208/s12248-011-9257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulitta J. B.; Landersdorfer C. B. (2011) Performance and robustness of the Monte Carlo importance sampling algorithm using parallelized S-ADAPT for basic and complex mechanistic models. AAPS J. 13, 212–26. 10.1208/s12248-011-9258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt E.; Limberg J.; Derendorf H. (1990) High-performance liquid chromatographic assay and erythrocyte partitioning of fleroxacin, a new fluoroquinolone antibiotic. J. Pharm. Biomed. Anal. 8, 67–71. 10.1016/0731-7085(90)80008-D. [DOI] [PubMed] [Google Scholar]
- Derendorf H. (1987) Erythrocyte binding of cephalosporins. J. Pharm. Pharmacol. 39, 129–31. 10.1111/j.2042-7158.1987.tb06959.x. [DOI] [PubMed] [Google Scholar]
- Gentry L. O.; Rodriguez G. G. (1990) Oral ciprofloxacin compared with parenteral antibiotics in the treatment of osteomyelitis. Antimicrob. Agents Chemother. 34, 40–3. 10.1128/AAC.34.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R. N.; Kennedy D. J.; Reilly P. M.; Luppen K. L.; Weinandt W. J.; Bollinger M. R.; Aguirre F.; Kodesch F.; Saeed A. M. (1987) Treatment of bone, joint, and soft-tissue infections with oral ciprofloxacin. Antimicrob. Agents Chemother. 31, 151–5. 10.1128/AAC.31.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]