Abstract
Solar-driven carbon dioxide (CO2) conversion to fuels and high-value chemicals can contribute to the better utilization of renewable energy sources. Photosynthetic (PS), photocatalytic (PC), photoelectrochemical (PEC), and photovoltaic plus electrochemical (PV+EC) approaches are intensively studied strategies. We aimed to compare the performance of these approaches using unified metrics and to highlight representative studies with outstanding performance in a given aspect. Most importantly, a statistical analysis was carried out to compare the differences in activity, selectivity, and durability of the various approaches, and the underlying causes are discussed in detail. Several interesting trends were found: (i) Only the minority of the studies present comprehensive metrics. (ii) The CO2 reduction products and their relative amount vary across the different approaches. (iii) Only the PV+EC approach is likely to lead to industrial technologies in the midterm future. Last, a brief perspective on new directions is given to stimulate discussion and future research activity.
Carbon dioxide (CO2) is one of the main greenhouse gases contributing to global climate change.1 According to the National Oceanic and Atmospheric Administration (United States), the global mean CO2 level reached 410 ppm in 2019.2 To cope with climate challenge, more than 170 nations signed the Paris agreement in 2016, committing to fight climate change by cutting CO2 emissions.3 Such a political ambition requires a paradigm shift, supported by technological breakthroughs. One such change is to consider CO2 as an abundant carbon source, instead of a pollutant. The turn-waste-into-wealth strategy will certainly play a key role in the green transformation of the chemical industry.4−6 There are numerous routes to convert CO2 to fuels and other chemicals. From the overall energy payback perspective, however, the most promising methods are those employing renewable energy. In this Focus Review, we focus on solar energy, which is regarded as a clean, abundant, and free renewable energy source. About 10% of the solar energy received on 0.3% of the Earth’s surface would be enough to fulfill the expected energy needs in 2050.7 Therefore, the combination of solar energy utilization and CO2 resources can be expected to produce fuels as well as value-added chemicals. Moreover, beyond their cost-effectiveness, such processes are environmentally friendly and carbon-neutral.8
The conversion of CO2 can lead to several different chemical/fuel products depending on the materials and/or methods employed, including carbon monoxide (CO), formic acid (HCOOH), methane (CH4), methanol (CH3OH), ethylene (C2H4), ethane (C2H6), propane (C3H8), ethanol (CH3CH2OH), acetic acid (CH3COOH), acetone, n-propanol, acetaldehyde, allyl alcohol, dimethyl ether, glycolaldehyde, hydroxyacetone, ethylene glycol, propionaldehyde, and glycerol.9 Although the carbon content of these products, at the current production level, accounts for only a fraction of the emitted CO2,10 the concept of solar-driven CO2 can be extended to fuel production in the future (especially for aviation where high energy density is inevitable), which accounts for a much larger carbon footprint.5
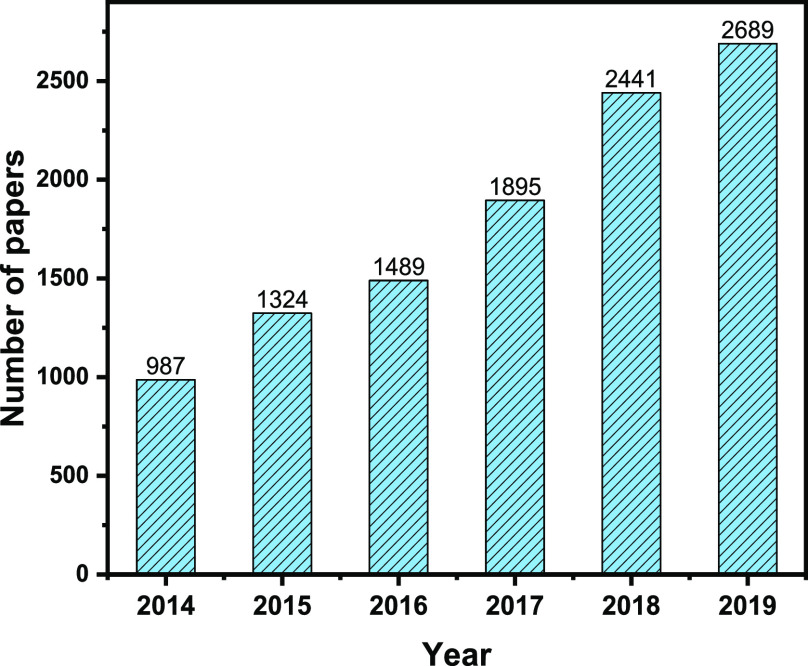
Since the discovery of photoinduced reduction of CO2 on semiconductors,11 enormous research efforts have been devoted to the solar-driven conversion of CO2, and the field has witnessed a renaissance in the past few years.7,8,12−30 We collected the number of papers published between 2014 and 2019 from the Web of Science database. There is a 3-fold increase in the number of published papers since 2014 (Figure 1), indicating the continuously growing research interest. In fact, this trend also follows the policy orientation of national governments and international funding agencies. Energy-X5 and Sunrise31 started as independent projects, supported by the European Union’s Horizon 2020 research and innovation program, focusing on the science and technology enabling efficient conversion of solar energy into chemicals. They are now merged into the “SUN-ERGY” program, to join forces under the umbrella of Horizon Europe, also in line with the Solar-Driven Chemistry Initiative of the European Chemical Society (EuChemS).32 In the United States, the Joint Center for Artificial Photosynthesis (JCAP) was established in 2010, aiming to find new and effective ways to produce fuels using only sunlight, water, and CO2. It is the largest research program in the United States dedicated to the advancement of solar-fuels generation science and technology.33 Other national programs are being implemented around the world, focusing on both fundamental science and technology development.
Figure 1.
Number of papers published in the years of 2014–2019. Data collected from Web of Science Core Collection on 2020-03-06; topic: (photo* or solar) and (CO2 or carbon dioxide) and (conversion or reduction).
Considering the current momentum of the field and the expectations of the funders (ultimately the society), there is a need to scrutinize the recent scientific and technological achievements. This exercise can shed light on what is competitive (and what remains in the land of promise) and also help to identify the most promising directions for newcomers to the field. There are a lot of books, book chapters, and review articles focusing on solar-driven CO2 reduction topics, and the aim of this Focus Review is not to repeat such information. In contrast, our approach is to compare precedent results, based on metrics that can be employed as overarching benchmarks through the various solar-driven CO2 conversion technologies. In addition, we highlight a few studies in which outstanding performances were achieved for a given metric.
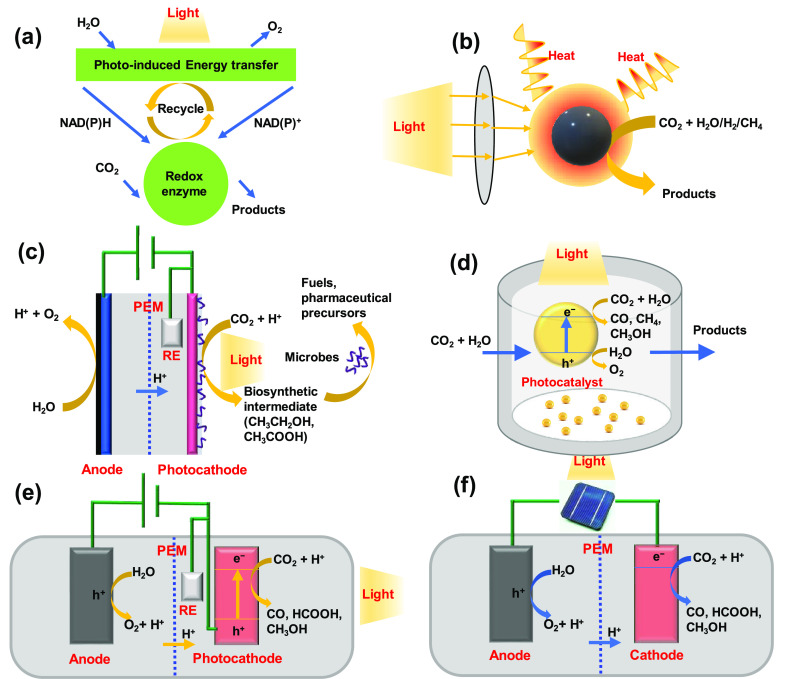
Different Solar-Driven CO2 Conversion Approaches. Solar-driven CO2 conversion methods can be categorized into biophotosynthetic, photothermal, microbial-photoelectrochemical, photosynthetic (PS), photocatalytic (PC), photoelectrochemical (PEC), photovoltaic plus electrochemical (PV+EC), etc. The classification and definitions of solar-driven CO2 conversion approaches involved in this review article are summarized in Table 1. The schematic illustrations of these systems together with the operational principles are also shown in Figure 2. Biophotosynthetic CO2 conversion mimics natural photosynthesis and therefore usually involves redox enzyme molecules as photocatalysts or artificial microbes for photosynthesis.34−40 The photothermal CO2 conversion approach uses high-temperature solar reactors, typically concentrated solar radiation, to split CO2, potentially offering high product formation rate.41−50 Microbial PEC CO2 conversion combines the advantages of semiconductor nanodevices and the high-selectivity biocatalysts, directly converting CO2 into fuels or chemicals.51−53 Among the above-mentioned pathways, PS, PC, PEC, and PV+EC approaches are more commonly studied, because they are mostly carried out under relatively mild conditions, such as low temperature and ambient pressure. These strategies will be discussed in detail later; here, only a brief overview is provided.
Table 1. Classifications and Definitions of Solar-Driven CO2 Conversion Approaches.
| category | definition |
|---|---|
| Biophotosynthetic | An approach that mimics natural photosynthesis, which usually involves redox enzyme molecules as photocatalysts or artificial microbes for photosynthesis |
| Photothermal | An approach that uses high-temperature solar reactors, typically employing concentrated solar radiation, to split CO2, potentially utilizing the entire solar spectrum and offering high product formation rate |
| Microbial-photoelectrochemical | Combines the advantages of semiconductor photoelectrodes and the high-selectivity microbe-based biocatalysts, directly converting CO2 into fuels or chemicals |
| Photosynthetic and photocatalytic (PS/PC) | Two sister approaches using particulate or molecular photocatalysts, either in solution or immobilized on a surface. This category includes both PC (ΔG < 0) and PS (ΔG > 0) processes, depending on the oxidation half reaction. Because of many similarities, they are discussed together herein, but in the light-to-fuel efficiency comparison (Figure 9b), only PS processes were selected to ensure fair comparison. |
| Photoelectrochemical (PEC) | Either one or both electrodes of the electrochemical cell is/are semiconductor photoelectrode(s). Photogenerated charge carriers drive either one or both half reactions. We included studies using the “buried junction” concept here, where a solar cell is covered by one or more catalyst(s) (and possibly a protecting layer) and this whole assembly acts as a photoelectrode. |
| Photovoltaic plus electrochemical (PV+EC) | The combination of PV cells with CO2 electrolysis in one device. This approach decouples the light-harvesting and the electrochemical conversion steps. |
Figure 2.
Schematic illustration of (a) biophotosynthetic, (b) photothermal, (c) microbial-photoelectrochemical, (d) photosynthetic and photocatalytic (PS/PC), (e) photoelectrochemical (PEC), and (f) photovoltaic plus electrochemical (PV+EC) approaches for CO2 conversion.
There are many studies using sunlight to convert CO2 over molecular or semiconductor photocatalysts, the so-called photosynthetic (PS) and photocatalytic (PC) processes. Notably, relevant chemical literature often does not differentiate between these two, although these reactions differ in their thermodynamics. PC processes are thermodynamically downhill (ΔG < 0) and are purely accelerated by the catalyst, whereas PS processes are thermodynamically unfavorable (ΔG > 0) and require photochemical energy input to occur. When CO2 reduction is paired with the oxygen evolution reaction, it is an uphill reaction (ΔG > 0); thus, it should be defined as a PS process.54,55 In contrast, if it is coupled with an anode process where a hole-scavenger is present, it can indeed be a PC process. This distinction is important, because there are different descriptors defining the performance in the two scenarios.54 While similar solar light harvesting, charge separation, and transportation processes occur, the surface reactions and recombination are very different in the two cases.19,55,56 Because of the many similarities, they are discussed together herein, except for the light-to-fuel conversion efficiency comparison.
Although hundreds of photocatalysts are reported yearly to demonstrate their effectiveness, many of these studies suffer from fundamental problems. Most of these studies focus only on the reduction part of the process such as the transformation of CO2 to CO, CH4, and HCOOH, but the coupled oxidation process (the other half of the story) is seldom discussed in detail. It has been a common practice to include sacrificial electron donors such as triethanolamine in a PC reaction to overcome both thermodynamic and kinetic limitations of the oxidation process. This practice, however, requires careful attention: (1) the process should be defined as PC rather than PS (see above); (2) the reported light-to-fuel conversion efficiencies might be inaccurate; (3) the oxidation of sacrificial donors may contribute to the products that are being considered as CO2-reduction products. There are at least two possible ways how a sacrificial donor can “contribute” to assumed CO2-reduction products: either the oxidation of the sacrificial electron donors directly produces C1 products, or the radical intermediates produced in the oxidation process have reductive abilities, which help to convert CO2. Therefore, if applied, it is very important to evaluate the fate of these sacrificial donors and their contributions to the overall yield of the products in the PC CO2 reduction reactions.57,58
Compared with the particle suspension-based PS and PC process, the photoelectrode-based PEC reduction of CO2 can integrate the advantages of photosynthesis and electrocatalysis.59 Based on which electrode acts as the light absorber, three different PEC configurations can be envisioned: photocathode–dark anode (shown in Figure 2e as an example), photoanode–dark cathode, and photocathode–photoanode. The fact that each photoelectrode can consist of multiple absorber layers to better cover the solar spectrum complicates the picture further. A sophisticated variant is the “buried junction” concept, where a solar cell is covered by one or more catalyst(s) (and possibly a protecting layer) and this whole assembly acts as a photoelectrode.60−63
Development in photovoltaic (PV) technologies over the past 5–10 years is eye-catching, with the record of light-to-electrical power conversion efficiency (PCE, which is the ratio between the incident solar photon energy and the electrical energy output) being continuously renewed.64 For example, the silicon-based single-junction PV cell could achieve the PCE of 26.7%; the III–V single-junction cells, such as GaAs, reached the PCE of as high as 29.1%, while the burgeoning perovskite-based cells could also achieve 21.6%. The multijunction cells, such as AlGaInP/AlGaAs/GaAs/GaInAs further increased the PCE to 47.1%.65,66 What is equally important, with the rapid growth of the PV industry, the price of the Si-based PV cells has declined sharply.67 Therefore, these low-cost and reliable silicon-based PV modules are widely available. PV cells can be combined with an electrolyzer, thus decoupling the light-harvesting (current generation) and the electrochemical conversion steps (PV+EC system). For the PV+EC systems discussed in this Focus Review, we excluded those studies where a solar cell covered by a catalyst acts as a photoelectrode for one half reaction (those are discussed in the PEC field). Only those scenarios are considered where the PV panel is the sole supplier of the electrochemical bias, and the CO2 conversion takes place on an electrode wired to the PV cell.63 The separation of the optical and electrical components allows a greater selection of materials and eliminates concerns of processing compatibilities and solution stability of the light-active components. Furthermore, in principle, it allows the use of high-quality (and expensive) electrocatalysts, because of the much higher operational current densities. This strategy has been successfully applied for water splitting to produce hydrogen. The price of renewable hydrogen has dropped to about €3.23 kg–1 considering the parameters relevant to Germany, which is already competitive with small and medium-size operations of conventional, fossil fuel-based processes. Considering the trend in the cost, €2.50 kg–1 seems realistic within a decade, which will be competitive with petrochemical approaches.63 We envision similar opportunities for PV+EC CO2 conversion, once mature electrolyzer technologies will be available.
Performance Metrics for PS/PC, PEC, and PV+EC Approaches. As data-mining becomes a major component of every research project, it is now more important than ever to report experimental data (and the drawn conclusions) in a manner that comparisons among laboratories can be easily made.68 While analyzing the papers from the past 5 years, we collected a broad set of performance metrics (Table 2), to see which ones allow the most meaningful comparisons for PS/PC, PEC, and PV+EC studies.
Table 2. Summarized Performance Metrics for PS/PC, PEC, and PV+EC Systems.
| PS/PC | PEC | PV+EC | |
|---|---|---|---|
| Performance metrics | Formation rate | Formation rate (current density) | Formation rate (current density) |
| Conversion | Potential/voltage | Potential/voltage | |
| Turnover number (TON) | |||
| Selectivity | Selectivity (Faradic efficiency, FE) | Selectivity (Faradic efficiency, FE) | |
| Quantum efficiency (QE) | Solar-to-fuel conversion efficiency (SFE) | Solar-to-fuel conversion efficiency (SFE) | |
| Durability | Incident photon-to-current conversion efficiency (IPCE) | Durability | |
| Absorbed photon-to-current conversion efficiency (APCE) | |||
| Durability |
Activity, selectivity, and durability are usually the main three aspects to evaluate the performance of different solar-driven CO2 conversion approaches. In PS/PC studies, product formation rate is the most commonly reported metric for evaluating the activity. In most cases, the formation rate is normalized over the weight of the catalyst or the geometrical area if it is immobilized on a substrate. Products, however, may vary in different catalytic systems. It is a worthwhile exercise to normalize the formation rate with the reaction stoichiometry (i.e., numbers of electrons transferred in the reaction); thus, comparisons can be reasonably made among different products (such analysis will be shown later). Quantum efficiency (QE) is another important component of the light-to-fuel efficiency, but it is not provided in all reports. For the PEC approach, the Faradaic efficiency (FE) is the most reported metric, often misleadingly interpreted as an activity descriptor. In addition, the onset potential of the reduction process (the potential/voltage at which the product detection measurement was carried out) and the corresponding normalized current density are also important metrics to be reported. Solar-to-fuel conversion efficiency (SFE) is a key metric, which is less reported in PEC systems. Strictly speaking, SFE is applicable only if no external bias is employed. Incident photon-to-current conversion efficiency (IPCE) and absorbed photon-to-current conversion efficiency (APCE) can also reflect the efficiency from different aspects.69 In PV+EC related systems, the performance metrics are similar to those of the PEC. The operation points (including voltage and current density) are usually provided, and SFE is more commonly reported.
Recent Development in PS/PC CO2 Conversion. In the past 5 years, numerous research articles have been published focusing on further improving the activity, selectivity, and durability for PS/PC CO2 reduction. There are also a lot of reviews and perspectives, such as a summary of photocatalyst development,7,8,16,20−22,24,27,28,70−91 design strategies for reactors,18,92,93 and the possibilities and challenges of solar fuel production.8,14,17,19,27,28,94−98 Here, we highlighted three studies on PS and PC CO2 conversion with outstanding performance in some regard (see bold values in Table 3).
Table 3. Selected Studies on PS/PC CO2 Conversion.
| PS | ||||||||
|---|---|---|---|---|---|---|---|---|
| catalyst | illumination conditions | reactant/solution | products | QE (%) | selectivity (%) | formation rate normalized (mmol e– gcat–1 h–1) | maximum test time (h) | ref. |
| Surface S and Br modified CoO/Co3O4 | 300 W Xe lamp, IR water filter, 500 mW cm–2 | H2O | CH4 | 2.3 at 405 nm | 98 | ∼80 | 9 | (99) |
| CuIn5S8 single-unit-cell layers | AM 1.5G filter, λ ≥ 400 nm, ∼50 mW cm–2 | H2O | CH4 | 0.79 | 100 | 0.0696 | 120 | (100) |
| (Ag@Cr)/Ga2O3 | 400 W high-pressure mercury lamp with a quartz filter | H2O | CO | 85.2 | 2.1 | 5 | (101) | |
| PC | ||||||||
|---|---|---|---|---|---|---|---|---|
| catalyst | illumination conditions | reactant/solution | products | QE (%) | selectivity (%) | formation rate normalized (mmol e- gcat–1 h–1) or TON | maximum test time (h) | ref. |
| Co–Co2P@NPC | 200 W white LED lamp | TEOA/H2O/MeCN, [Ru(bpy)3]Cl2·6H2O | CO | 79.1 | ∼70 | 18 | (102) | |
| RuP/C3N4 | 400 W Hg lamp, λ > 400 nm | DMA/TEOA | HCOOH | 5.7 at 400 nm | TON > 1000 | (103) | ||
| Iridium(III) complexes | Blue LED light, 0.43 mW cm–2 | TEOA/MeCN | CO | 10 | TON > 265 | 10 days | (104) | |
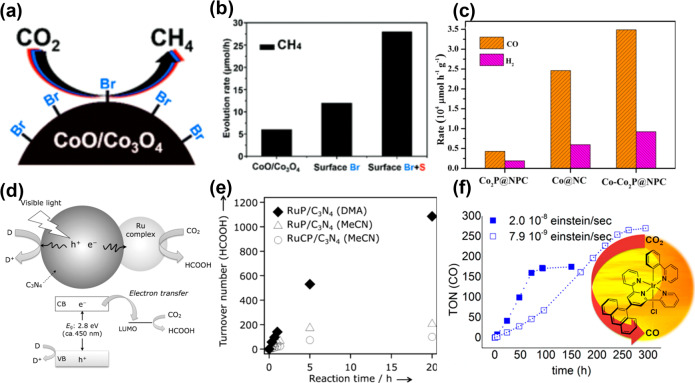
CO2 reduction to CH4 with high formation rate and selectivity was reported, when surface activated cobalt oxide nanoparticles were used as catalyst.99 As shown in Figure 3a, treatment with N-bromosuccinimide resulted in the formation of Co3O4 with coordinated Br on the surface, therefore enhancing the catalytic efficiency. The formation rate of CH4 was further enhanced by surface modification with sulfur, reaching 10 mmol g–1 h–1 (normalized formation rate of 80 mmol e– g–1 h–1) with a QE of 2.3% at 405 nm and a selectivity of 98% (Figure 3b). In another study, a heterogeneous hybrid catalyst of Co and Co2P nanoparticles was embedded in carbon nanolayers codoped with N and P (activities shown in Figure 3c) which was combined with a homogeneous Ru-based complex photosensitizer, allowing high CO formation rate.102 In atomically thin layers of sulfur-deficient CuIn5S8 (containing charge-enriched Cu–In dual sites), the formation of a stable Cu–C–O–In intermediate at the Cu–In dual sites was the key feature determining selectivity.100 As a result, the CuIn5S8 single-unit-cell layers achieved nearly 100% selectivity for visible-light-driven CO2 reduction to CH4, with a formation rate of 8.7 μmol g–1 h–1. A hybrid system of a ruthenium complex and carbon nitride (C3N4) was shown to selectively convert CO2 to HCOOH (Figure 3d,e).103 As for molecular systems, terpyridine modifications of an iridium(III) photocatalysts with a combined 2-phenylpyridine (ppy) and 2,2′:6′,2″-terpyridine (tpy) ligand have been investigated and yielded a turnover number (TON) of up to 265 with a QE of 0.10 (Figure 3f).104 It is worth pointing out that this catalytic system showed high durability (over 10 day operation without obvious decay of activity).
Figure 3.
(a) Schematic illustration of N-bromosuccinimide treated cobalt oxide nanoparticles and (b) CH4 formation rate over catalysts with different surface treatments. Reprinted with permission from ref (99). Copyright 2019 Royal Society of Chemistry. (c) Comparison of CO2 reduction performances of different catalysts. Reprinted with permission from ref (102). Copyright 2019 Wiley-VCH. (d) Schematic illustration of CO2 reduction using a Ru complex/C3N4 hybrid photocatalyst and (e) the turnover number of HCOOH production as a function of irradiation time using different photocatalysts and solvents. Reprinted with permission from ref (103). Copyright 2019 Wiley-VCH. (f) The turnover number of CO evolution as a function of irradiation time over modified iridium(III) photocatalyst. Reprinted from ref (104). Copyright 2017 American Chemical Society.
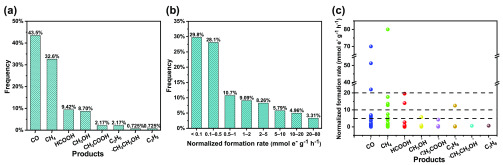
To get a statistically validated picture of PS/PC CO2 conversion studies, 138 cases were analyzed among those papers published since 2014. The selection criterion was the availability of two or more of the important performance indicators in the study. The majority of these studies (>100) employed various sacrificial agents; therefore, they belong to the PC category. The results are shown in Figure 4. For the product distribution, only the major products (selectivity > 30%) of each study were counted. The most common products formed are CO and CH4, which together account for over 75% of the major products. In addition, HCOOH and CH3OH also represent 9.4% and 8.7%, respectively. Other products, such as CH3COOH, C2H6, CH3CH2OH, and C3H8, were seldom reported as major products, which is consistent with the results in the selected studies we have highlighted. To transparently compare the activity of the catalysts in these studies, the reported formation rates have been normalized with the electron-transfer number of the given product, enabling comparisons among different products. Among the above 138 cases, 121 demonstrated unambiguous formation rate data, which were selected for the subsequent analysis. As shown in Figure 4b, the formation rates concentrated within a lower range of 0–0.5 mmol e– g–1 h–1, accounting for about 50% of the studies. There are 4 cases reported formation rates ranging from 20 to 80 mmol e– g–1 h–1. We further analyzed the normalized formation rate distribution of different products (Figure 4c and Table S1). Only CO and CH4 have been produced with a formation rate greater than 20 mmol e– g–1 h–1. Interestingly, although almost 10% of the cases had HCOOH as the major product, most of them reported the formation rate lower than 5 mmol e– g–1 h–1. This pattern suggests that there is a greater chance of reoxidation of HCOOH to CO2 (back reaction on the very same catalyst, driven by the photogenerated holes), compared to that for the gas-phase products, which rapidly move away from the catalyst surface.
Figure 4.
Statistical analysis of PS/PC CO2 conversion studies: (a) product distribution, (b) normalized formation rate distribution, and (c) normalized formation rate distribution of different products.
Recent Development in PEC CO2 Conversion. The number of studies employing PEC CO2 conversion approach has also grown rapidly in the past 5 years. Some outstanding examples are highlighted in Table 4.
Table 4. Selected Studies on PEC CO2 Conversion.
| Photocathode–Dark Anode | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| cathode | anode | illumination conditions | electrolyte | potential and current density | products | FE (%) | SFE (%) | maximum test time (h) | ref |
| Bi Nanosheets | Graphite | 1 Sun | NaHCO3 | –1.1 V vs RHE, 18 mA cm–2 | HCOOH | ∼100 | 1.5 | 12 | (105) |
| In0.4Bi0.6/MAPbI3 | Pt | 1 Sun | KHCO3 | –0.6 V vs RHE, ∼5.5 mA cm–2 | HCOOH | ∼100 | 1.5 | (106) | |
| TiO2-protected Cu2O–Re(tBu-bipy)(CO)3Cl | Pt | Xe lamp with KG 3 and AM 1.5 G filters | Re(tBu-bipy)(CO)3Cl and MeOH | –1.73 V vs Fc+/Fc, ∼1.5 mA cm–2 | CO | ∼100 | 5.5 | (107) | |
| Li-doped CuFeO2 | Graphite | 1 Sun | pyridine acetate buffer | –0.63 V vs SCE, ∼0.6 mA cm–2 | CH3OH | 96.7 | 1.5 | (108) | |
| CuFeO2/CuO | Pt | 1 Sun | KHCO3 | 0.15 V vs RHE, ∼1.5 mA cm–2 | HCOOH | >90 | 1–1.2 | 7 days | (109) |
| Photoanode–Dark Cathode | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| cathode | anode | illumination condition | electrolyte | potential and current density | products | FE (%) | SFE (%) | maximum test time (h) | ref |
| Pd/C-coated Ti mesh | GaAs/InGaP/TiO2/NiOx | 1 Sun | Anolyte: KOH; catholyte: KHCO3 | Cathode: ∼−0.8 V vs Ag/AgCl, 8.5 mA cm–2 | HCOOH | >94 | ∼10 | 3 | (9) |
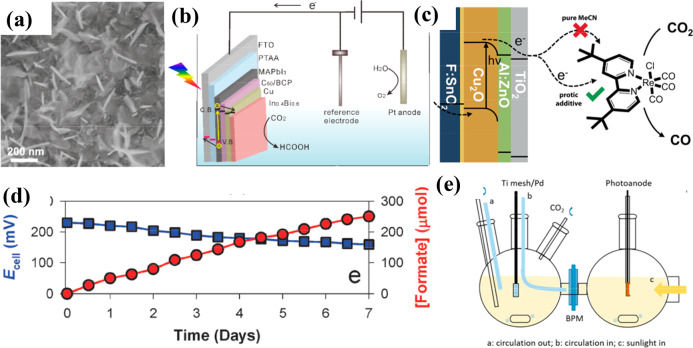
Mesoporous bismuth nanosheets have been prepared by the cathodic transformation of atomic-thick bismuth oxycarbonate nanosheets, which showed selective CO2 reduction to HCOO– with high current density (−1.1 V vs RHE, ∼18 mA cm–2, FE ≈100%) and operation stability (12 h). Moreover, Bi nanosheets were integrated with Ir/C dark anode in full cells and achieved a solar-to-formate conversion efficiency of 1.5%. The CO2 reduction performance was rationalized by the 2D mesoporous nanosheet morphology with an enlarged surface, abundant under-coordinated Bi sites, and structural robustness (Figure 5a).105
Figure 5.
(a) SEM image of reduced mesoporous Bi nanosheets. Reprinted with permission from ref (105). Copyright 2018 Wiley-VCH. (b) Full cell configuration containing In0.4Bi0.6-coated perovskite photocathode. Reprinted from ref (106). Copyright 2019 American Chemical Society. (c) Schematic of the PEC CO2 reduction process involving protected Cu2O photocathodes and a Re-based molecular catalyst. Reprinted with permission from ref (107). Copyright 2015 Royal Society of Chemistry. (d) Changes in Ecell and HCOOH production with a wired CuFeO2/CuO and Pt couple under illumination without external bias. Reprinted with permission from ref (109). Copyright 2015 Royal Society of Chemistry. (e) The scheme of photoanode-dark anode configuration for CO2 conversion. Reprinted from ref (9). Copyright 2016 American Chemical Society.
The application of hybrid organic–inorganic and fully inorganic perovskites in PEC processes has also been a hot topic. For example, a novel photocathode was prepared by coating an In0.4Bi0.6 alloy layer on a MAPbI3 PV cell (as illustrated in Figure 5b), which achieved a current density of 5.5 mA cm–2 at −0.6 V vs RHE, producing HCOOH at nearly 100% FE for 1.5 h.106 A TiO2-protected Cu2O photocathode was paired with a molecular rhenium bipyridyl catalyst. At −1.73 V vs Fc+/Fc, the system showed a current density of ∼1.5 mA cm–2 and FECO of nearly 100% (Figure 5c).107 This kind of configuration is not a standard PEC system as we discussed above because the light absorber and active sites are separated, and the buried junction together with the external electric power provides the bias.
There are also reports on PEC CO2 reduction to alcohols, for example, CH3OH was synthesized at a Li-doped CuFeO2 thin-film photocathode with a FECH3OH of 96.7% at −0.63 V vs SCE.108 Durability is another objective of the studies: a PEC cell, containing CuFeO2/CuO photocathode and Pt anode couples, could produce HCOOH for over 1 week at a solar-to-formate energy conversion efficiency of ∼1% (FE > 90%) without any external bias (Figure 5d).109 CuFeO2/CuO bulk heterojunction films were also capable of converting CO2 into C1–C6 aliphatic acid anions under simulated sunlight in the absence of any sacrificial chemicals or electrical bias, which shows that larger molecules can also be formed via PEC C–C coupling.110 The photoanode–dark cathode configuration has also been heavily investigated. For example, a solar-driven CO2 reduction cell was constructed encompassing a tandem GaAs/InGaP/TiO2/Ni photoanode, a Pd/C nanoparticle-coated Ti mesh cathode,9 and a bipolar membrane to allow for steady-state operation with a separate catholyte and anolyte. At the operational current density of 8.5 mA cm–2 without external bias, the cathode exhibited <100 mV overpotential and >94% FE for the reduction of CO2 to formate with SFE as high as 10%.
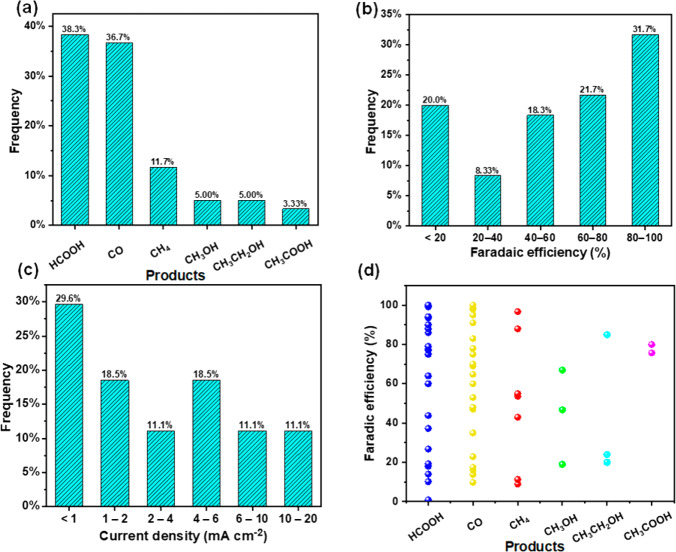
What is common in most of the highlighted studies is the very high selectivity. Note that almost 100% FE was reported for three different products (CO, HCOOH, and CH3OH). To further elaborate on this matter, statistical analysis of the PEC CO2 conversion studies has also been conducted (Figure 6). Sixty cases were collected that provided clear FE data for corresponding CO2 conversion products, which is employed as the key metric for comparing the PEC behavior herein.
Figure 6.
Statistical analysis of PEC CO2 conversion studies: (a) product distribution, (b) FE distribution, (c) current density (under 1 Sun) distribution, and (d) FE distribution of different products.
For product distribution, HCOOH and CO are the most common major products reported, together accounting for 75% of the studies, followed by CH3OH (11.7%), CH4 (5%), CH3CH2OH (5%), and CH3COOH (3.33%), which is consistent with the results of the highlighted studies. For the FE of the product formed in the largest amount, most of the studies reported FE higher than 60%, in which there are 32% with FE higher than 80%. There are still 20% of the studies reported, with FEs lower than 20%. To compare the activity, we also analyzed the current density distribution. More than 45% of the studies reported current density lower than 2 mA cm–2, while only 11% reported higher than 10 mA cm–2. For the FE distribution for different products, we do not see any cluster formation, which means that none of the products tends to form more selectively than others. In fact, there is a rather even distribution of the FE values for all products, which suggests that the selectivity of a given product is affected more by the electrode material and the PEC condition, rather than the product itself. It is easy to understand that different electrode materials greatly affect the product because the reducing power of the electrons in the PEC system is defined by the conduction band energy of the photocathode. The PEC condition, such as the electrolyte, also affect the surface chemistry and the intermediate species. Note that similarly high FE values were found for the highly reduced products (e.g., CH3OH, C2H5OH, CH4, etc.) to those obtained for CO and HCOOH. The detailed product distribution of PEC CO2 conversion studies within different FE ranges is shown in Table S2.
Recent Development in PV+EC CO2 Conversion. Based on the successful H2 evolution studies employing PV+EC systems, this configuration has attracted much attention also for CO2 reduction, especially in the past two years. Here we must make a distinction between (i) integrated systems, where the two functions are incorporated in the same unit, and those (ii) coupled ones, where regular PV panels are DC–DC connected to regular electrochemical cells. In our analysis, only the integrated systems were considered to ensure a fair comparison with the PS/PC and PEC approaches. In Table 5, we highlight some representative studies with high SFE and/or durability.
Table 5. Representative Studies on PV+EC CO2 Conversion.
| light absorber | anode | cathode | illumination conditions | electrolyte | products | operation point | FE (%) | SFE (%) | maximum test time (h) | ref |
|---|---|---|---|---|---|---|---|---|---|---|
| GaInP/GaInAs/Ge | CuO/SnO2 | CuO/SnO2 | 1 Sun | Anolyte: CsOH, Catholyte: CsHCO3 | CO | 2.38 V, – 0.55 V vs RHE, 11.6 mA cm–2 | 81 | 14.4 | 5 | (111) |
| GaInP/GaInAs/Ge | Sr2GaCoO5 | Ag | 1 Sun | NaNO3 | CO | 2.26 V, 3.54 mA cm–2 | 85–89 | 15.6 | 19 | (112) |
| Triple-junction GaAs (InGaP/GaAs/Ge) solar cell | Zn | Au | 1 Sun | Anolyte: KOH with zinc acetate, catholyte: KHCO3 | CO | 1.96 V, 10 mA cm–2 | ∼92 | ∼16.9 | 24 | (113) |
| GaInP/GaInAs/Ge | Ni or Pt | Ag/GDE | 1 Sun | Anolyte: KOH; Catholyte: KOH | CO | 2.23 V, – 0.6 V vs RHE, 14.4mA cm–2 | ∼100 | 19.1 | 150 | (114) |
Atomic layer deposition of SnO2 was performed on CuO nanowires for narrowing the product distribution of CO2 reduction, thus yielding predominantly CO. The prepared catalyst was employed as both the cathode and anode for complete CO2 electrolysis. In the resulting device, the electrodes were separated by a bipolar membrane, and a GaInP/GaInAs/Ge photovoltaic cell was used to drive the solar-driven splitting of CO2 into CO and oxygen with a solar-to-CO efficiency of 13.4% and overall SFE of 14.4%. The operating current density, selectivity toward CO, and solar-to-CO efficiency remained almost stable during 5 h of electrolysis.111 In another study, a CO2 reduction system was integrated, achieving an average solar-to-CO efficiency of 13.9% and SFE of 15.6% with no appreciable performance degradation in 19 h of operation.112
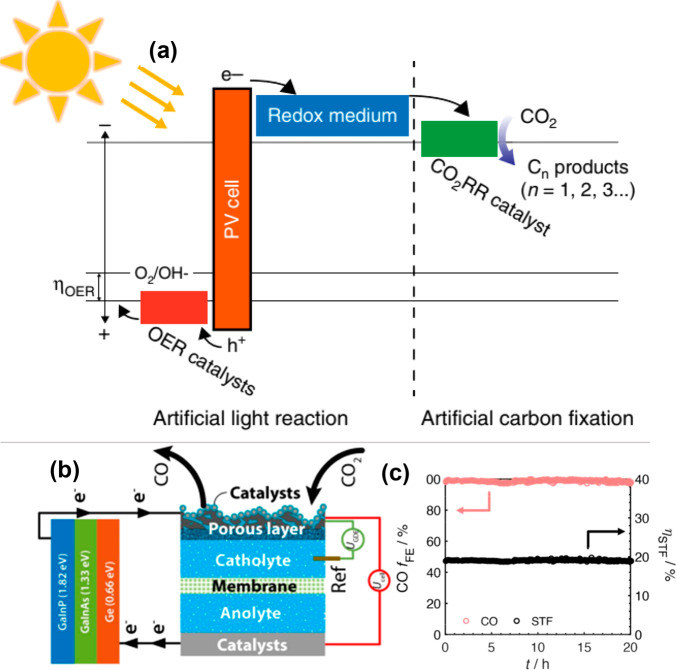
In another example, a two-step, redox-medium-assisted solar-driven CO2 electroreduction system was developed by incorporating a Zn/Zn(II) redox mediator that acts as the electron carrier during the photosynthesis. In the light reaction, the solar-driven oxygen evolution and Zn(II) reduction store electrons in the Zn/Zn(II) medium. The carbon fixation releases the stored electrons and leads to an unassisted electrochemical reduction of CO2. The energy diagram of each reaction part is shown in Figure 7a.113 This redox-medium-assisted system enables a solar-to-CO conversion efficiency of 15.6% under 1 Sun illumination. In addition, in a very recent study, solar-driven CO2 reduction to CO with 19% solar-to-CO efficiency under 1 Sun illumination in a gas diffusion electrode (GDE) flow cell has been reported.114 The use of a reverse assembled GDE (Figure 7b) prevented transition from a wetted to a flooded catalyst bed and allowed the device to operate stably for >150 h with no loss in efficiency. The FECO and SFE over a 20 h duration are shown in Figure 7c.
Figure 7.
(a) Energy diagram of each part in a redox-medium-assisted system. Reprinted with permission from ref (113). Copyright 2018 Springer Nature. (b) Illustration of a wire connection between the triple-junction cell and GDE cell and (c) CO Faradaic efficiency and solar-to-fuel efficiency over 20 h duration. Reprinted from ref (114). Copyright 2020 American Chemical Society.
As also highlighted by one of the Reviewers, the relative surface areas of PV and EC components are often significantly different, but this is often not clearly explained in the papers. In a practical device, the PV cells with a much larger area will be required compared to the area of the EC component, because of the relatively low energy density of solar irradiation. Importantly, an EC component can be cost-effective when it is smaller and runs at higher current densities (such as those with GDE cells). This mismatch, however, can bring confusion to the readers if the reported values are not concise. There have been some bad practices in reporting current density and SFE in the PV+EC systems. PV cells with a much larger area were integrated with electrodes with a smaller area, while the current density was improperly normalized with the area of the electrodes, resulting in misleadingly large current densities or SFEs. On the other hand, it is also possible to use electrodes with an area larger than that of the PV cells, especially when using expensive PV cells and relatively cheap electrode materials. In these cases, the overpotential can be very small, because of operating at low current density. Finally, in some cases the SFE was obtained under irradiance significantly lower than 1 Sun, resulting in higher SFE values, which is unlikely to scale with the light intensity.
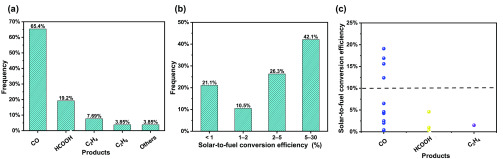
The above highlighted studies all reported CO as the main product with high SFEs. As for the statistics, 26 cases were collected, in which 19 cases demonstrated clear SFEs (Figure 8). For the product distribution, CO is the most common product, accounting for 65.4% alone. It is followed by HCOOH with 19.2%. Most of the studies reported SFE greater than 5%, accounting for 42.1%. Only 21.1% of the cases reported SFE less than 1%. For those reporting SFEs greater than 10%, all the main products are CO, which are highlighted in the above discussion. The statistical data is shown in Table S3.
Figure 8.
Statistical analysis of PV+EC CO2 conversion studies: (a) product distribution, (b) SFE distribution, and (c) SFE distribution of different products.
Comparisons of the Key Performance Metrics between PS/PC, PEC, and PV+EC Approaches for Solar-Driven CO2 Conversion. We have not found any literature precedence that compared the performance metrics among different approaches for solar-driven CO2 reduction. These subdisciplines, however, cannot be considered as isolated fields, and their comparison is of great importance to analyze intrinsic differences and similarities. As demonstrated in the above sections, there are different performance metrics for PS/PC, PEC, and PV+EC systems, among which, product distribution, light-to-fuel conversion efficiency, and maximum test time were selected as the indicators of selectivity, activity, and durability, respectively.
Inclusion and exclusion criteria:
-
(i)
Product Distribution. The inclusion criterion follows that of the above statistical studies: only the major products (selectivity > 30%) of a given study were counted.
-
(ii)
Light-to-fuel Efficiency. In principle, the cases included in the product distribution analysis were included here except those that did not provide clear light-to-fuel conversion efficiency data. In many PS/PC studies, measurements were performed under monochromatic illumination making the efficiency metrics higher than that measured under simulated sunlight or full-arc illumination. These issues, however, had only a negligible effect on the comparison, as shown in the following discussion. Those studies using sacrificial agents in the performance evaluation were excluded in the comparison of light-to-fuel efficiency, to ensure that water oxidation is the other half reaction. For PEC studies, only studies without external bias were taken into account in the light-to-fuel conversion efficiency comparison. For PV+EC studies, SFE (or light-to-fuel-efficiency in some cases) data were already obtained in the above sections.
-
(iii)
Maximum Reported Test Time. All cases were included here except those that did not provide clear maximum test time data.
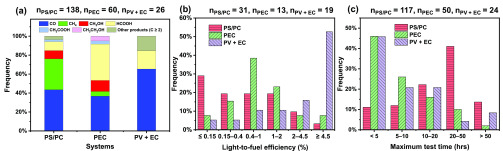
The comparison of product distribution is shown in Figure 9a. Overall, the products of PS/PC and PEC studies are more broadly distributed than those in PV+EC studies with a distinctly higher frequency of CO. The reason for this is that most of the PV+EC studies are proof of concept focusing on device fabrication or system validation, using commercial electrocatalysts, such as Ag115 and Au116 on which selective CO2 reduction to CO has been widely reported. The gas products (mainly CO and CH4) together account for nearly 80% in PS/PC studies, while they account for only about 40% in PEC studies, which might be associated with the factor that in PS/PC systems, both reduction and oxidation happen on the same particle, while in PEC systems they are spatially separated. The unfavored generation of liquid products in the PS/PC system may be plausibly further consumed by the photogenerated holes involving oxidation reaction conducted at the same particle surface. In addition, the frequency of HCOOH in PS/PC studies is lower than those of PEC and PV+EC plausibly because HCOOH, as one of the thermodynamically prior products, might be oxidized by the photogenerated holes or derived oxidizing intermediates, whereas this process is avoided to a great extent in PEC and PV+EC systems.
Figure 9.
Comparisons of (a) product distribution, (b) light-to-fuel conversion efficiency, and (c) longest measurements in PS/PC, PEC, and PV+EC systems.
The activities of the three approaches are compared by light-to-fuel efficiencies, as shown in Figure 9b. The differences are striking! Most of the light-to-fuel efficiencies in PS studies (PC were not included in this analysis) are located in low-value ranges, with 29.0% located between 0 and 0.15% and 19.4% located between 0.15 to 0.4%, accounting for 48.4% together. For the PEC studies, the majority is less than 2%, accounting for 61.6%. While for that of PV+EC, the light-to-fuel efficiencies are more concentrated in the high-value range (≥4.5%). There is an obvious trend that PS studies frequently reported relatively lower light-to-fuel efficiencies while those of PEC are somewhat higher, and those of PV+EC are further improved.
For the comparison of durability (shown in Figure 9c), most of the PEC and PV+EC studies reported the longest measurement with the maximum test time less than 10 h, accounting for more than 50%. Although fewer cases reported more than 50 h of durability, some of those still have good stability. Moreover, we found that PS/PC studies reported a higher frequency of more than 20 h durability. Generally, the instability in PS/PC systems is caused by photocorrosion resulting from the reduction/oxidation of the photoactive material by photogenerated electrons and holes. For that of PEC systems, not only photocorrosion but also electrocorrosion and electrolyte degradation are considerable challenges for long-term durability. The lack of many long-duration PV+EC studies is somewhat surprising, because PV cells have a very long lifetime, while over 100–1000 h stability was also demonstrated for EC systems. We speculate that more work has to be done on the integration to realize achievable durability for the combined system.
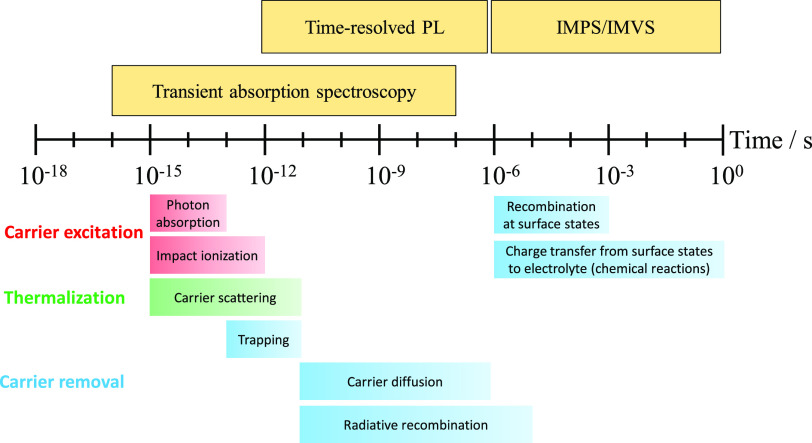
For the sake of simplicity, we compare the most important descriptors behind these trends in Table 6. As seen, the reasons are complex and convoluted; therefore, at this point, we discuss only one overarching aspect of all these areas, namely, the timescale of the different elementary processes. In Figure 10, we present the typical timescale of photoinduced processes occurring in semiconductors and at semiconductor interfaces. Most importantly, the timescale of the chemical reactions (especially the CO2 reduction reactions involving multielectron and multiproton transfer) is in the microsecond-to-second regime. This means that a substantially long (photo)electron lifetime is necessary for this process. Unfortunately, charge carrier recombination occurs at a much faster timescale (depending on the mechanism from subpicosecond to microsecond). This mismatch already indicates that high light-to-fuel conversion efficiencies cannot be realistically expected from PS and PEC systems, unless cocatalysts can be found, which can properly “store” electrons. There is precedence in the literature, where complex PS/PC assemblies allowed charge carrier lifetime on the order of microseconds.117 This lifetime enabled different redox reactions, although not those involving the transfer of multiple electrons and protons. Specifically, in a recent study on CsPbBr3 perovskite, nanocubes facilitated photodriven C–C coupling, where both charge carriers were rapidly (∼50 ps) extracted from the photoexcited perovskite NCs to reactant molecules. The separated charge carriers lived for more than 0.8 μs, enabling a radical mechanism to form the C–C bonds.118 At the same time, corrosion processes are also induced in the semiconductors via charge carrier trapping, posing a great threat for PS and PEC methods.119 Such corrosive processes inside the semiconductors are typically faster than the CO2 reduction reaction, where charge transfer is required from the electrode surface to the substrate. This is not the case for the PV+EC method, where charge carriers are rapidly extracted from the PV cell (on the nanosecond–picosecond) timescale.
Table 6. Summary of the Differences among PS/PC, PEC, and PV+EC Systems from Six Aspects.
| PS/PC systems | PEC systems | PV+EC systems | |
|---|---|---|---|
| Light absorption | One or more light absorbers are needed (see tandem and z-scheme configurations). | Either one (photocathode or photoanode) or two photoactive electrodes. The individual photoelectrodes can also be multicomponent. | Tailored photovoltaic cells can be designed (from single- to multijunction cells), to provide the necessary cell voltage. |
| Charge carrier collection | No need for carrier collection, but photogenerated holes and electrons need to reach the respective surface sites. | Charge carrier trapping at defect sites at the electrode/electrolyte interface hinders charge carrier collection. | Rapid charge carrier collection is achieved in the PV cell. |
| Charge transfer (reaction) | Both reactions proceed on the same particles. Preferably different sites for the two half reactions. Back reactions are possible. The rates of the two half reactions have to match. | Slow charge carrier transfer to the substrate or mediator from the electrode surface, compared to the timescale of charge carrier recombination. | A separate electrochemical interface is responsible for the chemical reaction. Well-known stable and active electrocatalysts can be employed. |
| Nano aspects | A high surface area is necessary to provide enough active sites for the reaction. High probability of surface recombination. | A high surface area is necessary to provide enough active sites for the reaction. High probability of surface recombination. | Nanostructured electrocatalysts can be used, without the detrimental surface recombination in the light absorber. |
| Stability | Intermediate stability, because of the presence of the solid/liquid interface. | Very difficult to achieve reasonable stability, because of the presence of current flow and the electrode/electrolyte interface. Different protective coatings seem to ensure certain improvements. | The stability is dictated only by the stability of the electrolyzer, as PV panels are stable for ages. Examples on the order of hundreds of hours are available. |
| Cost | Cheap experimental setup or device, but expensive multifunctional catalyst materials are needed. | More expensive and sophisticated cell designs are necessary, especially in the case of continuous flow processes. If a cocatalyst is employed, large amounts are needed because of the identical surface area of the light absorber and the electrochemical interface. | Relatively expensive system cost. Much smaller electrochemically active area is needed (compared to the size of the PV) and thus less electrocatalysts, membranes, etc. have to be used. It is also possible to select high-performance PV cells with a smaller area (under concentrated light) and electrodes with a larger area. |
Figure 10.
Typical timescale of different photoinduced processes in semiconductors, together with the methods that are employed to monitor them. PL, photoluminescence; IMPS/IMVS, intensity-modulated photocurrent/photovoltage spectroscopy.
Finally, we also found that often different products formed via the different approaches even with similar catalysts. Taking CuxO-based catalysts as an example, several studies are listed in Table 7. There is a variety of products including CO, CH4, C2H4, C2H6, HCOOH, CH3COOH, and CH3OH. In PS and PEC systems, the conduction band energy of the photocathode defines the energy of the photoelectrons, while in the PV+EC systems, it is dictated by the electrode potential. This important difference also means that while in electrocatalysis the reaction rate (i.e., current density) and the reducing power of the electrons (i.e., the electrode potential) are inherently coupled (see the Butler–Volmer equation), this is not the case for PEC.120 This simple fact can be a major contributor to the observed differences in the product distributions and a major opportunity for PEC-based methods in the future.
Table 7. Representative Studies on Solar-Driven CO2 Conversion Using CuxO-Based Catalysts.
The same catalyst may play different roles in different scenarios. Taking Au-based catalysts as an example, three studies are shown in Table 8. In a PS study, Au nanoparticles (NPs) were used as a catalyst for the conversion of CO2 and H2O into C1–C3 hydrocarbons under visible light irradiation.127 The Au NPs possess a strong localized surface plasmon resonance (LSPR) band centered around 520 nm, which enables the generation of energetic electron–hole carriers under green light for the reduction of CO2 and oxidation of H2O, resulting in the main products of hydrocarbons. In two PEC studies, Au cocatalyst can lead to main products CO and CH3CH2OH in different studies.60,128 In such cases, beyond facilitating charge transfer, the cocatalyst also affects the energetics of the electrode/electrolyte interface. In the highlighted PV+EC study, the main product is CO, where Au acted as an electrocatalyst.113
Table 8. Representative Studies on Solar-Driven CO2 Conversion Using Au-Based Catalysts.
Summary. Over the past years, the fundamental understanding of the solar-driven CO2 conversion reaction has improved substantially,129−136 and deactivation mechanisms have also been studied.137,138 Integration and validation of reactors and systems are also ongoing.111,114,125,139−146 With this Focus Review, our aim was to provide an overview of the state-of-the-art solar-driven CO2 conversion approaches. We have presented for the first time a statistical analysis of activity, selectivity, and durability of PS/PC, PEC, and PV+EC systems. The results indicate that (i) only the minority of studies present all those metrics, which fully describe the performance of a given system; (ii) the CO2 reduction products and their distribution are different in the different scenarios, and (iii) at this stage only the PV+EC approach shows a performance (especially in terms of activity and durability), which can lead to industrial technologies in the near future (see Figure 9 and Table 6).
Based on the statistical analysis, several questions and problems were raised:
-
(i)
Unbalanced research efforts. More than 80% of the studies focused on PS/PC CO2 conversion, most of which discussed catalyst synthesis and the corresponding laboratory performance evaluation. Very recently, critical comments were made on the doping of graphene with a plethora of elements for enhanced electrocatalytic effect.147 We see a very similar trend in solar-driven PS/PC CO2 conversion, namely that hundreds of “doped photocatalysts” are being tested with little outcome.
-
(ii)
Avoid the pitfalls. As discussed in the introduction of PC systems, many studies employ sacrificial electron donors, without evaluating the fate of these agents. It is strongly required to carry out a complete study on the overall solar-driven reactions, not only the CO2 reduction half of it. Another critical issue is to confirm that CO2 is the actual source of the carbon-containing products. Isotopic 13CO2 labeling is an efficient technique for this purpose. Quantitative detection of the evolved oxygen can also help quantifying the performance. Moreover, a control test without CO2 but in the presence of H2O would be a cost-effective, yet preliminary approach to confirm/exclude participation of carbon residues (on active sites) in CO2 reduction.
-
(iii)
Lack of complete data set. Only a minority of the studies contain metrics which fully describe the performance of a given system. While it is encouraging to see many studies being carried out in this area, telling the whole story is essential. This not only demonstrates the reliability of the work but also ensures reproducibility. Finally, reporting the appropriate metrics helps to make clear comparisons with other studies, shows the research status we have reached, and reminds how far we are from industrial applications.
By taking a careful look at the metrics presented above (especially concerning SFE values), we have to conclude that only the PV+EC systems have the potential to become an industrial technology in the near and midterm future.148 Several trends support this notion, most importantly: (i) The rapid improvements of PCE and a decrease in the cost of PV cells. In fact, the price of solar electricity has decreased to a level that in over 20 countries translates to grid parity. (ii) The continuous progress in developing practical CO2 electrolyzers.149 Particularly, there are emerging trends in the employment of gas diffusion electrodes (GDEs), aiming to eliminate limitations arising from slow mass transport and small turnover number at the active sites.150,151 The use of GDEs enabled the achievement of operational parameters of great industrial significance; unfortunately, not yet all of them are for the same system. These are (i) current densities higher than 300 mA cm–2, (ii) cell voltages in the 2.5–3.0 V range (translating to energy efficiencies higher than 50%), (iii) close to 100% FE for CO and HCOOH, and (iv) over 5000 h durability.
Future Directions. Selectivity is the metric which seems to be the most addressable if we better understand the nature of active sites.152−158 Atomic-level understanding of the active sites and transformation mechanisms under realistic working conditions is a prerequisite for the rational design of photoactive materials with high selectivity. For example, the (110) facet of a single-crystal Cu2O particle is active for photodriven CO2 reduction to methanol while the (100) facet is inert. The oxidation state of the active sites changes from Cu(I) toward Cu(II) because of CO2 and H2O coadsorption and changes back to Cu(I) after CO2 conversion under visible light illumination.156
Hybrid photoelectrode assemblies offer a platform to rationally design materials for solar-driven CO2 conversion. We need to consider the following processes. (i) Light absorption: it is essential to harness a reasonable portion of the solar spectrum. (ii) Charge transport: the semiconductor shall have high charge carrier mobility. The amount of bulk and surface traps shall also be minimized. (iii) Charge transfer kinetics: facile charge transfer from the semiconductor to either the CO2 molecules in the solution, or to a mediator (either immobilized or in the solution), is required. (iv) Stability and robustness are definitely major concerns, where chemical, electrochemical, and photocorrosion all have to be considered. Considering these very complex requirements, which have to be met by a photoelectrode, it is not too surprising that no single material could meet all of these so far. In biological systems, one can find complex architectures with components that have precisely defined functionality and complementarity. As a bioinspired approach, some of the limitations of the individual components can be overcome by the rational design and assembly of hybrid PS/PC and PEC materials, where the different functionalities are decoupled. For example, CsPbBr3@zeolitic imidazolate framework nanocomposites have been reported to exhibit enhanced CO2 reduction activity due to the addition of zeolitic imidazolate framework with its original CO2 capturing ability and the role of acting as a cocatalyst.159 In addition, it was demonstrated by the example of Cu2O that when a highly conductive scaffold is introduced into the photoelectrode, the charge carrier transport can be enhanced, and larger photocurrents can be harvested.160,161 Similar trends have also been discovered in organic photoelectrodes.162 This strategy greatly broadened the range of the catalyst and light absorber selection by reasonably combining the attractive features of each component.
Nanoscale aspects are important to enhance the current density to a level that makes practical significance. The field of nanostructured photoelectrodes is surprisingly an unexplored area. While the PS/PC163 and the EC communities have built solid and coherent knowledge on the effect of catalyst size at the nanoscale, there is no systematic study on this matter for PEC processes. In fact, the long-standing theory of PEC builds on thick electrode films (with film thickness of over several micrometers).164 Systematic study of nanoscale effects in PEC would be indeed important, because they affect all the important processes, such as light absorption, charge carrier transport, band bending, and charge transfer. Similar considerations are also valid for the effect of morphology. Despite some very informative studies on Si micro-165,166 and nanowires,167 there is a lack of knowledge whether there is an ideal morphology for PEC applications. In addition, the consequence of drastically increased surface/bulk ratio (which is the case for nanomaterials) on the contribution of surface functional groups to the materials’ property has remained unknown.
Plasmonic catalysis has become an emerging avenue in CO2 conversion by the promise of these particles to harness visible light as hot carriers and their intrinsic catalytic activity toward CO2 reduction. Noble metal NPs, in particular, allow the integration of strong visible-wavelength plasmonic excitation with surface activation of CO2 and therefore represent a novel and promising class of photocatalysts for CO2 reduction.8 The fundamental understanding of plasmon-assisted CO2 reduction processes, however, is still lacking. In plasmonic CO2 reduction reaction, catalytic activity, reaction pathways, and selectivity are expected to not only depend on the properties of the metal and metal/adsorbate interactions but also possibly be tuned by light excitation.168−170
Buried junction is another promising approach, because if properly designed, it can integrate the benefits of PV and EC technologies.171 In practice, the employment of buried junction in integrated PEC cells can overcome the instability issue. The PV components in the buried junction can act as more efficient and stable light absorbers than most of the single semiconductors when physical contact with the aqueous environment is avoided.172 This strategy might provide a new direction toward the enhanced durability of PEC systems.
Concentrated sunlight can enable high current density operation for both the PEC and PV+EC approaches. In these cases, the solar power input can be concentrated up to 500 Suns, which in combination with proper PV cells or photoelectrodes can allow current densities similar to those in industrial electrolyzers. For water splitting, 0.88 A cm–2 current density was demonstrated with an STH efficiency of over 15%, under concentrated solar irradiation (up to 474 kW m–2).173 Similar integration of photoanodes or PV cells can be envisioned with CO2 reduction GDEs on the cathode side. Such studies are in progress in our laboratory and will be communicated soon.
Value-added anode processes are also worth more consideration. Most CO2 reduction approaches couple cathodic CO2 reduction with the anodic oxygen evolution reaction (OER), resulting in approximately 90% of the electricity input being consumed by the OER in the EC scenario.174 This issue can be addressed by coupling other anodic oxidation reactions with less electricity needs and probably higher-value products. For example, oxidation of glycerol (a byproduct of biodiesel and soap production) can lower electricity consumption up to 53%, thus reducing the operating costs and carbon footprint. In addition, value-added products can be produced, including glyceraldehyde and lactic acid.174 This strategy might also offer opportunities to the PC and PEC approaches as well, because there will be no need to cope with the difficult OER, and in fact, thermodynamically downhill processes can be designed.
PV+EC systems can be further improved through component development, because to date, the focus was mostly on catalysts. A recent study has demonstrated that the largest voltage losses may occur at the membrane or the membrane electrode assembly rather than at the catalyst layers in their flow cell.175 As another example, we note that a solid electrolyte may be a better alternative, because the formed liquid products are in a mixture with the dissolved salts in liquid electrolytes, requiring energy-intensive downstream separation. However, in the solid electrolyte, the generated cations (such as H+) and anions (such as HCOO–) are combined to form pure product solutions without mixing with other ions.154 In addition, the development of reactors for EC CO2 utilization is also required, to optimize such characteristics as geometrical configuration, construction material, heat exchange, and mixing and flow characteristics.18,176
Acknowledgments
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant Agreement Nos. 716539 and 899747). This research was partially supported by the “Széchenyi 2020” program in the framework of GINOP-2.3.2-15-2016-00013 “Intelligent materials based on functional surfaces–from syntheses to applications” project. The authors thank Dr. Bíborka Janáky-Bohner for her help with manuscript preparation and Prof. Krishnan Rajeshwar (UT Arlington, United States and Prof. Balázs Endrődi (University of Szeged, Hungary) for their comments on an earlier version of the manuscript.
Biographies
Dr. Jie He is a postdoctoral research fellow at the University of Szeged, Hungary. He obtained his Ph.D. from Hunan University, China in 2018. His research interest includes integration and validation of continuous flow photoelectrochemical CO2 conversion cell and development of novel photoactive materials for water splitting. https://orcid.org/0000-0001-5481-6986
Prof. Csaba Janáky is an associate professor and Principal Investigator of the Photoelectrochemistry Research Group at the University of Szeged, Hungary. His research group focuses on converting CO2 to useful chemicals, utilizing solar energy. His ERC supported activity spans through the materials aspects of photoelectrochemistry to electrochemical cell development. https://orcid.org/0000-0001-5965-5173
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsenergylett.0c00645.
Statistical data of PS, PEC, and PV+EC systems (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Jacobson T. A.; Kler J. S.; Hernke M. T.; Braun R. K.; Meyer K. C.; Funk W. E. Direct Human Health Risks of Increased Atmospheric Carbon Dioxide. Nat. Sustain. 2019, 2 (8), 691–701. 10.1038/s41893-019-0323-1. [DOI] [Google Scholar]
- ESRL Global Monitoring Division - Global Greenhouse Gas Reference Network. https://www.esrl.noaa.gov/gmd/ccgg/trends/global.html (accessed 2020-03-19).
- Rogelj J.; Den Elzen M.; Höhne N.; Fransen T.; Fekete H.; Winkler H.; Schaeffer R.; Sha F.; Riahi K.; Meinshausen M. Paris Agreement Climate Proposals Need a Boost to Keep Warming Well below 2°C. Nature 2016, 534 (7609), 631–639. 10.1038/nature18307. [DOI] [PubMed] [Google Scholar]
- De Luna P.; Hahn C.; Higgins D.; Jaffer S. A.; Jaramillo T. F.; Sargent E. H. What Would It Take for Renewably Powered Electrosynthesis to Displace Petrochemical Processes?. Science 2019, 364 (6438), 3506. 10.1126/science.aav3506. [DOI] [PubMed] [Google Scholar]
- Nørskov J. K.; Weckhuysen B.; Centi G.; et al. Research Needs towards Sustainable Production of Fuels and Chemicals; Energy X, 2019. September. Downloaded from: https://www.energy-x.eu/wp-content/uploads/2019/09/Energy_X_Research-needs-report.pdf. [Google Scholar]
- Villadsen S. N. B.; Fosbøl P. L.; Angelidaki I.; Woodley J. M.; Nielsen L. P.; Møller P. The Potential of Biogas; the Solution to Energy Storage. ChemSusChem 2019, 12 (10), 2147–2153. 10.1002/cssc.201900100. [DOI] [PubMed] [Google Scholar]
- Ran J.; Jaroniec M.; Qiao S. Z. Cocatalysts in Semiconductor-Based Photocatalytic CO2 Reduction: Achievements, Challenges, and Opportunities. Adv. Mater. 2018, 30 (7), 1704649. 10.1002/adma.201704649. [DOI] [PubMed] [Google Scholar]
- Yu S.; Wilson A. J.; Kumari G.; Zhang X.; Jain P. K. Opportunities and Challenges of Solar-Energy-Driven Carbon Dioxide to Fuel Conversion with Plasmonic Catalysts. ACS Energy Lett. 2017, 2 (9), 2058–2070. 10.1021/acsenergylett.7b00640. [DOI] [Google Scholar]
- Zhou X.; Liu R.; Sun K.; Chen Y.; Verlage E.; Francis S. A.; Lewis N. S.; Xiang C. Solar-Driven Reduction of 1 Atm of CO2 to Formate at 10% Energy-Conversion Efficiency by Use of a TiO2-Protected III-V Tandem Photoanode in Conjunction with a Bipolar Membrane and a Pd/C Cathode. ACS Energy Lett. 2016, 1 (4), 764–770. 10.1021/acsenergylett.6b00317. [DOI] [Google Scholar]
- Grim R. G.; Huang Z.; Guarnieri M. T.; Ferrell J. R.; Tao L.; Schaidle J. A. Transforming the Carbon Economy: Challenges and Opportunities in the Convergence of Low-Cost Electricity and Reductive CO2 Utilization. Energy Environ. Sci. 2020, 13 (2), 472–494. 10.1039/C9EE02410G. [DOI] [Google Scholar]
- Halmann M. Photoelectrochemical Reduction of Aqueous Carbon Dioxide on P-Type Gallium Phosphide in Liquid Junction Solar Cells. Nature 1978, 275, 115–116. 10.1038/275115a0. [DOI] [Google Scholar]
- Kim W.; McClure B. A.; Edri E.; Frei H. Coupling Carbon Dioxide Reduction with Water Oxidation in Nanoscale Photocatalytic Assemblies. Chem. Soc. Rev. 2016, 45 (11), 3221–3243. 10.1039/C6CS00062B. [DOI] [PubMed] [Google Scholar]
- White J. L.; Baruch M. F.; Pander J. E.; Hu Y.; Fortmeyer I. C.; Park J. E.; Zhang T.; Liao K.; Gu J.; Yan Y.; Shaw T. W.; Abelev E.; Bocarsly A. B. Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes. Chem. Rev. 2015, 115 (23), 12888–12935. 10.1021/acs.chemrev.5b00370. [DOI] [PubMed] [Google Scholar]
- Tu W.; Zhou Y.; Zou Z. Photocatalytic Conversion of CO2 into Renewable Hydrocarbon Fuels: State-of-the-Art Accomplishment, Challenges, and Prospects. Adv. Mater. 2014, 26 (27), 4607–4626. 10.1002/adma.201400087. [DOI] [PubMed] [Google Scholar]
- Ulmer U.; Dingle T.; Duchesne P. N.; Morris R. H.; Tavasoli A.; Wood T.; Ozin G. A. Fundamentals and Applications of Photocatalytic CO2 Methanation. Nat. Commun. 2019, 10 (1), 3169. 10.1038/s41467-019-10996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.; Tong X.; Yu P.; Du W.; Wu J.; Rao H.; Wang Z. M. Carbon Dioxide Photo/Electroreduction with Cobalt. J. Mater. Chem. A 2019, 7 (28), 16622–16642. 10.1039/C9TA03892B. [DOI] [Google Scholar]
- Wang C.; Sun Z.; Zheng Y.; Hu Y. H. Recent Progress in Visible Light Photocatalytic Conversion of Carbon Dioxide. J. Mater. Chem. A 2019, 7 (3), 865–887. 10.1039/C8TA09865D. [DOI] [Google Scholar]
- Khan A. A.; Tahir M. Recent Advancements in Engineering Approach towards Design of Photo-Reactors for Selective Photocatalytic CO2 Reduction to Renewable Fuels. J. CO2 Util. 2019, 29, 205–239. 10.1016/j.jcou.2018.12.008. [DOI] [Google Scholar]
- Chang X.; Wang T.; Gong J. CO2 Photo-Reduction: Insights into CO2 Activation and Reaction on Surfaces of Photocatalysts. Energy Environ. Sci. 2016, 9 (7), 2177–2196. 10.1039/C6EE00383D. [DOI] [Google Scholar]
- Liu X.; Inagaki S.; Gong J. Heterogeneous Molecular Systems for Photocatalytic CO2 Reduction with Water Oxidation. Angew. Chem., Int. Ed. 2016, 55 (48), 14924–14950. 10.1002/anie.201600395. [DOI] [PubMed] [Google Scholar]
- Xiong J.; Song P.; Di J.; Li H. Ultrathin Structured Photocatalysts: A Versatile Platform for CO2 Reduction. Appl. Catal., B 2019, 256, 117788. 10.1016/j.apcatb.2019.117788. [DOI] [Google Scholar]
- Ye L.; Deng Y.; Wang L.; Xie H.; Su F. Bismuth-Based Photocatalysts for Solar Photocatalytic Carbon Dioxide Conversion. ChemSusChem 2019, 12 (16), 3671–3701. 10.1002/cssc.201901196. [DOI] [PubMed] [Google Scholar]
- Xia T.; Long R.; Gao C.; Xiong Y. Design of Atomically Dispersed Catalytic Sites for Photocatalytic CO2 Reduction. Nanoscale 2019, 11 (23), 11064–11070. 10.1039/C9NR03616D. [DOI] [PubMed] [Google Scholar]
- Wu H. L.; Li X. B.; Tung C. H.; Wu L. Z. Semiconductor Quantum Dots: An Emerging Candidate for CO2 Photoreduction. Adv. Mater. 2019, 31 (36), 1900709. 10.1002/adma.201900709. [DOI] [PubMed] [Google Scholar]
- Marques Mota F.; Kim D. H. From CO2 Methanation to Ambitious Long-Chain Hydrocarbons: Alternative Fuels Paving the Path to Sustainability. Chem. Soc. Rev. 2019, 48 (1), 205–259. 10.1039/C8CS00527C. [DOI] [PubMed] [Google Scholar]
- Yang J.; Guo Y.; Lu W.; Jiang R.; Wang J. Emerging Applications of Plasmons in Driving CO2 Reduction and N2 Fixation. Adv. Mater. 2018, 30 (48), 1802227. 10.1002/adma.201802227. [DOI] [PubMed] [Google Scholar]
- Sun Z.; Talreja N.; Tao H.; Texter J.; Muhler M.; Strunk J.; Chen J. Catalysis of Carbon Dioxide Photoreduction on Nanosheets: Fundamentals and Challenges. Angew. Chem., Int. Ed. 2018, 57 (26), 7610–7627. 10.1002/anie.201710509. [DOI] [PubMed] [Google Scholar]
- Stolarczyk J. K.; Bhattacharyya S.; Polavarapu L.; Feldmann J. Challenges and Prospects in Solar Water Splitting and CO2 Reduction with Inorganic and Hybrid Nanostructures. ACS Catal. 2018, 8 (4), 3602–3635. 10.1021/acscatal.8b00791. [DOI] [Google Scholar]
- Wu J.; Huang Y.; Ye W.; Li Y. CO2 Reduction: From the Electrochemical to Photochemical Approach. Adv. Sci. 2017, 4 (11), 1700194. 10.1002/advs.201700194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K.; Peng B.; Peng T. Recent Advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6 (11), 7485–7527. 10.1021/acscatal.6b02089. [DOI] [Google Scholar]
- Faber C; Allahverdiyeva-Rinne Y.; Artero V.; Baraton L.; Barbieri A.; Bercegol H.; Fleischer M.; Huynhthi H.; Kargul J.; Lepaumier H.; Lopez L.; Magnuson A.. Solar Energy for a Circular Economy: Technological Roadmap; SUNRISE, 2020. Downloaded from: https://sunriseaction.com/wp-content/uploads/2020/02/Roadmap_February_2020.pdf. [Google Scholar]
- EuChemS Call for Proposals: Solar-driven chemistry 2019/2020. https://www.euchems.eu/proposals-solar-driven-chemistry/ (accessed 2020-03-19).
- Yano J.; Haber J. A.; Gregoire J. M.; Friebel D.; Nilsson A.; Houle F. JCAP Research on Solar Fuel Production at Light Sources. Synchrotron Radiat. News 2014, 27 (5), 14–17. 10.1080/08940886.2014.952208. [DOI] [Google Scholar]
- Kuk S. K.; Ham Y.; Gopinath K.; Boonmongkolras P.; Lee Y.; Lee Y. W.; Kondaveeti S.; Ahn C.; Shin B.; Lee J. K.; Jeon S.; Park C. B. Continuous 3D Titanium Nitride Nanoshell Structure for Solar-Driven Unbiased Biocatalytic CO2 Reduction. Adv. Energy Mater. 2019, 9 (25), 1900029. 10.1002/aenm.201900029. [DOI] [Google Scholar]
- Ye J.; Yu J.; Zhang Y.; Chen M.; Liu X.; Zhou S.; He Z. Light-Driven Carbon Dioxide Reduction to Methane by Methanosarcina Barkeri-CdS Biohybrid. Appl. Catal., B 2019, 257, 117916. 10.1016/j.apcatb.2019.117916. [DOI] [Google Scholar]
- Shen Q.; Huang X.; Liu J.; Guo C.; Zhao G. Biomimetic Photoelectrocatalytic Conversion of Greenhouse Gas Carbon Dioxide: Two-Electron Reduction for Efficient Formate Production. Appl. Catal., B 2017, 201, 70–76. 10.1016/j.apcatb.2016.08.008. [DOI] [Google Scholar]
- Amao Y.; Kataoka R. Methanol Production from CO2 with the Hybrid System of Biocatalyst and Organo-Photocatalyst. Catal. Today 2018, 307, 243–247. 10.1016/j.cattod.2017.12.029. [DOI] [Google Scholar]
- Yadav R. K.; Oh G. H.; Park N. J.; Kumar A.; Kong K. J.; Baeg J. O. Highly Selective Solar-Driven Methanol from CO2 by a Photocatalyst/Biocatalyst Integrated System. J. Am. Chem. Soc. 2014, 136 (48), 16728–16731. 10.1021/ja509650r. [DOI] [PubMed] [Google Scholar]
- Yaashikaa P. R.; Senthil Kumar P.; Varjani S. J.; Saravanan A. A Review on Photochemical, Biochemical and Electrochemical Transformation of CO2 into Value-Added Products. J. CO2 Util. 2019, 33, 131–147. 10.1016/j.jcou.2019.05.017. [DOI] [Google Scholar]
- Yadav R. K.; Baeg J. O.; Kumar A.; Kong K. J.; Oh G. H.; Park N. J. Graphene-BODIPY as a Photocatalyst in the Photocatalytic-Biocatalytic Coupled System for Solar Fuel Production from CO2. J. Mater. Chem. A 2014, 2 (14), 5068–5076. 10.1039/c3ta14442a. [DOI] [Google Scholar]
- Wang L.; Wang Y.; Cheng Y.; Liu Z.; Guo Q.; Ha M. N.; Zhao Z. Hydrogen-Treated Mesoporous WO3 as a Reducing Agent of CO2 to Fuels (CH4 and CH3OH) with Enhanced Photothermal Catalytic Performance. J. Mater. Chem. A 2016, 4 (14), 5314–5322. 10.1039/C5TA10180H. [DOI] [Google Scholar]
- Meng X.; Wang T.; Liu L.; Ouyang S.; Li P.; Hu H.; Kako T.; Iwai H.; Tanaka A.; Ye J. Photothermal Conversion of CO2 into CH4 with H2 over Group VIII Nanocatalysts: An Alternative Approach for Solar Fuel Production. Angew. Chem., Int. Ed. 2014, 53 (43), 11478–11482. 10.1002/anie.201404953. [DOI] [PubMed] [Google Scholar]
- Jia J.; O’Brien P. G.; He L.; Qiao Q.; Fei T.; Reyes L. M.; Burrow T. E.; Dong Y.; Liao K.; Varela M.; Pennycook S. J.; Hmadeh M.; Helmy A. S.; Kherani N. P.; Perovic D. D.; Ozin G. A. Visible and Near-Infrared Photothermal Catalyzed Hydrogenation of Gaseous CO2 over Nanostructured Pd@Nb2O5. Adv. Sci. 2016, 3 (10), 1600189. 10.1002/advs.201600189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M. N.; Lu G.; Liu Z.; Wang L.; Zhao Z. 3DOM-LaSrCoFeO6-δ as a Highly Active Catalyst for the Thermal and Photothermal Reduction of CO2 with H2O to CH4. J. Mater. Chem. A 2016, 4 (34), 13155–13165. 10.1039/C6TA05402A. [DOI] [Google Scholar]
- Xu M.; Hu X.; Wang S.; Yu J.; Zhu D.; Wang J. Photothermal Effect Promoting CO2 Conversion over Composite Photocatalyst with High Graphene Content. J. Catal. 2019, 377, 652–661. 10.1016/j.jcat.2019.08.010. [DOI] [Google Scholar]
- Ghoussoub M.; Xia M.; Duchesne P. N.; Segal D.; Ozin G. Principles of Photothermal Gas-Phase Heterogeneous CO2 Catalysis. Energy Environ. Sci. 2019, 12 (4), 1122–1142. 10.1039/C8EE02790K. [DOI] [Google Scholar]
- Chen G.; Gao R.; Zhao Y.; Li Z.; Waterhouse G. I. N.; Shi R.; Zhao J.; Zhang M.; Shang L.; Sheng G.; Zhang X.; Wen X.; Wu L.; Tung C.; Zhang T. Alumina-Supported CoFe Alloy Catalysts Derived from Layered-Double-Hydroxide Nanosheets for Efficient Photothermal CO2 Hydrogenation to Hydrocarbons. Adv. Mater. 2018, 30 (3), 1704663. 10.1002/adma.201704663. [DOI] [PubMed] [Google Scholar]
- O’Brien P. G.; Ghuman K. K.; Jelle A. A.; Sandhel A.; Wood T. E.; Loh J. Y. Y.; Jia J.; Perovic D.; Singh C. V.; Kherani N. P.; Mims C. A.; Ozin G. A. Enhanced Photothermal Reduction of Gaseous CO2 over Silicon Photonic Crystal Supported Ruthenium at Ambient Temperature. Energy Environ. Sci. 2018, 11 (12), 3443–3451. 10.1039/C8EE02347F. [DOI] [Google Scholar]
- Wu D.; Deng K.; Hu B.; Lu Q.; Liu G.; Hong X. Plasmon-Assisted Photothermal Catalysis of Low-Pressure CO2 Hydrogenation to Methanol over Pd/ZnO Catalyst. ChemCatChem 2019, 11 (6), 1598–1601. 10.1002/cctc.201802081. [DOI] [Google Scholar]
- Ren J.; Ouyang S.; Xu H.; Meng X.; Wang T.; Wang D.; Ye J. Targeting Activation of CO2 and H2 over Ru-Loaded Ultrathin Layered Double Hydroxides to Achieve Efficient Photothermal CO2 Methanation in Flow-Type System. Adv. Energy Mater. 2017, 7 (5), 1601657. 10.1002/aenm.201601657. [DOI] [Google Scholar]
- Guzman M. S.; Rengasamy K.; Binkley M. M.; Jones C.; Ranaivoarisoa T. O.; Singh R.; Fike D. A.; Meacham J. M.; Bose A. Phototrophic Extracellular Electron Uptake Is Linked to Carbon Dioxide Fixation in the Bacterium Rhodopseudomonas Palustris. Nat. Commun. 2019, 10 (1), 1355. 10.1038/s41467-019-09377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo H. M. Solar-to-Chemical and Solar-to-Fuel Production from CO2 by Metabolically Engineered Microorganisms. Curr. Opin. Biotechnol. 2017, 45, 1–7. 10.1016/j.copbio.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Liu C.; Gallagher J. J.; Sakimoto K. K.; Nichols E. M.; Chang C. J.; Chang M. C. Y.; Yang P. Nanowire-Bacteria Hybrids for Unassisted Solar Carbon Dioxide Fixation to Value-Added Chemicals. Nano Lett. 2015, 15 (5), 3634–3639. 10.1021/acs.nanolett.5b01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh F. E. Photocatalysis versus Photosynthesis: A Sensitivity Analysis of Devices for Solar Energy Conversion and Chemical Transformations. ACS Energy Lett. 2017, 2 (2), 445–453. 10.1021/acsenergylett.6b00665. [DOI] [Google Scholar]
- Rajeshwar K.; Thomas A.; Janaky C. Photocatalytic Activity of Inorganic Semiconductor Surfaces: Myths, Hype, and Reality. J. Phys. Chem. Lett. 2015, 6 (1), 139–147. 10.1021/jz502586p. [DOI] [PubMed] [Google Scholar]
- Xiao M.; Wang Z.; Luo B.; Wang S.; Wang L. Enhancing Photocatalytic Activity of Tantalum Nitride by Rational Suppression of Bulk, Interface and Surface Charge Recombination. Appl. Catal., B 2019, 246, 195–201. 10.1016/j.apcatb.2019.01.053. [DOI] [Google Scholar]
- Kamat P. V.; Jin S. Semiconductor Photocatalysis: “Tell Us the Complete Story!. ACS Energy Lett. 2018, 3 (3), 622–623. 10.1021/acsenergylett.8b00196. [DOI] [Google Scholar]
- Kramm U. I.; Marschall R.; Rose M. Pitfalls in Heterogeneous Thermal, Electro- and Photocatalysis. ChemCatChem 2019, 11 (11), 2563–2574. 10.1002/cctc.201900137. [DOI] [Google Scholar]
- Chang X.; Wang T.; Yang P.; Zhang G.; Gong J. The Development of Cocatalysts for Photoelectrochemical CO2 Reduction. Adv. Mater. 2019, 31 (31), 1804710. 10.1002/adma.201804710. [DOI] [PubMed] [Google Scholar]
- Song J. T.; Ryoo H.; Cho M.; Kim J.; Kim J. G.; Chung S.-Y.; Oh J. Nanoporous Au Thin Films on Si Photoelectrodes for Selective and Efficient Photoelectrochemical CO2 Reduction. Adv. Energy Mater. 2017, 7 (3), 1601103. 10.1002/aenm.201601103. [DOI] [Google Scholar]
- Pastor E.; Le Formal F.; Mayer M. T.; Tilley S. D.; Francàs L.; Mesa C. A.; Grätzel M.; Durrant J. R. Spectroelectrochemical Analysis of the Mechanism of (Photo)Electrochemical Hydrogen Evolution at a Catalytic Interface. Nat. Commun. 2017, 8 (1), 14280. 10.1038/ncomms14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Shi J.; Zi W.; Wang P.; Liu S. F. Recent Advances in Photoelectrochemical Applications of Silicon Materials for Solar-to-Chemicals Conversion. ChemSusChem 2017, 10 (22), 4324–4341. 10.1002/cssc.201701674. [DOI] [PubMed] [Google Scholar]
- Rongé J.; Bosserez T.; Martel D.; Nervi C.; Boarino L.; Taulelle F.; Decher G.; Bordiga S.; Martens J. A. Monolithic Cells for Solar Fuels. Chem. Soc. Rev. 2014, 43 (23), 7963–7981. 10.1039/C3CS60424A. [DOI] [PubMed] [Google Scholar]
- Nayak P. K.; Mahesh S.; Snaith H. J.; Cahen D. Photovoltaic Solar Cell Technologies: Analysing the State of the Art. Nat. Rev. Mater. 2019, 4 (4), 269–285. 10.1038/s41578-019-0097-0. [DOI] [Google Scholar]
- Green M. A.; Dunlop E. D.; Hohl-Ebinger J.; Yoshita M.; Kopidakis N.; Ho-Baillie A. W. Y. Solar Cell Efficiency Tables (Version 55). Prog. Photovoltaics 2020, 28 (1), 3–15. 10.1002/pip.3228. [DOI] [Google Scholar]
- Best Research-Cell Efficiency Chart | Photovoltaic Research. https://www.nrel.gov/pv/cell-efficiency.html (accessed 2020-03-19).
- Liu Z.; Sofia S. E.; Laine H. S.; Woodhouse M.; Wieghold S.; Peters I. M.; Buonassisi T. Revisiting Thin Silicon for Photovoltaics: A Technoeconomic Perspective. Energy Environ. Sci. 2020, 13 (1), 12–23. 10.1039/C9EE02452B. [DOI] [Google Scholar]
- Endrődi B.; Bencsik G.; Darvas F.; Jones R.; Rajeshwar K.; Janáky C. Continuous-Flow Electroreduction of Carbon Dioxide. Prog. Energy Combust. Sci. 2017, 62, 133–154. 10.1016/j.pecs.2017.05.005. [DOI] [Google Scholar]
- Kumaravel V.; Bartlett J.; Pillai S. C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5 (2), 486–519. 10.1021/acsenergylett.9b02585. [DOI] [Google Scholar]
- Akhundi A.; Habibi-Yangjeh A.; Abitorabi M.; Rahim Pouran S. Review on Photocatalytic Conversion of Carbon Dioxide to Value-Added Compounds and Renewable Fuels by Graphitic Carbon Nitride-Based Photocatalysts. Catal. Rev.: Sci. Eng. 2019, 61 (4), 595–628. 10.1080/01614940.2019.1654224. [DOI] [Google Scholar]
- Hiragond C.; Ali S.; Sorcar S.; In S.-I. In Hierarchical Nanostructured Photocatalysts for CO2 Photoreduction. Catalysts 2019, 9 (4), 370. 10.3390/catal9040370. [DOI] [Google Scholar]
- Christoforidis K. C.; Fornasiero P. Photocatalysis for Hydrogen Production and CO2 Reduction: The Case of Copper-Catalysts. ChemCatChem 2019, 11 (1), 368–382. 10.1002/cctc.201801198. [DOI] [Google Scholar]
- Tjandra A. D.; Huang J. Photocatalytic Carbon Dioxide Reduction by Photocatalyst Innovation. Chin. Chem. Lett. 2018, 29 (6), 734–746. 10.1016/j.cclet.2018.03.017. [DOI] [Google Scholar]
- Ma Y.; Wang Z.; Xu X.; Wang J. Review on Porous Nanomaterials for Adsorption and Photocatalytic Conversion of CO2. Chin. J. Catal. 2017, 38 (12), 1956–1969. 10.1016/S1872-2067(17)62955-3. [DOI] [Google Scholar]
- Huang Z.; Teramura K.; Asakura H.; Hosokawa S.; Tanaka T. Recent Progress in Photocatalytic Conversion of Carbon Dioxide over Gallium Oxide and Its Nanocomposites. Curr. Opin. Chem. Eng. 2018, 20, 114–121. 10.1016/j.coche.2018.02.012. [DOI] [Google Scholar]
- Sun H.; Wang S. Research Advances in the Synthesis of Nanocarbon-Based Photocatalysts and Their Applications for Photocatalytic Conversion of Carbon Dioxide to Hydrocarbon Fuels. Energy Fuels 2014, 28 (1), 22–36. 10.1021/ef401426x. [DOI] [Google Scholar]
- Low J.; Cheng B.; Yu J.; Jaroniec M. Carbon-Based Two-Dimensional Layered Materials for Photocatalytic CO2 Reduction to Solar Fuels. Energy Storage Mater. 2016, 3, 24–35. 10.1016/j.ensm.2015.12.003. [DOI] [Google Scholar]
- Chen X.; Jin F. Photocatalytic Reduction of Carbon Dioxide by Titanium Oxide-Based Semiconductors to Produce Fuels. Front. Energy. 2019, 13 (2), 207–220. 10.1007/s11708-019-0628-9. [DOI] [Google Scholar]
- Abdullah H.; Khan M. M. R.; Ong H. R.; Yaakob Z. Modified TiO2 Photocatalyst for CO2 Photocatalytic Reduction: An Overview. J. CO2 Util. 2017, 22, 15–32. 10.1016/j.jcou.2017.08.004. [DOI] [Google Scholar]
- Shehzad N.; Tahir M.; Johari K.; Murugesan T.; Hussain M. A Critical Review on TiO2 Based Photocatalytic CO2 Reduction System: Strategies to Improve Efficiency. J. CO2 Util. 2018, 26, 98–122. 10.1016/j.jcou.2018.04.026. [DOI] [Google Scholar]
- Xie H.; Wang J.; Ithisuphalap K.; Wu G.; Li Q. Recent Advances in Cu-Based Nanocomposite Photocatalysts for CO2 Conversion to Solar Fuels. J. Energy Chem. 2017, 26 (6), 1039–1049. 10.1016/j.jechem.2017.10.025. [DOI] [Google Scholar]
- Xiong Z.; Lei Z.; Li Y.; Dong L.; Zhao Y.; Zhang J. A Review on Modification of Facet-Engineered TiO2 for Photocatalytic CO2 Reduction. J. Photochem. Photobiol., C 2018, 36, 24–47. 10.1016/j.jphotochemrev.2018.07.002. [DOI] [Google Scholar]
- Chen D.; Zhang X.; Lee A. F. Synthetic Strategies to Nanostructured Photocatalysts for CO2 Reduction to Solar Fuels and Chemicals. J. Mater. Chem. A 2015, 3 (28), 14487–14516. 10.1039/C5TA01592H. [DOI] [Google Scholar]
- Razzaq A.; In S. TiO2 Based Nanostructures for Photocatalytic CO2 Conversion to Valuable Chemicals. Micromachines 2019, 10 (5), 326. 10.3390/mi10050326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. Q.; Xu Y. J. Photocatalytic Conversion of CO2 over Graphene-Based Composites: Current Status and Future Perspective. Nanoscale Horiz. 2016, 1 (3), 185–200. 10.1039/C5NH00113G. [DOI] [PubMed] [Google Scholar]
- Shen M.; Zhang L.; Shi J. Converting CO2 into Fuels by Graphitic Carbon Nitride-Based Photocatalysts. Nanotechnology 2018, 29, 412001. 10.1088/1361-6528/aad4c8. [DOI] [PubMed] [Google Scholar]
- Zeng S.; Kar P.; Thakur U. K.; Shankar K. A Review on Photocatalytic CO2 Reduction Using Perovskite Oxide Nanomaterials. Nanotechnology 2018, 29, 052001. 10.1088/1361-6528/aa9fb1. [DOI] [PubMed] [Google Scholar]
- Voiry D.; Shin H. S.; Loh K. P.; Chhowalla M. Low-Dimensional Catalysts for Hydrogen Evolution and CO2 Reduction. Nat. Rev. Chem. 2018, 2 (1), 105. 10.1038/s41570-017-0105. [DOI] [Google Scholar]
- Li X.; Wen J.; Low J.; Fang Y.; Yu J. Design and Fabrication of Semiconductor Photocatalyst for Photocatalytic Reduction of CO2 to Solar Fuel. Sci. China Mater. 2014, 57 (1), 70–100. 10.1007/s40843-014-0003-1. [DOI] [Google Scholar]
- Shi R.; Waterhouse G. I. N.; Zhang T. Recent Progress in Photocatalytic CO2 Reduction Over Perovskite Oxides. Sol. RRL 2017, 1 (11), 1700126. 10.1002/solr.201700126. [DOI] [Google Scholar]
- Low J.; Cheng B.; Yu J. Surface Modification and Enhanced Photocatalytic CO2 Reduction Performance of TiO2: A Review. Appl. Surf. Sci. 2017, 392, 658–686. 10.1016/j.apsusc.2016.09.093. [DOI] [Google Scholar]
- Ola O.; Maroto-Valer M. M. Review of Material Design and Reactor Engineering on TiO2 Photocatalysis for CO2 Reduction. J. Photochem. Photobiol., C 2015, 24, 16–42. 10.1016/j.jphotochemrev.2015.06.001. [DOI] [Google Scholar]
- Das S.; Wan Daud W. M. A. Photocatalytic CO2 Transformation into Fuel: A Review on Advances in Photocatalyst and Photoreactor. Renewable Sustainable Energy Rev. 2014, 39, 765–805. 10.1016/j.rser.2014.07.046. [DOI] [Google Scholar]
- Kumar S.; Jain S.; Yadav Lamba B.; Kumar P. Epigrammatic Status and Perspective of Sequestration of Carbon Dioxide: Role of TiO2 as Photocatalyst. Sol. Energy 2018, 159, 423–433. 10.1016/j.solener.2017.11.007. [DOI] [Google Scholar]
- Ali S.; Flores M. C.; Razzaq A.; Sorcar S.; Hiragond C. B.; Kim H. R.; Park Y. H.; Hwang Y.; Kim H. S.; Kim H.; Gong E. H.; Lee J.; Kim D.; In S. Gas Phase Photocatalytic CO2 Reduction, “A Brief Overview for Benchmarking.. Catalysts 2019, 9 (9), 727. 10.3390/catal9090727. [DOI] [Google Scholar]
- Roy N.; Suzuki N.; Terashima C.; Fujishima A. Recent Improvements in the Production of Solar Fuels: From CO2 Reduction to Water Splitting and Artificial Photosynthesis. Bull. Chem. Soc. Jpn. 2019, 92 (1), 178–192. 10.1246/bcsj.20180250. [DOI] [Google Scholar]
- Neaţu Ş.; Maciá-Agulló J. A.; Garcia H. Solar Light Photocatalytic CO2 Reduction: General Considerations and Selected Bench-Mark Photocatalysts. Int. J. Mol. Sci. 2014, 15 (4), 5246–5262. 10.3390/ijms15045246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh I. Conversion of Carbon Dioxide into Methanol - A Potential Liquid Fuel: Fundamental Challenges and Opportunities (a Review). Renewable Sustainable Energy Rev. 2014, 31, 221–257. 10.1016/j.rser.2013.11.045. [DOI] [Google Scholar]
- Choi J. Y.; Lim C. K.; Park B.; Kim M.; Jamal A.; Song H. Surface Activation of Cobalt Oxide Nanoparticles for Photocatalytic Carbon Dioxide Reduction to Methane. J. Mater. Chem. A 2019, 7 (25), 15068–15072. 10.1039/C9TA04323C. [DOI] [Google Scholar]
- Li X.; Sun Y.; Xu J.; Shao Y.; Wu J.; Xu X.; Pan Y.; Ju H.; Zhu J.; Xie Y. Selective Visible-Light-Driven Photocatalytic CO2 Reduction to CH4 Mediated by Atomically Thin CuIn5S8 Layers. Nat. Energy 2019, 4 (8), 690–699. 10.1038/s41560-019-0431-1. [DOI] [Google Scholar]
- Pang R.; Teramura K.; Asakura H.; Hosokawa S.; Tanaka T. Effect of Thickness of Chromium Hydroxide Layer on Ag Cocatalyst Surface for Highly Selective Photocatalytic Conversion of CO2 by H2O. ACS Sustainable Chem. Eng. 2019, 7 (2), 2083–2090. 10.1021/acssuschemeng.8b04665. [DOI] [Google Scholar]
- Xu Y.; Mo J.; Fu Z. C.; Liu S.; Yang Z.; Fu W. F. An Exceptionally Efficient Co-Co2P@N, P-Codoped Carbon Hybrid Catalyst for Visible Light-Driven CO2-to-CO Conversion. Chem. - Eur. J. 2018, 24 (34), 8596–8602. 10.1002/chem.201801465. [DOI] [PubMed] [Google Scholar]
- Kuriki R.; Sekizawa K.; Ishitani O.; Maeda K. Visible-Light-Driven CO2 Reduction with Carbon Nitride: Enhancing the Activity of Ruthenium Catalysts. Angew. Chem., Int. Ed. 2015, 54 (8), 2406–2409. 10.1002/anie.201411170. [DOI] [PubMed] [Google Scholar]
- Genoni A.; Chirdon D. N.; Boniolo M.; Sartorel A.; Bernhard S.; Bonchio M. Tuning Iridium Photocatalysts and Light Irradiation for Enhanced CO2 Reduction. ACS Catal. 2017, 7 (1), 154–160. 10.1021/acscatal.6b03227. [DOI] [Google Scholar]
- Yang H.; Han N.; Deng J.; Wu J.; Wang Y.; Hu Y.; Ding P.; Li Y.; Li Y.; Lu J. Selective CO2 Reduction on 2D Mesoporous Bi Nanosheets. Adv. Energy Mater. 2018, 8 (35), 1801536. 10.1002/aenm.201801536. [DOI] [Google Scholar]
- Chen J.; Yin J.; Zheng X.; Ait Ahsaine H.; Zhou Y.; Dong C.; Mohammed O. F.; Takanabe K.; Bakr O. M. Compositionally Screened Eutectic Catalytic Coatings on Halide Perovskite Photocathodes for Photoassisted Selective CO2 Reduction. ACS Energy Lett. 2019, 4 (6), 1279–1286. 10.1021/acsenergylett.9b00751. [DOI] [Google Scholar]
- Schreier M.; Gao P.; Mayer M. T.; Luo J.; Moehl T.; Nazeeruddin M. K.; Tilley S. D.; Grätzel M. Efficient and Selective Carbon Dioxide Reduction on Low Cost Protected Cu2O Photocathodes Using a Molecular Catalyst. Energy Environ. Sci. 2015, 8 (3), 855–861. 10.1039/C4EE03454F. [DOI] [Google Scholar]
- Yuan J.; Yang L.; Hao C. Communication—Lithium-Doped CuFeO2 Thin Film Electrodes for Photoelectrochemical Reduction of Carbon Dioxide to Methanol. J. Electrochem. Soc. 2019, 166 (14), H718–H720. 10.1149/2.0921914jes. [DOI] [Google Scholar]
- Kang U.; Choi S. K.; Ham D. J.; Ji S. M.; Choi W.; Han D. S.; Abdel-Wahab A.; Park H. Photosynthesis of Formate from CO2 and Water at 1% Energy Efficiency via Copper Iron Oxide Catalysis. Energy Environ. Sci. 2015, 8 (9), 2638–2643. 10.1039/C5EE01410G. [DOI] [Google Scholar]
- Kang U.; Yoon S. H.; Han D. S.; Park H. Synthesis of Aliphatic Acids from CO2 and Water at Efficiencies Close to the Photosynthesis Limit Using Mixed Copper and Iron Oxide Films. ACS Energy Lett. 2019, 4 (9), 2075–2080. 10.1021/acsenergylett.9b01281. [DOI] [Google Scholar]
- Schreier M.; Héroguel F.; Steier L.; Ahmad S.; Luterbacher J. S.; Mayer M. T.; Luo J.; Grätzel M. Solar Conversion of CO2 to CO Using Earth-Abundant Electrocatalysts Prepared by Atomic Layer Modification of CuO. Nat. Energy 2017, 2 (7), 17087. 10.1038/nenergy.2017.87. [DOI] [Google Scholar]
- Zhou L. Q.; Ling C.; Zhou H.; Wang X.; Liao J.; Reddy G. K.; Deng L.; Peck T. C.; Zhang R.; Whittingham M. S.; Wang C.; Chu C.; Yao Y.; Jia H. A High-Performance Oxygen Evolution Catalyst in Neutral-pH for Sunlight-Driven CO2 Reduction. Nat. Commun. 2019, 10 (1), 4081. 10.1038/s41467-019-12009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Liu J.; Wang Y.; Wang Y.; Zheng G. Efficient Solar-Driven Electrocatalytic CO2 Reduction in a Redox-Medium-Assisted System. Nat. Commun. 2018, 9 (1), 5003. 10.1038/s41467-018-07380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W.; Richter M. H.; Sullivan I.; Larson D. M.; Xiang C.; Brunschwig B. S.; Atwater H. A.; Xiang C.; Brunschwig B. S.; Atwater H. A. CO2 Reduction to CO with 19% Efficiency in a Solar-Driven Gas Diffusion Electrode Flow Cell under Outdoor Solar Illumination. ACS Energy Lett. 2020, 5 (2), 470–476. 10.1021/acsenergylett.9b02576. [DOI] [Google Scholar]
- Ma M.; Liu K.; Shen J.; Kas R.; Smith W. A. In Situ Fabrication and Reactivation of Highly Selective and Stable Ag Catalysts for Electrochemical CO2 Conversion. ACS Energy Lett. 2018, 3 (6), 1301–1306. 10.1021/acsenergylett.8b00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S.; Jin R.; Jin R. Opportunities and Challenges in CO2 Reduction by Gold- and Silver-Based Electrocatalysts: From Bulk Metals to Nanoparticles and Atomically Precise Nanoclusters. ACS Energy Lett. 2018, 3 (2), 452–462. 10.1021/acsenergylett.7b01104. [DOI] [Google Scholar]
- Kodaimati M. S.; McClelland K. P.; He C.; Lian S.; Jiang Y.; Zhang Z.; Weiss E. A. Viewpoint: Challenges in Colloidal Photocatalysis and Some Strategies for Addressing Them. Inorg. Chem. 2018, 57 (7), 3659–3670. 10.1021/acs.inorgchem.7b03182. [DOI] [PubMed] [Google Scholar]
- Wang K.; Lu H.; Zhu X.; Lin Y.; Beard M. C.; Yan Y.; Chen X. Ultrafast Reaction Mechanisms in Perovskite Based Photocatalytic C-C Coupling. ACS Energy Lett. 2020, 5 (2), 566–571. 10.1021/acsenergylett.9b02714. [DOI] [Google Scholar]
- Samu G. F.; Balog Á.; De Angelis F.; Meggiolaro D.; Kamat P. V.; Janáky C. Electrochemical Hole Injection Selectively Expels Iodide from Mixed Halide Perovskite Films. J. Am. Chem. Soc. 2019, 141 (27), 10812–10820. 10.1021/jacs.9b04568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranek R. Selectivity of Chemical Conversions: Do Light-Driven Photoelectrocatalytic Processes Hold Special Promise?. Angew. Chem., Int. Ed. 2019, 58 (47), 16724–16729. 10.1002/anie.201908654. [DOI] [PubMed] [Google Scholar]
- Li H.; Zhang X.; MacFarlane D. R. Carbon Quantum Dots/Cu2O Heterostructures for Solar-Light-Driven Conversion of CO2 to Methanol. Adv. Energy Mater. 2015, 5 (5), 1401077. 10.1002/aenm.201401077. [DOI] [Google Scholar]
- Brito J. F.; Genovese C.; Tavella F.; Ampelli C.; Boldrin Zanoni M. V.; Centi G.; Perathoner S. CO2 Reduction of Hybrid Cu2O-Cu/Gas Diffusion Layer Electrodes and Their Integration in a Cu-based Photoelectrocatalytic Cell. ChemSusChem 2019, 12 (18), 4274–4284. 10.1002/cssc.201901352. [DOI] [PubMed] [Google Scholar]
- Kim J. H.; Magesh G.; Kang H. J.; Banu M.; Kim J. H.; Lee J.; Lee J. S. Carbonate-Coordinated Cobalt Co-Catalyzed BiVO4/WO3 Composite Photoanode Tailored for CO2 Reduction to Fuels. Nano Energy 2015, 15, 153–163. 10.1016/j.nanoen.2015.04.022. [DOI] [Google Scholar]
- Chang X.; Wang T.; Zhao Z. J.; Yang P.; Greeley J.; Mu R.; Zhang G.; Gong Z.; Luo Z.; Chen J.; Cui Y.; Ozin G. A.; Gong J. Tuning Cu/Cu2O Interfaces for the Reduction of Carbon Dioxide to Methanol in Aqueous Solutions. Angew. Chem., Int. Ed. 2018, 57 (47), 15415–15419. 10.1002/anie.201805256. [DOI] [PubMed] [Google Scholar]
- Huan T. N.; Dalla Corte D. A.; Lamaison S.; Karapinar D.; Lutz L.; Menguy N.; Foldyna M.; Turren-Cruz S. H.; Hagfeldt A.; Bella F.; Fontecave M.; Mougel V. Low-Cost High-Efficiency System for Solar-Driven Conversion of CO2 to Hydrocarbons. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (20), 9735–9740. 10.1073/pnas.1815412116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb Nath N. C.; Choi S. Y.; Jeong H. W.; Lee J. J.; Park H. Stand-Alone Photoconversion of Carbon Dioxide on Copper Oxide Wire Arrays Powered by Tungsten Trioxide/Dye-Sensitized Solar Cell Dual Absorbers. Nano Energy 2016, 25, 51–59. 10.1016/j.nanoen.2016.04.025. [DOI] [Google Scholar]
- Yu S.; Jain P. K. Plasmonic Photosynthesis of C1-C3 Hydrocarbons from Carbon Dioxide Assisted by an Ionic Liquid. Nat. Commun. 2019, 10 (1), 2022. 10.1038/s41467-019-10084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara N.; Kamimura S.; Tsubota T.; Ohno T. Photoelectrochemical CO2 Reduction by a P-Type Boron-Doped g-C3N4 Electrode under Visible Light. Appl. Catal., B 2016, 192, 193–198. 10.1016/j.apcatb.2016.03.055. [DOI] [Google Scholar]
- Mateo D.; Asiri A. M.; Albero J.; García H. The Mechanism of Photocatalytic CO2 Reduction by Graphene-Supported Cu2O Probed by Sacrificial Electron Donors. Photochem. Photobiol. Sci. 2018, 17 (6), 829–834. 10.1039/C7PP00442G. [DOI] [PubMed] [Google Scholar]
- Ci C.; Carbó J. J.; Neumann R.; Graaf C. De; Poblet J. M. Photoreduction Mechanism of CO2 to CO Catalyzed by a Rhenium(I)-Polyoxometalate Hybrid Compound. ACS Catal. 2016, 6 (10), 6422–6428. 10.1021/acscatal.6b01638. [DOI] [Google Scholar]
- Zheng Y.; Zhang W.; Li Y.; Chen J.; Yu B.; Wang J.; Zhang L.; Zhang J. Energy Related CO2 Conversion and Utilization: Advanced Materials/Nanomaterials, Reaction Mechanisms and Technologies. Nano Energy 2017, 40, 512–539. 10.1016/j.nanoen.2017.08.049. [DOI] [Google Scholar]
- Poudyal S.; Laursen S. Photocatalytic CO2 Reduction by H2O: Insights from Modeling Electronically Relaxed Mechanisms. Catal. Sci. Technol. 2019, 9 (4), 1048–1059. 10.1039/C8CY02046A. [DOI] [Google Scholar]
- Karamian E.; Sharifnia S. On the General Mechanism of Photocatalytic Reduction of CO2. J. CO2 Util. 2016, 16, 194–203. 10.1016/j.jcou.2016.07.004. [DOI] [Google Scholar]
- Kang H. Y.; Nam D. H.; Yang K. D.; Joo W.; Kwak H.; Kim H. H.; Hong S. H.; Nam K. T.; Joo Y. C. Synthetic Mechanism Discovery of Monophase Cuprous Oxide for Record High Photoelectrochemical Conversion of CO2 to Methanol in Water. ACS Nano 2018, 12 (8), 8187–8196. 10.1021/acsnano.8b03293. [DOI] [PubMed] [Google Scholar]
- Uzunova E. L.; Seriani N.; Mikosch H. CO2 Conversion to Methanol on Cu(I) Oxide Nanolayers and Clusters: An Electronic Structure Insight into the Reaction Mechanism. Phys. Chem. Chem. Phys. 2015, 17 (16), 11088–11094. 10.1039/C5CP01267H. [DOI] [PubMed] [Google Scholar]
- Yin G.; Huang X.; Chen T.; Zhao W.; Bi Q.; Xu J.; Han Y.; Huang F. Hydrogenated Blue Titania for Efficient Solar to Chemical Conversions: Preparation, Characterization, and Reaction Mechanism of CO2 Reduction. ACS Catal. 2018, 8 (2), 1009–1017. 10.1021/acscatal.7b03473. [DOI] [Google Scholar]
- Meister S.; Reithmeier R. O.; Tschurl M.; Heiz U.; Rieger B. Unraveling Side Reactions in the Photocatalytic Reduction of CO2: Evidence for Light-Induced Deactivation Processes in Homogeneous Photocatalysis. ChemCatChem 2015, 7 (4), 690–697. 10.1002/cctc.201402984. [DOI] [Google Scholar]
- Sommers J. M.; Alderman N. P.; Viasus C. J.; Gambarotta S. Revisiting the Behaviour of BiVO4 as a Carbon Dioxide Reduction Photo-Catalyst. Dalt. Trans. 2017, 46 (19), 6404–6408. 10.1039/C7DT00414A. [DOI] [PubMed] [Google Scholar]
- Sriramagiri G. M.; Ahmed N.; Luc W.; Dobson K. D.; Hegedus S. S.; Jiao F. Toward a Practical Solar-Driven CO2 Flow Cell Electrolyzer: Design and Optimization. ACS Sustainable Chem. Eng. 2017, 5 (11), 10959–10966. 10.1021/acssuschemeng.7b02853. [DOI] [Google Scholar]
- Endrődi B.; Kecsenovity E.; Samu A.; Darvas F.; Jones R. V.; Török V.; Danyi A.; Janáky C. Multilayer Electrolyzer Stack Converts Carbon Dioxide to Gas Products at High Pressure with High Efficiency. ACS Energy Lett. 2019, 4 (7), 1770–1777. 10.1021/acsenergylett.9b01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain F.; Tang P.; Carretero N. M.; Andreu T.; Gerling L. G.; Voz C.; Arbiol J.; Morante J. R. A Prototype Reactor for Highly Selective Solar-Driven CO2 Reduction to Synthesis Gas Using Nanosized Earth-Abundant Catalysts and Silicon Photovoltaics. Energy Environ. Sci. 2017, 10 (10), 2256–2266. 10.1039/C7EE01747B. [DOI] [Google Scholar]
- Haas T.; Krause R.; Weber R.; Demler M.; Schmid G. Technical Photosynthesis Involving CO2 Electrolysis and Fermentation. Nat. Catal. 2018, 1 (1), 32–39. 10.1038/s41929-017-0005-1. [DOI] [Google Scholar]
- Ren D.; Loo N. W. X.; Gong L.; Yeo B. S. Continuous Production of Ethylene from Carbon Dioxide and Water Using Intermittent Sunlight. ACS Sustainable Chem. Eng. 2017, 5 (10), 9191–9199. 10.1021/acssuschemeng.7b02110. [DOI] [Google Scholar]
- Deng W.; Zhang L.; Dong H.; Chang X.; Wang T.; Gong J. Achieving Convenient CO2 Electroreduction and Photovoltage in Tandem Using Potential-Insensitive Disordered Ag Nanoparticles. Chem. Sci. 2018, 9 (32), 6599–6604. 10.1039/C8SC02576B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. J.; Piao G.; Kim S.; Yang S. Y.; Park Y.; Han D. S.; Shon H. K.; Hoffmann M. R.; Park H. High-Efficiency Solar Desalination Accompanying Electrocatalytic Conversions of Desalted Chloride and Captured Carbon Dioxide. ACS Sustainable Chem. Eng. 2019, 7 (18), 15320–15328. 10.1021/acssuschemeng.9b02640. [DOI] [Google Scholar]
- Gurudayal; Bullock J.; Srankó D. F.; Towle C. M.; Lum Y.; Hettick M.; Scott M. C.; Javey A.; Ager J. Efficient Solar-Driven Electrochemical CO2 Reduction to Hydrocarbons and Oxygenates. Energy Environ. Sci. 2017, 10 (10), 2222–2230. 10.1039/C7EE01764B. [DOI] [Google Scholar]
- Wang L.; Sofer Z.; Pumera M. Will Any Crap We Put into Graphene Increase Its Electrocatalytic Effect?. ACS Nano 2020, 14 (1), 21–25. 10.1021/acsnano.9b00184. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Xiang C. Comparative Analysis of Solar-to-Fuel Conversion Efficiency: A Direct, One-Step Electrochemical CO2 Reduction Reactor versus a Two-Step, Cascade Electrochemical CO2 Reduction Reactor. ACS Energy Lett. 2018, 3 (8), 1892–1897. 10.1021/acsenergylett.8b01077. [DOI] [Google Scholar]
- Higgins D.; Hahn C.; Xiang C.; Jaramillo T. F.; Weber A. Z. Gas-Diffusion Electrodes for Carbon Dioxide Reduction: A New Paradigm. ACS Energy Lett. 2019, 4 (1), 317–324. 10.1021/acsenergylett.8b02035. [DOI] [Google Scholar]
- Szczesny J.; Ruff A.; Oliveira A. R.; Pita M.; Pereira I. A. C.; De Lacey A. L.; Schuhmann W. Electroenzymatic CO2 Fixation Using Redox Polymer/Enzyme-Modified Gas Diffusion Electrodes. ACS Energy Lett. 2020, 5, 321–327. 10.1021/acsenergylett.9b02436. [DOI] [Google Scholar]
- Liu K.; Smith W. A.; Burdyny T. Introductory Guide to Assembling and Operating Gas Diffusion Electrodes for Electrochemical CO2 Reduction. ACS Energy Lett. 2019, 4 (3), 639–643. 10.1021/acsenergylett.9b00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Liu B.; Ren Y.; Yuan Y.; Zhao H.; Yang H.; Liu S. Hydrogenated Nanotubes/Nanowires Assembled from TiO2 Nanoflakes with Exposed {111} Facets: Excellent Photo-Catalytic CO2 Reduction Activity and Charge Separation Mechanism between (111) and (111) Polar Surfaces. J. Mater. Chem. A 2019, 7 (24), 14761–14775. 10.1039/C9TA00736A. [DOI] [Google Scholar]
- Wan L.; Zhou Q.; Wang X.; Wood T. E.; Wang L.; Duchesne P. N.; Guo J.; Yan X.; Xia M.; Li Y. F.; Jelle A. A.; Ulmer U.; Jia J.; Li T.; Sun W.; Ozin G. A. Cu2O Nanocubes with Mixed Oxidation-State Facets for (Photo)Catalytic Hydrogenation of Carbon Dioxide. Nat. Catal. 2019, 2 (10), 889–898. 10.1038/s41929-019-0338-z. [DOI] [Google Scholar]
- Gao C.; Meng Q.; Zhao K.; Yin H.; Wang D.; Guo J.; Zhao S.; Chang L.; He M.; Li Q.; Zhao H.; Huang X.; Gao Y.; Tang Z. Co3O4 Hexagonal Platelets with Controllable Facets Enabling Highly Efficient Visible-Light Photocatalytic Reduction of CO2. Adv. Mater. 2016, 28 (30), 6485–6490. 10.1002/adma.201601387. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Chen X.; Fang M.; Chen J.; Li Y.; Xie Z.; Kuang Q.; Zheng L. Photo-Induced Au-Pd Alloying at TiO2 {101} Facets Enables Robust CO2 Photocatalytic Reduction into Hydrocarbon Fuels. J. Mater. Chem. A 2019, 7 (3), 1334–1340. 10.1039/C8TA09412H. [DOI] [Google Scholar]
- Wu Y. A.; McNulty I.; Liu C.; Lau K. C.; Liu Q.; Paulikas A. P.; Sun C. J.; Cai Z.; Guest J. R.; Ren Y.; Stamenkovic V.; Curtiss L. A.; Liu Y.; Rajh T. Facet-Dependent Active Sites of a Single Cu2O Particle Photocatalyst for CO2 Reduction to Methanol. Nat. Energy 2019, 4 (11), 957–968. 10.1038/s41560-019-0490-3. [DOI] [Google Scholar]
- Zhao Y.; Wei Y.; Wu X.; Zheng H.; Zhao Z.; Liu J.; Li J. Graphene-Wrapped Pt/TiO2 Photocatalysts with Enhanced Photogenerated Charges Separation and Reactant Adsorption for High Selective Photoreduction of CO2 to CH4. Appl. Catal., B 2018, 226, 360–372. 10.1016/j.apcatb.2017.12.071. [DOI] [Google Scholar]
- Xia C.; Zhu P.; Jiang Q.; Pan Y.; Liang W.; Stavitski E.; Alshareef H. N.; Wang H. Continuous Production of Pure Liquid Fuel Solutions via Electrocatalytic CO2 Reduction Using Solid-Electrolyte Devices. Nat. Energy 2019, 4 (9), 776–785. 10.1038/s41560-019-0451-x. [DOI] [Google Scholar]
- Kong Z. C.; Liao J. F.; Dong Y. J.; Xu Y. F.; Chen H. Y.; Kuang D.-B.; Su C. Y. Core@shell CsPbBr3@zeolitic Imidazolate Framework Nanocomposite for Efficient Photocatalytic CO2 Reduction. ACS Energy Lett. 2018, 3 (11), 2656–2662. 10.1021/acsenergylett.8b01658. [DOI] [Google Scholar]
- Kecsenovity E.; Endrödi B.; Tóth P. S.; Zou Y.; Dryfe R. A. W.; Rajeshwar K.; Janáky C. Enhanced Photoelectrochemical Performance of Cuprous Oxide/Graphene Nanohybrids. J. Am. Chem. Soc. 2017, 139 (19), 6682–6692. 10.1021/jacs.7b01820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kecsenovity E.; Endrodi B.; Pápa Z.; Hernádi K.; Rajeshwar K.; Janáky C. Decoration of Ultra-Long Carbon Nanotubes with Cu2O Nanocrystals: A Hybrid Platform for Enhanced Photoelectrochemical CO2 Reduction. J. Mater. Chem. A 2016, 4 (8), 3139–3147. 10.1039/C5TA10457B. [DOI] [Google Scholar]
- Kormányos A.; Hursán D.; Janáky C. Photoelectrochemical Behavior of PEDOT/Nanocarbon Electrodes: Fundamentals and Structure-Property Relationships. J. Phys. Chem. C 2018, 122 (25), 13682–13690. 10.1021/acs.jpcc.8b00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh F. E. Inorganic Nanostructures for Photoelectrochemical and Photocatalytic Water Splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. 10.1039/C2CS35266D. [DOI] [PubMed] [Google Scholar]
- Rajeshwar K.Electron Transfer at Semiconductor-Electrolyte Interfaces. Electron Transfer in Chemistry; Balzani V., Ed.; Wiley- VCH: Weinheim, 2001. [Google Scholar]
- Boettcher S. W.; Spurgeon J. M.; Putnam M. C.; Warren E. L.; Turner-Evans D. B.; Kelzenberg M. D.; Maiolo J. R.; Atwater H. A.; Lewis N. S. Energy-Conversion Properties of Vapor-Liquid-Solid-Grown Silicon Wire-Array Photocathodes. Science 2010, 327 (5962), 185–187. 10.1126/science.1180783. [DOI] [PubMed] [Google Scholar]
- Warren E. L.; Atwater H. a.; Lewis N. S. Silicon Microwire Arrays for Solar Energy-Conversion Applications. J. Phys. Chem. C 2014, 118 (2), 747–759. 10.1021/jp406280x. [DOI] [Google Scholar]
- Goodey A. P.; Eichfeld S. M.; Lew K.-K.; Redwing J. M.; Mallouk T. E. Silicon Nanowire Array Photelectrochemical Cells. J. Am. Chem. Soc. 2007, 129 (41), 12344–12345. 10.1021/ja073125d. [DOI] [PubMed] [Google Scholar]
- Panayotov D. A.; Frenkel A. I.; Morris J. R. Catalysis and Photocatalysis by Nanoscale Au/TiO2: Perspectives for Renewable Energy. ACS Energy Lett. 2017, 2 (5), 1223–1231. 10.1021/acsenergylett.7b00189. [DOI] [Google Scholar]
- Negrín-Montecelo Y.; Comesaña-Hermo M.; Khorashad L. K.; Sousa-Castillo A.; Wang Z.; Pérez-Lorenzo M.; Liedl T.; Govorov A. O.; Correa-Duarte M. A. Photophysical Effects hehind the Efficiency of Hot Electron Injection in Plasmon-Assisted Catalysis: The Joint Role of Morphology and Composition. ACS Energy Lett. 2020, 5 (2), 395–402. 10.1021/acsenergylett.9b02478. [DOI] [Google Scholar]
- Creel E. B.; Corson E. R.; Eichhorn J.; Kostecki R.; Urban J. J.; McCloskey B. D. Directing Selectivity of Electrochemical Carbon Dioxide Reduction Using Plasmonics. ACS Energy Lett. 2019, 4 (5), 1098–1105. 10.1021/acsenergylett.9b00515. [DOI] [Google Scholar]
- Spitler M. T.; Modestino M. A.; Deutsch T. G.; Xiang C. X.; Durrant J. R.; Esposito D. V.; Haussener S.; Maldonado S.; Sharp I. D.; Parkinson B. A.; Ginley D. S.; Houle F. A.; Hannappel T.; Neale N. R.; Nocera D. G.; McIntyre P. C. Practical Challenges in the Development of Photoelectrochemical Solar Fuels Production. Sustain. Energy Fuels 2020, 4 (3), 985–995. 10.1039/C9SE00869A. [DOI] [Google Scholar]
- Xu P.; Huang T.; Huang J.; Yan Y.; Mallouk T. E. Dye-Sensitized Photoelectrochemical Water Oxidation through a Buried Junction. Proc. Natl. Acad. Sci. U. S. A. 2018, 115 (27), 6946–6951. 10.1073/pnas.1804728115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tembhurne S.; Nandjou F.; Haussener S. A Thermally Synergistic Photo-Electrochemical Hydrogen Generator Operating under Concentrated Solar Irradiation. Nat. Energy 2019, 4 (5), 399–407. 10.1038/s41560-019-0373-7. [DOI] [Google Scholar]
- Verma S.; Lu S.; Kenis P. J. A. Co-Electrolysis of CO2 and Glycerol as a Pathway to Carbon Chemicals with Improved Technoeconomics Due to Low Electricity Consumption. Nat. Energy 2019, 4 (6), 466–474. 10.1038/s41560-019-0374-6. [DOI] [Google Scholar]
- Salvatore D.; Berlinguette C. P. Voltage Matters When Reducing CO2 in an Electrochemical Flow Cell. ACS Energy Lett. 2020, 5 (1), 215–220. 10.1021/acsenergylett.9b02356. [DOI] [Google Scholar]
- Castro S.; Albo J.; Irabien A. Photoelectrochemical Reactors for CO2 Utilization. ACS Sustainable Chem. Eng. 2018, 6 (12), 15877–15894. 10.1021/acssuschemeng.8b03706. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.