Abstract
Coronaviruses are a group of enveloped viruses with single-stranded non-segmented positive-sense RNA genomes. In December 2019, SARS-CoV-2 appeared in China for the first time and quickly spread throughout the world. Although certain medications suggested for other afflictions tend to be potentially effective for curing the infection, there is no approved vaccination or drug available for this virus yet. Comprehension of the disease molecular pathogenesis could provide useful tools for COVID-19 patients in surveillance, prognosis, treatment, vaccine development and therapeutic targeting. The present research aims to summarize the association in COVID-19 patients between molecular dimensions of comorbidities with clinical and preclinical information.
Developing an ACE2 inhibitor could be a possible therapeutic target. Plasmin is another possible candidate both in diagnosis and treatment areas. All predicted biomarkers must be validated either through randomized clinical trials or experimental assays before clinical application in patients.
Key Words: Cancer, ardiovascular, hronic obstructive pulmonary disease, COVID-19, iabetes mellitus, ypertension
Introduction
On December 2019, a novel zoonotic COVID-19 belonging to the Orthocoronavirinae subfamily and distinct from Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) was reported first in Wuhan, China, which became a global emergence (1). COVID-19 could cause symptoms ranging from common cold to Acute Respiratory Distress Syndrome (ARDS). Despite the high transmission and mortality rate (2.1%), there is no vaccine or treatment developed yet (2). Severe conditions tend to occur in older males and patients with one or more co-morbidities such as diabetes mellitus (DM), hypertension, cerebrovascular disease, and chronic obstructive pulmonary disease (COPD) (3). The aim of the present study is to summarize the correlation between molecular aspects of comorbidities and clinical evidences in COVID-19 patients to provide resources in vaccine production and therapeutic targeting.
Chronic obstructive pulmonary disease association with COVID-19 severity
Patients with COPD are predisposed to a significant, over five-fold enhanced risk of severe COVID-19 infection (4). The connecting link between preexistence of COPD and COVID-19 severity is possibly angiotensin converting enzyme 2 (ACE2). ACE2 is an integral membrane glycoprotein that is constitutively expressed by the epithelial cells of the pneumocytes, nephrocytes, and endothelium. To regulate both the cross-species and human-to-human transmissions of SARS-CoV, the spike protein receptor-binding domain (RBD) in SARS-CoV can interact with its host receptor, ACE2. Well knowledge about SARS-CoV and its high homology with COVID-19 strongly suggest that COVID-19 uses ACE2 as its receptor to bind with RBD (particularly Gln493) to provide suitable interaction with its human host cell. Moreover, identification of other critical residues suggests that COVID-19 recruit multiple capacities (e.g. Asn501) for human-to-human transmission. It could justify the high transmission entity of COVID-19 (5). These findings provide insights into receptor usage as possible preventive measures and therapeutic targets in COPD patients infected with COVID-19. Single cell immune profiling describes interferon- MAPK signaling pathway as the major blood immune response for viral infection such as COVID-19 (1, 6, 7). High expression level of FOS and IRF27 (as MAPK transcription factors) suggest that there can be a candidate marker gene for cured COVID-19 patients (1). Another transcriptomic study reveals strong crosslink between COVID-19 pathogenesis and excessive cytokine release such as CCL2/MCP-1, CXCL10/IP-10, CCL3/MIP-1A, and CCL4/MIP1B. Furthermore, COVID-19 activates P53 signaling pathway in lymphocytes which is a possible reason for lymphopenia in COVID-19 patients (8).
A comprehensive computational analysis described that IL6, TNF, CXCL8, IFNG, CCL5, IL10, CCL2, ICAM1, CXCL1 and CXCR4 are involved in lung disease of high-risk patients of COVID-19 as top key genes and the predicted drug treatment is Plerixafor (9). All above would suggest a possible biomarker in COVID-19 management.
Diabetes mellitus in the context of COVID-19
Cellular innate immunity, the first line of defense against COVID-19, is extensively harmed in patients with uncontrolled DM (10).
ACE2 is responsible to convert angiotensin II into angiotensin 1–7 that leads to vasodilation. DM is linked to activation of renin-angiotensin-aldosterone system (RAAS) in various tissues. Moreover, patients with DM widely use ACE inhibitors (ACEi) and angiotensin receptor blockers (ARBs), which have benefit in COVID-19 alleviation (11). We can conclude that, application of ARB is speculated as a potential therapeutic target for COVID-19 (12). Both types I and II diabetes are associated with elevated plasmin(ogen) levels in plasma (13). Studies suggest that high level of Furin in plasma could lead to dysmetabolic conditions particularly DM (14). As we will discuss below, Furin level of plasma would be elevated by COVID-19 infection. We can conclude that, Furin is an important key in COVID-19 clinical severity predisposed by diabetes.
A meta-analysis investigation proposed IL, TNF, CXCL8, IL10, CCL2, ICAM1, IFNG, IL2, FN1 and CXCR4 as top key genes for the diabetic high-risk patients and introduced Plerixafor, Quinine, Pentoxifylline, and Rapamycin as repurposed drugs in DM patients infected by COVID-19 (9).
Cardiovascular and hypertension precondition COVID-19
Cardiovascular and hypertension are two other ACE2 associated diseases which adversely affect the COVID-19 prognosis (15). ACE2 cleaves angiotensin II, a peptide with multiple actions that promote cardiovascular disease. RASS blocker and ACEi are widely used in cardiovascular disease and hypertension treatment. Patients with cardiovascular disease are predisposed to more severe COVID-19 infection (16).
The top ten major genes for hypertension high-risk patients includes: VEGFA, IL6, TNF, CCL2, MMP9, ALB, IL10, PTGS2, CXCL8, CASP3 and the predicted drugs were Paclitaxel, Thalidomide, and Rapamycin. The key genes involved in heart disease high-risk group of COVID-19 were IL6, TNF, CXCL8, CCL2, MAPK1 EGFR, ICAM1, CCL5, CXCR4, AGT and the predicted drugs were Plerixafor, Afatinib, Gefitinib, Paclitaxel, and Cortisol (9).
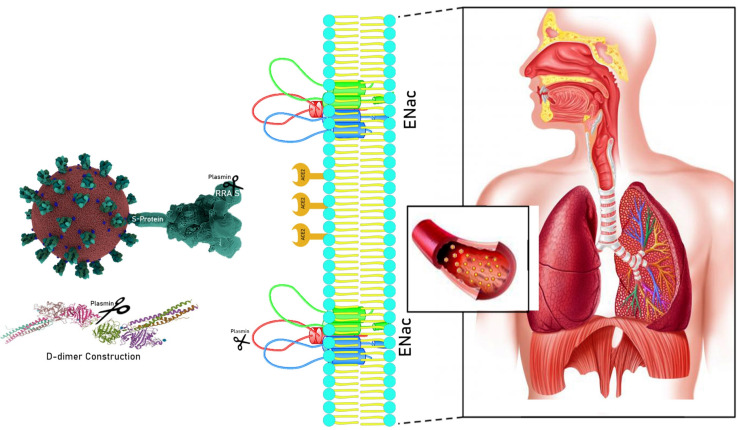
Systemic thrombosis in circulatory system and hemorrhage results in multi-organ failure in COVID-19 patients. Hyperfibrinolysis is a characteristic of COVID-19 resulting from increased level of D-dimer (as a coagulation index) (17, 18). COVID-19 uses its receptor-binding domain structure in the Spike (S) protein to attach to the host ACE2. The Furin-like cleavage site (682RRAR/S686) exclusively exists in the S1/S2 protease cleavage site of the COVID-19 virus (not in SARS-CoV). The S1 site of the Spike protein is responsible for attachment to the human host cell ACE2 receptor, whereas the S2 region is responsible for the fusion of the viral RNA to human cell membranes (19). Plasmin, and TMPRSS proteases may cleave the S-protein of coronaviruses which can increase virus entrance into the bronchial epithelial cells (20). Plasmin cleaves human γENaC subunit in trypsin, chymotrypsin, prostasin, and elastases (21). Increased level of plasmin results in hyperproduction of γENaC in the collecting tubule, which enhances salt retention, contributing to renal hypertension (22). On the other hand, γENaC proteins are located in the apical surfaces of respiratory epithelium. Since γENaC is responsible for Na+ entrance to epithelial cells and plasmin cleaves γENaC both on respiratory and vein epithelium [Figure 1]. All those events lead to dehydration of respiratory epithelium and hypertension, two major events in severe COVID-19 (23, 24). In-vitro studies demonstrated a serine protease inhibitor for plasmin and TMPRSS2 which can block S-protein of COVID-19 entry to the host cells (25). Assessment of plasmin(ogen) levels and its enzymatic activity could be appropriate biomarkers for COVID-19 severity in addition to resultant D-dimer (18). We can conclude that, elevated levels of plasmin(ogen) may be a bridge between COVID-19 severity and hypertension and ARDS predisposing disease which could be a good purpose in both prognosis and treatment area.
Figure 1.
Role of plasmin(ogen) in molecular pathogenesis of COVID-19. Plasmin break down Furin-like cleavage site on 682RRAR/S686 of COVID-19 S-glycoprotein underlying increase the ability of attachment between COVID-19 and its human host cell receptor, ACE2, result in virus attachment and fusion. Plasmin also can cleave fibrin and produce D-dimer both in bronchoalveolar lavage fluid and plasma, result in hemorrhage. γENaC is located on apical surface of alveolar epithelium and is responsible for Na+ entrance to epithelial cells. Plasmin cleaves γENaC both on respiratory epithelium and endothelium of veins results in dehydration of respiratory epithelium and hypertension
Cancer high-risk patient of COVID-19
Investigations reveal that patients suffering from cancer are more susceptible to COVID-19 infection and have a poorer prognosis due to immunosuppressive state caused by neoplasm and anticancer therapies (26). A comprehensive gene-disease association study introduces VEGFA, STAT3, IL6, TNF, MAPK3, MAPK1, CASP3, MMP9, PTGS, and EGF as important genes in cancer high-risk patients of COVID-19 and offers Gentamicin, Hydroxychloroquine, Sorafenib, Sulindac, Thalidomide, Erlotinib, and Vandetanib as drugs in this group (9).
The antiviral effects of interferons are largely mediated by the JAK–STAT signaling pathway (27). Also, Jak2 mutations describe clinical significances in myeloproliferative neoplasms and leukemia (28). Baricitinib, a tumor necrosis factor (TNF) antagonist which acts as Jak1 and Jak2 inhibitor, is widely used in the treatment of rheumatoid arthritis (29). All above, we speculate that application of Baricitinib could be a possible effective treatment in patients who are positive for Jak mutations and are infected with COVID-19 (30, 31). Approximately 80% of patients with COVID-19 can clear virus largely through interferons and remain asymptomatic or with mild symptoms. We do not recommend Baricitinib or other Jak inhibitors for this group of patients. However, in moderate to severe cases, the maximum load of virus occurs 7 days after symptom onset, and later that we can clinically observe the severe phase of the disease due to hyper inflammation which is so-called “Cytokine storm”. Cytokine storm occurs through enhanced levels of interferons α and β and IL-6, all of which are involved in JAK–STAT signaling pathway (32). These evidences suggest that, JAK–STAT pathway inhibition (such as Baricitinib), may be an appropriate treatment approach in hospitalized patients (moderate to severe cases) (33). These findings are consistent with the similar studies on SARS-CoV and MERS-CoV (34, 35).
In our present study, gene-disease association related to COVID-19 symptoms, clinical outcomes, and risk factors were studied. Since the only treatment of COVID-19 is supportive, understanding the molecular pathogenesis of the COVID-19 infections in susceptible patients could lead to achieve both diagnostic biomarkers in early stage of the disease and targeted therapeutic markers. Developing an ACE2 inhibitor could be a possible therapeutic target. Plasmin is another possible candidate both in diagnosis and treatment areas. All predicted biomarkers must be validated either through randomized clinical trials or through experimental assays before being used in patients.
Conflict of interests:
None.
Acknowledgments
This study was supported financially by Mashhad University of Medical Sciences, Mashhad, Iran (Grant# 981856).
References
- 1.Huang L, Shi Y, Gong B, Jiang L, Liu X, Yang J, et al. Blood single cell immune profiling reveals the interferon-MAPK pathway mediated adaptive immune response for COVID-19. medRxiv. 2020 [Google Scholar]
- 2.Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020;395(10223):514–23. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tetro JA. Is COVID-19 receiving ADE from other coronaviruses? Microbes and Infection. 2020;22(2):72–3. doi: 10.1016/j.micinf.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respiratory Medicine. 2020 doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. Journal of virology. 2020;94:7. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unterholzner L. The interferon response to intracellular DNA: why so many receptors? Immunobiology. 2013;218(11):1312–21. doi: 10.1016/j.imbio.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Kroczynska B, Mehrotra S, Arslan AD, Kaur S, Platanias LC. Regulation of interferon-dependent mRNA translation of target genes. Journal of Interferon & Cytokine Research. 2014;34(4):289–96. doi: 10.1089/jir.2013.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerging Microbes & Infections. 2020;9(1):761–70. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar S. COVID-19: A drug repurposing and biomarker identification by using comprehensive gene-disease associations through protein-protein interaction network analysis. 2020 [Google Scholar]
- 10.Ma RCW, Holt RIG. COVID-19 and diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2020 Apr ; doi: 10.1111/dme.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet Respiratory Medicine. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Medicine. 2020:1–5. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray EC, Miller RG, Demko JE, Costacou T, Kinlough CL, Demko CL, et al. Urinary plasmin (ogen) as a prognostic factor for hypertension. Kidney international reports. 2018;3(6):1434–42. doi: 10.1016/j.ekir.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez C, Rysä J, Almgren P, Nilsson J, Engström G, Orho-Melander M, et al. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. Journal of internal medicine. 2018;284(4):377–87. doi: 10.1111/joim.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Hu W, Ling J, Mo P, Zhang Y, Jiang Q, et al. Hypertension and Diabetes Delay the Viral Clearance in COVID-19 Patients. medRxiv. 2020 [Google Scholar]
- 16.South AM, Diz D, Chappell MC. COVID-19, ACE2 and the Cardiovascular Consequences. American Physiological Society Rockville, MD. 2020 doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji H-L, Zhao R, Matalon S, Matthay MA. Elevated plasmin (ogen) as a common risk factor for COVID-19 susceptibility. Physiological Reviews. 2020 doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutard B, Valle C, de Lamballerie X, Canard B, Seidah N, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Research. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kam Y-W, Okumura Y, Kido H, Ng LF, Bruzzone R, Altmeyer R. Cleavage of the SARS coronavirus spike glycoprotein by airway proteases enhances virus entry into human bronchial epithelial cells in vitro. PloS one. 2009;4(11) doi: 10.1371/journal.pone.0007870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao R, Ali G, Nie HG, Chang Y, Bhattarai D, Su X, et al. Plasmin improves oedematous blood-gas barrier by cleaving epithelial sodium channels. British Journal of Pharmacology. 2020 doi: 10.1111/bph.15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oxlund CS, Buhl KB, Jacobsen IA, Hansen MR, Gram J, Henriksen JE, et al. Amiloride lowers blood pressure and attenuates urine plasminogen activation in patients with treatment–resistant hypertension. Journal of the American Society of Hypertension. 2014;8(12):872–81. doi: 10.1016/j.jash.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Kleyman TR, Kashlan OB, Hughey RP. Epithelial Na+ channel regulation by extracellular and intracellular factors. Annual review of physiology. 2018;80:263–81. doi: 10.1146/annurev-physiol-021317-121143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. The Lancet Respiratory Medicine. 2020 doi: 10.1016/S2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. The Lancet Oncology. 2020;21(3):335–7. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Bona D, Cippitelli M, Fionda C, Cammà C, Licata A, Santoni A, et al. Oxidative stress inhibits IFN-α-induced antiviral gene expression by blocking the JAK–STAT pathway. Journal of hepatology. 2006;45(2):271–9. doi: 10.1016/j.jhep.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 28.Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24(6):1128. doi: 10.1038/leu.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genovese MC, Kremer J, Zamani O, Ludivico C, Krogulec M, Xie L, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243–52. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- 30.Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. COVID-19: combining antiviral and anti-inflammatory treatments. The Lancet Infectious Diseases. 2020 doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Favalli EG, Biggioggero M, Maioli G, Caporali R. Baricitinib for COVID-19: a suitable treatment? The Lancet Infectious Diseases. 2020 doi: 10.1016/S1473-3099(20)30262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. The Lancet. 2020;395(10223):e30–e1. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cameron MJ, Ran L, Xu L, Danesh A, Bermejo-Martin JF, Cameron CM, et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. Journal of virology. 2007;81(16):8692–706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Channappanavar R, Fehr AR, Zheng J, Wohlford-Lenane C, Abrahante JE, Mack M, et al. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. The Journal of clinical investigation. 2019;129(9) doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]