Abstract
Backgrounds
Engineering yeast as a consolidated bioprocessing (CBP) microorganism by surface assembly of cellulosomes has been aggressively utilized for cellulosic ethanol production. However, most of the previous studies focused on Saccharomyces cerevisiae, achieving efficient conversion of phosphoric acid-swollen cellulose (PASC) or microcrystalline cellulose (Avicel) but not carboxymethyl cellulose (CMC) to ethanol, with an average titer below 2 g/L.
Results
Harnessing an ultra-high-affinity IM7/CL7 protein pair, here we describe a method to engineer Pichia pastoris with minicellulosomes by in vitro assembly of three recombinant cellulases including an endoglucanase (EG), an exoglucanase (CBH) and a β-glucosidase (BGL), as well as a carbohydrate-binding module (CBM) on the cell surface. For the first time, the engineered yeasts enable efficient and direct conversion of CMC to bioethanol, observing an impressive ethanol titer of 5.1 g/L.
Conclusions
The research promotes the application of P. pastoris as a CBP cell factory in cellulosic ethanol production and provides a promising platform for screening the cellulases from different species to construct surface-assembly celluosome.
Keywords: Cellulosome, Pichia pastoris, Consolidate bioprocessing (CBP), Carboxymethyl cellulose (CMC), Bioethanol
Background
Cellulosic biomass derived from low-value agricultural and wood pulping wastes is likely the most abundant renewable resource in the world [1–3]. In the past decades, production of bioethanol from cellulose has increasingly attracted attention due to the low cost and environmental friendliness. However, conversion of cellulose into fermentable sugars capable of utilization by microbes is still challenging [2, 4], largely limiting the industrial production of cellulosic bioethanol. In a traditional process, cellulosic biomass is degraded by commercial cellulases followed by microbial fermentation, leading to the production is time consuming and expensive [5–7]. To address the issue, several new strategies have been developed such as secretory expression of cellulases by bacteria [8], in vivo assembly of cellulosomes [9, 10] within microorganisms [11–13], as well as the consolidated bioprocessing (CBP) that combines enzyme production, cellulose hydrolysis, and biological fermentation into a single process [14, 15].
Yeast, especially for Saccharomyces cerevisiae, has been widely considered as an ideal CBP candidate for ethanol production due to its high ethanol productivity and strong ethanol tolerance [16]. In early studies, cellulases were cell-secreted in S. cerevisiae culture medium or independently displayed on the cell surface [17, 18], but the bioethanol yields were often quite low. Currently, the works demonstrated that in vivo or in vitro assembly of multiple cellulases on the S. cerevisiae cell surface in a structure termed cellulosome [19, 20] can significantly increase the ethanol yields [21–24]. In nature, the cellulosome is a complicated multi-enzyme machine produced by many cellulolytic microorganisms [25, 26]. Those methods required displaying multiple components on the yeast surface, including heterogeneous dockerin–cohesin pairs, carbohydrate-binding modules (CBMs) and appropriated bacterial cellulases, leading to low displaying efficiency sometimes. To date, microcrystalline cellulose (Avicel) or phosphoric acid-swollen cellulose (PASC) has been successfully utilized as the substrate for yeast fermentation, though the ethanol yields cannot meet the requirement of industrial production. Moreover, carboxymethyl cellulose (CMC) is difficult to be converted by S. cerevisiae, possibly because of its ultra-high viscosity that weakens the diffusion of hydrolysis products and influences the ethanol fermentation [27, 28]. Collectively, more effort is needed to achieve the goal of industrial production of cellulosic ethanol using yeast as the CBP cell factory.
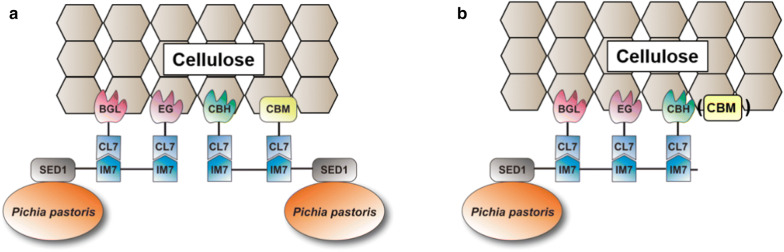
Recently, we developed an indirect Pichia pastoris surface-display method [29] that can simply display various enzymes with an average efficiency ten times higher than that of commonly used S. cerevisiae surface-display methods. Compared with S. cerevisiae, P. pastoris can achieve a much higher cell density in fermentation [1]. In practical applications, P. pastoris has been successfully applied in whole-cell biocatalysis for biodiesel production [30]. Therefore, we believe that P. pastoris is more suitable for catalyzing the reactions in greater viscosity, such as conversion of the high-viscosity CMC to ethanol. In this work, we want to expand our approach for construction of minicellulosomes on the P. pastoris cell surface, and then employ the engineered yeasts to produce cellulosic bioethanol. First, we harnessed an ultra-high-affinity IM7/CL7 protein pair [31] rather than the conventional dockerin–cohesin pairs for cellulosome assembly. In this system, the CL7 tag that engineered from the E. coli Colicin E7 DNase (CE7) retains the ultra-high-binding affinity (KD ≈ 10−14–10−17 M) with its inhibitor Immunity protein 7 (IM7). According to the design (Fig. 1), the IM7 scaffoldin proteins were repeatedly displayed for twice or three times, generating Y-IM2 and Y-IM3 yeasts, respectively.
Fig. 1.
In vitro assembly of minicellulosomes on the P. pastoris cell surface. The ultra-high-affinity IM7/CL7 protein pair was used as the dockerin–cohesin pair for the yeast display system. The IM7 proteins were repeatedly displayed for (a) twice or (b) three times on the yeast cell surface. The three cellulases including an endoglucanase (EG), an exoglucanase (CBH) and a β-glucosidase (BGL), as well as a carbohydrate-binding module (CBM) were fused with an N-terminal CL7 tag and recombinantly expressed in E. coli
Diverse cellulases including an exo-mode cellobiohydrolase (CBH) from Yarrowia lipolytica [32], an endoglucanase (EG) from Clostridium thermocellum DSM1237 [33], a glucose-tolerant β-glucosidase (BGL) from Thermoanaerobacterium thermosaccharolyticum DSM 571 [34], and a CBM from Thermobifida fusca [35] were fused with N-terminal CL7 tags and recombinantly expressed in Escherichia coli. After that, the engineered P. pastoris yeasts were in vitro incubated with the E. coli lysates containing cellulases and CBM, leading to the assembly of minicellulosomes on cell surface. The cellulase activity assay indicated that Y-IM2 and Y-IM3 were able to hydrolyze microcrystalline cellulose (Avicel), phosphoric acid-swollen cellulose [PASC (86.2)] or carboxymethyl cellulose (CMC) to reducing sugars, with the enzyme activity comparable to or higher than free cellulases.
Finally, we employed the engineered yeasts as CBP cell factories to directly break down and ferment Avicel, PASC or CMC, producing ethanol with a titer of 2.5 g/L for Avicel and 1.2 g/L for PASC, respectively. Surprisingly, CMC is preferred for bioethanol fermentation, achieving an impressive ethanol titer of 5.1 g/L. To the best of our knowledge, this is the first time an engineered yeast can efficiently and directly transfer CMC to bioethanol. Moreover, the P. pastoris yeast with minicellulosomes can be lyophilized as the compound cellulases without loss of enzyme activity, showing great potential for industrial applications. Taken together, we develop a promising CBP platform for cellulose hydrolysis and bioethanol production by engineering the P. pastoris with surface-display minicellulosomes.
Results
Repeatedly displaying IM7 scaffoldins on the P. pastoris cell surface
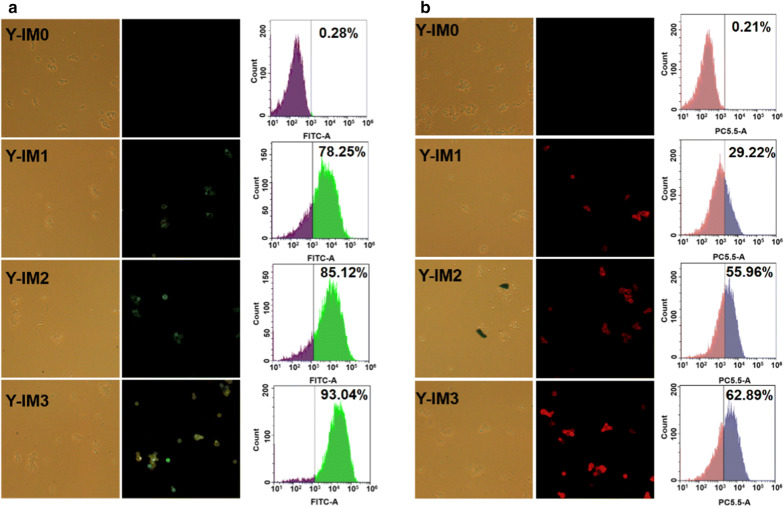
In conventional yeast cell surface-display methods, the dockerin–cohesin pairs from bacterial cellulosomes are adopted, in which the dockerin is roughly a 10-kDa calcium-binding module that non-covalently associates with the scaffoldin (cohesin) at affinity in the sub-nM (~ 10−6 M) range [19]. In this work, the ultra-high-affinity IM7/CL7 protein pair (Fig. 1) was harnessed for cellulosomes’ assembly [31]. The 16 KDa CL7 is a catalytically inactive mutant of E. coli Colicin E7 (CE7) DNase with a pretty low KD (~ 10−14–10−17 M) toward its binding 10 KDa partner immunity protein 7 (IM7). Based on the IM7/CL7 system, we recently developed an indirect P. pastoris surface-display method, achieving a tenfold increase in the display efficiency [29]. We believed that the ultra-strong protein–protein interaction between IM7 and CL7 would be helpful for cellulosome assembly. As shown in Fig. 1, the yeast surface anchor protein SED1 from S. cerevisiae without its signal sequence was fused to the IM7 scaffoldins. The surface localization of IM7 scaffoldins was confirmed by immunofluorescence microscopy and FACS (Fig. 2a). As a control, the wild-type Y-IM0 yeast without modification was not immunostained, whereas the Y-IM1, Y-IM2 and Y-IM3 variants were all in green color in the presence of mouse anti-HA monoclonal antibodies and FITC-conjugated goat anti-mouse antibodies. These results indicated that all engineered yeasts displayed the IM7 scaffoldins on the cell surface. As expected, the display efficiency was elevated with increasing the numbers of IM7. Furthermore, the engineered yeasts were all in red color in the presence of CL7 tagged mCherry fluorescent proteins (Fig. 2b). The FACS (Fig. 2b) confirmed that repeatedly anchoring the IM7 proteins can enhance the display efficiency. Compared with current yeast surface-display systems, in which less than 50% of cells were positively stained by immunofluorescence [21], over 90% positive cells were observed by our method. The huge increase in display efficiency was attributed to the use of ultra-high-affinity IM7/CL7 protein pair.
Fig. 2.
Fluorescence microscopy and flow cytometry analysis of scaffoldin surface display. All the yeast cells were treated with (a) mouse anti-HA tag monoclonal antibodies together with FITC (fluorescein isothiocyanate)-conjugated goat anti-mouse IgG antibodies, or with (b) CL7-mCheery red fluorescent proteins
In vitro assembly of minicelluolosomes on the P. pastoris cell surface
Previously, researchers assembled functional minicellulosomes in vitro on the S. cerevisiae yeast cell surface by incubation of the engineered yeast that has a chimeric scaffoldin [9, 10] or two miniscaffoldins [20, 21] with exogenous recombinant cellulases. There are two strategies for cellulosome assembly, in vitro or in vivo modes. Using the in vitro strategy, researchers obtained twofold higher bioethanol yields [12, 13, 23, 24], possibly due to the metabolic load was lowered in these yeast strains since they had no need to express and secrete the cellulases. Thus, here we also chose the in vitro mode to construct minicelluolosomes on the P. pastoris cell surface. Three different cellulases, including a CBH from Yarrowia lipolytica, an EG from Clostridium thermocellum DSM1237, a BGL from Thermoanaerobacterium thermosaccharolyticum DSM 571, as well as a CBM from Thermobifida fusca, were fused with N-terminal CL7 tags and recombinantly expressed and purified from E. coli. Notably, we had also tried to add the CL7 tag in the C-terminus, but some of cellulases were not expressed well. Based on our experiences, fusing the CL7 tag in the N-terminus can usually promote the solubility and production of the target proteins. Finally, the purified enzymes were incubated with Y-IM2 or Y-IM3 yeast.
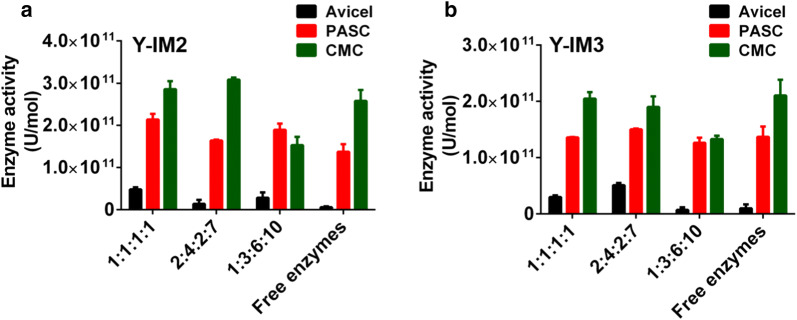
To prove construction of minicellulosomes, Avicel, PASC (86.2), or CMC was utilized as the substrate for enzyme activity assay according to the protocol [31]. This experiment was for preliminary screening the optimal ratio of each enzyme. We adjusted the ratio of EG, CBH, BGL and CBM at 1:1:1:1, 2:4:2:7 and 1:3:6:10, respectively. Meanwhile, the sample of free cellulases at 1:1:1:1 was used as the control [36]. The rational of the cellulases ratio is based on the previous reports [12, 37] as well as our own preliminary screening experiments. The data (Fig. 3) indicate that the enzyme activity of Y-IM2 and Y-IM3 is comparable to or higher than that of free cellulases. Importantly, we must point out that the enzyme activity shown in Fig. 3 was detected by determination of the reducing sugars within the first 30 min. Therefore, it does not equal to the hydrolysis capacity of yeast cells during the whole fermentation process, which had been investigated below. As expected, both minicellulosomes and the free cellulases showed higher activity toward CMC and PASC than Avicel (Fig. 3), though the improvement of the enzyme activity caused by minicellulosomes toward Avicel was more obvious (~ 2.6-fold). Based on these results, we chose 1:1:1:1 and 2:4:2:7 as the optimized ratios for the following fermentation experiments.
Fig. 3.
Enzyme activity of minicellulosomes on the cell surface of (a) Y-IM2 or (b) Y-IM3 yeast against Avicel, PASC and CMC at the different ratios of EG, CBH, BGL and CBM. The free cellulases were used as a control. It should be noted that this enzyme activity assay was detected by determination of the reducing sugars within the first 30 min
Direct fermentation of celluloses to ethanol
Direct ethanol fermentation from Avicel, PASC (86.2) or CMC was examined using the Y-IM2 and Y-IM3 yeast variants with E. coli lysate containing cellulases and CBM (Fig. 4a, b). The data demonstrated that the ethanol titer quickly increased within 60 h for all three substrates. Besides, Y-IM2 was better as the ethanol producer than Y-IM3 toward Avicel and PASC, achieving the highest ethanol yield at 2.5 g/L for Avicel and 1.2 g/L for PASC (Fig. 4c), respectively. In the previous studies of S. cerevisiae, Avicel or PASC was always the better substrate than CMC, yet the average ethanol production was lower than 2 g/L. Herein, employing our P. pastoris system obtained the same-level ethanol production when using Avicel or PASC as the substrate. Surprisingly, CMC was the best carbon source for both Y-IM2 and Y-IM3, achieving a highest ethanol yield of 5.1 g/L by Y-IM3 (Fig. 4c). As a control, the wild-type Y-IM0 yeast without engineering was used, which almost showed no ethanol production (less than 0.3 g/L) (Fig. 4c, left columns). This result indicated that the assembly of cellulosomes on Y-IM2 or Y-IM3 yeast cell surface was effective.
Fig. 4.
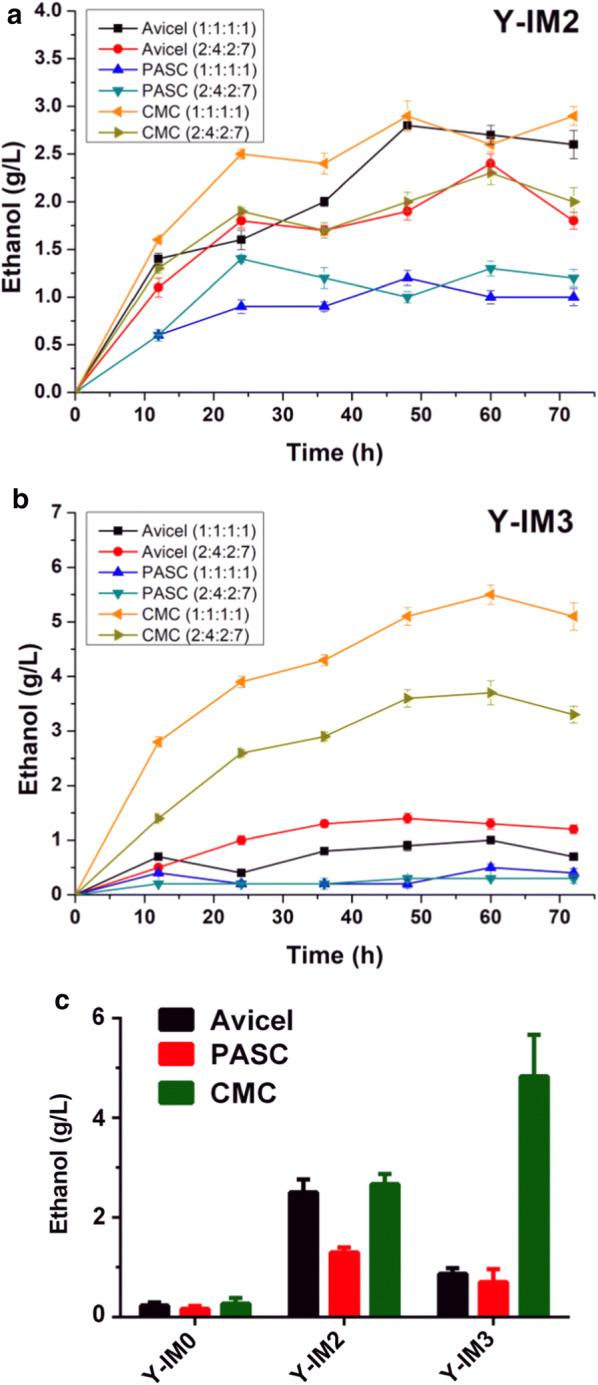
Direct ethanol production from three cellulose substrates using different yeast variants. Time profiles of the ethanol production from the yeast (a) Y-IM2 or (b) Y-IM3. The numbers in the bracket represented the ratios of EG, CBH, BGL and CBM used in the experiment. c The highest ethanol production of each cellulose substrates was shown. As the control, the wild-type Y-IM0 yeast was treated with E. coli lysate containing the enzymes
Moreover, we investigated the synergistic effect on CMC hydrolysis by examination of the reducing sugars produced in the fermentation. The data in Fig. 5 showed that the sugar concentration in Y-IM3 was higher than Y-IM2 after 12 h, indicating that more sugars were available for consumption by Y-IM3. In other words, the rate of CMC hydrolysis for Y-IM3 was higher than Y-IM2 during the whole fermentation process, confirming that the synergy effect increased with the number of IM7 scaffoldins being increased. These data are consistent with the ethanol production result (Fig. 4).
Fig. 5.
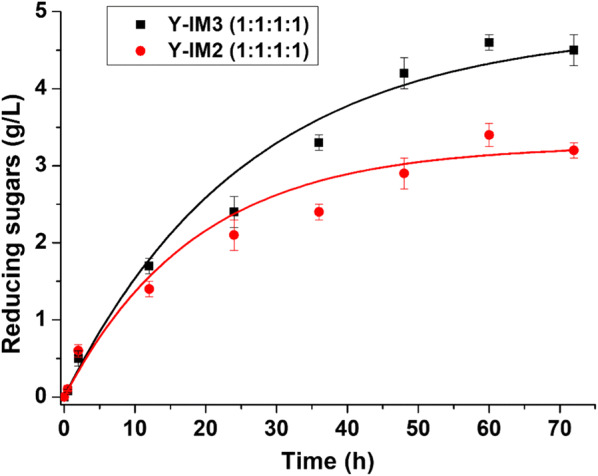
Whole-cell hydrolysis of CMC by the yeast Y-IM2 and Y-IM3 during the fermentation process
Lyophilization of yeast cells as the compound cellulases
The commercial cellulase is often supplied as the compound of three types of cellulases including EG, CBH and BGL. The annual consumption of cellulases is very huge in many industrial fields [1], particularly in the breeding industry. As known to all, P. pastoris is considered as a GRAS (generally recognized as safe) microorganism by FDA and has been employed to produce diverse human peptides and proteins. In addition, P. pastoris has a strong cell wall and outer membrane capable of serving as a stable biomaterial for enzyme immobilization. Inspired by this knowledge, we believe that the P. pastoris with surface-display minicellulosomes might be directly used as the compound cellulases for industrial needs. To this end, we initially detected whether the dead yeasts with surface-display cellulosomes have the enzyme activity or not. The results (Additional file 1: Fig. S6) demonstrated that the catalytical activity was unchanged within 3 months when the yeast cells were stored at − 20 °C. Thereafter, the Y-IM2 and Y-IM3 with minicellulosomes were lyophilized for long-term storage. No loss of enzyme activity was observed when recovering the yeast cells in the buffer solution, proving that the lyophilized P. pastoris can be utilized as the compound cellulases. Most importantly, such kind of lyophilized P. pastoris with cellulosomes can be rapidly produced at large scale and low cost, showing great potential in industry.
Discussion
Plenty of works have demonstrated that assembly of cellulosomes on the S. cerevisiae cell surface can enable it as a CBP cell factory to produce ethanol from cellulose. However, the application of such engineering strategy was limited largely due to the low display efficiency of cellulases and/or the high metabolic burden of the host yeast. To enhance the bioethanol production, several attempts have been made such as screening the dockerin–cohesin pairs [22], introducing the double-layered scaffoldins [23, 24], and adjusting the cellulase species and ratios [21, 37], etc. However, the minicellusomes in these works were constructed through hydrophobic interaction, hydrogen bond, or disulfide bond, leading to relatively low surface-display efficiency and poor stability. In addition, the engineering S. cerevisiae only produced less than 2 g/L ethanol from the conversion of Avicel or PASC. No obvious bioethanol was produced from CMC due to its ultra-high viscosity might cause problems in the fermentation.
In this study, to improve the display efficiency and availability of CMC, we describe a new strategy by engineering P. pastoris with tightly linked cell surface minicellulosomes. Compared with current yeast surface-display systems, the repeatedly anchoring IM7 scaffoldins on the P. pastoris cell surface demonstrated significantly enhanced display efficiencies, from ~ 50 to ~ 90%. The minicellulosomes were efficiently assembled by the introduction of an ultra-high-affinity IM7/CL7 protein pair [31]. Specially, four components including EG, CBHI, BGL and CBM from distinct bacteria were fused with N-terminal CL7 tags, purified from E. coli, and in vitro incubated with Y-IM2 or Y-IM3 variant at different ratios, resulting in comparable or better catalytic activity than that of free enzymes. We further investigated the synergy effect on CMC hydrolysis by determination of the reducing sugars produced by Y-IM2 and Y-IM3 in the fermentation, showing that more sugars were available for consumption by Y-IM3 than Y-IM2. This result confirmed that the synergy effect was enhanced by increasing the number of IM7 scaffoldins. Moreover, the engineered P. pastoris strains were lyophilized for long-term storage without cellulase activity losses, showing great potential as the compound cellulases instead of commercial cellulases in industry.
At last, we harnessed the engineered P. pastoris with minicellulosomes as the CBP cell factory to directly convert Avicel, PASC and CMC to ethanol. The highest ethanol yield was 2.5 g/L for Avicel and 1.2 g/L for PASC, which were comparable to or higher than those obtained by S. cerevisiae previously. More importantly, these results indicated that CMC was preferred for bioethanol fermentation in our P. pastoris system, achieving a highest titer of 5.1 g/L. To the best of our knowledge, it is the first successful work that realized efficient production of ethanol from CMC by yeast. Compared with the commonly used bioethanol producer S. cerevisiae, P. pastoris has been able to reach a much higher cell density in fermentation. It is therefore suitable for catalyzing the high-viscosity cellulose substrate such as CMC, which was consistent with what we have observed.
In this work, we found that optimizing the ratio of various cellulases would affect the cellulosome assembly and the ethanol production quite a lot. In future, higher cellulosic ethanol production might be achieved by further combinatorial optimization of the cellulase species and ratios.
Conclusion
Taking advantage of the ultra-high-affinity IM7/CL7 system, we develop an efficient method capable of in vitro assembling minicellulosomes on the P. pastoris cell surface. The engineered yeasts with cellulosomes can be cost-effectively produced at large scale and lyophilized as the compound cellulases, showing great potential in industrial applications. For the first time, the engineered P. pastoris enabled efficient production of ethanol from direct conversion of CMC, achieving an impressive ethanol titer of 5.1 g/L. Collectively, the research promotes the application of P. pastoris as a CBP cell factory in cellulosic ethanol production.
Methods
Strains and media
E. coli DH5α was used as the host for DNA manipulations, and E. coli BL21(DE3) was the host for recombinant expression of CL7 tagged cellulases or CBM domains. P. pastoris strain GS115 and the vector pPICZαA were obtained from Invitrogen (Carlsbad, CA, USA). The vectors pET23a-T, pET23a-CL7, and pCDNA3.1-mCherry were constructed and stored in our laboratory previously [24]. The genes encoding exo-mode cellobiohydrolases (CBH) from Yarrowia lipolytica, endoglucanases (EG) from Clostridium thermocellum DSM1237, glucose-tolerant β-glucosidase (BGL) from Thermoanaerobacterium thermosaccharolyticum DSM 571, as well as CBM from Thermobifida fusca were synthesized by Sangon Biotech (Shanghai, China). The gene sequences are shown in the Additional file 1. E. coli strains were grown in LB medium (1% tryptone, 0.5% yeast extract, 1% NaCl) supplied with 100 μg/ml ampicillin. P. pastoris yeasts were grown first in YPD plates (1% yeast extract, 2% peptide, and 2% glucose) supplemented with 100 µg/mL of Zeocin, and then cultured in BMGY/BMMY medium base (20.0 g/L peptone, 10.0 g/L yeast extract, 100 mmol PBS broth, pH 6.0).
Construction of plasmids
The plasmids pET23a-CL7-EG, pET23a-CL7-CBHI, pET23a-CL7-BGL, and pET23a-CL7-CBM were constructed by insertion of the corresponding genes into pET23a(+) vectors (Invitrogen, USA). The plasmid pPICZαA-HA-Im7-SED1 that produces P. pastoris Y-IM1 was described in the previous work [29]. The plasmids of Y-IM2 and Y-IM3 were constructed based on Y-IM1 plasmid by repeatedly inserting IM7 for twice or three times, namely pPICZαA-HA-2XIm7-SED1 and pPICZαA-HA-3XIm7-SED1, respectively. In addition, a “GGGGS”2 liker was added between each IM7 units. All the P. pastoris yeast plasmids were digested with Pme1 and transformed into yeast cells to integrate the target genes into the yeast chromosome. The detailed protocol, schemes (Additional file 1: Figs. S1–S5), and primer pairs (Additional file 1: Table S1) are given in Additional file 1.
Yeast surface-display and E. coli expression
All the P. pastoris yeast plasmids were digested with Pme1 and transformed into GS115 competent cells. Transformants were first isolated by incubation at 28 °C for 48 h on YPD plates supplemented with 100 µg/mL of Zeocin. Then, five to ten single colonies of transformants were inoculated in 20 mL of BMGY in 250-mL flasks and cultivated at 28 °C under 200 rpm. After 24 h, the cells were centrifuged at 5000×g for 5 min, resuspended in 20 mL of BMMY medium containing 1% (v/v) methanol and continued to grow at 28 °C, 200 rpm for 24 h. To express CL7 tagged proteins in E. coli, 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the cells when the cells were grown to an OD600 of 0.6. Then, the cells were grown at 18 °C for 12 h. The E. coli cells collected were resuspended in PBS buffer containing 200 mM NaCl and 10 mM CaCl2 (pH 7.4) and then sonicated on ice for 20 min. The cell lysates were either purified by Ni–NTA affinity columns, or directly incubated with the engineered yeast strains in fermentation experiments.
Fluorescence microscopy and flow cytometric analysis
The yeast strains including Y-IM0, Y-IM1, Y-IM2, and Y-IM3 were harvested and washed twice by ice-cold water, resuspended and blocked in 1 mL PBS buffer (200 mM NaCl, pH 7.4) with 1 mg/mL BSA for 1 h at 4 °C with rotation. Then, 1 µL of mouse anti-HA tag monoclonal antibodies or 5 µg of CL7–mCherry fluorescent proteins were added to the cell suspension of 1000 µL and then incubated at room temperature with rotation for 2 h. In the next, the cells were washed three times with PBS and resuspended in 200 µL of PBS with the addition of 1 µL of FITC-conjugated goat anti-mouse IgG(H + L) antibodies, followed by incubation of them at room temperature for 1 h with rotation. Finally, the cells were washed three times with PBS, resuspended in 1 mL of PBS and examined by a fluorescence microscopy (IX73, Olympus, Tokyo, Japan). Flow cytometric analysis (FACS) was analyzed with a flow cytometer (CytoFLEX, Beckman Coulter, Suzhou, China) to estimate the percentage of the fluorescence positive yeast cells.
In vitro assembly of minicellulosome and enzyme activity assay
The induced Y-IM2 and Y-IM3 strains were mixed with the purified recombinant CL7 tagged enzymes in 100 mM Tris–HCl buffer with 10 mM CaCl2 (pH 8.0) at various ratios, and kept for 2 h at 4 °C for minicellulosome assembly [23, 24]. The enzyme activity of cellulosome or free cellulases against Avicel or PASC or CMC was detected by 3, 5-dinitrosaloculoc acid (DNS) assay [36]. The PASC (86.2) was prepared from Avicel (Sangon Biotech, Shanghai, China) as described previously [38]. Minicellulosomes or free cellulases were incubated with 0.1% cellulose substrate in 50 mM citrate buffer (pH 4.8) with 10 mM CaCl2 at 50 °C for 30 min. After addition of DNS and boiling for 10 min, the reducing sugars were detected at 540 nm. One unit of the enzyme activity was defined as the amount of enzyme that released 1 mol of product from the cellulose substrate at 50 °C in 1 min.
Fermentation
After induction, the Y-IM0, Y-IM2 and Y-IM3 strains were washed twice with YP medium (1% yeast extract, 2% peptone, 10 mM CaCl2). Then, they were incubated with E. coli lysates containing enzymes at various ratios in the same buffer, and kept for 4 h at 4 °C to allow cellulosome assembly. Next, yeast cells with minicellulosomes were cultivated in YP medium with 1% cellulose substrate (Avicel, PASC, or CMC) to an OD600 of 50. Fermentation was performed anaerobically 100-mL flask at 30 °C with agitation at 250 rpm. The ethanol concentration was analyzed by an ethanol biosensor M-100 (Shellman Life Science, Shenzhen, China) supplied with polyaniline film-immobilized ***alcohol oxidase, which had been proven and in good agreement with the results of standard method obtained by gas chromatography [39]. Meanwhile, the reducing sugar concentration was determined by the biosensor instrument.
Lyophilization of the yeast cells
The freshly induced YM-2 and YM-3 yeast cells were incubated with E. coli lysates containing EG, CBH, BGL and CBM at 1:1:1:1 in 100 mM Tris–HCl buffer with 10 mM CaCl2 (pH 8.0), and kept for 2 h at room temperature. The above mixtures were centrifuged for 10 min at 8000 rpm. After that, the cell pellets were collected and lyophilized at − 70 °C under vacuum using a LABCONCO freeze drier (Kansas City, Missouri, USA).
Supplementary information
Additional file 1. Additional figures and table.
Acknowledgements
The authors thank Professor. Shihui Yang in the Hubei University (Wuhan, China) to supply the ethanol biosensor instrument.
Abbreviations
- PASC
Acid-swollen cellulose
- Avicel
Microcrystalline cellulose
- CMC
Carboxymethyl cellulose (CMC)
- CBP
Consolidated bioprocessing
- CBM
Carbohydrate-binding module
- CBH
Exo-mode cellobiohydrolases
- EG
Endoglucanases
- BGL
Glucose-tolerant β-glucosidase
- FACS
Flow cytometric analysis
Authors’ contributions
LXM and YL designed the experiments. CD, JQ and XPW performed the enzyme activity assay and detection experiments of bioethanol production. LXC, WLS and STL constructed and plasmids and purified the recombinant enzymes. CD and YL analyzed the data. YL and JQ wrote the paper. KW gave help to edit the paper and participated in patent application. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NO. 2190070220), China Postdoctoral Science Foundation (NO. 2019M662573) and 3551 Optics Valley Talent Schema.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
A patent involved in ethanol production using Pichia pastoris with surface-display minicellulosomes was submitted by the institute of authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ce Dong and Jie Qiao contributed equally to this work
Contributor Information
Jie Qiao, Email: jieqiao@hubu.edu.cn.
Lixin Ma, Email: malixing@hubu.edu.cn.
Yi Liu, Email: yiliu0825@hubu.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13068-020-01749-1.
References
- 1.Sarsaiya S, Jain A, Kumar Awasthi S, Duan Y, Kumar Awasthi M, Shi J. Microbial dynamics for lignocellulosic waste bioconversion and its importance with modern circular economy, challenges and future perspectives. Bioresour Technol. 2019;291:121905. doi: 10.1016/j.biortech.2019.121905. [DOI] [PubMed] [Google Scholar]
- 2.Prasad RK, Chatterjee S, Mazumder PB, Gupta SK, Sharma S, Vairale MG, Datta S, Dwivedi SK, Gupta DK. Bioethanol production from waste lignocelluloses: a review on microbial degradation potential. Chemosphere. 2019;231:588–606. doi: 10.1016/j.chemosphere.2019.05.142. [DOI] [PubMed] [Google Scholar]
- 3.Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ, Jr, Hallett JP, Leak DJ, Liotta CL, et al. The path forward for biofuels and biomaterials. Science. 2006;311:484–489. doi: 10.1126/science.1114736. [DOI] [PubMed] [Google Scholar]
- 4.Lambertz C, Garvey M, Klinger J, Heesel D, Klose H, Fischer R, Commandeur U. Challenges and advances in the heterologous expression of cellulolytic enzymes: a review. Biotechnol Biofuels. 2014;7:135–150. doi: 10.1186/s13068-014-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynd LR, Laser MS, Bransby D, Dale BE, Davison B, Hamilton R, Himmel M, Keller M, McMillan JD, Sheehan J, Wyman CE. How biotech can transform biofuels. Nat Biotechnol. 2008;26:169–172. doi: 10.1038/nbt0208-169. [DOI] [PubMed] [Google Scholar]
- 6.Zaldivar J, Nielsen J, Olsson L. Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol. 2001;56:17–34. doi: 10.1007/s002530100624. [DOI] [PubMed] [Google Scholar]
- 7.Wyman CE. Twenty years of trials, tribulations, and research progress in bioethanol technology: selected key events along the way. Appl Biochem Biotechnol. 2001;91–93:5–21. doi: 10.1385/ABAB:91-93:1-9:5. [DOI] [PubMed] [Google Scholar]
- 8.Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66:506–577. doi: 10.1128/MMBR.66.3.506-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayer EA, Lamed R. The cellulosome saga: Early history. In: Uversky V, Kataeva IA, editors. cellulosome. Nova Science Publishers Inc: New York; 2006. pp. 11–46. [Google Scholar]
- 10.Bayer EA, Lamed R, White BA, Flint HJ. From cellulosomes to cellulosomics. Chem Rec. 2008;8:364–377. doi: 10.1002/tcr.20160. [DOI] [PubMed] [Google Scholar]
- 11.Ben-David Y, Morais S, Stern J, Mizrahi I, Bayer EA. Cell-surface display of designer cellulosomes by Lactobacillus plantarum. Method Enzymol. 2019;617:241–263. doi: 10.1016/bs.mie.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Tsai SL, Goyal G, Chen W. Surface display of a functional minicellulosome by intracellular complementation using a synthetic yeast consortium and its application to cellulose hydrolysis and ethanol production. Appl Environ Microbiol. 2010;76:7514–7520. doi: 10.1128/AEM.01777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai SL, Oh J, Singh S, Chen R, Chen W. Functional assembly of minicellulosomes on the Saccharomyces cerevisiae cell surface for cellulose hydrolysis and ethanol production. Appl Environ Microbiol. 2009;75:6087–6093. doi: 10.1128/AEM.01538-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson DG, McBride JE, Shaw AJ, Lynd LR. Recent progress in consolidated bioprocessing. Curr Opin Biotechnol. 2012;23:396–405. doi: 10.1016/j.copbio.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Lynd LR, van Zyl WH, McBride JE, Laser M. Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol. 2005;16:577–583. doi: 10.1016/j.copbio.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Alper H, Moxley J, Nevoigt E, Fink GR, Stephanopoulos G. Engineering yeast transcription machinery for improved ethanol tolerance and production. Science. 2006;314:1565–1568. doi: 10.1126/science.1131969. [DOI] [PubMed] [Google Scholar]
- 17.Den Haan R, Rose SH, Lynd LR. van Zyl WH/Hydrolysis and fermentation of amorphous cellulose by recombinant Saccharomyces cerevisiae. Metab Eng. 2007;9:87–94. doi: 10.1016/j.ymben.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Fujita Y, Ito J, Ueda M, Fukuda H, Kondo A. Synergistic saccharification, and direct fermentation to ethanol, of amorphous cellulose by use of an engineered yeast strain codisplaying three types of cellulolytic enzyme. Appl Environ Microbiol. 2004;70:1207–1212. doi: 10.1128/AEM.70.2.1207-1212.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SP, Bayer EA, Czjzek M. Continually emerging mechanistic complexity of the multi-enzyme cellulosome complex. Curr Opin Struct Biol. 2017;44:151–160. doi: 10.1016/j.sbi.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Fontes CM, Gilbert HJ. Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu Rev Biochem. 2010;79:655–681. doi: 10.1146/annurev-biochem-091208-085603. [DOI] [PubMed] [Google Scholar]
- 21.Tang H, Wang J, Wang S, Shen Y, Petranovic D, Hou J, Bao X. Efficient yeast surface-display of novel complex synthetic cellulosomes. Microb Cell Fact. 2018;17:122–135. doi: 10.1186/s12934-018-0971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai SL, DaSilva NA, Chen W. Functional display of complex cellulosomes on the yeast surface via adaptive assembly. ACS Synth Biol. 2013;2:14–21. doi: 10.1021/sb300047u. [DOI] [PubMed] [Google Scholar]
- 23.Fan L-H, Zhang Z-J, Yu X-Y, Xue Y-X, Wang M-M, Tan T-W. In vitro assembly of minicellulosomes with two scaffoldins on the yeast cell surface for cellulose saccharification and bioethanol production. Process Biochem. 2013;48:430–437. doi: 10.1016/j.procbio.2013.01.012. [DOI] [Google Scholar]
- 24.Fan LH, Zhang ZJ, Yu XY, Xue YX, Tan TW. Self-surface assembly of cellulosomes with two miniscaffoldins on Saccharomyces cerevisiae for cellulosic ethanol production. Proc Natl Acad Sci USA. 2012;109:13260–13265. doi: 10.1073/pnas.1209856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamed R, Setter E, Bayer EA. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J Bacteriol. 1983;156:828–836. doi: 10.1128/JB.156.2.828-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayer EA, Kenig R, Lamed R. Adherence of Clostridium thermocellum to cellulose. J Bacteriol. 1983;156:818–827. doi: 10.1128/JB.156.2.818-827.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin X, Wu L, Huang S, Qin Y, Qiu X, Lou H. Effect of lignin-based amphiphilic polymers on the cellulase adsorption and enzymatic hydrolysis kinetics of cellulose. Carbohydr Polym. 2019;207:52–58. doi: 10.1016/j.carbpol.2018.11.070. [DOI] [PubMed] [Google Scholar]
- 28.Weimer PJ, Lopez-Guisa JM, French AD. Effect of cellulose fine structure on kinetics of its digestion by mixed ruminal microorganisms in vitro. Appl Environ Microbiol. 1990;56:2421–2429. doi: 10.1128/AEM.56.8.2421-2429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S, Qiao J, Lin S, Liu Y, Ma L. A highly efficient indirect P. pastoris surface display method based on the CL7/Im7 ultra-high-affinity system. Molecules. 2019;24:1483–1497. doi: 10.3390/molecules24081483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarzhans JP, Luttermann T, Geier M, Kalinowski J, Friehs K. Towards systems metabolic engineering in Pichia pastoris. Biotechnol Adv. 2017;35:681–710. doi: 10.1016/j.biotechadv.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Vassylyeva MN, Klyuyev S, Vassylyev AD, Wesson H, Zhang Z, Renfrow MB, Wang H, Higgins NP, Chow LT, Vassylyev DG. Efficient, ultra-high-affinity chromatography in a one-step purification of complex proteins. Proc Natl Acad Sci USA. 2017;114:e5138–e5147. doi: 10.1073/pnas.1704872114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei H, Wang W, Alahuhta M, Vander Wall T, Baker JO, Taylor LE, 2nd, Decker SR, Himmel ME, Zhang M. Engineering towards a complete heterologous cellulase secretome in Yarrowia lipolytica reveals its potential for consolidated bioprocessing. Biotechnol Biofuels. 2014;7:148–162. doi: 10.1186/s13068-014-0148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leis B, Held C, Bergkemper F, Dennemarck K, Steinbauer R, Reiter A, Mechelke M, Moerch M, Graubner S, Liebl W, et al. Comparative characterization of all cellulosomal cellulases from Clostridium thermocellum reveals high diversity in endoglucanase product formation essential for complex activity. Biotechnol Biofuels. 2017;10:240–256. doi: 10.1186/s13068-017-0928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao L, Pang Q, Xie J, Pei J, Wang F, Fan S. Enzymatic properties of Thermoanaerobacterium thermosaccharolyticum beta-glucosidase fused to Clostridium cellulovorans cellulose binding domain and its application in hydrolysis of microcrystalline cellulose. BMC Biotechnol. 2013;13:101–109. doi: 10.1186/1472-6750-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forsberg Z, Rohr AK, Mekasha S, Andersson KK, Eijsink VG, Vaaje-Kolstad G, Sorlie M. Comparative study of two chitin-active and two cellulose-active AA10-type lytic polysaccharide monooxygenases. Biochemistry. 2014;53:1647–1656. doi: 10.1021/bi5000433. [DOI] [PubMed] [Google Scholar]
- 36.Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23:257–270. doi: 10.1016/0168-1656(92)90074-J. [DOI] [Google Scholar]
- 37.Goyal G, Tsai SL, Madan B, DaSilva NA, Chen W. Simultaneous cell growth and ethanol production from cellulose by an engineered yeast consortium displaying a functional mini-cellulosome. Microb Cell Fact. 2011;10:89–97. doi: 10.1186/1475-2859-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang YH, Cui J, Lynd LR, Kuang LR. A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: evidence from enzymatic hydrolysis and supramolecular structure. Biomacromol. 2006;7:644–648. doi: 10.1021/bm050799c. [DOI] [PubMed] [Google Scholar]
- 39.Kuswandi B, Irmawati T, Hidayat MA, Jayus AM. A simple visual ethanol biosensor based on alcohol oxidase immobilized onto polyaniline film for halal verification of fermented beverage samples. Sensors. 2014;14:2135–2149. doi: 10.3390/s140202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional figures and table.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its additional files.