Abstract
Pastoralist children in the Ethiopian Somali Regional State (ESRS) are at high risk for undernutrition and intestinal parasitic infections (IPIs). We assessed the nutritional status and its association with IPIs in 500 children <5 years of age in a clustered cross‐sectional study in Adadle district, ESRS. Stool samples were microscopically examined for IPIs and biomarkers for iron and vitamin A status, anthropometry, and food variety score (FVS) were assessed. Median (interquartile range [IQR]) FVS was 2.0 (2.0, 4.0), and 35% of children were exclusively breastfed up to age 6 months. Prevalence of stunting, wasting, underweight and mid‐upper arm circumference (MUAC) <12.5 cm was 30, 34, 40, and 16%, respectively. Median (IQR) haemoglobin, ferritin, and retinol‐binding protein concentrations were 9.5 g dL‐1 (8.2, 10.9), 6.2 μg L‐1 (4.0, 10.2), and 0.8 μmol L−1 (0.67, 0.91), respectively. Prevalence of anaemia, iron, and vitamin A deficiency was 75, 91, and 30%, respectively. IPIs' prevalence was 47%; the most prevalent IPIs were Giardia lamblia (22%) and Ascaris lumbricoides (15%). Giardial infections but not A. lumbricoides increased the risk for MUAC <12.5 cm (adjusted odds ratio [aOR]: 3.50, 95% confidence interval [CI] [2.21, 5.54]). The odds for anaemia were 97% (aOR: 0.03, 95% CI [0.03, 0.07]) and 89% (aOR: 0.11, 95% CI [0.11, 0.23]) less for children with FVS >2 or with exclusive breastfeeding up to 6 months, respectively. Undernutrition and IPIs are alarmingly high in <5 years of age children in ESRS. Giardial infections and low nutritional adequacy of the diet seem to be major contributing factors to the precarious nutritional status and should be addressed by appropriate interventions.
Keywords: Anaemia, children <5 years, Ethiopian Somali Regional State, intestinal parasitic infection, micronutrient deficiencies, pastoralist, undernutrition
Key messages
The burden of undernutrition (including wasting, stunting, underweight, anaemia, iron, and vitamin A deficiency) and intestinal parasitic infections is enormously high in children <5 years of age in the Ethiopian Somali Regional State and appropriate treatment and prevention measures are urgently needed.
Undernutrition as indicated by low mid‐upper arm circumference was strongly positively associated with giardial infections and age but negatively associated with milk consumption.
Exclusive breastfeeding for 6 months and a higher food variety were associated with a massively reduced risk for anaemia.
1. INTRODUCTION
Although undernutrition in Ethiopian children <5 years of age has steadily decreased since 2000, it remains widespread, particularly in certain regions. In the Ethiopian Somali Regional State (ESRS), stunting and underweight have been reported to be 27 and 29%, respectively, and the wasting prevalence of 23% is countrywide the highest value and substantially higher than the country's average wasting prevalence of 10% (Central Statistical Agency/Ethiopia & ICF International, 2016). Undernutrition also includes micronutrient deficiencies. For children <5 years of age living in the ESRS, data on micronutrient deficiencies assessed by biomarkers are unavailable, except for anaemia. In the most recent demographic health survey, 83% of the children <5 years of age were anaemic, and the anaemia prevalence in ESRS increased by 14% from 2011 to 2016 (Central Statistical Agency/Ethiopia & ICF International, 2012, 2016). The aetiology of anaemia is multifactorial; it is estimated that half of the anaemia in sub‐Saharan Africa is due to iron deficiency (ID; Kassebaum et al., 2014). Hence, a substantial number of children <5 years of age in ESRS is likely to be iron deficient, but no specific data is available. Similarly, data for vitamin A deficiency (VAD) in children from ESRS is missing. Nationwide data excluding ESRS indicates that VAD is a serious public health problem in Ethiopia with almost 40% of young children having deficient serum retinol levels (Demissie, Ali, Mekonen, Haider, & Umeta, 2010).
Undernutrition, which has been found to be an underlying cause in a third of deaths in children <5 years (Black et al., 2008), results from the interaction between inadequate diet and infectious disease. It is associated with intestinal parasitic infections (IPIs) of helminths and protozoa (Crompton & Nesheim, 2002; de Gier, Campos Ponce, van de Bor, Doak, & Polman, 2014; Hall, Hewitt, Tuffrey, & de Silva, 2008; Stanley, 2003), which are often a major public health problem in children <5 years in developing countries (Hotez, Fenwick, Savioli, & Molyneux, 2009). In various regions in Ethiopia, high IPIs prevalence ranging from 30–75% have been reported in preschool children <5 years of age (Asfaw & Goitom, 2000; Nyantekyi et al., 2010) and school children (Abdi, Nibret, & Munshea, 2017; Abera & Nibret, 2014; Alemayehu et al., 2017; Feleke, 2016; Gelaw et al., 2013; Nguyen et al., 2012). It is assumed that infections with soil‐transmitted helminths and protozoa are as prevalent in ESRS as in other Ethiopian regions, but no data is available.
The arid to semiarid ESRS is widely inhabited by pastoralist. The amount and types of foods available to the pastoralists are strongly influenced by ecological and cultural factors as well as economic opportunities and constraints. Therefore, undernutrition is a concern in pastoralist populations, especially in young children (Galvin, 1992). In pastoralist communities, poor nutritional status of children due inadequate diet is often exacerbated by lack of potable water and unsanitary conditions, which contribute to increased rates of IPIs (Bechir, Schelling, Hamit, Tanner, & Zinsstag, 2012). The preferred staple of pastoral populations is milk from livestock, and their diet has been reported to be generally low in energy and micronutrients but adequate in protein (Iannotti & Lesorogol, 2014; Sellen, 1996). Pastoralists, such as the ones in the ESRS, have been neglected in nutritional research, and detailed up‐to‐date data on nutritional status is missing. This study therefore aims to assess the nutritional status and its association with IPIs in children <5 years of age living in the Adadle district of the Somali region, Ethiopia. This study is part of the Jigjiga One Health Initiative, which aims to improve different aspects of human and animal health and of the environmental wellbeing among pastoralists in the ESRS. The data of this study will be important to design and target approaches to improve the nutritional status of young children in ESRS.
2. MATERIALS AND METHODS
2.1. Study setting
A clustered cross‐sectional study was carried out in Adadle district in the Somali regional state, Eastern Ethiopia. Adadle is located in the lowlands of the Wabe Shabale River subbasin 17 km from the major town Gode. Most of the inhabitants in Adadle are Muslim pastoralists, who mainly depend on their own animal products for consumption and income generation. Altitude is 300–500 m; temperature is between 18–38°C, and rainfall approximately 300 mm per year (Gebre‐Mariam, 2007).
2.2. Sample size and household selection
To determine the sample size, we adjusted for clustering of households within sub‐Kebeles or pastoralist camps (cluster unit), assuming an intraclass correlation coefficient of 0.2, a prevalence of underweight of 25%, and 10 eligible children per cluster (Bennett, Woods, Liyanage, & Smith, 1991). A total sample size of 500 children would allow to estimate the prevalence with a precision defined as one half length of the 95% confidence interval of 6 percentage points (i.e., 3.2). As part of a three‐level random selection process, first, out of the 15 Kebeles (lowest administrative unit) in the Adadle Woreda (district) in ESRS, six Kebeles (Buursaredo, Gabal, Boholoxagare, Higlo, Harsogand, and Malkasallax) were selected proportional to their size using an existing population list. Two Kebeles were excluded because of security reasons, and seven were excluded due to logistical reasons. As a second step, random geographical coordinates were generated for the selection of sub‐Kebeles within each Kebele (Jean‐Richard et al., 2015). Third, within a sub‐Kebele, households were systematically selected by throwing a pencil. The data collectors then walked to the edge of the sub‐Kebele into the direction to which the pencil pointed, numbering all the households on the way (Brogan, Flagg, Deming, & Waldman, 1994). A random number was chosen to identify one household as a starting household for the cluster of the pointed direction. Data collectors then continued to the right side until the targeted number of samples per cluster was reached. In household with two and more eligible children, all the children were enrolled. Camps were selected as a cluster, and all eligible children <5 years of age in the camp were enrolled. All children aged 1–5 years who started consumption of complementary foods were enrolled. Children whose mother/caregivers reported a severe illness within the last 2 weeks prior to the study were excluded.
2.3. Data collection
Trained fieldworkers collected the data at the beginning of the dry season in 2016, locally named xagaa, using a previously tested questionnaire including sociodemographic status of the children's family, drinking water source and sanitation (toilet type and waste disposal), milk consumption in the family (milk source and consumption habit), breastfeeding practice, and a 24‐hr food recall. As birth records rarely exist in pastoralist population, age of the children was assessed by the number of breastfeeding months for children <2 years of age and still receiving breast milk. For children ≥2 years of age, age was verified by the number of rainy seasons through which the child lived using a local pastoralist seasonal calendar. Breastfeeding practice were assessed by asking whether the child has been exclusively breastfed up to 6 months and whether the mother was aware of the World Health Organization (WHO) recommendation of exclusive breastfeeding up to 6 months of age. The 24‐hr food recall embedded in the questionnaire recorded any food item or beverage consumed by the child the day before the interview but did not consider quantities of consumed foods and beverages. To assess nutritional adequacy of the diet, a food variety score (FVS) was calculated based on the number of different food items eaten during the day before the day of the interview as previously published (Hatloy, Torheim, & Oshaug, 1998). All food items were given the same weight. Beverages and condiments (e.g., salt or bouillon cubes) were not considered as food items and therefore not included in the calculation.
2.4. Anthropometry measurements
Weight and height were measured in duplicate and recorded as the average of the two measurements using a digital balance scale and a wooden measuring board, respectively. For the weighing, children able and willing to stand alone were asked to stand in the middle of the scale without touching anything. For children, who were not willing to stand alone, first the weight of their mother/caregiver alone was measured then the mother/caregiver holding the child was measured. The weight of the mother/caregiver alone was then deducted from the weight of the mother/caregiver holding the child. Children were weighted with light clothes and without shoes to the nearest 0.5 kg. The accuracy of the weighing scale was monitored daily against known weights. The height of the children was measured without shoes, with heels together, arms to the sides, legs straight, shoulders relaxed, and the head looking straight ahead. The height was measured to the nearest 1.0 cm. Z scores for weight‐for‐age (WAZ), weight‐for‐height (WHZ), and height‐for‐age (HAZ) were calculated using WHO Anthro software (Version 3.2.2.1). Stunting, underweight, and wasting were defined as HAZ, WAZ, and WHZ, <‐2 standard deviations, respectively. Mid‐upper arm circumference (MUAC) was measured in duplicate using a measuring tape to the nearest 1 mm. Low/insufficient MUAC was defined as MUAC <12.5 cm (WHO, 2013) and used as the main indicator for undernutrition.
2.5. Blood sampling, biochemical analysis, and applied cut‐offs
Venous whole‐blood samples from the study children were collected directly into trace element–free lithium heparin tubes (Sarstedt). In addition, capillary blood using a finger prick was collected to assess haemoglobin (Hb) with a portable HemoCue 301+ analyser (HemoCue AB). Samples were immediately stored in a refrigerated cool box and transported to the laboratory units in the Adadle Health Center. The samples were then centrifuged (3,000 × g for 10 min at room temperature) using a portable centrifuge (LW Scientific). Plasma aliquots were stored at −20°C until they were shipped on dry ice with an international courier to a laboratory in Germany for the analysis of plasma ferritin (PF), soluble transferrin receptor (sTfR), retinol‐binding protein (RBP), C‐reactive protein (CRP), and α1‐acid glycoprotein (AGP) using a sandwich enzyme‐linked immunosorbent assay (Erhardt, Estes, Pfeiffer, Biesalski, & Craft, 2004). Liquicheck Trilevel (Bio‐Rad Laboratories Inc.) was used as control material with each run of analysis, and measured values were within acceptable ranges as specified by the manufacturer. Severe, moderate, and mild anaemia was defined as; Hb <7.0, Hb = 7.0–9.9, and Hb = 10.0–10.9 g dl−1, respectively. ID was defined as PF <12 μg L−1 or sTfR >8.3 mg L−1. In the children who provided both a capillary and venous blood sample, ID anaemia was defined as Hb <11.0 g dl−1 plus PF <12 μg L−1 and/or sTfR >8.3 mg L−1 (Erhardt et al., 2004; WHO/UNICEF/UNU, 2001). Parents of the children with Hb levels <7.0 gdl−1 were instructed to take the child to the health facility for follow‐up care. VAD was defined as RBP <0.7 μmol L−1; children with ≥0.7 μmol L−1 but <1.05 μmol L−1 were considered as at risk for VAD (WHO, 2011). AGP and CRP concentrations for healthy children were defined as <1 g L−1 and <5 mg L−1, respectively (Thurnham et al., 2010). Serum ferritin was corrected for inflammation based on CRP and AGP using the Thurnham et al., 2010 regression coefficients (Thurnham et al., 2010).
2.6. Stool sample collection and processing
Stool samples were collected using coded plastic containers given to the mothers/caregivers of the children with detailed instructions of how to collect fresh stool samples. Qualitative single Kato–Katz thick smears were prepared from each stool sample and examined under a microscope for the presence of soil‐transmitted helminth (Ascaris lumbricoides, hookworm, and Trichuris trichiura) and Schistosoma mansoni eggs (Katz, Chaves, & Pellegrino, 1972). In addition, stool smears based on sodium acetate–acetic acid‐formalin (SAF)‐fixed stool samples were examined to detect protozoal vegetative forms and cysts and helminth eggs and larvae (Utzinger et al., 2010). All enrolled children received Albendazole or Mebendazole for treatment.
2.7. Ethical considerations
The study was conducted according to the declaration of Helsinki, and ethical clearance was obtained from the Review Committee of the University of Jigjiga in Ethiopia (JJU‐RERC 002/2016) and the Swiss Ethics Committee of Northwest and Central Switzerland (Ethikkommision Nordwest‐ und Zentralschweiz; EKNZ BASEC UBE‐req. 2016‐00204) prior to initiation of the study. A material transfer agreement was established by the Food, Medicine and Health Care Authority of Ethiopia for the shipment of blood and faecal samples from Ethiopia to Switzerland. All the parents/caregivers of the participating children gave oral and written consent prior to the study enrollment of their children.
2.8. Statistical analysis
Descriptive statistics were done with Stata (Intercooled Stata® Version 14, College Station, TX). The main anthropometric indicator for undernutrition was MUAC <12.5 cm (WHO, 2013) because other anthropometric indicators, such as stunting or underweight, were affected by the difficulty of recording the exact age of the children from their mothers due to the lack of a birth certificate, or by the weight measurements that was only measured to the nearest 0.5 kg. Also, in contrast to height, MUAC can be measured more easily and reliably in children <5 years old. Multivariable logistic regression analysis including the covariates age, sex, milk consumption, Giardia lamblia, A. lumbricoides, FVS, and exclusive breastfeeding up to 6 months of age (EBF) were conducted. Odds ratio were adjusted for clustering on a village level using generalized estimating equation models for binary outcomes, logit link, and independent correlation structure. The generalized estimating equation models were fitted using the geeglm package of the statistical environment R (v 3.5.0).
3. RESULTS
3.1. Participant characteristics
A total of 500 children aged between 1–5 years were enrolled from six Kebeles of Adadle Woreda in ESRS (Figure S1). For eight children, no data was collected due to loss of follow‐up after consent signing. The questionnaire and anthropometric measurements were therefore completed for 492 children. In total, 453 capillary blood samples, 404 venous blood samples, and 387 stool samples were collected and analysed. For 371 children, both capillary and venous blood samples were available. Gender was equally distributed, and approximately one‐tenth of the children were <2 years of age whereas the remaining children were between 2 and 5 years of age (Table 1). All mothers, of whom the clear majority was illiterate, spoke Somali language. The main source of drinking water was river water. Households mainly used outdoor nature toilet facilities, and waste was dumped in the open space. Almost all households had milk from their own sources, and almost none of them did boil the milk before consumption.
Table 1.
Sociodemographic characteristics, food variety score, and breastfeeding pattern of children <5 years of age living in Adadle Woreda, Somali region, Ethiopia (data collected in the dry season 2016)
| Characteristic | % (n = 492) |
|---|---|
| Age of the children | |
| <2 years | 8.3 |
| ≥2 years | 91.7 |
| Sex | |
| Female | 50.8 |
| Male | 49.2 |
| Literacy of the mothera | |
| Illiterate | 97.6 |
| First to eighth grade | 2.4 |
| Source of drinking waterb | |
| Dug well | 16.5 |
| River/bond | 83.5 |
| Types of household toiletc | |
| Pit latrines | 2.2 |
| Outdoor in nature | 97.8 |
| Household waste disposald | |
| Dumped in the river | 95.9 |
| Burned | 4.1 |
| Source of milke | |
| Own source | 96.5 |
| Bought from market | 3.5 |
| Milk consumption habit | |
| Boiled | 3.0 |
| Raw | 97.0 |
| Food variety score | |
| ≤2 food items | 55.1 |
| >2 food items | 44.9 |
| Exclusive breastfeeding | |
| Exclusively breastfed up to 6 months of life | 35.0 |
| Not exclusively breastfed up to 6 months of life | 65.0 |
Answer options in the questionnaire were: Illiterate, First to eigth grade, and above ninth grade.
Answer options in the questionnaire were: Tap, tube well/borehole, dug well, spring water, river, tank truck, and rain water.
Answer options in the questionnaire were: Pit latrines, composed latrines, bucket toilet, hanging toilet, and outdoor in nature.
Answer options in the questionnaire were: Dumped in the compound, dumped in the open space, dumped in the river, and burned.
Answer options in the questionnaire were: Own source, bought from market, relatives, and other sources.
3.2. Food variety score and breastfeeding practices
Almost all children (96%) had eaten a type of staple food in the 24 hr recall. The staples were mainly cereals and included whole wheat or wheat flour (44%), maize (20%), rice (18%), potato (8%), sorghum (4%), and pasta (2%). Consumption of vegetables was low; only 14 and 26% of the children consumed tomato and onions, respectively. No fruits rich in vitamin C or A were consumed. Banana was the only fruit that was consumed by 2% of the children. Milk and milk products were consumed by 42% of the children; 37% and 5% consumed milk and yoghurt, respectively. Goat and sheep meat was consumed by 6% of the children, and 39% consumed oils and fats. Tea consumption with milk was reported in 51% of the children. The median (interquartile range) FVS was low with 2.0 (2.0, 4.0), and most of the children (34%) consumed only two food items (Figure 1). In total, >50% of the children consumed only one or two food item (Table 1). The percentage of EBF was only 35%. The other 65% received animal milk, tea with milk, or solid foods before the age of 6 months. Thirty‐six (36) % of the mothers were aware of the WHO recommendation of exclusive breastfeeding during the first 6 months of life.
Figure 1.
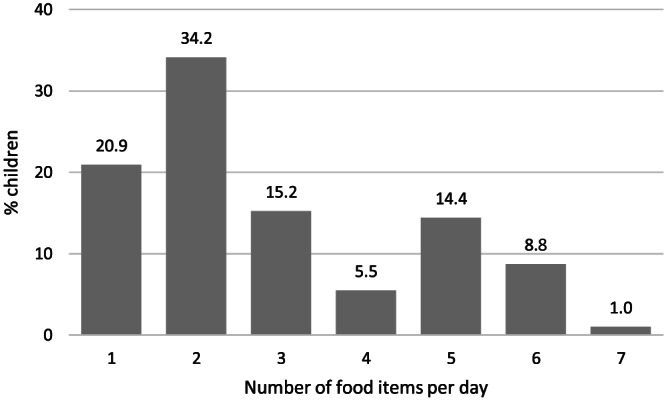
Number of food items consumed by children <5 years of age living in Adadle Woreda, Somali region, Ethiopia, derived from a 24‐hr food recall in the dry season 2016 (n = 492). Food items include all foods consumed the day prior to the food recall but do not include beverages or condiments (e.g., salt or bouillon cubes)
3.3. Nutritional status and intestinal parasitic infections
Stunting, wasting, and underweight were 30, 34, and 40%, respectively, with median Z scores for HAZ, WHZ, and WAZ clearly below −1 (Table 2). The biomarker measurements for iron, vitamin A, and inflammation status were also shown in Table 2. Values indicate poor iron and vitamin A status, and very high prevalence of anaemia, ID, IDA, and VAD was observed. Anaemia (75%) was mainly moderate anaemia (51%) followed by mild (16%) and severe anaemia (8%). Overall, IDA prevalence was 73%. Looking only at the anaemic children who provided both capillary and venous blood samples, 97% had IDA. The median (interquartile range) for the inflammation markers CRP and AGP used to correct the PF values was 0.4 mg L‐1 (0.2, 1.3) and 0.7 g L‐1 (0.5, 0.9), respectively. VAD was found in 30% of the children (Table 2), and 58% of the children were at risk for VAD.
Table 2.
Anthropometric, iron, vitamin A and inflammation status among children <5 years of age living in Adadle Woreda, Somali region, Ethiopia, overall and by gender (data collected in the dry season 2016)
| Variable | All children | Boys | Girls |
|---|---|---|---|
| Height (cm)a | 91 (84, 98) | 91 (84, 98) | 91 (84, 99) |
| Weight (kg)a | 12 (10, 13) | 12 (10, 13) | 12 (10, 13) |
| HAZa | −1.5 (−2.2, −1.0) | −1.7 (−2.3, −1.2) | −1.3 (−2.0, −0.9) |
| WAZa | −1.8 (−2.7, −1.0) | −1.8 (−3.0, −1.0) | −1.6 (−2.6, −0.8) |
| WHZa | −1.2 (−2.5, 0.0) | −1.4 (−2.9, −0.2) | −1.0 (−2.5, 0.1) |
| Insufficient MUAC <12.5 cma | 15.9% | 13.6% | 18.0% |
| Hb (g dl−1)b | 9.5 (8.2, 10.9) | 9.4 (8.2, 10.9) | 9.7 (8.2, 11.0) |
| Anaemia (Hb <11.0 g dl−1)b | 75.1% | 76.2% | 73.9% |
| PF (μg L−1)c | 6.2 (4.0, 10.2) | 5.9 (4.0, 9.4) | 6.4 (4.0, 10.5) |
| sTfR (mg L‐1)c | 21.1 (13.2, 33.1) | 21.5 (13.3, 34.3) | 20.1 (13.1, 32.1) |
| Iron deficiency (PF <12 μg L−1 and/or sTfR >8.3 mg L‐1)c | 91.1% | 90.8% | 91.4% |
| Iron deficiency anaemia (Hb <11.0 g dl ‐1 + PF <12 μg L−1 and/or sTfR >8.3 mg L‐1)d | 72.8% | 75.3% | 70.3% |
| RBP (μmol L‐1)c | 0.8 (0.67, 0.91) | 0.8 (0.67, 0.91) | 0.8 (0.67, 0.92) |
| Vitamin A deficiency (RBP <0.7 μmol)c | 30.2% | 29.7% | 30.6% |
| Elevated CRP >5 mg L−1 c | 10.6% | 10.3% | 11.0% |
| Elevated AGP >1 g L−1 c | 19.1% | 17.4% | 20.6% |
Note. Values represent median (interquartile range), unless indicated otherwise.
Abbreviations: AGP, α‐1‐acid glycoprotein; CRP, C‐reactive protein; HAZ, height‐for‐age z score; Hb, haemoglobin; MUAC, mid‐upper arm circumference; PF, plasma ferritin; RBP, retinol‐binding protein; sTfR, soluble transferrin receptor; WAZ, weight‐for‐age z score; WHZ, weight‐for‐height z score.
n = 492.
n = 453.
n = 404.
n = 371.
The prevalence of IPIs is summarized in Table 3. The overall prevalence of IPIs was high with 47% children having at least a single infection. The most prevalent IPIs were protozoan infections with G. lamblia (22%) followed by helminthic infections with A. lumbricoides (15%).
Table 3.
Prevalence of intestinal parasitic infections among children <5 years of age living in Adadle Woreda, Somali region, Ethiopia (data collected in the dry season 2016)
| % (n = 387) | 95% CI | |
|---|---|---|
| Overall | ||
| Parasite‐free | 53.2 | 46.2, 60.1 |
| Single‐parasite | 42.1 | 36.7, 47.7 |
| Poly‐parasite | 4.7 | 2.5, 8.4 |
| Protozoa | ||
| Giardia lamblia | 22.0 | 16.2, 29.1 |
| Entamoeba histolytica | 4.1 | 2.2, 7.8 |
| Entamoeba dispar | 0.3 | 0.0, 1.8 |
| Entamoeba coli | 1.0 | 0.3, 3.0 |
| Helminths | ||
| Ascaris lumbricoides | 14.7 | 7.1, 28.0 |
| Trichuris trichiura | 0.5 | 0.1, 1.8 |
| Hookworms | 1.6 | 0.7, 3.3 |
| Hymenolepis nana | 3.1 | 1.3, 7.1 |
| Taenia spp. | 2.1 | 0.9, 4.8 |
| Enterobius vermicularis | 1.8 | 0.8, 3.9 |
Children infected with G. lamblia were 3.5 times more likely to be undernourished as indicated by low MUAC compared with noninfected children (Table 4). Remarkably, ID was associated with a reduced risk of G. lamblia infection. No significant associations were found between G. lamblia infection and anaemia or VAD. For A. lumbricoides, we did not find any association with undernutrition. Low MUAC was associated with no milk consumption, children <2 years of age, and female children. The odds of having low MUAC was 49% less in children consuming milk than in children not consuming milk. Children who consumed more than two food groups had anaemia and ID odds that were 97% and 90% lower than children who consumed two or less than two food items (Table 4). The odds for anaemia in children who had EBF were 89% less than in children who were not EBF, and there was a tendency for lower odds for ID in EBF children. Furthermore, the odds of being anaemic were higher in the children <2 years of age. Children consuming more than two food items or consuming milk tended to have lower odds for VAD than their peers consuming one or two food items or not consuming milk, respectively.
Table 4.
Logistic regression analysis of associations between mid‐upper arm circumference, anaemia, iron and vitamin A deficiency and sociodemographic characteristics, food variety score, milk consumption, selected intestinal parasitic infections, and exclusive breastfeeding (data collected in the dry season 2016)
| Variables | MUAC <12.5 cm | Anaemia (Hb <11.0 g dl−1) | Iron deficiency (PF <12 μg L−1 and/or sTfR >8.3 mg L‐1) | Vitamin A deficiency (RBP <0.7 μmol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (n/N)b | aORa | aCI95 | % (n/N)b | aORa | aCI95 | % (n/N)b | aORa | aCI95 | % (n/N)b | aORa | aCI95 | |
| Age | ||||||||||||
| <2 years | 31.7 (13/41) | 88.6 (31/35) | 87.9 (29/33) | 33.3 (11/33) | ||||||||
| ≥2 years | 14.4 (65/451) | 0.41 | 0.20, 0.83 | 73.9 (309/418) | 0.24 | 0.22, 0.95 | 91.4 (339/371) | 1.08 | 0.37, 3.15 | 29.9 (111/371) | 0.92 | 0.34, 2.51 |
| Sex | ||||||||||||
| female | 18.0 (45/250) | 73.9 (164/222) | 91.4 (191/209) | 30.6 (64/209) | ||||||||
| Male | 13.6 (33/242) | 0.54 | 0.30, 0.95 | 76.2 (176/231) | 1.61 | 1.55, 2.42 | 90.8 (177/195) | 0.90 | 0.32, 2.57 | 29.7 (58/195) | 0.98 | 0.64, 1.49 |
| Food variety score | ||||||||||||
| ≤2 food items | 17.0 (46/271) | 95.5 (231/242) | 96.4 (216/224) | 32.6 (73/224) | ||||||||
| >2 food items | 14.5 (32/221) | 0.96 | 0.64, 1.46 | 51.7 (109/111) | 0.03 | 0.03, 0.07 | 84.4 (152/180) | 0.10 | 0.03, 0.38 | 27.2 (49/180) | 0.68 | 0.44, 1.04 |
| Milk consumption | ||||||||||||
| No | 18.9 (54/285) | 74.3 (197/265) | 90.1 (191/212) | 34.4 (73/212) | ||||||||
| Yes | 11.6 (24/207) | 0.51 | 0.30, 0.87 | 76.1 (143/188) | 0.76 | 0.41, 1.30 | 92.2 (177/192) | 1.14 | 0.47, 2.76 | 25.5 (49/192) | 0.61 | 0.35, 1.06 |
| Giardia lamblia infection | ||||||||||||
| Negative | 12.6 (38/302) | 78.9 (221/280) | 94.1 (239/254) | 31.5 (80/254) | ||||||||
| Positive | 32.9 (28/85) | 3.50 | 2.21, 5.54 | 75.9 (60/79) | 0.93 | 0.18, 1.80 | 86.4 (57/66) | 0.45 | 0.24, 0.83 | 30.3 (20/66) | 0.91 | 0.44, 1.88 |
| Ascaris lumbricoides infection | ||||||||||||
| Negative | 17.0 (56/330) | 77.0 (238/309) | 91.7 (244/266) | 31.6 (84/266) | ||||||||
| Positive | 17.5 (10/57) | 1.38 | 0.82‐2.32 | 86.0 (43/50) | 0.79 | 0.35‐1.39 | 96.3 (52/54) | 1.35 | 0.38‐4.81 | 29.6 (16/54) | 0.87 | 0.54‐1.4 |
| EBF first six months of age | ||||||||||||
| No | 16.2 (52/320) | 91.9 (262/285) | 95.2 (257/270) | 33.0 (89/270) | ||||||||
| Yes | 15.1 (26/172) | 0.93 | 0.51‐1.7 | 46.4 (78/168) | 0.11 | 0.11‐0.23 | 82.8 (111/134) | 0.58 | 0.31‐1.09 | 24.6 (33/134) | 0.77 | 0.51‐1.18 |
Abbreviations: aOR, adjusted odds ratio; aCI95, adjusted 95% confidence interval; EBF, exclusive breastfeeding; Hb, haemoglobin; PF, plasma ferritin; RBP, retinol‐binding protein; sTfR, soluble transferrin receptor.
aOR were calculated by adjusting for clustering within households and administrative units (Kebeles) as a fixed effect.
n/N, n = number of children that are positive for the dependent variable in the respective category of the independent variable; N = total number of children in the category of the independent variable.
4. DISCUSSION
To our knowledge, this is the first study collecting detailed data about the nutritional status of children <5 years of age living in the ESRS including nutritional biomarkers, anthropometrics, and IPIs. In the present study, high prevalence of both undernutrition and IPIs were observed. This high prevalence of IPIs is not surprising as in our study area, systematic mass anti‐helminthic drug administration or deworming programs are not implemented, and infected children are only occasionally treated based on clinical suspicion. Several previous studies have reported high prevalence of IPIs among Ethiopian preschool and school children (Abdi et al., 2017; Abera & Nibret, 2014; Alemayehu et al., 2017; Mahmud et al., 2013; Nguyen et al., 2012; Yami, Mamo, & Kebede, 2011); however, data on under five children in ESRS was not available. Depending on the area where the previous studies were conducted, the type of the most prevalent IPIs is different from our study as protozoa and helminths follow a focal distribution depending on climate and environment. For example, in a study conducted in the Tigray region, Northern Ethiopia, the prevalence of enteric parasites in children <5 years of age (48%) was comparable with our study (47%). In contrast to our study, the most prevalent parasites were Entamoeba histolytica and Hymenolepis nana (Asfaw & Goitom, 2000). In our study, low MUAC, used as a proxy for undernutrition, was significantly associated with G. lamblia. Similarly, Giardial infections but not helminthic infections were strongly associated with underweight, wasting, and stunting in a previous study in Zimbabwean school children (Loewenson, Mason, & Patterson, 1986). In another study in Chad, undernutrition in pastoralist children was significantly associated with IPIs (Bechir, Schelling, Hamit, et al., 2012).
We found an extremely high prevalence of ID (91%), as measured as low PF and/or elevated sTfR, suggesting a lack of iron in the diet of the children. Interestingly, ID was associated with a decreased risk of infections with G. lamblia. ID has been associated with protection against several infectious diseases including protection against G. duodenalis in Rwandan children (Danquah, Gahutu, Ignatius, Musemakweri, & Mockenhaupt, 2014; Prentice, 2008). For intestinal and extracellular parasites, such as G. lamblia and G. duodenalis, a low iron status of the host may generate an unfavourable environment and interfere with the parasite's metabolism and replication (Duncombe, Bolin, Davis, Fagan, & Davis, 1980) and could be considered as a host mechanism to withhold iron from the parasite (Prentice, 2008). Anaemia in our study was mainly IDA as indicated by the high prevalence of IDA (73%) almost equal to the anaemia prevalence (75%). To a limited extent, inflammation might have contributed to anaemia as well as indicated by elevated CRP and AGP levels in 11 and 19% of the children, respectively. We used both of the inflammation markers to correct the ferritin concentrations. However, due to the already very low ferritin concentration in almost all children, the effect of the correction on median ferritin concentration and prevalence of ID and IDA was negligible. The anaemia prevalence in our study (75%) was 8% lower than that reported in the most recent DHS for ESRS (Central Statistical Agency/Ethiopia & ICF International, 2016). This difference might be due to a general improvement in nutritional status in ESRS or due to seasonal differences in diet patterns influencing Hb levels. In pastoralist communities, meat consumption is increased during rainy season compared with the dry season as condition for animals are more favourable; hence, more livestock products are available; and particularly, milk yields are higher (Sadler & Catley, 2009). This leads to an increased consumption of milk but also an increased meat consumption as incomes are better from selling milk and milk products. On the other side, the dry season is characterized by a higher availability of staples and a decrease of livestock products, especially milk and milk products (Hassen, Ismail, Haile, & Legese, 2013). We conducted our study at the beginning of the dry season shortly after the rainy season. Therefore, we were likely to capture a slightly improved nutritional status influenced by the increased milk and meat consumption during the rainy season. In our study, children who were EBF up to 6 months of age were less likely to be anaemic. This has also been documented in other studies where EBF in the first 6 months of life was associated with reduced risk of anaemia and ID in infants (Marques, Taddei, Lopez, & Braga, 2014; Uyoga et al., 2016). This is likely because EBF reduces diarrheal disease (and other infections) that accompany early introduction of complementary foods, especially in resource‐limited areas, such as ESRS, where water and sanitation facilities are inadequate. The iron concentration and bioavailability in the diet of young children from resource‐poor areas is often inappropriate to meet their requirements (Gibson, Bailey, Gibbs, & Ferguson, 2010); therefore, children in ESRS who become anaemic in infancy due to the early introduction of complementary foods will unlikely catch up their Hb levels during toddlerhood. Our findings support the recommendation for EBF up to 6 months in this setting. Most of our study children were not EBF up to 6 months of age (65%), and maternal knowledge about the importance of EBF was scarce; 36% of mothers were aware of the WHO recommendation of EBF. Programs on maternal nutrition education are therefore needed to promote EBF in our population.
The mean FVS was very low; most of the children consumed only one or two food items the day before the interview. The consumed foods were mostly staples, which are not a good source of iron or other micronutrients (Gibson et al., 2010). Meat, a good iron source, was rarely consumed because as mentioned before, during the dry season, availability of staples but not meat is high. Our results clearly stress that lack of micronutrient‐dense foods and low nutritional adequacy of the diet are serious issues in pastoralists of the ESRS and a contributing factor to undernutrition. Anaemia was strongly negatively associated with FVS and the odds of anaemia in children who consumed more than two food items were 97% less than in children consuming one or two food items. Similarly to our study, a previous study on predictors of anaemia in Tanzanian children <5 years of age found that nonconsumption of meat, fruits, and vegetables is significantly associated with anaemia, indicating that a more divers diet is beneficial for Hb levels (Kejo, Petrucka, Martin, Kimanya, & Mosha, 2018). Our measurement of RBP indicates that 30% of the children in the ESRS are vitamin A deficient. Milk and milk products are the main animal sources for vitamin A (Codjia, 2001) and frequently consumed by pastoralist communities particularly during the rainy season (Galvin, 1992). In children from nomadic Chadian pastoralists, serum retinol concentrations were higher than in children from settled pastoralists consuming less milk and milk products (Bechir et al., 2012). Goat and cow milk were reported to be important sources of vitamin A in Chadian pastoralist, and the retinol concentrations of milk significantly correlated with the human serum retinol concentration (Zinsstag et al., 2002). Our data was collected shortly after the rainy season characterized by high milk consumption; hence, prevalence of VAD might be higher at the end of the dry season when milk and milk products have been scarcely available for a longer period. A previous study in pastoralists not only showed a strong seasonal fluctuation of milk consumption but also of human retinol status as the retinol concentrations in blood were strongly associated with season (Crump et al., 2017). In our study, milk and milk products were consumed by 42% of the children, and there was a tendency (adjusted 95% confidence interval [0.35, 1.06]) that the odds of having VAD in children consuming milk were lower than those for children not consuming milk, indicating the importance of milk consumption for vitamin A status. Additionally, milk consumption was associated with less undernutrition as indicated by better MUAC. The vital nutritional profile of milk and dairy products providing protein, essential fatty acids, and critical micronutrients, such as vitamin A, vitamin B12 and calcium can help to prevent undernutrition (Murphy & Allen, 2003). In pastoralists in Samburu, Kenya, milk contributed to only 10% of the overall energy intake but to ≥50% of the vitamin A, C, and B12 intake (Iannotti & Lesorogol, 2014). Milk intake in low‐income countries has been associated with improved growth indicators, micronutrient status, and cognitive performance in undernourished children (Dror & Allen, 2011).
The strengths of our study include the holistic approach using biomarkers, anthropometry, and IPIs analysis to gather the first comprehensive data on the nutritional status of children <5 years of age in ESRS and the factors influencing undernutrition. We had a large sample size covering several Kebeles and were able to collect and analyse biological samples in a challenging logistical and infrastructural setting. Although the number of enrolled children per cluster was higher than assumed for the sample size determination, we were able to estimate the prevalences with the anticipated precision as the estimated intracluster correlation coefficient was lower than expected (e.g., .024 for anaemia and .048 for VAD using analysis of variance estimates; for further intra cluster coefficients, see Table S1). Our approach was that parasitic infections increase the risk of undernutrition based on evidence where helminthic infections were strongly associated with undernutrition (Smith & Brooker, 2010). The relationship between infections and nutritional status is however complex, and it is also possible that undernutrition increases the susceptibility to infections: and hence the risk of infections. One limitation in our study was the difficult collection of accurate data on birth dates due to missing birth certificates and high illiteracy in mothers. This had an impact on the analysis of parameters that required a reliable age measurement. In our study, we therefore decided not to use MUAC‐for‐age Z scores as an indicator for undernutrition, and WAZ and HAZ in Table 2 need to be interpreted with caution. Due to constraints with the equipment, body weight was only measured to the nearest 0.5 kg. This affected WHZ, and therefore, we decided not to use WHZ as a key indicator for undernutrition and did not conduct a regression analysis on it. Between 10 to 20% of the children did not provide a blood or stool sample. This could have introduced bias. Consequently, we compared the sociodemographic characteristics of children with missing samples with those with complete data. We did not found noteworthy differences (see Table S2) indicating that our study was not affected by such a bias. Infrastructural constraints only allowed us to conduct qualitative analysis of IPIs using single Kato Katz and SAF smears, and food intake was based on a single 24‐hr recall that did not allow us to capture day‐to‐day and seasonal variations, and which could be influenced by recall bias.
To conclude, our findings show that in pastoralist communities in ESRS, prevalence of undernutrition and IPIs is alarmingly high in children <5 years of age. Undernutrition defined as low MUAC was associated with g iardial infections and milk consumption, and anaemia with exclusive breastfeeding and low nutritional adequacy of the diet. A holistic approach to improve nutrition, promote maternal education, as well as to reduce IPIs is therefore urgently needed in this region. Such an approach should include (a) education on breastfeeding practice, dietary diversification, and good hygiene; (b) enhanced infrastructure to provide safe drinking water, environmental sanitation, and healthcare access; (c) regular deworming and facilitated access to helminth and protozoa treatments; and 4) nutritional interventions to provide adequate amounts of micronutrients, particularly during periods of food shortage, for example, seasonal droughts. The latter could include provision of micronutrient powders (e.g., to fortify milk) and capacity building on food conservation for animal source foods so that they can be stored for use during the dry season.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
CONTRIBUTIONS
KO, JZ, ES, JH, and CIC designed the study; KO, RS, AU, and SA managed study sites and sample collection; KO, JZ, ES, JH, and CIC analysed study data, KO and CIC wrote the first draft; and all the authors reviewed and approved the manuscript.
Supporting information
Figure S1: Sampling diagram
Table S1: Intra cluster correlation coefficients. Estimated using R's ICCbin package.
Table S2: Socio‐demographic characteristics stratified by missing values for iron biomarkers (serum ferritin and soluble transferrin receptor) or intestinal parasites.
ACKNOWLEDGMENT
Special thanks goes to the mothers of the children who allowed their children to participate in the study, local guiders who aided to find the pastoralist camps, data collectors, and the laboratory staff in Freiburg, Germany, who helped for the biomarker analysis.
This study was part of Jigjiga One Health Initiative project funded by the Swiss Agency for Development and Cooperation (SDC) under the grant number 7F‐09057.01.02.
Osman KA, Zinsstag J, Tschopp R, et al. Nutritional status and intestinal parasites among young children from pastoralist communities of the Ethiopian Somali region. Matern Child Nutr. 2020;16:e12955 10.1111/mcn.12955
REFERENCES
- Abdi, M. , Nibret, E. , & Munshea, A. (2017). Prevalence of intestinal helminthic infections and malnutrition among schoolchildren of the Zegie Peninsula, northwestern Ethiopia. Journal of Infection and Public Health, 10(1), 84–92. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/27026133, 10.1016/j.jiph.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Abera, A. , & Nibret, E. (2014). Prevalence of gastrointestinal helminthic infections and associated risk factors among schoolchildren in Tilili town, northwest Ethiopia. Asian Pacific Journal of Tropical Medicine, 7(7), 525–530. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25063281. 10.1016/S1995-7645(14)60088-2 [DOI] [PubMed] [Google Scholar]
- Alemayehu, B. , Tomass, Z. , Wadilo, F. , Leja, D. , Liang, S. , & Erko, B. (2017). Epidemiology of intestinal helminthiasis among school children with emphasis on Schistosoma mansoni infection in Wolaita zone, Southern Ethiopia. BMC Public Health, 17(1), 587 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28633651. 10.1186/s12889-017-4499-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfaw, S. T. , & Goitom, L. (2000). Malnutrition and enteric parasitoses among under‐five children in Aynalem Village, Tigray. Ethiopian Journal of Health Development, 14, 67–76. [Google Scholar]
- Bechir, M. , Schelling, E. , Hamit, M. A. , Tanner, M. , & Zinsstag, J. (2012). Parasitic infections, anemia and malnutrition among rural settled and mobile pastoralist mothers and their children in Chad. EcoHealth, 9(2), 122–131. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/22160444, 10.1007/s10393-011-0727-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechir, M. , Schelling, E. , Kraemer, K. , Schweigert, F. , Bonfoh, B. , Crump, L. , … Zinsstag, J. (2012). Retinol assessment among women and children in sahelian mobile pastoralists. EcoHealth, 9(2), 113–121. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/22825749, 10.1007/s10393-012-0781-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, S. , Woods, T. , Liyanage, W. M. , & Smith, D. L. (1991). A simplified general method for cluster‐sample surveys of health in developing countries. World Health Statistics Quarterly, 44(3), 98–106. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/1949887 [PubMed] [Google Scholar]
- Black, R. E. , Allen, L. H. , Bhutta, Z. A. , Caulfield, L. E. , de Onis, M. , Ezzati, M. , … Rivera, J. (2008). Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet, 371(9608), 243–260. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18207566. S0140‐6736(07)61690‐0 [pii], 10.1016/S0140-6736(07)61690-0 [DOI] [PubMed] [Google Scholar]
- Brogan, D. , Flagg, E. W. , Deming, M. , & Waldman, R. (1994). Increasing the accuracy of the Expanded Programme on Immunization's cluster survey design. Annals of Epidemiology, 4(4), 302–311. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/7921320, 10.1016/1047-2797(94)90086-8 [DOI] [PubMed] [Google Scholar]
- Central Statistical Agency/Ethiopia, & ICF International . (2012). Ethiopia demographic and health survey 2011. Retrieved from Addis Ababa, Ethiopia: http://dhsprogram.com/pubs/pdf/FR255/FR255.pdf
- Central Statistical Agency/Ethiopia, & ICF International . (2016). Ethiopia demographic and health survey 2016—Key indicators. Retrieved from Addis Ababa, Ethiopia: http://dhsprogram.com/pubs/pdf/PR81/PR81.pdf
- Codjia, G. (2001). Food sources of vitamin A and provitamin A specific to Africa: An FAO perspective. Food and Nutrition Bulletin, 22(4), 357–360. [Google Scholar]
- Crompton, D. W. , & Nesheim, M. C. (2002). Nutritional impact of intestinal helminthiasis during the human life cycle. Annual Review of Nutrition, 22, 35–59. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/12055337, 10.1146/annurev.nutr.22.120501.134539 [DOI] [PubMed] [Google Scholar]
- Crump, L. , Bechir, M. , Ngandolo, B. N. , Daugla, D. M. , Hattendorf, J. , & Zinsstag, J. (2017). Seasonal dynamics of human retinol status in mobile pastoralists in Chad. Acta Tropica, 166, 280–286. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/27919689, 10.1016/j.actatropica.2016.11.040 [DOI] [PubMed] [Google Scholar]
- Danquah, I. , Gahutu, J. B. , Ignatius, R. , Musemakweri, A. , & Mockenhaupt, F. P. (2014). Reduced prevalence of Giardia duodenalis in iron‐deficient Rwandan children. Tropical Medicine & International Health, 19(5), 563–567. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/24898273, 10.1111/tmi.12284 [DOI] [PubMed] [Google Scholar]
- de Gier, B. , Campos Ponce, M. , van de Bor, M. , Doak, C. M. , & Polman, K. (2014). Helminth infections and micronutrients in school‐age children: A systematic review and meta‐analysis. American Journal of Clinical Nutrition, 99(6), 1499–1509. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/24740209, 10.3945/ajcn.113.069955 [DOI] [PubMed] [Google Scholar]
- Demissie, T. , Ali, A. , Mekonen, Y. , Haider, J. , & Umeta, M. (2010). Magnitude and distribution of vitamin A deficiency in Ethiopia. Food and Nutrition Bulletin, 31(2), 234–241. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/20707229, 10.1177/156482651003100206 [DOI] [PubMed] [Google Scholar]
- Dror, D. K. , & Allen, L. H. (2011). The importance of milk and other animal‐source foods for children in low‐income countries. Food and Nutrition Bulletin, 32(3), 227–243. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/22073797, 10.1177/156482651103200307 [DOI] [PubMed] [Google Scholar]
- Duncombe, V. M. , Bolin, T. D. , Davis, M. , Fagan, M. R. , & Davis, A. E. (1980). The effect of iron deficiency, protein deficiency and dexamethasone on infection, re‐infection and treatment of Giardia muris in the mouse. The Australian Journal of Experimental Biology and Medical Science, 58(1), 19–26. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/7447792, 10.1038/icb.1980.2 [DOI] [PubMed] [Google Scholar]
- Erhardt, J. G. , Estes, J. E. , Pfeiffer, C. M. , Biesalski, H. K. , & Craft, N. E. (2004). Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C‐reactive protein by an inexpensive, sensitive, and simple sandwich enzyme‐linked immunosorbent assay technique. Journal of Nutrition, 134(11), 3127–3132. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/15514286 [DOI] [PubMed] [Google Scholar]
- Feleke, B. E. (2016). Nutritional status and intestinal parasite in school age children: A comparative cross‐sectional study. International Journal Of Pediatrics, 2016, 1962128. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/27656219, 10.1155/2016/1962128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin, K. A. (1992). Nutritional ecology of pastoralists in dry tropical Africa. American Journal of Human Biology, 4(2), 209–221. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/28524353, 10.1002/ajhb.1310040206 [DOI] [PubMed] [Google Scholar]
- Gebre‐Mariam, A. (2007). The critical issue of land ownership. Violent conflict between the Abdalla Tolomogge and the Awlihan in Godey Zone, Somali Region, Ethiopia. Bern: NCCR North‐South. [Google Scholar]
- Gelaw, A. , Anagaw, B. , Nigussie, B. , Silesh, B. , Yirga, A. , Alem, M. , … Gelaw, B. (2013). Prevalence of intestinal parasitic infections and risk factors among schoolchildren at the University of Gondar Community School, Northwest Ethiopia: A cross‐sectional study. BMC Public Health, 13, 304 Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/23560704, 10.1186/1471-2458-13-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, R. S. , Bailey, K. B. , Gibbs, M. , & Ferguson, E. L. (2010). A review of phytate, iron, zinc, and calcium concentrations in plant‐based complementary foods used in low‐income countries and implications for bioavailability. Food and Nutrition Bulletin, 31(2 Suppl), S134–S146. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/20715598 [DOI] [PubMed] [Google Scholar]
- Hall, A. , Hewitt, G. , Tuffrey, V. , & de Silva, N. (2008). A review and meta‐analysis of the impact of intestinal worms on child growth and nutrition. Maternal & Child Nutrition, 4(Suppl 1), 118–236. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/18289159, 10.1111/j.1740-8709.2007.00127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassen, A. , Ismail, A. , Haile, A. , & Legese, G. (2013). Analysis of sheep and goat value chains in Shinelle district, Somali Region, Ethiopia (2nd ed.). Addis Ababa, Ethiopia and Nairobi, Kenya: ICARDA/ILRI. [Google Scholar]
- Hatloy, A. , Torheim, L. E. , & Oshaug, A. (1998). Food variety—A good indicator of nutritional adequacy of the diet? A case study from an urban area in Mali, West Africa. European Journal of Clinical Nutrition, 52(12), 891–898. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9881884 [DOI] [PubMed] [Google Scholar]
- Hotez, P. J. , Fenwick, A. , Savioli, L. , & Molyneux, D. H. (2009). Rescuing the bottom billion through control of neglected tropical diseases. Lancet, 373(9674), 1–1575. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19410718. 10.1016/S0140-6736(09)60233-6 [DOI] [PubMed] [Google Scholar]
- Iannotti, L. , & Lesorogol, C. (2014). Animal milk sustains micronutrient nutrition and child anthropometry among pastoralists in Samburu, Kenya. American Journal of Physical Anthropology, 155(1), 66–76. Retrieved from <Go to ISI>://WOS:000340477700005. 10.1002/ajpa.22547 [DOI] [PubMed] [Google Scholar]
- Jean‐Richard, V. , Crump, L. , Abicho, A. A. , Abakar, A. A. , Mahamat, A. 2nd , Bechir, M. , … Zinsstag, J. (2015). Estimating population and livestock density of mobile pastoralists and sedentary settlements in the south‐eastern Lake Chad area. Geospatial Health, 10(1), 307 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26054513. 10.4081/gh.2015.307 [DOI] [PubMed] [Google Scholar]
- Kassebaum, N. J. , Jasrasaria, R. , Naghavi, M. , Wulf, S. K. , Johns, N. , Lozano, R. , … Murray, C. J. (2014). A systematic analysis of global anemia burden from 1990 to 2010. Blood, 123(5), 615–624. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24297872. 10.1182/blood-2013-06-508325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz, N. , Chaves, A. , & Pellegrino, J. (1972). A simple device for quantitative stool thick‐smear technique in Schistosomiasis mansoni . Revista do Instituto de Medicina Tropical de São Paulo, 14(6), 397–400. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/4675644 [PubMed] [Google Scholar]
- Kejo, D. , Petrucka, P. M. , Martin, H. , Kimanya, M. E. , & Mosha, T. C. (2018). Prevalence and predictors of anemia among children under 5 years of age in Arusha District, Tanzania. Pediatric Health Med Ther, 9, 9–15. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29443328. 10.2147/PHMT.S148515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenson, R. , Mason, P. R. , & Patterson, B. A. (1986). Giardiasis and the nutritional status of Zimbabwean schoolchildren. Annals of Tropical Paediatrics, 6(1), 73–78. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2428298 [DOI] [PubMed] [Google Scholar]
- Mahmud, M. A. , Spigt, M. , Mulugeta Bezabih, A. , Lopez Pavon, I. , Dinant, G. J. , & Blanco Velasco, R. (2013). Risk factors for intestinal parasitosis, anaemia, and malnutrition among school children in Ethiopia. Pathogens and global health, 107(2), 58–65. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23683331. 10.1179/2047773213Y.0000000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques, R. F. , Taddei, J. A. , Lopez, F. A. , & Braga, J. A. (2014). Breastfeeding exclusively and iron deficiency anemia during the first 6 months of age. Revista da Associação Médica Brasileira (1992), 60(1), 18–22. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24918847 [DOI] [PubMed] [Google Scholar]
- Murphy, S. P. , & Allen, L. H. (2003). Nutritional importance of animal source foods. Journal of Nutrition, 133(11 Suppl 2), 3932S–3935S. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14672292. 10.1093/jn/133.11.3932S [DOI] [PubMed] [Google Scholar]
- Nguyen, N. L. , Gelaye, B. , Aboset, N. , Kumie, A. , Williams, M. A. , & Berhane, Y. (2012). Intestinal parasitic infection and nutritional status among school children in Angolela, Ethiopia. Journal of Preventive Medicine and Hygiene, 53(3), 157–164. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23362622 [PMC free article] [PubMed] [Google Scholar]
- Nyantekyi, L. A. , Legesse, M. , Belay, M. , Tadesse, K. , Manaye, K. , Macias, C. , & Erko, B. (2010). Intestinal parasitic infections among under‐five children and maternal awareness about the infections in Shesha Kekele, Wondo Genet, Southern Ethiopia. Ethiopian Journal of Health Development, 24, 185–190. [Google Scholar]
- Prentice, A. M. (2008). Iron metabolism, malaria, and other infections: What is all the fuss about? Journal of Nutrition, 138(12), 2537–2541. Retrieved from <Go to ISI>://000261038300040. 10.3945/jn.108.098806 [DOI] [PubMed] [Google Scholar]
- Sadler, K. , & Catley, A. (2009). Milk matters: The role and value of milk in the diets of Somali pastoralist children in Liben and Shinile, Ethiopia.
- Sellen, D. W. (1996). Nutritional status of Sub‐Saharan Africa pastoralists: A review of the literature. Nomadic Peoples, 39, 107–134. [Google Scholar]
- Smith, J. L. , & Brooker, S. (2010). Impact of hookworm infection and deworming on anaemia in non‐pregnant populations: A systematic review. Tropical Medicine & International Health, 15(7), 776–795. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20500563. 10.1111/j.1365-3156.2010.02542.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, S. L. Jr. (2003). Amoebiasis. Lancet, 361(9362), 1025–1034. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12660071. 10.1016/S0140-6736(03)12830-9 [DOI] [PubMed] [Google Scholar]
- Thurnham, D. I. , McCabe, L. D. , Haldar, S. , Wieringa, F. T. , Northrop‐Clewes, C. A. , & McCabe, G. P. (2010). Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta‐analysis. American Journal of Clinical Nutrition, 92(3), 546–555. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20610634. 10.3945/ajcn.2010.29284 [DOI] [PubMed] [Google Scholar]
- Utzinger, J. , Botero‐Kleiven, S. , Castelli, F. , Chiodini, P. L. , Edwards, H. , Kohler, N. , … Marti, H. (2010). Microscopic diagnosis of sodium acetate‐acetic acid‐formalin‐fixed stool samples for helminths and intestinal protozoa: A comparison among European reference laboratories. Clinical Microbiology and Infection, 16(3), 267–273. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19456836. 10.1111/j.1469-0691.2009.02782.x [DOI] [PubMed] [Google Scholar]
- Uyoga, M. A. , Karanja, S. , Paganini, D. , Cercamondi, C. I. , Zimmermann, S. A. , Ngugi, B. , … Zimmermann, M. B. (2016). Duration of exclusive breastfeeding is a positive predictor of iron status in 6‐ to 10‐month‐old infants in rural Kenya. Maternal & Child Nutrition. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27896919, 13 10.1111/mcn.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2011). Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Retrieved from https://www.who.int/vmnis/indicators/retinol.pdf
- WHO . (2013). Updates on the management of severe acute malnutrition in infants and children. Retrieved from Geneva: [PubMed]
- WHO/UNICEF/UNU (2001). Iron deficiency anaemia assessment, prevention, and control: A guide for programme managers. Geneva: WHO. [Google Scholar]
- Yami, A. , Mamo, Y. , & Kebede, S. (2011). Prevalence and predictors of intestinal helminthiasis among school children in Jimma zone; A cross‐sectional study. Ethiopian Journal of Health Sciences, 21(3), 167–174. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22434996 [PMC free article] [PubMed] [Google Scholar]
- Zinsstag, J. , Schelling, E. , Daoud, S. , Schierle, J. , Hofmann, P. , Diguimbaye, C. , … Tanner, M. (2002). Serum retinol of Chadian nomadic pastoralist women in relation to their livestocks' milk retinol and beta‐carotene content. International Journal for Vitamin and Nutrition Research, 72(4), 221–228. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12214559. 10.1024/0300-9831.72.4.221 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Sampling diagram
Table S1: Intra cluster correlation coefficients. Estimated using R's ICCbin package.
Table S2: Socio‐demographic characteristics stratified by missing values for iron biomarkers (serum ferritin and soluble transferrin receptor) or intestinal parasites.


