Abstract
Fragile X syndrome (FXS) is a neurodevelopmental disorder characterized by intellectual disabilities and a plethora of neuropsychiatric symptoms. FXS is the leading monogenic cause of autism spectrum disorder (ASD), which is defined clinically by repetitive and/or restrictive patterns of behavior and social communication deficits. Epilepsy and anxiety are also common in FXS and ASD. Serotonergic neurons directly innervate and modulate the activity of neurobiological circuits altered in both disorders, providing a rationale for investigating serotonin receptors (5-HTRs) as targets for FXS and ASD drug discovery. Previously we unveiled an orally active aminotetralin, (S)-5-(2'-fluorophenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine (FPT), that exhibits partial agonist activity at 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs and that reduces repetitive behaviors and increases social approach behavior in wild-type mice. Here we report that in an Fmr1 knockout mouse model of FXS and ASD, FPT is prophylactic for audiogenic seizures. No FPT-treated mice displayed audiogenic seizures, compared to 73% of vehicle-treated mice. FPT also exhibits anxiolytic-like effects in several assays and increases social interactions in both Fmr1 knockout and wild-type mice. Furthermore, FPT increases c-Fos expression in the basolateral amygdala, which is a preclinical effect produced by anxiolytic medications. Receptor pharmacology assays show that FPT binds competitively and possesses rapid association and dissociation kinetics at 5-HT1ARs and 5-HT7Rs, yet has slow association and rapid dissociation kinetics at 5-HT2CRs. Finally, we reassessed and report FPT’s affinity and function at 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs. Collectively, these observations provide mounting support for further development of FPT as a pharmacotherapy for common neuropsychiatric symptoms in FXS and ASD.
Keywords: fragile X syndrome, autism spectrum disorder, Fmr1, serotonin receptor, anxiolytic, anticonvulsant, audiogenic seizures, sex differences
Introduction
Fragile X Syndrome (FXS) is the leading monogenic cause of intellectual disability and autism spectrum disorder (ASD), which is defined clinically by the presence of repetitive and/or restrictive patterns of behavior and social communication deficits.1 Up to 31% of individuals with FXS who are also diagnosed with ASD have epilepsy, and most exhibit other neuropsychiatric symptoms, including sensory hypersensitivities, hyperactivity, and anxiety.2−4 One study reported that an astonishing 98% of adolescents and young adults with FXS and ASD have an anxiety disorder.3
FXS is caused by hypermethylation of the FMR1 gene that ultimately prevents expression of FMRP, an RNA binding protein that regulates translation of over 800 mRNAs, including many causally linked to ASD.5,6 Despite a trove of neurobiological knowledge about FXS and ASD discovered in the last several decades, the development of safe and effective medications for FXS and ASD remains an urgent need. There are no FDA-approved drug therapies for FXS, and medications for ASD, the antipsychotics risperidone and aripiprazole, are approved only to treat irritability.7
Central inhibitory–excitatory dyshomeostasis is the predominant pathophysiological theory of FXS and ASD, based on observations, for example, of deficiencies in GABA receptors and neural circuitry.2,8−11 Serotonin (5-hydroxytryptamine, 5-HT) neurons directly innervate and modulate the activity of GABAergic and glutamatergic neurons,12−14 and selective 5-HT reuptake inhibitors (SSRIs) have treated millions of neurotypical individuals with anxiety disorders and obsessive-compulsive disorder. Several new lines of evidence suggest that targeting the 5-HT system is effective for treating neuropsychiatric symptoms in FXS and ASD.15−18 A retrospective study found that SSRIs were one of the most commonly used pharmacotherapies for psychiatric symptoms in adults with FXS,19 and a recent randomized, double-blind, placebo-controlled clinical trial showed that the SSRI sertraline improves fine motor skills and social participation in children with FXS.17 Also, a new study showed that mutating SHANK3—variants of which are causal risk factors for ASD—in a cynomolgus monkey produces core ASD phenotypes that are alleviated by the SSRI fluoxetine.20
The 5-HT system might also be targeted to treat epilepsy. Depleting 5-HT increases seizure severity,21 and sertraline or optogenetic stimulation of raphe nuclei dose-dependently prevents respiratory arrest induced by audiogenic seizures or proconvulsants, respectively, in seizure-prone mice.22,23 SSRIs, however, indiscriminately and indirectly activate all 5-HT receptors (5-HTRs); targeting individual 5-HTRs is a more precise strategy for effecting therapeutic outcomes. With few exceptions, many studies show that activating 5-HT1ARs, either systemically or via direct injections into the hippocampus, attenuates seizures induced by both GABA antagonists and glutamate agonists in wild-type animals.24−27 Also, 5-HT7R antagonism appears to prevent audiogenic seizures in mouse models.28 Two recent, though small, clinical trials reported that the selective 5-HT2CR agonist lorcaserin (Belviq) effectively treats seizures in childhood-onset epilepsies.29,30 These data provide compelling evidence that distinct 5-HTRs can modulate critical inhibitory and excitatory neural circuitry that translates behaviorally. Few clinical studies, however, have focused on 5-HT1ARs, and no studies have focused on 5-HT2CRs or 5-HT7Rs as targets for treating neuropsychiatric symptoms in FXS or ASD.
5-HT1ARs are densely expressed in the hippocampus, amygdala, and neocortex and are the predominant inhibitory 5-HTR subtype, decreasing cAMP production via activation of Gαi proteins and subsequent inhibition of adenylyl cyclases; the cAMP system is targeted by FMRP and altered in FXS and ASD.5,31 5-HT1ARs are expressed pre- and postsynaptically where they regulate cellular activity via modulation of inwardly rectifying potassium channels, which also are targets of FMRP.5 Moreover, activation of 5-HT1ARs promotes prosocial behavior, and agonists are used to treat anxiety.32,33 Recently, Chugani et al. reported that the 5-HT1AR partial agonist buspirone (Buspar) reduces repetitive behaviors in children with ASD, and several subsequent clinical studies confirmed the anxiolytic effectiveness of buspirone in ASD.34−36 Buspirone, however, has substantial off-target activity as a dopamine D2-type antagonist,37 which limits its clinical utility given well-known side effects, including sedation.38 5-HT2CRs, which directly regulate the activity of GABAergic neurons, are modestly expressed in the cortex and amygdala and are densely expressed in the striatum, a neural system that regulates social and motor behaviors.39−41 5-HT7Rs, relative to 5-HT1ARs and 5-HT2CRs, are limited in their distribution in the mammalian brain. They are expressed in midline neural structures, including the lateral septum, thalamus, and hypothalamus42 and are located on GABAergic and glutamatergic neurons43,44 where they regulate cellular activity by coupling to Gαs and Gα12 proteins,45 increasing cAMP and Rho signaling, respectively. Like the cAMP system, several molecular components of the Rho pathway are regulated by FMRP.5 Finally, 8-OH-DPAT, a 5-HT1AR agonist that decreases adenylyl cyclase activity, or LP-211, a noncompetitive antagonist of 5-HT7R activation of adenylyl cyclase and cAMP formation that appears to act as a 5-HT7R agonist in vivo,46,47 corrects mGluR-dependent long-term depression abnormalities in Fmr1 knockout mice.48
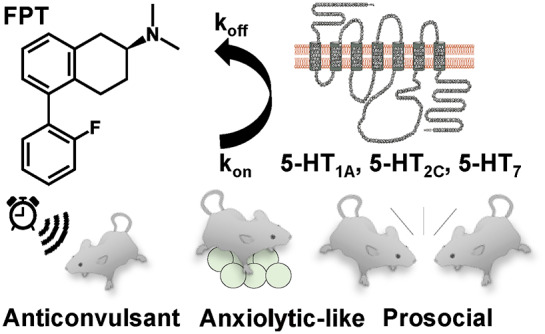
We previously reported that (S)-5-(2′-fluorophenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine (FPT), an orally active medication candidate that partially activates 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs, increases social interactions in C57BL/6J mice and eliminates idiopathic repetitive jumping in C58/J mice in a dose-dependent manner, with optimal effects at 5.6 mg/kg.49 Importantly, FPT did not alter locomotor behavior, showing its effects were not mediated by sedation. FPT, however, had not been tested in a genetic model useful for the study of FXS or ASD, such as Fmr1 knockout mice. Herein, we characterized the effects of FPT, at the 5.6 mg/kg dose, in Fmr1 knockout and wild-type mice, on several neurobehavioral measures with translational relevance for epilepsy, anxiety, repetitive behaviors, social behaviors, and spatial working memory. Mice were assessed, in novel environments, for audiogenic seizure susceptibility, social approach behavior, marble-burying behavior, grooming, rearing, spontaneous alternation performance, exploratory behavior, and locomotor activity. In addition, we evaluated the effects of FPT on expression of the immediate early gene, c-Fos, in several neural systems implicated in anxiety, social behavior, and memory, and tested the effects of FPT on c-Fos expression in the inferior colliculus—a midbrain auditory structure recently shown to be necessary for the audiogenic seizure phenotype in Fmr1 knockout mice50—after Fmr1 knockout mice were briefly exposed to a seizure-eliciting alarm. Current experiments used the more active S-enantiomer of FPT obtained via a chiral synthesis strategy, whereas our previous report used single enantiomer FPT obtained via HPLC separation of the racemate.49 Thus, we reassessed FPT’s affinity and function at 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs. Finally, we characterized FPT’s kinetic binding parameters at each of these 5-HTRs.
Results
FPT Prevented Audiogenic Seizures in Fmr1 Knockout Mice
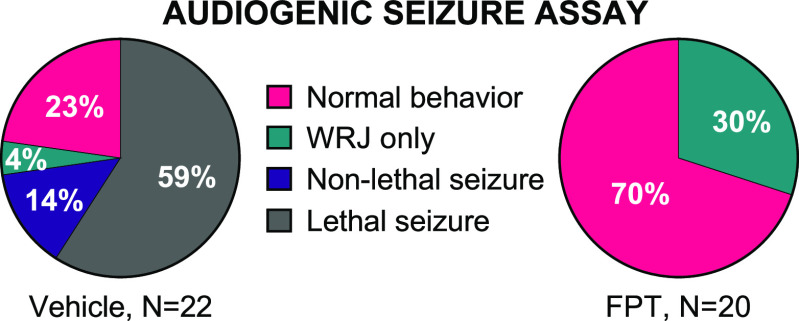
Fmr1 knockout mice are hypersensitive to acoustic stimuli, akin to sensory hypersensitivity in individuals with FXS.50 For example, Fmr1 knockout mice are prone to audiogenic seizures that are elicited by continuous, high-decibel (e.g., 120 dB), high-frequency noise. Behaviors elicited by this auditory stimulus follow a sequential pattern that begins with a startle response soon after the noise onset, followed by freezing, then wild-running and jumping (WRJ) that can progress to tonic-clonic seizures and then to respiratory arrest. The entire sequence lasts for approximately 2 min. Consistent with previous findings,51,52 we observed that juvenile (P23−P25) Fmr1 knockout mice were more susceptible to audiogenic seizures compared to adult (>P60) Fmr1 knockout mice (Fisher’s exact test, P < 0.0001). Figure 1 (left) shows data from vehicle-treated, juvenile Fmr1 knockout mice; Figure S1 shows data from vehicle-treated, adult Fmr1 knockout mice. Because juvenile Fmr1 knockout mice more readily exhibit the audiogenic seizure phenotype, they were used for testing the anticonvulsant effects of FPT. [Juvenile wild-type mice (N = 4) did not display audiogenic seizures (data not shown).] FPT prevented audiogenic seizures in juvenile Fmr1 knockout mice; effects were highly significant (Figure 1, right; Fisher’s exact test, P < 0.0001, compared to vehicle). All FPT-treated mice survived exposure to the auditory stimulus, and most showed no signs of abnormal behavior, including freezing or WRJ during the exposure. [A PDF-enriched media video showing a representative audiogenic seizure test of three vehicle-treated next to three FPT-treated juvenile Fmr1 knockout mice is available upon request.] Early in testing, we observed increased audiogenic seizure susceptibility in juvenile male compared to female Fmr1 knockout mice (Figure S2, P < 0.02); FPT’s anticonvulsant effects did not discriminate between sexes.
Figure 1.
FPT is an anticonvulsant in Fmr1 knockout mice. The majority of vehicle-treated Fmr1 knockout mice (ages P23–P25) had lethal audiogenic seizures. FPT blocked audiogenic seizures in all mice tested; results were highly significant (****, P < 0.0001, relative to vehicle). WRJ = wild running and jumping. Non-lethal seizure = recovered from tonic-clonic seizure.
FPT Increased Social Interactions in Fmr1 Knockout and Wild-Type Mice
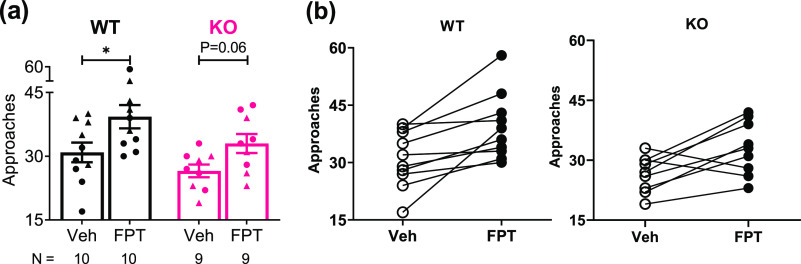
Two cage-mate mice that were the same sex, genotype, and age, one treated with vehicle and one treated with FPT, were placed in a novel open-field, and social approach behavior was scored by an observer blind to treatment. There was a significant treatment effect (F(1, 34) = 10.54, P = 0.0026) and genotype effect (F(1, 34) = 5.421; P = 0.0260) on social interactions. As shown in Figure 2a, FPT-treated wild-type mice exhibited significantly more social approaches than vehicle-treated wild-type mice (P = 0.0116, d = 1.05) with similar effects in Fmr1 knockout mice (P = 0.0596, d = 1.13). Suggestive of minor social deficits, the number of social approaches exhibited by vehicle-treated Fmr1 knockout mice was 14% less than vehicle-treated wild-type mice (P = 0.1879, d = 0.71). After FPT treatment, this difference vanished. As shown in Figure 2b, closer examination of the 10 wild-type pairs revealed that every FPT-treated mouse initiated more social interactions than the vehicle-treated mouse within each pair. Similarly, of the 9 Fmr1 knockout pairs, 7 of the FPT-treated mice initiated more social interactions than vehicle-treated mice within each pair. Notably, we also tested mice in the three-chamber social interaction test as described,53 but we did not observe a significant social preference in any group (data not shown), consistent with some other reports.54
Figure 2.
FPT increases social approach behavior. Shown are the number of social approaches in an open-field from paired wild-type (WT) or Fmr1 knockout (KO) mice, one treated with vehicle (Veh) and the other FPT. (a) Vehicle-treated KO mice tended to exhibit fewer social approaches than vehicle-treated WT mice. FPT significantly increased social interactions in WT mice, relative to vehicle, and increased social interactions in KO mice, normalizing social approaches to vehicle-treated WT levels. (b) Data from (a) unmasked to show social approach results of the individual pairs of mice. These data show that each FPT-treated wild-type mouse exhibited more social approaches than its vehicle-treated pair, and 7 of 9 FPT-treated Fmr1 knockout mice exhibited more social approaches than their vehicle-treated pairs.
FPT Decreased Marble-Burying in Fmr1 Knockout and Wild-Type Mice
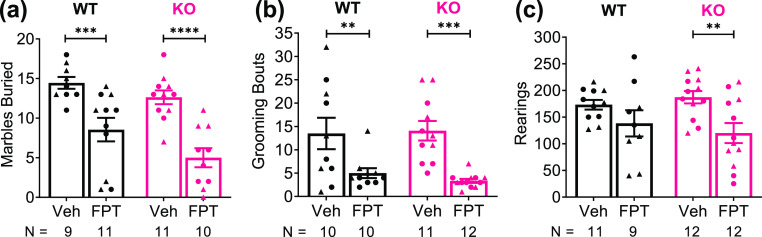
Marble-burying in mice is an ethologically relevant, repetitive behavior that has been used to model obsessive-compulsive behavior and anxiety.55,56 Several approved anxiolytic medications reduce marble-burying in mice.56 There was a significant treatment effect (F(1, 37) = 34.68, P < 0.0001) and genotype effect (F(1, 37) = 5.425, P = 0.0254) on marble-burying. As shown in Figure 3a, FPT reduced marble-burying in Fmr1 knockout and wild-type mice, and the effects were highly significant (P < 0.0001, d = 2.27, Fmr1 knockout; P = 0.001, d = 1.53, wild-type, relative to vehicle). FPT-treated Fmr1 knockout mice showed significantly less marble-burying than FPT-treated wild-type mice (P = 0.0332, d = 0.80). Picture S1 shows a representative example of the effect of FPT on marble-burying behavior in Fmr1 knockout and wild-type mice.
Figure 3.
FPT produces anxiolytic-like effects in wild-type (WT) and Fmr1 knockout (KO) mice. (a) FPT, relative to vehicle (Veh), significantly decreased marble-burying and (b) repetitive grooming in both WT and KO mice. (c) FPT significantly reduced rearing in KO mice and tended to decrease rearing in WT mice.
FPT Decreased Repetitive Grooming in Fmr1 Knockout and Wild-Type Mice
Recent studies purport that grooming in a novel environment may be indicative of translationally relevant repetitive behavior, anxiety, or obsessive-compulsive behavior.57,58 There was a significant treatment effect on grooming (F(1, 39) = 23.68, P < 0.0001). As shown in Figure 3b, FPT reduced grooming in Fmr1 knockout and in wild-type mice; the effects were large and highly significant (P = 0.0003, d = 2.14, Fmr1 knockout, and P = 0.0055, d = 1.08, wild-type, relative to vehicle). Closer examination of grooming behaviors (Figure S3) revealed a significant treatment effect (F(1, 39) = 6.496, P = 0.0149) and a near significant interaction (F(1, 39) = 3.951, P = 0.0539) in the analysis of back grooming. FPT significantly reduced back grooming in Fmr1 knockout mice (P = 0.0019, d = 1.23), but not wild-type mice (P = 0.7033). There also was a significant treatment effect on ear grooming (F(1, 39) = 8.731, P = 0.0053). FPT significantly reduced ear grooming in Fmr1 knockout mice (P = 0.007, d = 1.27). There was no significant main effect of treatment, genotype, or an interaction in the analysis of belly grooming, though there were trends for treatment (F(1, 39) = 2.425, P = 0.1275) and an interaction (F(1, 39) = 3.498, P = 0.0690). There were no main effects on the variability of nose grooming (F(1, 39) ≤ 0.8449).
FPT Decreased Repetitive Rearing in Fmr1 Knockout Mice
Rearing in a novel open-field may be indicative of a repetitive behavior.59 There was a significant treatment effect on rearing (F(1, 40) = 9.707, P = 0.0034). As shown in Figure 3c, FPT reduced rearing in Fmr1 knockout mice by 36% (P = 0.0041, d = 1.25, relative to vehicle). There was a 20% reduction in rearing in FPT-treated wild-type mice. However, the effect was medium-sized, and the difference was not statistically significant (P = 0.1579, d = 0.62, relative to vehicle).
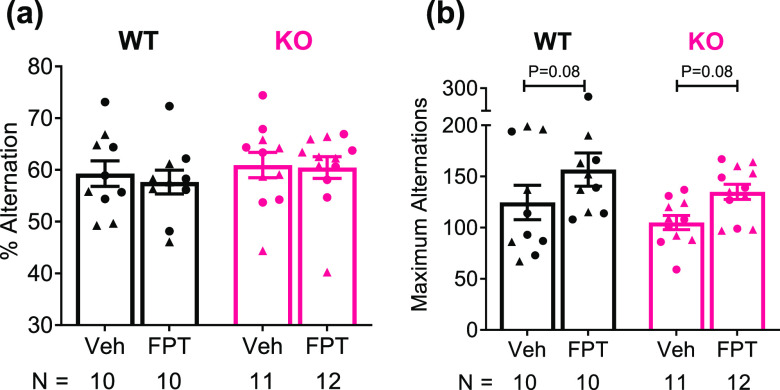
FPT Did Not Impact Spontaneous Alternation Performance but Increased Exploratory Activity in a Y-Maze
Spontaneous alternation can be used to assess hippocampus-sensitive, spatial working memory and can be used to assess perseverative behavior, in cases where animals repeatedly turn in one direction when they enter the center of the maze.60,61 We did not observe any effects on spontaneous alternation performance of the independent variables we evaluated (Figure 4a). There was, however, a significant treatment effect (F(1, 39) = 6.473, P = 0.0150) and a trend for a genotype effect (F(1, 39) = 2.882, P = 0.0975) on the maximum number of alternations. As shown in Figure 4b, compared to vehicle, FPT increased arm entries in Fmr1 knockout mice by 26%, a large effect, though results were not statistically significant (P = 0.0798, d = 1.23). FPT also increased arm entries in wild-type mice by 26%, but given the variability in the data, the effect was medium-sized, and results were not statistically significant (P = 0.0794, d = 0.61).
Figure 4.
FPT does not affect spontaneous alternation performance in a Y-maze in either wild-type (WT) or Fmr1 knockout (KO) mice, but it tends to increase exploratory behavior. (a) There were no effects of genotype or treatment on spontaneous alternation performance. (b) FPT, relative to vehicle (Veh), tended to increase the number of arm entries by WT and Fmr1 KO mice.
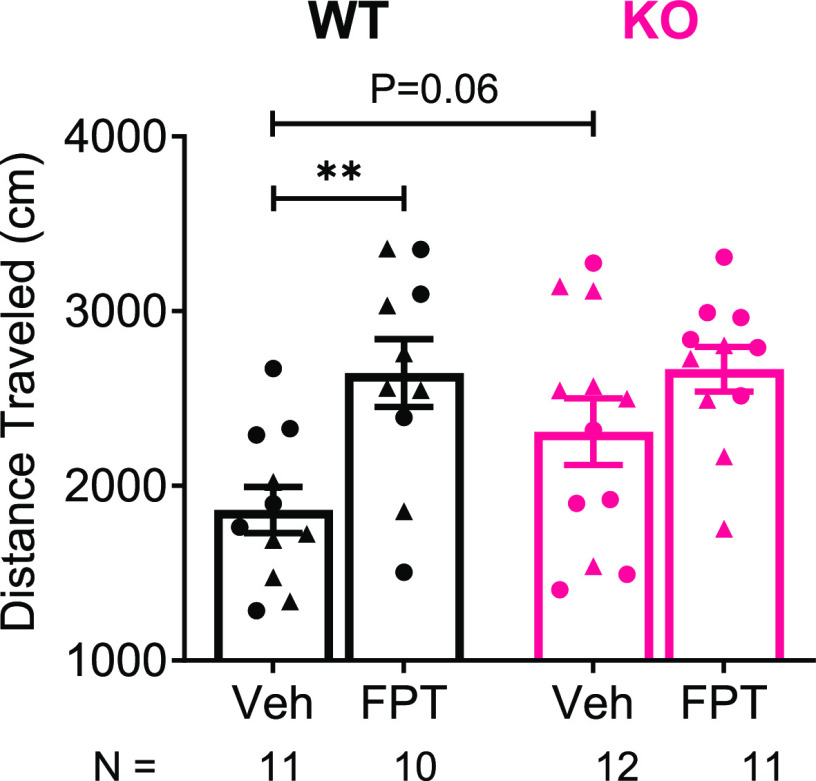
FPT Increased Locomotor Activity in Wild-Type but Not Fmr1 Knockout Mice
Like previous observations, Fmr1 knockout mice displayed greater locomotor activity relative to wild-type mice, suggestive of hyperactivity which is common in individuals with FXS;2 however, the effects were only modest. There was a significant treatment effect (F(1, 40) = 11.97, P = 0.0013) and a trend for a main effect of genotype (F(1, 40) = 2.041, P = 0.1609) on locomotor activity. As shown in Figure 5, locomotor activity was 19% higher in vehicle-treated Fmr1 knockout mice compared to vehicle-treated wild-type mice. The effect was large, and the difference was nearly significant (P = 0.0562, d = 0.80). FPT increased locomotion by ∼25% in wild-type mice (P = 0.0021, d = 1.47), and there was a nonsignificant trend of increased locomotion in Fmr1 knockout mice (P = 0.1239, d = 0.64). We previously showed that FPT did not alter locomotor activity in adult, male C57BL/6 or C58/J mice at doses up to 5.6 mg/kg,49 so its effects on locomotion likely suggest a drug by wild-type mouse strain interaction.
Figure 5.
FPT increases locomotor activity in an open-field. FPT, relative to vehicle (Veh), significantly increased locomotor behavior in wild-type (WT) mice, and there was a trend to increase locomotor activity in Fmr1 knockout (KO) mice. KO mice tended to exhibit increased locomotor activity relative to WT mice.
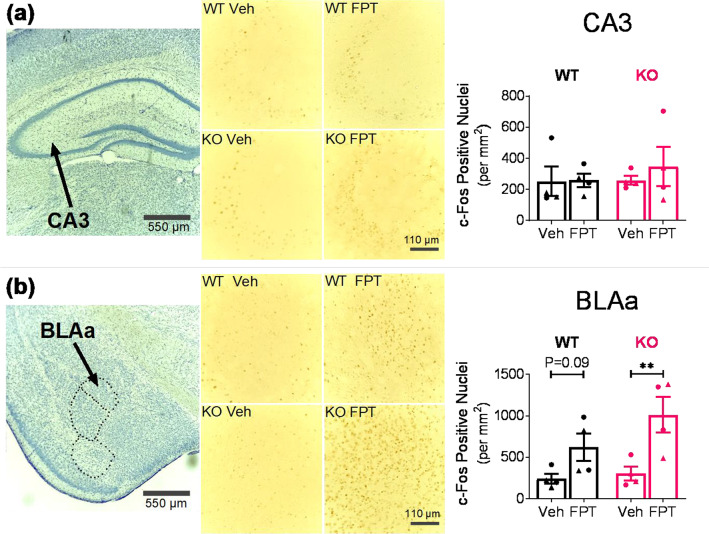
FPT Increased c-Fos Expression in the Basolateral Amygdala of Adult Fmr1 Knockout and Wild-Type Mice Resting in Their Home Cages
On the basis of the distribution of FMRP in the adult mouse brain62 and considering neural systems implicated in cognitive and neuropsychiatric symptoms (e.g., anxiety, sensory hypersensitivity, and social deficits) present in FXS and ASD,63−65 we evaluated the effects of FPT on c-Fos expression in the hippocampus (CA1, CA3, and dentate gyrus (DG)), the basolateral amygdala (ventral (BLAv), posterior (BLAp), and anterior (BLAa)), the somatosensory (SS) cortex, the hypothalamus (periventricular hypothalamic nucleus, intermediate (PVi), posterior hypothalamic nucleus (PH), and dorsomedial nucleus of the hypothalamus (DMH)), the paraventricular nucleus of the thalamus (PVT), and the ventral retrosplenial area (RSPv). Shown in Figure 6a,b are results from analyzes of c-Fos expression in CA3 and the BLAa, respectively. There were no main effects of the independent variables on c-Fos in CA3; however, there was a significant treatment effect in BLAa (F(1, 12) = 14.22, P = 0.0027). FPT increased c-Fos levels in the BLAa of Fmr1 knockout mice by 234% relative to vehicle, a statistically significant difference with a large effect (P = 0.0046, d = 2.18). In the BLAa of wild-type mice, FPT increased c-Fos expression by 157% relative to vehicle. The effect was large, but the difference was not statistically significant (P = 0.0871, d = 1.54). As shown in Table S1, FPT did not significantly alter c-Fos expression in any other brain region examined; although, in every brain region assessed in Fmr1 knockout mice, the number of c-Fos positive cells was higher after FPT treatment.
Figure 6.
Effects of FPT on c-Fos expression in the dorsal hippocampus (a) and basolateral amygdala (b) in wild-type (WT) and Fmr1 knockout (KO) mice. Top left and bottom left: Representative 4× magnification images of cresyl violet stained dorsal hippocampus and amygdala, respectively. Top middle and bottom middle: Representative 20× magnification images of DAB-stained c-Fos in CA3 and BLAa, respectively, from WT and KO mice after treatment with vehicle (Veh) or FPT. Top right: FPT did not significantly increase c-Fos expression in CA3. Bottom right: FPT significantly increased c-Fos expression in the BLAa of KO mice and tended to increase c-Fos expression in the BLAa of WT mice.
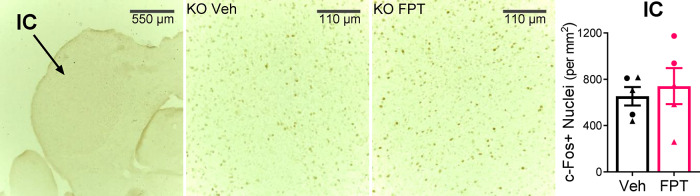
FPT Did Not Alter c-Fos Expression in the Inferior Colliculus of Juvenile Fmr1 Knockout Mice Briefly Exposed to a 120 dB Alarm
A recent study showed that absence of FMRP in the inferior colliculus is required for the audiogenic seizure phenotype in Fmr1 knockout mice.50 We examined c-Fos expression in the inferior colliculus in juvenile Fmr1 knockout mice, pretreated with vehicle or FPT, that were exposed for 30 s to a 120 dB alarm. As shown in Figure 7, there was no effect of FPT on c-Fos expression in the inferior colliculus (P = 0.63).
Figure 7.
Effects of FPT on c-Fos expression in the inferior colliculus (IC) of juvenile Fmr1 knockout mice exposed for 30 s to a 120 dB alarm. FPT, relative to vehicle (Veh), did not affect the number of c-Fos positive cells.
FPT Displayed Short Residence Time and Partial Agonist Activity with Varying Potencies and Efficacies at 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs
The Ki, EC50, and Emax values for FPT at 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs are shown in Table 1 and are similar to results we previously reported.49 Results from canonical intracellular signaling assays are shown in Figure S4. To further clarify the nature of FPT’s interaction with 5-HT7Rs, where it displays weak agonist efficacy, functional antagonism experiments were performed whereby 5-carboxamidotryptamine (5-CT), a full-efficacy 5-HT1AR and 5-HT7R agonist, was used to stimulate 5-HT7Rs in the presence or absence of varying concentrations of FPT. These studies revealed a Kb value for FPT of 27.9 ± 2.5 nM (mean ± SEM; pA2 = 7.56 ± 0.04). FPT did not alter the Emax of 5-CT, consistent with the profile of a 5-HT7R competitive antagonist (Figure S4).
Table 1. In Vitro Affinity and Functional Activity of FPT at 5-HT1ARs, 5-HT2CRs, and 5-HT7Rsa.
| receptor | Ki | EC50 | Emax |
|---|---|---|---|
| 5-HT1A | 4.0 ± 0.1b | 37.4 ± 6.8 | 84 ± 1.4 |
| 5-HT2C | 243 ± 23 | 307 ± 76 | 68.4 ± 3.6 |
| 5-HT7 | 5.3 ± 2b | 4.2 ± 2.1 | 21.8 ± 3.9 |
Affinities are reported as the equilibrium dissociation constants (Ki) in nM, determined from radioligand competition binding assays. Functional potency is expressed as the EC50 (nM) and efficacy as Emax (% normalized to the full agonists 5-CT for 5-HT1ARs, 5-HT7Rs or 5-HT for 5-HT2CRs). Data were derived from at least three independent experiments.
Data are also reported in Perry et al. 2020.96
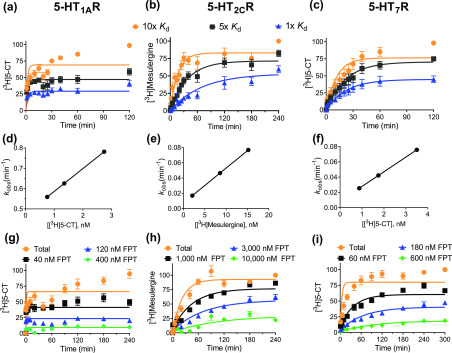
To establish the kinetic parameters for unlabeled FPT receptor binding, the association (kon) and dissociation (koff) rate constants for [3H]5-CT binding to 5-HT1ARs and 5-HT7Rs and those for [3H]mesulergine binding to 5-HT2CRs were determined (Table 2, Figure 8a–c). To the best of our knowledge, the kon and koff of [3H]5-CT binding to human 5-HT1ARs and [3H]mesulergine to human 5-HT2CRs are reported here for the first time, while the koff of [3H]5-CT at 5-HT7Rs has been reported at guinea pig66 and human receptors.67 The koff of [3H]5-CT at 5-HT7Rs reported here at 23 °C (koff = 0.007 ± 0.002 min–1) is comparable to findings by To et al. for the guinea pig homologue at 23 °C, but it is slower than that reported by Satala et al. for the human receptor at 37 °C, consistent with binding kinetics being thermosensitive processes.
Table 2. Kinetic Binding Parameters of Radioligands and Unlabeled FPT at 5-HT1ARs, 5-HT2CRs, and 5-HT7Rsa.
| receptor | compound | residence time | koff | kon | Kd |
|---|---|---|---|---|---|
| 5-HT1A | [3H]5-CT | 3 | 0.32 ± 0.06 | 1.3 × 108 ± 3.3 × 107 | 2.5 |
| FPT | 2 | 0.50 ± 0.04 | 2.8 × 107 ± 8.6 × 106 | 18 | |
| 5-HT2C | [3H]mesulergine | 143 | 0.007 ± 0.001 | 4.1 × 106 ± 2.2 × 105 | 1.7 |
| FPT | 5 | 0.186 ± 0.018 | 2.5 × 105 ± 1.9 × 104 | 732 | |
| 5-HT7 | [3H]5-CT | 143 | 0.007 ± 0.002 | 2.3 × 107 ± 2.9 × 106 | 0.3 |
| FPT | 4 | 0.26 ± 0.12 | 4.3 × 107 ± 1.8 × 107 | 6.2 |
koff (min–1) and kon (M–1·min–1) were derived from 3–4 independent experiments. Residence time was calculated as 1/koff and was rounded to the nearest minute, while the kinetically determined Kd was calculated using mean kinetic parameters and the equation Kd = koff/kon, presented in nM.
Figure 8.
Determination of radioligand and FPT kinetic binding parameters at 5-HT1ARs, 5-HT2CRs and 5-HT7Rs. (a–c) Association kinetics of three concentrations of radioligand (10×, 5×, and 1× Kd) at (a) 5-HT1ARs, (b) 5-HT2CRs, and (c) 5-HT7Rs. (d–f) Plot of the observed association rate versus radioligand concentration yields a linear relationship for 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs, indicative of ligand binding to a single site. (g–i) Competition kinetics of varying concentrations of unlabeled FPT in the presence of 5–10 nM radioligand at 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs. Data are organized in columns by receptor, and in rows by assay format. Association curves were derived from 3–5 independent experiments, normalized where the specific binding of radioligand at the latest time point is 100%, and binding at time 0 is fixed to 0%. Data reporting the kobs (kobs = (kon × [radioligand]) + koff) are presented in singlet form from an individual association binding experiment.
The kinetically derived Kd (koff/kon) of radioligands assessed here, calculated from the mean kinetic parameters, are in close agreement with those determined at equilibrium for 5-HT2CRs and 5-HT7Rs.49,68 The Kd of [3H]5-CT at 5-HT1ARs is, however, higher than most reports.69 To validate that radioligand binding followed the law of mass action, the observed association rate, kobs, was plotted as a function of radioligand concentration. The relationship between kobs and radioligand binding was linear (r2 ≥ 0.98) with the slope and y-intercept in agreement with the kon and koff, respectively, for all receptors, consistent with the law of mass action (Figure 8d–f).
Following the determination of radioligand receptor binding kinetic parameters, the kon and koff of unlabeled FPT binding to 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs (Table 2) were determined via competitive kinetic methods. There was a near significant difference in the koff of FPT at 5-HT1ARs compared to that of [3H]5-CT (P = 0.063). The koff of FPT was higher than that of the radioligands at 5-HT2CRs and 5-HT7Rs, consistent with the monotonic approach to equilibrium observed in competition kinetic assays (Figure 8h–i).70 The residence time of FPT at 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs was relatively brief. Welch’s ANOVA revealed a significant main effect (W(2.000, 3.530) = 16.60, P = 0.016), though individual comparisons revealed only a near significant difference between 5-HT1AR and 5-HT2CR residence times (P = 0.0557). The association rate of FPT at 5-HT2CRs was slower than its rates at 5-HT1ARs and 5-HT7Rs, explaining its lower affinity at 5-HT2CRs.
Discussion
This project revealed that FPT, a 5-HTR modulator, prevents audiogenic seizures in juvenile Fmr1 knockout mice. In addition to its prominent anticonvulsant activity, FPT also increases social interactions and reduces anxiety-like, repetitive behaviors with large effect sizes in adult Fmr1 knockout and wild-type mice, without reducing locomotor activity. The therapeutic-like effects of FPT observed in both Fmr1 knockout and wild-type mice show that genetic knockout of Fmr1 does not impact FPT’s pharmacodynamics. This is crucial information that validates FPT’s translational potential, as it provides evidence for normally functioning neurobiological systems (e.g., 5-HTRs) in FXS that can be targeted to treat neuropsychiatric symptoms.71 Importantly, reiterating from the Introduction, serotonergic drugs, specifically SSRIs, can treat certain neuropsychiatric symptoms in FXS and ASD (e.g., obsessive-compulsive behavior and anxiety) that are often comorbid with core symptoms [see https://www.fraxa.org/medication-reference-guide/]. The efficacy of SSRIs in FXS and ASD, however, remains contentious, and no medications are approved to treat core symptoms. New and improved medications are needed, and results from this project highlight that other serotonergic compounds may be useful for treating FXS and/or ASD.
We observed phenotypic differences between mutant and wild-type mice in three assays [audiogenic seizures (robustly), social approach behaviors (weakly), and locomotor behavior (modestly)], and FPT normalized behavior in Fmr1 knockout mice in each of them. Also, FPT’s anxiolytic-like effects in mutant and wild-type animals provide evidence that FPT may be an effective medication for anxiety in individuals with FXS and ASD and also for anxiety in neurotypical individuals. In the context of our previous observations that FPT is orally active, is brain penetrable, eliminates idiopathic, repetitive jumping in C58/J mice, and increases social interactions in C57BL/6J mice,49 our new findings provide mounting evidence to advance FPT (or compounds with similar pharmacology) to clinical trials evaluating efficacy to treat neuropsychiatric symptoms in FXS and ASD.
Our reassessment of FPT’s functional activity (EC50, Emax) at 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs is in close agreement with our initial assessment49 with minor deviations. For example, in the present studies, we observed that FPT has greater efficacy to stimulate 5-HT1ARs and somewhat less efficacy to stimulate 5-HT2CRs and 5-HT7Rs. Regarding 5-HT7Rs, we used the same stable cell line as in ref (49) and verified receptor binding site densities were relatively unchanged. We clarify that the control 5-HT7R agonist in ref (49), AS-19, is a modest partial agonist relative to the full agonist, 5-CT, used here (data not shown), which reasonably explains the small discrepancy in FPT’s 5-HT7R efficacy from our previous report. Also, here we used FPT synthesized by chiral methods to ensure enantiomeric purity. We conclude that FPT is a low-efficacy, partial agonist at 5-HT7Rs and a moderate- to high-efficacy partial agonist at 5-HT1ARs and 5-HT2CRs.
Receptor kinetics experiments indicate that FPT binds rapidly and reversibly with similar rates at 5-HT1ARs and 5-HT7Rs, and while its short residence time (<5 min) is conserved at 5-HT2CRs, the rate of association is slower, potentially limiting its action at 5-HT2CRs in vivo. However, our initial pharmacokinetics data suggest FPT likely engages central 5-HT2CRs at the 5.6 mg/kg dose.49 The kinetically determined Kds and the Kis derived from equilibrium competitive binding experiments are similar, but some disagreement exists. It is well-established that the time required for a radioligand to reach equilibrium depends on the concentration and koff of both the radioligand and the unlabeled competitor. When the radioligand dissociates slower than the competitor (k2 ≪ k4), as observed here for 5-HT2CR and 5-HT7Rs, the time to equilibrium for the IC50 of a competitive binding curve is 1.75/k2 at high concentrations of radioligand (∼4 h for [3H]mesulergine at 5-HT2CRs and [3H]5-CT at 5-HT7Rs). However, the time to equilibrium for the periphery of the curve is 3.5/k2 (∼8 h), since radioligand rarely binds at high concentrations of competitor. Therefore, it is likely that our competition assays approached, but did not fully reach, equilibrium at 90 min, explaining the similar but nonidentical affinity estimates.72 Another discrepancy arises between the equilibrium Ki, kinetic Kd, and EC50 of FPT at 5-HT1ARs. Interestingly, the association of [3H]5-CT to 5-HT1ARs resembles the profile of a ligand initially stabilizing a high affinity state of the 5-HT1AR, before quickly converting to a lower affinity state. This behavior is also observed for agonist but not antagonist binding at β-adrenergic receptors70,73 and may explain the variability in potency between assay formats.
The anticonvulsant activity of FPT in Fmr1 knockout mice is remarkable. Its triad of activity at 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs may underlie this effect, based on the following evidence. Selective 5-HT1AR agonists have been shown to prevent, reduce onset latency, and/or decrease the severity of seizures in multiple animal models.25,26 Furthermore, 5-HT1AR PET imaging is used to identify epileptic foci prior to surgical resections because of decreased 5-HT1AR expression in the medial temporal lobe of epileptic patients.74 Regarding 5-HT2CRs, 5-HT2CR knockout mice exhibit increased seizure susceptibility,75 and recent clinical trials report that lorcaserin (Belviq), a relatively selective 5-HT2CR agonist, treats seizures in children with Dravet syndrome and other severe cases of epilepsy.29,30 Finally, it has been reported that, for a series of compounds, activity to attenuate audiogenic seizures in DBA/2J mice closely correlates with affinity and antagonist activity at 5-HT7Rs.28 As is the case for most G protein-coupled receptor orthosteric ligands that are weak partial agonists, we expected that FPT may behave as an antagonist in the presence of a full agonist at 5-HT7Rs. Indeed, we confirmed this. As shown in the Supporting Information (Figure S4d), FPT competitively antagonized 5-CT activation of 5-HT7Rs coupled to stimulation of cAMP production.
Because the c-Fos gene can be activated by multiple independent signaling pathways (e.g., G protein-coupled receptors, ligand-gated Ca2+ channels, receptor tyrosine kinases), c-Fos is widely used as a marker of neuronal activation, including in Fmr1 knockout mice.76 Exposure to an auditory alarm induces c-Fos expression in the inferior colliculus, a midbrain auditory neural system.77 We therefore tested whether FPT blocks expression of c-Fos in the inferior colliculus of Fmr1 knockout mice briefly exposed to the auditory alarm used to elicit audiogenic seizures. FPT, relative to vehicle, did not alter the number of c-Fos positive cells in the inferior colliculus. Although a negative finding, it aligns with a previous report that c-Fos expression in the inferior colliculus does not reflect the auditory hypersensitivity phenotype of Fmr1 knockout mice,77 despite excitatory neurons in the inferior colliculus being necessary for audiogenic seizures in these mice.50 Thus, c-Fos expression alone in the inferior colliculus is not a viable biomarker for this phenotype.
Since serotonergic neurons project to the inferior colliculus78 and enhance GABAergic activity there,79 we might expect FPT to work in a similar way. If so, then we would not be able to distinguish between c-Fos induced by activation of excitatory pathways caused by seizure-eliciting auditory stimuli and c-Fos induced by activation of inhibitory pathways that prevent audiogenic seizures. In other words, we can only speculate that FPT might increase c-Fos expression in GABAergic neurons that cannot be distinguished, without dual-labeling, from an auditory stimulus inducing c-Fos expression in glutamatergic neurons.
We are continuing our investigation of the sex difference in audiogenic seizure susceptibility wherein juvenile male were more susceptible than juvenile female Fmr1 knockout mice. Nevertheless, FPT was equally efficacious as an anticonvulsant in both sexes. Likewise, we are investigating the developmental implications, as, regardless of sex, adult Fmr1 knockout mice were much less susceptible to audiogenic seizures.
FPT increased social interactions in Fmr1 knockout and wild-type mice in an open-field, akin to prosocial effects observed after treatment with other 5-HT1AR agonists in a different preclinical model of ASD.32 Although social withdrawal and social communication difficulties are characteristic of ASD and FXS,80 we only observed minor deficits in open-field social interactions in Fmr1 knockout mice. This could be explained by the fact that we tested social interactions of cage mates. Though, published results of social behaviors in Fmr1 knockout mice are inconsistent, with some reports showing deficits and others reporting none.81,82 Nevertheless, FPT increased social interactions in 100% of paired wild-type mice and 78% of paired Fmr1 knockout mice.
We used the open-field, social interaction test because it can also provide information about anxiety-like behavior. Exposure to a novel environment, such as the open-field, can be anxiogenic; because anxiety and social behavior are incompatible, an increase in social behavior in the open field suggests a decrease in anxiety.83 Results from the open-field social interaction test provide additional evidence that FPT produces anxiolytic effects coincident with prosocial effects. We reiterate that anxiety disorders are extremely common in individuals with FXS and ASD,3 and core ASD symptoms such as repetitive behaviors and social deficits might be causally related to severe anxiety.
The marble-burying test has translational validity for assessing a novel compound’s usefulness to reduce repetitive anxiety- or compulsive-like behaviors.55,56 The near elimination of marble-burying caused by FPT demonstrates that FPT attenuates these behaviors in both Fmr1 knockout mice and wild-type mice. Activation of 5-HT1ARs produces anxiolytic effects, as evidenced by the FDA-approved, partial agonist buspirone (Buspar), and anxiolytics that affect the serotonin system, including 5-HT1AR agonists and SSRIs, reliably reduce marble-burying.56 FPT’s anxiolytic-like effects, therefore, might be attributed to its 5-HT1AR agonist activity. Several studies report that Fmr1 knockout mice exhibit hyperlocomotion,2 which we also observed, but FPT tended to potentiate this phenotype. Thus, the efficacy of FPT to reduce marble-burying (as well as rearing and grooming) was not due to sedative effects.
FPT did not enhance or impair spontaneous alternation performance, a hippocampus-dependent spatial working memory task.84 Interestingly, the classic 5-HT1AR agonist, 8-OH-DPAT, decreases spontaneous alternation performance,61 providing evidence that FPT’s broad neurobehavioral effects may not be solely attributed to 5-HT1AR activation. The lack of a genotype effect on spontaneous alternation performance is consistent with previous observations of Fmr1 knockout mice.71,85 We did not observe an increase in grooming bouts in Fmr1 knockout relative to wild-type mice, nor did we observe excessive back grooming in Fmr1 knockout mice, reported by others.86 However, we did observe a trend of increased belly grooming in Fmr1 knockout mice. Grooming observed in a novel environment is an example of a repetitive behavior that may also be an indication of anxiety,58 and FPT robustly reduced grooming in Fmr1 knockout mice. FPT also tended to increase the maximum number of alternations in Fmr1 knockout mice, which could be indicative of increased exploratory activity or decreased neophobia.
To help delineate the neurobiological effects of FPT, we tested its effects on c-Fos expression in several neural systems—including systems implicated in anxiety, social behavior, and cognition—in mice at rest in their home cages. Of the brain structures we analyzed, we observed that FPT significantly increased c-Fos expression only in the basolateral amygdala. This effect is consistent with reports of other compounds with anxiolytic and/or prosocial effects. The antipsychotic olanzapine reduces anxiety-like behaviors in socially isolated rats while concomitantly increasing c-Fos expression in the amygdala.87 The prosocial entactogen, 5-HT releasing drug methylenedioxymethamphetamine (MDMA), increases c-Fos expression in the amygdala, an effect blocked by antagonists of 5-HT1ARs.88 Also, a selective, postsynaptic 5-HT1AR agonist dose-dependently increases c-Fos in several neural systems in the rat brain, though analysis of the amygdala was not reported.89 Thus, FPT may increase amygdala c-Fos expression through its agonist activity at 5-HT1ARs.
We acknowledge we have not ascertained the mechanism(s) of action responsible for FPT’s behavioral activities; with respect to this, FPT’s polypharmacology at known and unknown targets creates a well-understood challenge. We are actively investigating reproducible biomarker(s) in Fmr1 knockout mice impacted by FPT. In conclusion, FPT is an anticonvulsant in Fmr1 knockout mice and exhibits robust anxiolytic and prosocial effects in both Fmr1 knockout and wild-type mice. Germane for drug development, FPT does not exhibit sedative effects, distinguishing it from several other medication candidates, such as arbaclofen, that had efficacy in Fmr1 knockout mice.90 Thus, FPT is a unique medication candidate for FXS and ASD.
Methods
(S)-5-(2′-Fluorophenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine, FPT
FPT hydrochloride used in all experiments was determined by HPLC to be >99% pure (Shimadzu Lab Solutions, Figure S5). Details for the chiral synthesis of FPT are shown in Scheme S1.
Animal Subjects and Husbandry
All experimental protocols involving mice were approved by the Mercer University Institutional Animal Care and Use Committee. Breeding pairs of FVB.129P2-Pde6b+Tyrc-chFmr1tm1Cgr/J (Fmr1 knockout, stock #004624) and FVB.129P2-Pde6b+Tyrc-ch/AntJ (wild-type, stock #004828) were procured from the Jackson Laboratory to develop colonies. All mice were fed and watered ad libitum. Mice were fed Purina LabDiet 5001, except for pregnant or nursing breeders and their litters, who were fed Purina LabDiet 5015 breeder’s chow. Male and female adult (P60–P180) mice were used for all tests, except for audiogenic seizure tests, which included juvenile (P23–P25) and adult mice.
Maintaining the breeding scheme employed by the Jackson Laboratory for these mice, 1–2 homozygous Fmr1 knockout females (P60–P240) were paired with an adult, hemizygous Fmr1 knockout male for breeding Fmr1 knockout mice, and 1–2 wild-type females (P60–P240) were paired with an adult, wild-type male for breeding wild-type mice. The Jackson Laboratory implements a genetic stability program to limit cumulative genetic drift by rebuilding foundation stocks from cryopreserved, pedigreed embryos every five generations. They recommend refreshing breeding colonies every 5–10 generations to minimize genetic drift, which we employed as part of our research design.
We maintained this breeding strategy for several reasons: (1) Homozygote female and hemizygote male Fmr1 knockout mice are fertile and produce viable offspring. This reduces the number of mice, since heterozygote mice are not generated. (2) Regarding maternal effects on behavior, our objective was to maximize phenotypes in the knockout mice, relative to wild-type mice. We report here that Fmr1 knockout mice bred from homozygote knockout females and hemizygote knockout males show no differences in repetitive behaviors (grooming, rearing, or marble-burying) compared to wild-type mice; however, Fmr1 knockout mice bred in our vivarium uphold a robust audiogenic seizure phenotype and show modest hyperactivity and mild deficits in social behavior. (3) Fmr1 knockout dams build nests and care for their pups, and we have observed no differences in the physical health of Fmr1 knockout litters, including weight and coat condition, relative to wild-type mice bred from wild-type dams and sires. (4) Critically, cortical electroencephalogram phenotypes recently observed in Fmr1 knockout mice (and in individuals with FXS, e.g., enhanced gamma band power in the auditory cortex) are consistent across multiple reports regardless of whether littermate controls are used,91 providing evidence that Fmr1 knockout phenotypes are not due to maternal care effects.
Litters were weaned at P20–P22 and separated into new home cages with littermates, matched for sex and genotype, and identified by ear punch. Genotype confirmation by PCR (Applied Biosystems 2720 Thermal Cycler) was performed for quality control (data available upon request), based on methods described by the Jackson Laboratory, using a ∼3 mm tail-snip obtained from each pup at weaning. Groups of 2–4 experimental mice were housed in polycarbonate cages (6 in. × 10 in. × 5 in.) with open-air, stainless-steel wire lids and nesting sheets (Bio-Serv) on a 12 h/12 h light/dark cycle.
Treatment Approach
All tests were conducted during the light cycle (07:00–19:00) from approximately 10:00−18:00. For all tests, mice were acclimated to the procedure room in their home cages ≥30 min prior to treatment with vehicle or FPT. For in vivo testing, FPT was dissolved in Milli-Q (MilliporeSigma) water, which also served as vehicle and sterile-filtered prior to administration intraperitoneally at 5.6 mg/kg (0.1 mL/10 g), an optimal dose determined from our previous mouse studies.49 Vehicle or FPT was administered 30 min prior to the start of all behavioral testing and 90 min before euthanasia for c-Fos experiments. At least one scorer was blind to treatment and genotype during all tests. Behavioral scorers were present in the procedure rooms, which were dimly lit relative to the vivarium, only during audiogenic seizure and social approach behavior tests. The number of animals tested per group (minimum of 9, i.e., in the social approach test) was based on a power analysis (https://clincalc.com/stats/samplesize.aspx) that considered variability reported in the literature and our own observations: α was set to 0.05, and power was set at 80%. Because we observed a sex difference in audiogenic seizure susceptibility after testing ∼10 vehicle-treated Fmr1 knockout mice (5 males and 5 females), we included sex as a separate grouping variable and therefore tested 22 vehicle-treated mice in this assay (12 females and 10 males).
Audiogenic Seizures
Mice treated with vehicle and mice treated with FPT were placed in adjacent, identical, clear polycarbonate boxes (18 in. × 8 in. × 8 in.), each covered with a plastic screen. After a 1 min acclimation period, mice were exposed to 120 dB alarm (RadioShack Kit #49–1010, doorstop alarm) for 5 min, held by hand directly above the boxes. An audiogenic seizure was defined and categorized as a tonic-clonic seizure with the animal making a full recovery afterward or a tonic-clonic seizure progressing to respiratory arrest. WRJ, often observed before tonic-clonic seizures in mice, was also documented. In cases where mice did not exhibit any detectable alterations in behavioral activity, “normal behavior” was documented. Boxes were cleaned vigorously with running water and were towel dried after each test.
Social Interactions
Littermates were paired for testing such that within the pair one mouse was administered FPT and the other was administered vehicle. Pairs were placed in an open-field box (18 in. × 8 in. × 8 in.) for a 10 min observation period. During this time, scorers recorded the number of social interactions, defined as the number of approaches one mouse initiated toward the other that resulted in direct bodily contact, including nose-to-nose, nose-to-torso, and/or nose-to-tail. A tally was kept for each mouse (vehicle or FPT) within the pair such that both mice served as test mice.
Locomotion and Rearing
Mice were placed in sound-attenuated, open-field chamber (10.75 in. × 10.75 in. × 8 in.), and behaviors were recorded automatically using an infrared photobeam array (Med Associates, model ENV-510S) for 10 min.
Marble-Burying
Mice were placed individually, for 10 min, in clean boxes (6 in. × 10 in. × 5 in.) containing 18 clear marbles atop fresh home-bedding. Marbles buried (i.e., marbles ≥ 50% covered with bedding) were calculated after the test session.
Spontaneous Alternation and Grooming
Mice were placed in the center of a Y-maze (Maze Engineers, single arm dimensions 2.75 in. × 13.75 in. × 8 in.), and automatic behavioral tracking and scoring began 30 s later (Noldus, Ethovision XT 14). Spontaneous alternations, maximum alternations, and the number of grooming bouts were recorded automatically for 15 min. Back, belly, nose and ear grooming were scored from video recordings after testing.
c-Fos Immunohistochemistry
Home-Cage Test
c-Fos immunohistochemistry with free-floating, 50 μm coronal brain sections was performed based on previously described methods.92 c-Fos primary antibody (1:1000) was a mouse monoclonal IgG from Abcam ([2H2], ab208942). The secondary antibody (1:200) was a biotinylated anti-mouse IgG from Vector Laboratories (Vectastain Elite kit). Details of immunohistochemistry steps are provided in the Supporting Information. Sections were visualized on an Echo Revolve R4 (Discover Echo) brightfield microscope in upright mode at 4× (PLCN4×NA 0.1, Olympus Life Science) and 20× (UPLFLN10×2PH/NA 0.5, Olympus Life Science) magnification at 170% zoom. Images were captured using the built-in 12MP CMOS Color camera from the Echo Revolve software. The 20× magnified images (475 μm × 440 μm = 0.209 mm2) were analyzed using ImageJ software (1.52a Java; 1.8.0_112 [64-bit]). Raw images (3226 × 3024 pixel2) were converted to 8-bit, followed by appropriate thresholding to identify c-Fos positive cells. Size was defined independently for each region (range: 800–3000 pixel2), and circularity was set between 0.3 and 1.0 for all brain regions. The density of c-Fos positive cells per 0.209 mm2 was averaged across three consecutive sections for each region and each brain. c-Fos positive cells per square millimeter were calculated for each brain region of every animal and then averaged over genotype and treatment.
Auditory Stimulus Test
Juvenile Fmr1 knockout mice were acclimated in their home cages in a quiet room where they received injections 30 min prior to being moved to a testing room. In the testing room, mice were placed in clear polycarbonate boxes (18 in. × 8 in. × 8 in.), each covered with a plastic screen. After a 1 min acclimation period, mice were exposed for 30 s to a 120 dB alarm (RadioShack Kit #49–1010, doorstop alarm) held by hand directly above the box; exposure for this duration did not elicit seizures in any of the mice tested. Mice were returned to their home cages in the quiet room for 60 min and then were prepared for c-Fos immunohistochemistry exactly as described above. c-Fos was examined in 1–5 sections of the inferior colliculus (external and central nucleus) from −4.855 to −5.155 mm with respect to Bregma (online Allen Mouse Brain Reference Atlas version 1, 2008).
In Vitro Pharmacology
HEK-293 cells (ATCC, CRL-1573) stably expressing the human 5-HT7(a)R (∼7 pmol/mg protein),49 CHO-K1 cells stably expressing the human 5-HT1AR (∼1 pmol/mg protein, generously provided by the Psychoactive Drug Screening Program),93 and HEK-293 cells transiently expressing human 5-HT2C-INIRs were used for in vitro experiments. All cells were grown as described,49 and stable cells were maintained in 400–500 μg/mL G418. All commercially available test compounds were from Tocris, Sigma-Aldrich, or Alfa Aesar.
The potency (EC50) and efficacy (Emax) of FPT at 5-HT1ARs and 5-HT7Rs was assessed using PerkinElmer’s LANCE Ultra cAMP immunoassay; potency and efficacy at 5-HT2CRs were assessed with Cisbio’s IP-One assay. All functional assays were conducted according to the manufacturers’ guidelines in 384-well plates. FPT and 5-CT maleate, a 5-HT1AR and 5-HT7R full efficacy agonist (used to calculate Emax), were tested at 0.1 nM to 10 μM concentrations, in triplicate, at 37 °C for 2 h using ∼300 cells/μL for 5-HT7Rs and for 1 h using ∼300 cells/μL and 0.3 μM forskolin (EC90) for 5-HT1ARs. For 5-HT2CRs, methods were as previously reported,49 with 5-HT hydrochloride used to calculate Emax. FRET was quantified using a Synergy H1 plate reader equipped with LANCE and HTRF filter cubes (BioTek). FPT 5-HT7R functional antagonism was determined by coincubation of 5-CT over seven concentrations (0.01 nM to 10 μM) in the presence of 100, 300, and 1000 nM FPT. The affinity of FPT at 5-HT1ARs, 5-HT7Rs ([3H]5-CT, 38.9–157.8 Ci/mmol, PerkinElmer), and 5-HT2CRs ([3H]mesulergine, 74.4–83 Ci/mmol, PerkinElmer) was determined by competition binding, as previously described.49
Determination of the Kinetic Binding Parameters of [3H]5-CT at 5-HT1ARs and 5-HT7Rs and of [3H]Mesulergine at 5-HT2CRs
The kinetics of [3H]5-CT binding to 5-HT1ARs and 5-HT7Rs, as well as [3H]mesulergine binding to 5-HT2CRs, were determined using methods previously described,94 with 250 μL total volume per well in a 96-well plate. The off-rate (koff, k2) was determined at 5-HT1ARs and 5-HT7Rs using ∼ Kd concentrations of [3H]5-CT. After a 2 h equilibration period at 23 °C, 5-HT (10 μM) was applied at 22 time points (data available upon request). The on-rate (kon, k1) of [3H]5-CT binding to 5-HT1ARs and 5-HT7Rs, and [3H]mesulergine binding to 5-HT2CRs, was determined via association kinetics using three concentrations of radioligand and a global fit analysis. These experiments simultaneously provided koff values for [3H]5-CT, which were pooled with the dissociation data determined for 5-HT1ARs and 5-HT7Rs; this method was also used for calculating the koff of [3H]mesulergine at 5-HT2CRs. Binding was initiated by the addition of membrane homogenate over 10–12 time-points. Bound and free radioligand were separated by rapid filtration via Whatman GF/B filters (Brandel), presoaked in 0.3% (w/v) polyethylenimine. Filters were oven-dried, and scintillation was detected with a Tri-Carb 2910TR liquid scintillation analyzer (PerkinElmer). Data points in each experiment were at least duplicated.
Determination of Kinetic Binding Parameters of Unlabeled FPT at 5-HT1ARs, 5-HT2CRs, and 5-HT7Rs
The kon (k3) and koff (k4) of unlabeled FPT was determined at 23 °C based on published methods.94 A single concentration of radioligand [5 nM (calculated) for 5-HT1ARs; 10 nM (calculated) for 5-HT2CRs and 5-HT7Rs] was coincubated with three concentrations of FPT. Membrane homogenate was added at 11–13 time points. Nonspecific binding was determined using 10 μM 5-HT (5-HT1ARs and 5-HT7Rs) or mianserin hydrochloride (5-HT2CRs). Equations used for calculations are provided in the Supporting Information.
Statistical Analyses and Figure Configurations
In vitro binding and function data were analyzed using nonlinear regression models in GraphPad Prism 8.2. One experimental test of FPT for functional activity at 5-HT2CRs was excluded from analysis, based on a two-sided Grubb’s test, reporting that it was a significant outlier (P < 0.05, z = 1.8). Details of the analyses used for dissociation and association kinetics experiments are provided in the Supporting Information. In vitro data show the mean ± SEM. An unpaired Student’s t-test was used to compare the koff of FPT to [3H]5-CT. Welch’s ANOVA was used to compare the residence time of FPT at all receptors, since the variance across receptors was unequal; Dunnett’s T3 post hoc correction for multiple comparisons was used to compare mean residence times between receptors.
In vivo data were also analyzed using GraphPad Prism 8.2. Fisher’s exact tests were used to evaluate audiogenic seizure results. An unpaired Student’s t-test was used to compare c-Fos expression in the inferior colliculus between juvenile Fmr1 knockout mice pretreated with vehicle or FPT and then exposed to a 120 dB alarm. Data from all other experiments were analyzed using two-way ANOVAs, followed by Fisher’s LSD test (as all comparisons were planned). One subject was excluded from the locomotor behavior analysis, based on a two-sided Grubb’s test, reporting that it was a significant outlier (P < 0.05, z = 2.4). Statistical significance was set to α < 0.05; however, we also discuss data where trends (P < 0.2) were observed.95 Cohen’s d scores were manually calculated and are reported to show effect sizes; a large effect size was set to d > 0.80. In Figures 2–7, bar graphs show means ± SEM, circles represent male subjects, and triangles represent female subjects. The asterisk marks *, **, ***, and **** represent P values of <0.05, <0.01, <0.001, <0.0001, respectively.
Acknowledgments
The authors thank Yiming Chen, Hima Patel, and Jiaxing Guo for help establishing early receptor kinetics experiments, ChungYu Chen, Donna Ha, Zachary Zeisler, and Rachel Lukavsky for help with in vivo experiments, and Dr. Nader Moniri for feedback on an early draft of the manuscript.
Glossary
Abbreviations
- FXS
fragile X syndrome
- ASD
autism spectrum disorder
- FPT
((S)-5-(2′-fluorophenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine
- 5-CT
5-carboxamidotryptamine
- 5-HT
5-hydroxytryptamine
- 5-HTR
serotonin receptor
- WRJ
wild running and jumping
- CPM
counts per minute
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.9b00101.
Figure S1, audiogenic seizure prevalence in adult Fmr1 knockout mice; Figure S2, sex differences in audiogenic seizure prevalence in juvenile Fmr1 knockout mice; Figure S3, effects of FPT on distinct types of grooming behavior; Figure S4, functional activity of FPT at 5-HTRs; Table S1, complete c-Fos results; Scheme S1, new synthetic scheme for FPT; Figure S5, HPLC purity of FPT; Picture S1, representative photo of marble-burying results; detailed methods for binding kinetics and c-Fos assays (PDF)
Author Contributions
J.L.A., A.B.C., T.S.S., and C.E.C. wrote the body of the manuscript, and all authors contributed to the editing of the complete manuscript. J.L.A. managed the mouse colonies and conducted all in vivo experiments, with help from Mercer University pharmacy and undergraduate students who served as blinded scorers of mouse behaviors. J.L.A. wrote the methods for in vivo experiments. A.B.C. conducted all in vitro experiments and wrote the corresponding methods. T.S.S. performed c-Fos immunohistochemistry experiments and wrote the corresponding methods. J.L.A., A.B.C., T.S.S., and C.E.C. analyzed data and generated figures. M.M. synthesized FPT. R.G.B. supervised the synthesis of FPT and in vitro experiments. C.E.C. conceived experiments and supervised in vivo, ex vivo, and in vitro experiments. All authors have given approval to the final version of the manuscript.
This work was supported by a CDMRP/DOD Idea Development Award W81XWH-17-1-0329 (C.E.C.) and W81XWH-17-1-0322 (R.G.B.), by research start-up funds from Mercer University, College of Pharmacy (C.E.C.), by a Fellowship from FRAXA Research Organization (C.E.C.), and by USPHS (NIH/NIDA) R01-DA047130 (R.G.B.).
R.G.B. and C.E.C. are shareholders in the start-up, biopharmaceutical company, Seropeutics LLC, cofounded by R.G.B. R.G.B. is named as inventor on the patent application, US20170081273A1, which discloses the new chemical entity FPT, jointly owned by Northeastern University and University of Florida.
The authors declare no competing financial interest.
After this paper was published ASAP March 9, 2020, additional changes were made throughout. The revised version was reposted March 11, 2020.
Supplementary Material
References
- American Psychiatric Association., and American Psychiatric Association. DSM-5 Task Force . (2013) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed., American Psychiatric Association, Washington, D.C. [Google Scholar]
- Hagerman R. J.; Berry-Kravis E.; Hazlett H. C.; Bailey D. B. Jr.; Moine H.; Kooy R. F.; Tassone F.; Gantois I.; Sonenberg N.; Mandel J. L.; Hagerman P. J. (2017) Fragile X syndrome. Nat. Rev. Dis Primers 3, 17065. 10.1038/nrdp.2017.65. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. E.; Kidd S. A.; Andrews H. F.; Budimirovic D. B.; Esler A.; Haas-Givler B.; Stackhouse T.; Riley C.; Peacock G.; Sherman S. L.; Brown W. T.; Berry-Kravis E. (2017) Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics 139, S194–S206. 10.1542/peds.2016-1159F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukmanji S.; Manji S. A.; Kadhim S.; Sauro K. M.; Wirrell E. C.; Kwon C. S.; Jette N. (2019) The co-occurrence of epilepsy and autism: A systematic review. Epilepsy Behav 98, 238–248. 10.1016/j.yebeh.2019.07.037. [DOI] [PubMed] [Google Scholar]
- Darnell J. C.; Van Driesche S. J.; Zhang C.; Hung K. Y.; Mele A.; Fraser C. E.; Stone E. F.; Chen C.; Fak J. J.; Chi S. W.; Licatalosi D. D.; Richter J. D.; Darnell R. B. (2011) FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261. 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano P.; Zhou X.; Astrovskaya I.; Turner T. N.; Wang T.; Brueggeman L.; Barnard R.; Hsieh A.; Snyder L. G.; Muzny D. M.; et al. (2019) Exome sequencing of 457 autism families recruited online provides evidence for autism risk genes. NPJ. Genom Med. 4, 19. 10.1038/s41525-019-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H.; Aran A.; Berry-Kravis E. (2019) Emerging pharmacological therapies in fragile X syndrome and autism. Curr. Opin. Neurol. 32, 635–640. 10.1097/WCO.0000000000000703. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L.; Merzenich M. M. (2003) Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain Behav. 2, 255–267. 10.1034/j.1601-183X.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt G. J.; Fatemi S. H. (2011) Alterations in GABAergic biomarkers in the autism brain: research findings and clinical implications. Anat. Rec. 294, 1646–1652. 10.1002/ar.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. B.; Valakh V. (2015) Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron 87, 684–698. 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWeerdt S. (2019) The signaling imbalance theory of autism, explained. https://www.spectrumnews.org/news/signaling-imbalance-theory-autism-explained/.
- Celada P.; Puig M. V.; Artigas F. (2013) Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosci. 7, 25. 10.3389/fnint.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R. (2011) Serotonergic regulation of neuronal excitability in the prefrontal cortex. Neuropharmacology 61, 382–386. 10.1016/j.neuropharm.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciranna L. (2006) Serotonin as a Modulator of Glutamate- and GABA-Mediated Neurotransmission: Implications in Physiological Functions and in Pathology. Current Neuropharmacology 4, 101–114. 10.2174/157015906776359540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani D. C.; Muzik O.; Behen M.; Rothermel R.; Janisse J. J.; Lee J.; Chugani H. T. (1999) Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann. Neurol. 45, 287–295. . [DOI] [PubMed] [Google Scholar]
- Muller C. L.; Anacker A. M. J.; Veenstra-VanderWeele J. (2016) The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 321, 24–41. 10.1016/j.neuroscience.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiss Hess L.; Fitzpatrick S. E.; Nguyen D. V.; Chen Y.; Gaul K. N.; Schneider A.; Lemons Chitwood K.; Eldeeb M. A.; Polussa J.; Hessl D.; Rivera S.; Hagerman R. J. (2016) A Randomized, Double-Blind, Placebo-Controlled Trial of Low-Dose Sertraline in Young Children With Fragile X Syndrome. J. Dev Behav Pediatr 37, 619–628. 10.1097/DBP.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J. (2020) Why serotonin medications may yet help children with autism. https://www.spectrumnews.org/opinion/viewpoint/why-serotonin-medications-may-yet-help-children-with-autism/.
- Berry-Kravis E.; Sumis A.; Hervey C.; Mathur S. (2012) Clinic-based retrospective analysis of psychopharmacology for behavior in fragile x syndrome. Int. J. Pediatr 2012, 843016. 10.1155/2012/843016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Z.; Zhao H.; Li B.; Yan S.; Wang L.; Tang Y.; Li Z.; Bai D.; Li C.; Lin Y.; Li Y.; Liu J.; Xu H.; Guo X.; Jiang Y. H.; Zhang Y. Q.; Li X. J. (2019) CRISPR/Cas9-mediated disruption of SHANK3 in monkey leads to drug-treatable autism-like symptoms. Hum. Mol. Genet. 28, 561–571. 10.1093/hmg/ddy367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan G. F.; Murray N. M.; Hajek M. A.; Richerson G. B. (2014) Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J. Physiol. 592, 4395–4410. 10.1113/jphysiol.2014.277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupal S.; Faingold C. L. (2006) Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia 47, 21–26. 10.1111/j.1528-1167.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Zhao H.; Zeng C.; Van Dort C.; Faingold C. L.; Taylor N. E.; Solt K.; Feng H.-J. (2018) Optogenetic activation of 5-HT neurons in the dorsal raphe suppresses seizure-induced respiratory arrest and produces anticonvulsant effect in the DBA/1 mouse SUDEP model. Neurobiol. Dis. 110, 47–58. 10.1016/j.nbd.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari A.; Davoudi S. (2017) The effect of sertraline and 8-OH-DPAT on the PTZ_induced seizure threshold: Role of the nitrergic system. Seizure 45, 119–124. 10.1016/j.seizure.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Pericic D.; Lazic J.; Jazvinscak Jembrek M.; Svob Strac D. (2005) Stimulation of 5-HT 1A receptors increases the seizure threshold for picrotoxin in mice. Eur. J. Pharmacol. 527, 105–110. 10.1016/j.ejphar.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Lopez-Meraz M. L.; Gonzalez-Trujano M. E.; Neri-Bazan L.; Hong E.; Rocha L. L. (2005) 5-HT1A receptor agonists modify epileptic seizures in three experimental models in rats. Neuropharmacology 49, 367–375. 10.1016/j.neuropharm.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Gariboldi M.; Tutka P.; Samanin R.; Vezzani A. (1996) Stimulation of 5-HT1A receptors in the dorsal hippocampus and inhibition of limbic seizures induced by kainic acid in rats. Br. J. Pharmacol. 119, 813–818. 10.1111/j.1476-5381.1996.tb15745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourson A.; Kapps V.; Zwingelstein C.; Rudler A.; Boess F. G.; Sleight A. J. (1997) Correlation between 5-HT7 receptor affinity and protection against sound-induced seizures in DBA/2J mice. Naunyn-Schmiedeberg's Arch. Pharmacol. 356, 820–826. 10.1007/PL00005123. [DOI] [PubMed] [Google Scholar]
- Tolete P.; Knupp K.; Karlovich M.; DeCarlo E.; Bluvstein J.; Conway E.; Friedman D.; Dugan P.; Devinsky O. (2018) Lorcaserin therapy for severe epilepsy of childhood onset: A case series. Neurology 91, 837–839. 10.1212/WNL.0000000000006432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin A.; Hamling K. R.; Knupp K.; Hong S.; Lee L. P.; Baraban S. C. (2017) Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain 140, 669–683. 10.1093/brain/aww342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. J.; Bhattacharyya A.; Lahvis G. P.; Yin J. C.; Malter J.; Davidson R. J. (2008) The cyclic AMP phenotype of fragile X and autism. Neurosci. Biobehav. Rev. 32, 1533–1543. 10.1016/j.neubiorev.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C.; Lin H. C.; Chan Y. H.; Gean P. W.; Yang Y. K.; Chen P. S. (2013) 5-HT1A-receptor agonist modified amygdala activity and amygdala-associated social behavior in a valproate-induced rat autism model. Int. J. Neuropsychopharmacol. 16, 2027–2039. 10.1017/S1461145713000473. [DOI] [PubMed] [Google Scholar]
- Celada P.; Bortolozzi A.; Artigas F. (2013) Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs 27, 703–716. 10.1007/s40263-013-0071-0. [DOI] [PubMed] [Google Scholar]
- Coleman D. M.; Adams J. B.; Anderson A. L.; Frye R. E. (2019) Rating of the Effectiveness of 26 Psychiatric and Seizure Medications for Autism Spectrum Disorder: Results of a National Survey. J. Child Adolesc. Psychopharmacol. 29, 107–123. 10.1089/cap.2018.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceranoglu T. A.; Wozniak J.; Fried R.; Galdo M.; Hoskova B.; DeLeon Fong M.; Biederman J.; Joshi G. (2019) A Retrospective Chart Review of Buspirone for the Treatment of Anxiety in Psychiatrically Referred Youth with High-Functioning Autism Spectrum Disorder. J. Child Adolesc. Psychopharmacol. 29, 28–33. 10.1089/cap.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani D. C.; Chugani H. T.; Wiznitzer M.; Parikh S.; Evans P. A.; Hansen R. L.; Nass R.; Janisse J. J.; Dixon-Thomas P.; Behen M. (2016) Efficacy of Low-Dose Buspirone for Restricted and Repetitive Behavior in Young Children with Autism Spectrum Disorder: A Randomized Trial. J. Pediatr. 170, 45–53.e4. 10.1016/j.jpeds.2015.11.033. [DOI] [PubMed] [Google Scholar]
- Sundaram H.; Newman-Tancredi A.; Strange P. G. (1992) Pharmacological characterisation of the 5-HT1A serotonin receptor using the agonist [3H]8-OH-DPAT, and the antagonist [3H]spiperone. Biochem. Soc. Trans. 20, 145S. 10.1042/bst020145s. [DOI] [PubMed] [Google Scholar]
- Canal C. E., Booth R. G., and Williams D. A. (2020) Drugs Used to Treat Mental, Behavioral, and Cognitive Disorders, in Foye’s Principles of Medicinal Chemistry (Roche V. F., Zito S. W., Lemke T. L., and Williams D. A., Eds.) pp 313–421, Wolters Kluwer, Philadelphia, PA. [Google Scholar]
- Canal C. E.; Murnane K. S. (2017) The serotonin 5-HT2C receptor and the non-addictive nature of classic hallucinogens. J. Psychopharmacol. 31, 127–143. 10.1177/0269881116677104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoida K.; Masseck O. A.; Deneris E. S.; Herlitze S. (2014) Gq/5-HT2c receptor signals activate a local GABAergic inhibitory feedback circuit to modulate serotonergic firing and anxiety in mice. Proc. Natl. Acad. Sci. U. S. A. 111, 6479–6484. 10.1073/pnas.1321576111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo M. V. (2016) Striatal Circuits as a Common Node for Autism Pathophysiology. Front. Neurosci. 10, 27. 10.3389/fnins.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisawa T.; Ishiyama T.; Ono M.; Ishibashi T.; Taiji M. (2013) Binding of lurasidone, a novel antipsychotic, to rat 5-HT7 receptor: analysis by [3H]SB-269970 autoradiography. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 40, 132–137. 10.1016/j.pnpbp.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Neumaier J. F.; Sexton T. J.; Yracheta J.; Diaz A. M.; Brownfield M. (2001) Localization of 5-HT(7) receptors in rat brain by immunocytochemistry, in situ hybridization, and agonist stimulated cFos expression. J. Chem. Neuroanat. 21, 63–73. 10.1016/S0891-0618(00)00092-2. [DOI] [PubMed] [Google Scholar]
- Tokarski K.; Kusek M.; Hess G. (2011) 5-HT7 receptors modulate GABAergic transmission in rat hippocampal CA1 area. J. Physiol. Pharmacol. 62, 535–540. [PubMed] [Google Scholar]
- Ciranna L.; Catania M. V. (2014) 5-HT7 receptors as modulators of neuronal excitability, synaptic transmission and plasticity: physiological role and possible implications in autism spectrum disorders. Front. Cell. Neurosci. 8, 250. 10.3389/fncel.2014.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanes P.; Lacivita E.; Rodriguez J.; Brea J.; Burgueno J.; Vela J. M.; Cadavid M. I.; Loza M. I.; Leopoldo M.; Castro M. (2013) The arylpiperazine derivatives N-(4-cyanophenylmethyl)-4-(2-diphenyl)-1-piperazinehexanamide and N-benzyl-4-(2-diphenyl)-1-piperazinehexanamide exert a long-lasting inhibition of human serotonin 5-HT7 receptor binding and cAMP signaling. Pharmacol. Res. Perspect. 1, e00013. 10.1002/prp2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund P. B.; Leopoldo M.; Caccia S.; Sarkisyan G.; Fracasso C.; Martelli G.; Lacivita E.; Berardi F.; Perrone R. (2010) LP-211 is a brain penetrant selective agonist for the serotonin 5-HT7 receptor. Neurosci. Lett. 481, 12–16. 10.1016/j.neulet.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L.; Spatuzza M.; D’Antoni S.; Bonaccorso C. M.; Trovato C.; Musumeci S. A.; Leopoldo M.; Lacivita E.; Catania M. V.; Ciranna L. (2012) Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of Fragile X syndrome. Biol. Psychiatry 72, 924–933. 10.1016/j.biopsych.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Canal C. E.; Felsing D. E.; Liu Y.; Zhu W.; Wood J. T.; Perry C. K.; Vemula R.; Booth R. G. (2015) An Orally Active Phenylaminotetralin-Chemotype Serotonin 5-HT7 and 5-HT1A Receptor Partial Agonist that Corrects Motor Stereotypy in Mouse Models. ACS Chem. Neurosci. 6, 1259–1270. 10.1021/acschemneuro.5b00099. [DOI] [PubMed] [Google Scholar]
- Gonzalez D.; Tomasek M.; Hays S.; Sridhar V.; Ammanuel S.; Chang C. W.; Pawlowski K.; Huber K. M.; Gibson J. R. (2019) Audiogenic Seizures in the Fmr1 Knock-Out Mouse Are Induced by Fmr1 Deletion in Subcortical, VGlut2-Expressing Excitatory Neurons and Require Deletion in the Inferior Colliculus. J. Neurosci. 39, 9852–9863. 10.1523/JNEUROSCI.0886-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G.; Osterweil E.; Rao B. S.; Smith G. B.; Auerbach B. D.; Chattarji S.; Bear M. F. (2007) Correction of fragile X syndrome in mice. Neuron 56, 955–962. 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q. J.; Rammal M.; Tranfaglia M.; Bauchwitz R. P. (2005) Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 49, 1053–1066. 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yang M.; Silverman J. L.; Crawley J. N. (2011) Automated Three-Chambered Social Approach Task for Mice. Current Protocols in Neuroscience 56, ns0826s56. 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan S. O.; Reynolds C. D.; Smith G. D.; Holley A. J.; Escobar B.; Chandler M. A.; Volquardsen M.; Jefferson T.; Pandian A.; Smith T.; Huebschman J.; Lugo J. N. (2017) Deletion of Fmr1 results in sex-specific changes in behavior. Brain and Behavior 7, e00800. 10.1002/brb3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolmarans W.; Scheepers I. M.; Stein D. J.; Harvey B. H. (2018) Peromyscus maniculatus bairdii as a naturalistic mammalian model of obsessive-compulsive disorder: current status and future challenges. Metab. Brain Dis. 33, 443–455. 10.1007/s11011-017-0161-7. [DOI] [PubMed] [Google Scholar]
- de Brouwer G.; Fick A.; Harvey B. H.; Wolmarans W. (2019) A critical inquiry into marble-burying as a preclinical screening paradigm of relevance for anxiety and obsessive-compulsive disorder: Mapping the way forward. Cogn Affect Behav Neurosci 19, 1–39. 10.3758/s13415-018-00653-4. [DOI] [PubMed] [Google Scholar]
- Zike I.; Xu T.; Hong N.; Veenstra-VanderWeele J. (2017) Rodent models of obsessive compulsive disorder: Evaluating validity to interpret emerging neurobiology. Neuroscience 345, 256–273. 10.1016/j.neuroscience.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff A. V.; Stewart A. M.; Song C.; Berridge K. C.; Graybiel A. M.; Fentress J. C. (2016) Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 17, 45–59. 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisaoka T.; Komori T.; Kitamura T.; Morikawa Y. (2018) Abnormal behaviours relevant to neurodevelopmental disorders in Kirrel3-knockout mice. Sci. Rep. 8, 1408. 10.1038/s41598-018-19844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol D. L.; Gold P. E.; Scavuzzo C. J. (2013) Use it and boost it with physical and mental activity. Hippocampus 23, 1125–1135. 10.1002/hipo.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda N.; Joel D. (2012) Animal models of obsessive-compulsive disorder: exploring pharmacology and neural substrates. Neurosci. Biobehav. Rev. 36, 47–63. 10.1016/j.neubiorev.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Zorio D. A.; Jackson C. M.; Liu Y.; Rubel E. W.; Wang Y. (2017) Cellular distribution of the fragile X mental retardation protein in the mouse brain. J. Comp. Neurol. 525, 818–849. 10.1002/cne.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalon A.; Bruns A.; Risterucci C.; Honer M.; Ballard T. M.; Ozmen L.; Jaeschke G.; Wettstein J. G.; von Kienlin M.; Kunnecke B.; Lindemann L. (2014) Chronic metabotropic glutamate receptor 5 inhibition corrects local alterations of brain activity and improves cognitive performance in fragile X mice. Biol. Psychiatry 75, 189–197. 10.1016/j.biopsych.2013.05.038. [DOI] [PubMed] [Google Scholar]
- Reinhard S. M.; Rais M.; Afroz S.; Hanania Y.; Pendi K.; Espinoza K.; Rosenthal R.; Binder D. K.; Ethell I. M.; Razak K. A. (2019) Reduced perineuronal net expression in Fmr1 KO mice auditory cortex and amygdala is linked to impaired fear-associated memory. Neurobiol. Learn. Mem. 164, 107042. 10.1016/j.nlm.2019.107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMayo M. M.; Young L. J.; Hickie I. B.; Song Y. J. C.; Guastella A. J. (2019) Circuits for social learning: A unified model and application to Autism Spectrum Disorder. Neurosci. Biobehav. Rev. 107, 388–398. 10.1016/j.neubiorev.2019.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To Z. P.; Bonhaus D. W.; Eglen R. M.; Jakeman L. B. (1995) Characterization and distribution of putative 5-ht7 receptors in guinea-pig brain. Br. J. Pharmacol. 115, 107–116. 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satala G.; Duszynska B.; Lenda T.; Nowak G.; Bojarski A. J. (2018) Allosteric Inhibition of Serotonin 5-HT7 Receptors by Zinc Ions. Mol. Neurobiol. 55, 2897–2910. 10.1007/s12035-017-0536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Canal C. E.; Cordova-Sintjago T. C.; Zhu W.; Booth R. G. (2017) Mutagenesis Analysis Reveals Distinct Amino Acids of the Human Serotonin 5-HT2C Receptor Underlying the Pharmacology of Distinct Ligands. ACS Chem. Neurosci. 8, 28–39. 10.1021/acschemneuro.6b00124. [DOI] [PubMed] [Google Scholar]
- Dunlop J.; Zhang Y.; Smith D. L.; Schechter L. E. (1998) Characterization of 5-HT1A receptor functional coupling in cells expressing the human 5-HT1A receptor as assessed with the cytosensor microphysiometer. J. Pharmacol. Toxicol. Methods 40, 47–55. 10.1016/S1056-8719(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Motulsky H. J.; Mahan L. C. (1984) The kinetics of competitive radioligand binding predicted by the law of mass action. Mol. Pharmacol. 25, 1–9. [PubMed] [Google Scholar]
- Zieba J.; Sinclair D.; Sebree T.; Bonn-Miller M.; Gutterman D.; Siegel S.; Karl T. (2019) Cannabidiol (CBD) reduces anxiety-related behavior in mice via an FMRP-independent mechanism. Pharmacol., Biochem. Behav. 181, 93–100. 10.1016/j.pbb.2019.05.002. [DOI] [PubMed] [Google Scholar]
- Hoare S. R. J.; Fleck B. A.; Williams J. P.; Grigoriadis D. E. (2020) The importance of target binding kinetics for measuring target binding affinity in drug discovery: a case study from a CRF1 receptor antagonist program. Drug Discovery Today 25, 7–14. 10.1016/j.drudis.2019.09.011. [DOI] [PubMed] [Google Scholar]
- Insel P. A.; Mahan L. C.; Motulsky H. J.; Stoolman L. M.; Koachman A. M. (1983) Time-dependent decreases in binding affinity of agonists for beta-adrenergic receptors of intact S49 lymphoma cells. A mechanism of desensitization. J. Biol. Chem. 258, 13597–13605. [PubMed] [Google Scholar]
- Didelot A.; Ryvlin P.; Lothe A.; Merlet I.; Hammers A.; Mauguiere F. (2008) PET imaging of brain 5-HT1A receptors in the preoperative evaluation of temporal lobe epilepsy. Brain 131, 2751–2764. 10.1093/brain/awn220. [DOI] [PubMed] [Google Scholar]
- Sejourne J.; Llaneza D.; Kuti O. J.; Page D. T. (2015) Social Behavioral Deficits Coincide with the Onset of Seizure Susceptibility in Mice Lacking Serotonin Receptor 2c. PLoS One 10, e0136494. 10.1371/journal.pone.0136494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. D.; Anacker A. M. J.; Kerr T. M.; Forsberg C. G.; Wang J.; Zhang B.; Veenstra-VanderWeele J. (2017) Effects of a social stimulus on gene expression in a mouse model of fragile X syndrome. Mol. Autism 8, 30. 10.1186/s13229-017-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Toth M. (2001) Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience 103, 1043–1050. 10.1016/S0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- Klepper A.; Herbert H. (1991) Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res. 557, 190–201. 10.1016/0006-8993(91)90134-H. [DOI] [PubMed] [Google Scholar]
- Wang H.-T.; Luo B.; Huang Y.-N.; Zhou K.-Q.; Chen L. (2008) Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear. Res. 236, 42–51. 10.1016/j.heares.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Fernandez B. A.; Scherer S. W. (2017) Syndromic autism spectrum disorders: moving from a clinically defined to a molecularly defined approach. Dialogues Clin. Neurosci. 19, 353–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan S. O.; Reynolds C. D.; Smith G. D.; Holley A. J.; Escobar B.; Chandler M. A.; Volquardsen M.; Jefferson T.; Pandian A.; Smith T.; Huebschman J.; Lugo J. N. (2017) Deletion of Fmr1 results in sex-specific changes in behavior. Brain Behav 7, e00800. 10.1002/brb3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur Y. S.; Huynh L. X.; Crusio W. E. (2006) Social behavior deficits in the Fmr1 mutant mouse. Behav. Brain Res. 168, 172–175. 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- File S. E. (1988) How good is social interaction as a test of anxiety? in Selected Models of Anxiety, Depression, and Psychosis (Simon P., Soubrié P., and Widlöcher D., Eds.), pp 151–166, Karger, New York. [Google Scholar]
- Spellman T.; Rigotti M.; Ahmari S. E.; Fusi S.; Gogos J. A.; Gordon J. A. (2015) Hippocampal-prefrontal input supports spatial encoding in working memory. Nature 522, 309–314. 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q. J.; Asafo-Adjei P. K.; Arnold H. M.; Brown R. E.; Bauchwitz R. P. (2004) A phenotypic and molecular characterization of the fmr1-tm1Cgr fragile X mouse. Genes, Brain Behav. 3, 337–359. 10.1111/j.1601-183X.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Carreno-Munoz M. I.; Martins F.; Medrano M. C.; Aloisi E.; Pietropaolo S.; Dechaud C.; Subashi E.; Bony G.; Ginger M.; Moujahid A.; Frick A.; Leinekugel X. (2018) Potential Involvement of Impaired BKCa Channel Function in Sensory Defensiveness and Some Behavioral Disturbances Induced by Unfamiliar Environment in a Mouse Model of Fragile X Syndrome. Neuropsychopharmacology 43, 492. 10.1038/npp.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisavljevic A.; Peric I.; Gass P.; Inta D.; Lang U. E.; Borgwardt S.; Filipovic D. (2019) Brain Sub/Region-Specific Effects of Olanzapine on c-Fos Expression of Chronically Socially Isolated Rats. Neuroscience 396, 46–65. 10.1016/j.neuroscience.2018.11.015. [DOI] [PubMed] [Google Scholar]
- Hunt G. E.; McGregor I. S.; Cornish J. L.; Callaghan P. D. (2011) MDMA-induced c-Fos expression in oxytocin-containing neurons is blocked by pretreatment with the 5-HT-1A receptor antagonist WAY 100635. Brain Res. Bull. 86, 65–73. 10.1016/j.brainresbull.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A.; Martel J. C.; Assie M. B.; Buritova J.; Lauressergues E.; Cosi C.; Heusler P.; Bruins Slot L.; Colpaert F. C.; Vacher B.; Cussac D. (2009) Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br. J. Pharmacol. 156, 338–353. 10.1111/j.1476-5381.2008.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C.; Wijetunge L.; Kinoshita M. N.; Shumway M.; Hammond R. S.; Postma F. R.; Brynczka C.; Rush R.; Thomas A.; Paylor R.; Warren S. T.; Vanderklish P. W.; Kind P. C.; Carpenter R. L.; Bear M. F.; Healy A. M. (2012) Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABAB receptors with arbaclofen. Sci. Transl. Med. 4, 152ra128. 10.1126/scitranslmed.3004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen T. H.; Lovelace J. W.; Ethell I. M.; Binder D. K.; Razak K. A. (2019) Developmental Changes in EEG Phenotypes in a Mouse Model of Fragile X Syndrome. Neuroscience 398, 126–143. 10.1016/j.neuroscience.2018.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal C. E.; Gold P. E. (2007) Different temporal profiles of amnesia after intra-hippocampus and intra-amygdala infusions of anisomycin. Behav. Neurosci. 121, 732–741. 10.1037/0735-7044.121.4.732. [DOI] [PubMed] [Google Scholar]
- Roth B. L.; Lopez E.; Patel S.; Kroeze W. K. (2000) The Multiplicity of Serotonin Receptors: Uselessly Diverse Molecules or an Embarrassment of Riches?. Neuroscientist 6, 252–262. 10.1177/107385840000600408. [DOI] [Google Scholar]
- Sykes D. A.; Dowling M. R.; Charlton S. J. (2010) Measuring receptor target coverage: a radioligand competition binding protocol for assessing the association and dissociation rates of unlabeled compounds. Current protocols in pharmacology Chapter 9, ph0914s50. 10.1002/0471141755.ph0914s50. [DOI] [PubMed] [Google Scholar]
- Amrhein V.; Greenland S.; McShane B. (2019) Scientists rise up against statistical significance. Nature 567, 305–307. 10.1038/d41586-019-00857-9. [DOI] [PubMed] [Google Scholar]
- Perry C. K.; Casey A. B.; Felsing D. E.; Vemula R.; Zaka M.; Herrington N. B.; Cui M.; Kellogg G. E.; Canal C. E.; Booth R. G. (2020) Synthesis of novel 5-substituted-2-aminotetralin analogs: 5-HT1A and 5-HT7 G protein-coupled receptor affinity, 3D-QSAR and molecular modeling. Bioorg. Med. Chem. 28, 115262. 10.1016/j.bmc.2019.115262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.