Abstract
To reduce the risk of spread of the novel coronavirus (COVID-19), the emerging protocols are advising for less physician-patient contact, shortening the contact time, and keeping a safe distance. It is recommended that unnecessary casting be avoided in the events that alternative methods can be applied such as in stable ankle fractures, and hindfoot/midfoot/forefoot injuries. Fiberglass casts are suboptimal because they require a follow up for cast removal while a conventional plaster cast is amenable to self-removal by submerging in water and cutting the cotton bandages with scissors. At present, only fiberglass casts are widely available to allow waterproof casting. To reduce the contact time during casting, a custom-made 3D printed casts/splints can be ordered remotely which reduces the number of visits and shortens the contact time while it allows for self-removal by the patient. The cast is printed after the limb is 3D scanned in 5-10 seconds using the commercially available 3D scanners. In contrast to the conventional casting, a 3D printed cast/splint is washable which is an advantage during an infectious crisis such as the COVID-19 pandemic.
Key Words: 3D Printing, Coronavirus, COVID-19, Orthopaedic cast, Orthopaedic splint
Introduction
To reduce the risk of spread of the novel coronavirus (COVID-19), the emerging protocols are advising for less physician-patient contact, shortening the contact time, and keeping a safe distance. It is recommended that unnecessary casting be avoided in the events that alternative methods can be applied such as in stable ankle fractures, and hindfoot/midfoot/forefoot injuries (1). Fiberglass casts are suboptimal because they require a follow up for cast removal while a conventional plaster cast is amenable to self-removal by submerging in water and cutting the cotton bandages with scissors. At present, only fiberglass casts are widely available to allow waterproof casting.
To reduce the contact time during casting, a custom-made 3D printed casts/splints can be ordered remotely which reduces the number of visits and shortens the contact time while it allows for self-removal by the patient. The cast is printed after the limb is 3D scanned in 5-10 seconds using the commercially available 3D scanners. In contrast to the conventional casting, a 3D printed cast/splint is washable which is an advantage during an infectious crisis such as the COVID-19 pandemic (2).
Recently, patient-specific casts and splints have been created using three-dimensional (3D) printing technologies (3). Materials used for this purpose with 3D printing include polyether ether ketone (PEEK) and its powder, polyethylene terephthalate glycol-modified (PETG), nylon 680, acrylonitrile butadiene styrene (ABS), polylactic acid (PLA) and carbon fiber infused polylactic acid (PLA) and related materials among others. Many of these materials have been approved for use by the Food and Drug Administration (FDA USA).
Multiple studies demonstrated that additive manufacturing and 3D printed casts and splints provide better comfort for the wearer by allowing better cleanliness, less skin problems, and improved function (2, 3). Given that, we investigated and compared the new 3D materials to the conventional ones used in casting of a limb, and elucidate whether the physical properties of these offer similar support and protection for patients [Figure 1-3].
Figure 1.
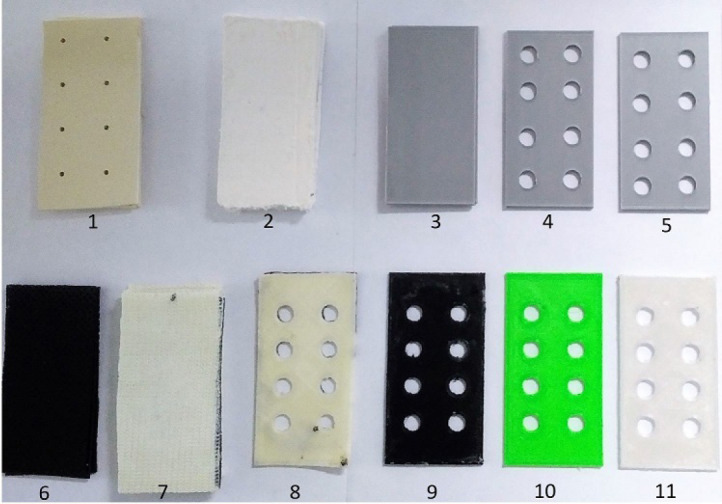
This is a general view of the tested materials. 1.Thermoplastic, 2.Plaster of Paris, 3.Silver PLA (without lattice), 4.Silver PLA (11% Lattice), 5.Silver PLA (11% Lattice), 6.lightweight Thermoplastic, 7.Fiberglass, 8.ABS, 9.PETG, 10.PLA, 11.Polycarbonate
Fiberglass, plaster and thermoplastic materials are approved by the FDA as Class I orthopedic devices. Considering these materials as the gold standard for casts and splints, we tested the non-inferiority of the 3D printing materials to see if their properties fall in the accepted range. A premarket notification application and FDA clearance is not required before marketing the device in the U.S if the device falls into a generic category of exempted class I device (4).
Our study showed that new materials used in 3D fabrication for casts and splints offer adequate physical properties with presumably better durability and safety profile that can alternatively be used for orthoses [Figure 4-6]. Among these, PLA had more than twice flexural and shear strength in compare to the other materials and even three times more the conventional cast materials [Table 1-3]. PLA is a polymerized structure from natural resources, which is environmental-friendly and biodegradable. Conventional cast materials specifically thermoplastics are polymerized from non-renewable petroleum reserves with unfavorable biodegradability index. Mechanical properties of synthetic polymers from natural monomers have improved in the last decade (5). Beside the superiority in ultimate strength, and elastic/plastic properties of the 3D printing materials, the cost was comparable between the conventional and 3D printing materials [Table 4].
Figure 4.
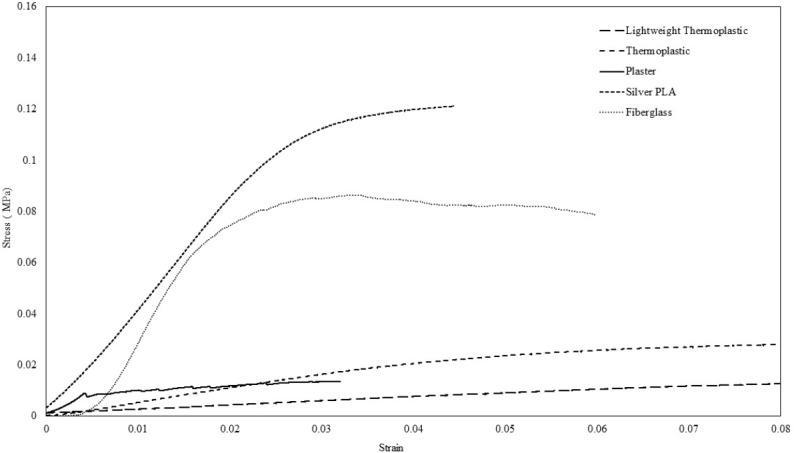
Strain/Stress is plotted for the following materials after the thickness was standardized to 3mm: Fiberglass, Plaster, Silver PLA infill 100% without any lattice, Thermoplastics and lightweight thermoplastics
Figure 6.
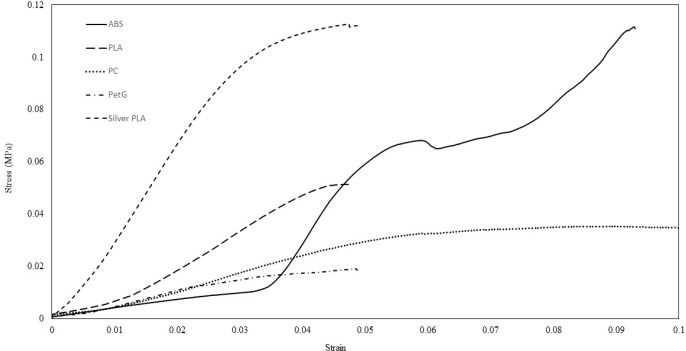
Strain/Stress is plotted for the 3D printing material with 80% infill and 11% lattice
Table 1.
Comparing test result mechanical properties
| MATERIAL | LATTICE | INFILL SHAPE | INFILL (%) | PEAK FORCE (N) | SHEAR STRENGTH (Kpa) |
|---|---|---|---|---|---|
| ABS | None | Square | 20 | 320 | 55.4 |
| ABS | 6.50% | Square | 60 | 257 | 44.5 |
| ABS | 11% | Honeycomb | 80 | 363 | 62.8 |
| PLA | None | Honeycomb | 20 | 370 | 64 |
| PLA | 6.50% | Honeycomb | 60 | 351 | 60.7 |
| PLA | 11 % - holes in mid | Honeycomb | 80 | 210 | 36.8 |
| PLA | 11% | Honeycomb | 80 | 280 | 48.4 |
| PC | 11% | Honeycomb | 80 | 192 | 33.2 |
| PetG | 11% | Honeycomb | 80 | 104 | 18 |
| Fiberglass | None | N/A | N/A | 942 | 84.4 |
| Fiberglass | None | N/A | N/A | 795 | 69.7 |
| Fiberglass | None | N/A | N/A | 1030 | 90.3 |
| Plaster | None | N/A | N/A | 111 | 12.8 |
| Plaster | None | N/A | N/A | 104 | 12.13 |
| Plaster | None | N/A | N/A | 103 | 12 |
| Thermoplastic | 11% | N/A | N/A | 174 | 30.1 |
| Thermoplastic | 11% | N/A | N/A | 170 | 29.8 |
| Thermoplastic | 11% | N/A | N/A | 180 | 31.5 |
| Silver PLA | None | Square | 100 | 661 | 114.4 |
| Silver PLA | None | Square | 100 | 620 | 108.7 |
| Silver PLA | 11% | Square | 100 | 680 | 119.2 |
| Silver PLA | 11% | Square | 100 | 615 | 106.4 |
| Lightweight Thermoplastic | None | N/A | N/A | 83 | 14.3 |
Table 3.
Testing the reliability of the results by repeating the test on 3 samples of each material
Table 4.
Comparing the price of different materials
| Material | Price | Unit |
|---|---|---|
| Scotchcast (Fiberglass) 4” | $16.84 | Per roll |
| PLA | $29.99 | 2.2 lbs. |
| ABS | $29.95 | 2.2 lbs. |
| Nylon | $24 | 1 lbs. |
| Polycarbonate | $49.99 | 2.2 lbs |
| Powder | $45-$75 | Kg |
It has been shown that 3D printing parameters have an impact on mechanical properties. This includes the layer thickness, orientation, raster angle, air gap, infill shape, bed temperature, nozzle temperature, cooling system, and infill density (6). Mentioned parameters have to be optimized to attain a cheap material with the highest shear strength or desired variables (7). By taking these steps, a simple arm splint prototype was made using PLA within the FDM 3D-printers (3).
Table 2.
Status of each material after being loaded
| Material | Configuration |
|---|---|
| ABS | Broke |
| PETG | Broke |
| PC | Broke |
| Fiberglass | (layers became apart) |
| PLA | Did not break - bent and delaminated (layers became apart) |
| Plaster of Paris | Broke |
| Thermoplastic | Did not break - only bent |
Figure 2.

Load Cell Specification is shown
Figure 3.

Three-point bending method used for mechanical properties testing
Figure 5.
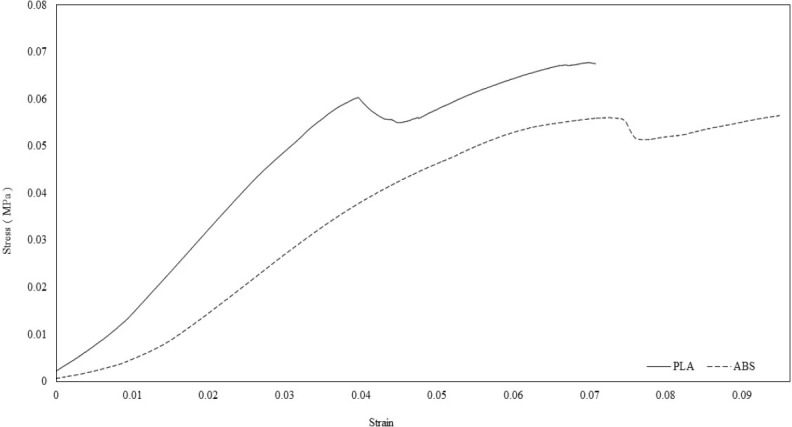
Strain/Stress is plotted for the PLA and ABS materials after the thickness was standardized to 3mm, with 20% infill and without lattice
References
- 1.Clinical guide for the management of trauma and orthopaedic patients during the coronavirus pandemic. Specialty guides for patient management during the coronavirus pandemic. 2020. Available at: URL: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-orthopaedic-trauma-and-coronavirus-v1-16-march-2020.pdf.
- 2.Hoogervorst P, Knox R, Tanaka K, Working ZM, El Naga AN, Herfat S, et al. A biomechanical comparison of fiberglass casts and 3-dimensional-printed, open-latticed, ventilated casts. Hand (N Y) 2019;27:1558944719831341. doi: 10.1177/1558944719831341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaya F, Pedro PS, Silva JL, D’Amato R, Heras ES, Juanes JA. Design of an orthopedic product by using additive manufacturing technology: the arm splint. J Med Syst. 2018;42(3):54. doi: 10.1007/s10916-018-0909-6. [DOI] [PubMed] [Google Scholar]
- 4.Medical device exemptions 510(k) and GMP requirements. U.S. Food and Drug Administration. 2019. Available at: URL: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/315.cfm?GMPPart=888#start.
- 5.Chiellini E, Cinelli P, Chiellini F, Imam SH. Environmentally degradable bio-based polymeric blends and composites. Macromol Biosci. 2004;4(3):218–31. doi: 10.1002/mabi.200300126. [DOI] [PubMed] [Google Scholar]
- 6.Samykano M, Selvamani S, Kadirgama K, Ngui W, Kanagaraj G, Sudhakar K. Mechanical property of FDM printed ABS: influenceof printing parameters. Int J Adv Manufact Technol. 2019;102(3):2779–96. [Google Scholar]
- 7.Ali M, Yerbolat G, Amangeldi S. Material optimization method in 3D printing. Proceedings of the 2018 IEEE International Conference on Advanced Manufacturing, ICAM 2018, Institute of Electrical and Electronics Engineers Inc; Taiwan: 2019. pp. 365–8. [Google Scholar]