Abstract
This paper aimed to examine the effect of breastfeeding on longitudinal patterns of common infections up to 2 years and respiratory symptoms up to 8 years. To assess the incidence and reoccurrence of infections and allergic symptoms in the first years of life among 1,603 children from the EDEN mother–child cohort, distinct longitudinal patterns of infectious diseases as well as skin rash and respiratory symptoms were identified by group‐based trajectory modelling. To characterize infections, we considered the parent‐reported number of cold/nasopharyngitis and diarrhoea from birth to 12 months and otitis and bronchitis/bronchiolitis from birth to 2 years. To characterize allergy‐related symptoms, we considered the parent‐reported occurrence of wheezing and skin rash from 8 months to 8 years and asthma from 2 to 8 years. Then associations between breastfeeding and these longitudinal patterns were assessed through adjusted multinomial logistic regression. Compared with never‐breastfed infants, ever‐breastfed infants were at a lower risk of diarrhoea events in early infancy as well as infrequent events of bronchitis/bronchiolitis throughout infancy. Only predominant breastfeeding duration was related to frequent events of bronchitis/bronchiolitis and infrequent events of otitis. We found no significant protective effect of breastfeeding on longitudinal patterns of cold/nasopharyngitis, skin rash, or respiratory symptoms. For an infant population with a short breastfeeding duration, on average, our study confirmed a protective effect of breastfeeding on diarrhoea events in early infancy, infrequent bronchitis/bronchiolitis and, to a lesser extent, infrequent otitis events up to 2 years but not on other infections, skin rash, or respiratory symptoms4.
Keywords: breastfeeding, infection, skin rash, wheezing, longitudinal pattern, birth cohort
Short abstract
In an infant population with a short breastfeeding duration and using longitudinal patterns of infection, skin rash and respiratory symptoms, on average, our study confirmed a protective effect of breastfeeding on diarrhea events in early infancy, infrequent bronchitis/bronchiolitis and, to a lesser extent,infrequent otitis events up to 2 years but not on cold/ nasopharyngitis, skin rash or respiratory symptoms.
Key messages
Although breastfeeding appears related to a lower risk of infections in young children worldwide, its benefits on allergy prevention remain more controversial.
The use of group‐based trajectory modeling allowed to identify the temporal evolution of infection and allergic events throughout childhood.
Even in a context of short breastfeeding duration and high hygienic conditions, breastfeeding is associated with lower risk of early gastro‐intestinal infections and of respiratory infections but not related to allergic symptoms.
1. INTRODUCTION
The World Health Organization recommends exclusive breastfeeding in the first 6 months of life or at least the first 4 months of life (World Health Organization, 2003). At birth, because of the small in utero exposure to antigens, the newborn's immune system is immature. Human breast milk contains biologically active substances such as lactoferrin, oligosaccharides, or maternal leukocytes, which are thought to not only protect the infant against infections but also promote the immune system's maturation (Field, 2006; Hanson et al., 2003).
A recent review emphasized a protective effect of breastfeeding on diarrhoea and respiratory infections (Victora et al., 2016), with an estimated prevention of 72% of hospitalizations for diarrhoea and 57% of respiratory infections related to breastfeeding as well as a protective effect on otitis media in children up to 2 years of age. Studies assessing effect of breastfeeding on otitis media were mostly from high‐income countries, and those assessing effect of breastfeeding on diarrhoea and respiratory infections were mostly from low‐ and middle‐income countries (Bowatte et al., 2015; Horta & Victora, 2013). Concerning allergic disorders, a recent review concluded a protective effect of breastfeeding on asthma, but the evidence was weaker for eczema and allergic rhinitis (Lodge et al., 2015). In this review, the protective effect of breastfeeding on allergic disorders was greater in low‐ than high‐income countries.
In high‐income countries, the preventive effect of breastfeeding on respiratory tract infections and allergies is less consistent across studies (Bion et al., 2016; Bowatte et al., 2015; Chiu et al., 2016; Lodge et al., 2015). In a cluster‐randomized trial on promotion of breastfeeding (Promotion of Breastfeeding Intervention Trial), breastfeeding was related to a reduced risk of gastrointestinal infections and atopic eczema in the first year of life (Kramer et al., 2001). However, most studies have reported infections and allergy‐related diseases as outcomes at a specific time point but not their longitudinal pattern throughout infancy and childhood. Assessing association of breastfeeding with a more longitudinal approach could allow for new insights into the timing and duration of the protective effect of breastfeeding on these outcomes.
In this context, the aim of this study was to examine the association between breastfeeding and the trajectories of infections up to 2 years and skin rash or respiratory symptoms up to 8 years.
2. METHODS
2.1. Study population
The EDEN mother–child study is a prospective cohort designed to assess prenatal and postnatal determinants of child growth, development, and health (Heude et al., 2016). In brief, 2,002 pregnant women were recruited in two French university hospitals, before 24 weeks of amenorrhea. Exclusion criteria were multiple pregnancies, known diabetes before pregnancy, illiteracy, and planning to move outside the region in the next 3 years. Written consent was obtained from both parents.
2.2. Breastfeeding
Information on breastfeeding was collected by questionnaires given to parents during the maternity stay and at ages 4, 8, and 12 months and 2 years of the child. The calculation of breastfeeding duration was previously described in detail (Betoko et al., 2013). For the present analysis, breastfeeding was defined as any breastfeeding when the infant received breast milk and as predominant breastfeeding when the only milk received by the infant was breast milk. Both breastfeeding definitions were assessed through their initiation (never vs. ever) and duration. The latter one was assessed as a continuous variable, but as the mean duration of breastfeeding is very short in France (Wagner et al., 2015) and in order to avoid confusion related to the term “long breastfeeding duration,” breastfeeding duration was also assessed as a categorical variable (<1 month, 1 to <4 months, and ≥4 months).
2.3. Infections, skin rash, and respiratory symptoms
Data on infections, skin rash, and respiratory symptoms were collected by questionnaires completed by parents at ages 4, 8, and 12 months of the child and then ages 2, 3, 4, 5, and 8 years.
For infection‐related outcomes, parents could report cold/nasopharyngitis (at ages 4, 8, and 12 months), diarrhoea (at ages 4, 8, and 12 months), otitis (at ages 4, 8, and 12 months and 2 years), and bronchitis/bronchiolitis (at ages 4, 8, and 12 months and 2 years). For skin rash and respiratory symptoms, parents could report skin rash (at ages 8 and 12 months and 2, 3, 4, 5, and 8 years), wheezing (at ages 8 and 12 months and 2, 3, 4, 5, and 8 years), and asthma (at ages 2, 3, 4, 5, and 8 years). At each of these ages, parents were asked to report whether the event had occurred since the last follow‐up and for infections, the number of episodes (1, 2, and ≥3) during the considered period.
2.4. Potential confounders
Family history of allergy is a known risk factor for allergy development (Lack, 2008). Because this family susceptibility results from an inappropriate reaction of the immune system, it is also an important factor to consider when assessing infections in infancy. Parental and sibling history of asthma, eczema, allergic rhinitis, and food allergy was collected during a face‐to‐face interview at 24 to 28 weeks of gestation. Infants were considered at risk of allergy if at least one parent or sibling had one of these allergic symptoms.
During the same interview, data on the study centre, maternal education level, family monthly income, and smoking status were collected. Parity, sex, gestational age, delivery mode, and maternal age were collected at birth from obstetric and paediatric records. The main type of childcare, age at first attendance at a collective care arrangement in the first year, and age at first introduction of solid food were collected from self‐administered questionnaires at ages 4, 8, and 12 months of the child.
2.5. Study samples
Children with missing data on birth weight were excluded from the analyses because they represented early lost to follow‐up (n = 103). Because analyses were run separately for infections and skin rash or respiratory symptoms, children with data at only one time point or less regarding any outcome were excluded (n = 232 for infections and n = 465 for skin rash and respiratory symptoms). Children with missing data on any breastfeeding were excluded (n = 1). Finally, we excluded all children with missing data on potential confounding variables (n = 63 for infections and n = 56 for skin rash and respiratory symptoms). Thus, our sample consisted of 1,603 children for the analysis of infections and 1,377 for the analysis of skin rash and respiratory symptoms.
2.6. Statistical analyses
Mothers included in the current analysis of infections were compared with their EDEN counterparts by Student t test and chi‐square test for continuous and categorical data, respectively.
Among children with at least two documented time points for the considered outcome, available data for the considered outcome at each time point were used to model the longitudinal patterns, by Nagin's method for group‐based trajectory modelling (GBTM; D. Nagin, 2005). The method is based on the underlying hypothesis that within a population, there are inherent groups that evolve according to different patterns. The groups are not directly identifiable or pre‐established by sets of characteristics but are statistically determined by each series of responses. Using the TRAJ procedure from SAS software, multiple models were created, varying in the number of groups and shapes (computed by polynomial equations). Age in months at each time point was the independent variable. For infection patterns, we modelled the number of episodes (none, 1, 2, and ≥3) during each period (CNORM model). For skin rash and respiratory symptom patterns, we modelled the occurrence of at least one event during each period (LOGIT model). To choose the most suitable model for each outcome, we used four decision criteria (Tables S2 and S3). A more complex model (b) has been preferred over a simpler model (a) only in case of higher Bayesian Information Criteria (BIC), defined as follows: 2*(BICmodelB − BICmodelA) > 10. Then, to identify the shape of patterns, we considered the Average Posterior Probability (≥0.7), the difference between the actual and the estimated prevalence (closest to 0) and the Odds of Correct Classification (>5). As suggested by Nagin and Odgers (2010), we also systematically verified that selected models were plausible in real life and therefore easily explainable.
Bivariate analyses between breastfeeding initiation or duration (in three categories), whatever the definition used, and longitudinal patterns of health outcomes involved chi‐square tests and are presented in Tables S4 to S7
The potential links between breastfeeding and longitudinal patterns of health outcomes were assessed using multinomial logistic regression analyses. Analyses were run separately for each definition of breastfeeding and each outcome. All multivariate analyses were adjusted for potential confounding factors, previously identified in literature: family history of allergy (at risk of allergy vs. not‐at‐risk), parity (multiparous vs. primiparous), sex (boys vs. girls), preterm birth, C‐section delivery, age at first attendance at a collective care arrangement (<4 months, 4 to <8 months, 8 to 12 months, and never attended within the first year), age at introduction of solid food (<4 months, 4 to <6 months, and ≥6 months), study centre (Nancy vs. Poitiers), maternal smoking during pregnancy, maternal education level (secondary school or less, high school, 2‐year university degree, and 5‐year university degree), maternal age at birth (<25 years, 25 to 29 years, 30 to 34 years, and >34 years), and monthly family income (≤€1,500, €1,501 to €2,300, €2,301 to €3,000, €3,001 to €3,800, and ≥€3,801).
As no interaction was highlighted between family history of allergies and breastfeeding (all p‐value ≥ .5), analyses were not stratified on family history of allergies.
p < .05 was considered statistically significant. All analyses were carried out using SAS version 9.4 (SAS, Cary, NC).
2.7. Availability of data and materials
The data underlying the findings cannot be made freely available because of ethical and legal restrictions because this study includes an important number of variables that, together, could be used to reidentify the participants based on a few key characteristics and then be used to access other personal data. Therefore, the French ethical authority strictly forbids making such data freely available. However, they can be obtained upon request from the EDEN principal investigator. Readers may contact barbara.heude@inserm.fr to request the data.
2.8. Ethic statement
The EDEN mother‐child cohort was approved by the Ethics Committee of the University Hospital of Kremlin‐Bicêtre on December 12, 2002, and data files were declared to the National Committee for Processed Data and Freedom.
3. RESULTS
The mothers included in our analyses of infections were compared with their nonincluded counterparts (Table S1). Briefly, nonincluded mothers were younger, with lower education level, lower family income, and initiate less breastfeeding than mothers included in the analyses. The nonincluded sample less frequently reported a family history of allergy. The characteristics of the study sample compared by breastfeeding duration categories are available in Table 1.
Table 1.
Characteristics of the study sample according to any breastfeeding duration (n = 1,603 children)
| N | Any breastfeeding duration | ||
|---|---|---|---|
| <1 month | 1 to 4 months | ≥4 months | |
| 523 | 510 | 570 | |
| Recruitment in Poitiers | 63.7% (333) | 42.2% (215) | 38.8% (221) |
| Familial history of allergy | 49.7% (260) | 52.7% (269) | 54.7% (312) |
| Primiparous mother | 44.2% (231) | 50.2% (256) | 43.9% (250) |
| Maternal smoking during pregnancy | 31.5% (165) | 25.1% (128) | 15.1% (86) |
| Maternal master's degree | 22.0% (115) | 33.5% (171) | 47.9% (273) |
| Maternal age at birth (years) | 29.4 (± 4.9) | 29.3 (± 4.7) | 30.5 (± 4.6) |
| Family monthly income | |||
| ≤€1,500 | 17.0% (89) | 11.2% (57) | 12.3% (70) |
| €1,501–2,300 | 37.3% (195) | 29.8% (152) | 22.8% (130) |
| €2,301–3,000 | 25.8% (135) | 28.4% (145) | 28.4% (162) |
| €3,001–3,800 | 13.0% (68) | 17.8% (91) | 19.6% (112) |
| €3,801 | 6.9% (36) | 12.7% (65) | 16.8% (96) |
| Boy | 53.2% (278) | 54.5% (278) | 48.9% (279) |
| Preterm birth | 5.2% (27) | 6.9% (35) | 4.0% (23) |
| C‐section delivery | 16.6% (87) | 16.5% (84) | 14.7% (84) |
| Age at first attendance to collective care arrangement | |||
| Before 4 months | 16.4% (86) | 21.4% (109) | 15.1% (86) |
| Between 4 and 8 months | 6.9% (36) | 11.6% (59) | 17.5% (100) |
| Between 8 and 12 years | 2.1% (11) | 4.1% (21) | 5.3% (30) |
| Never | 74.6% (390) | 62.9% (321) | 62.1% (354) |
|
Age at solid food introduction % (n) or mean (±SD) |
3.9 (±1.7) | 4.2 (±1.6) | 5.0 (±1.4) |
Abbreviation: SD, standard deviation.
3.1. Longitudinal patterns of infections in infancy
The optimal pattern model for describing diarrhoea patterns in infancy was a four‐group model with a square shape for each pattern (Figure 1a). The first pattern (9% of children) was characterized by only early events, before age 8 months, and labelled “Only early.” The second pattern (10% of children) was characterized by recurrent events throughout infancy and labelled “High throughout infancy.” The third pattern (43% of children) was characterized by a first event after 4 months and labelled “Lagged occurrence.” The last pattern (38% of children) was characterized by no diarrhoea and labelled “Never.”
Figure 1.
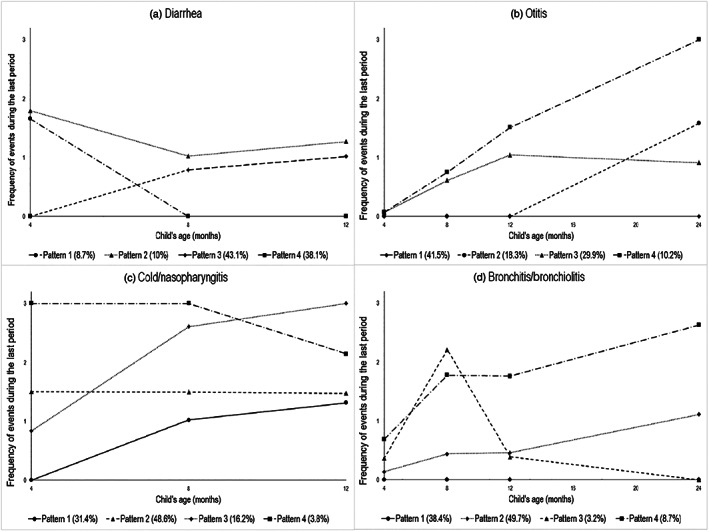
Longitudinal patterns of diarrhoea, otitis, cold/nasopharyngitis, and bronchitis/bronchiolitis up to 2 years (n = 1,603). Pattern legends: (a) Diarrhoea: (1) “Only early,” (2) “High throughout infancy,” (3) “Lagged occurrence,” and (4) “Never”; (b) Otitis: (1) “Never,” (2) “Lagged occurrence,” (3) “Infrequent occurrence,” and (4) “Increasing throughout infancy”; (c) Cold/nasopharyngitis: (1) “Lagged occurrence,” (2) “Moderate throughout infancy,” (3) “Increasing throughout infancy,” and (4) “High throughout infancy”; (d) Bronchitis/bronchiolitis: (1) “Never,” (2) “Infrequent occurrence,” (3) “Peak in early infancy,” and (4) “Increasing throughout infancy”
The optimal pattern model for describing otitis patterns in infancy was a four‐group model with a constant shape for the first pattern and a square shape for the three other patterns (Figure 1b). The first pattern (42% of children) was characterized by no otitis event throughout infancy and labelled “Never.” The second pattern (18% of children) was characterized by a first event after 12 months and labelled “Lagged occurrence.” The third pattern (30% of children) was characterized by increasing events in the first year, with their number remaining moderate up to 2 years, and labelled “Infrequent occurrence.” The last pattern (10% of children) was characterized by increasing events throughout infancy, with a quite high number, and labelled “Increasing throughout infancy.”
The optimal pattern model for describing cold/nasopharyngitis patterns in infancy was a four‐group model with a linear shape for the second and fourth patterns and a square shape for the first and third patterns (Figure 1c). The first pattern (31% of children) was characterized by a first event after age 4 months and labelled “Lagged occurrence.” The second pattern (49% of children) was characterized by moderate number of events throughout infancy and labelled “Moderate throughout infancy.” The third pattern (16% of children) was characterized by an increased number of events throughout infancy and labelled “Increasing throughout infancy.” The last pattern (4% of children) was characterized by a high number of events in early infancy and labelled “High in early frequency.”
The optimal pattern model for describing bronchitis/bronchiolitis patterns in infancy was a four‐group model with a cubic shape for the first, second, and fourth patterns and a square shape for the third (Figure 1d). The first pattern (38% of children) was characterized by no event throughout infancy and labelled “Never.” The second pattern (50% of children) was characterized by increasing events, with their number remaining low, and labelled “Infrequent occurrence.” The third pattern (3% of children) was characterized by peak events at 8 months and labelled “Peak in early infancy.” The last pattern (9% of children) was characterized by increasing events throughout infancy and labelled “Increasing throughout infancy.”
3.2. Longitudinal patterns of skin rash and respiratory symptoms in childhood
The optimal pattern model for describing skin rash patterns in childhood was a five‐group model with a square shape for all patterns except the fourth one, which had a cubic shape (Figure 2c). The first pattern (61% of children) was characterized by no skin rashes throughout childhood and labelled “Never.” The second pattern (12% of children) was characterized by a decreasing occurrence of skin rash and labelled “Decreasing throughout childhood.” The third pattern (13% of children) was characterized by an increasing occurrence of events and labelled “Increasing throughout childhood.” The fourth pattern (4% of children) was characterized by a high peak between 2 and 3 years and labelled “Strong peak in early childhood.” The last pattern (10% of children) was characterized by a high occurrence of skin rash throughout childhood and labelled “High throughout childhood.”
Figure 2.
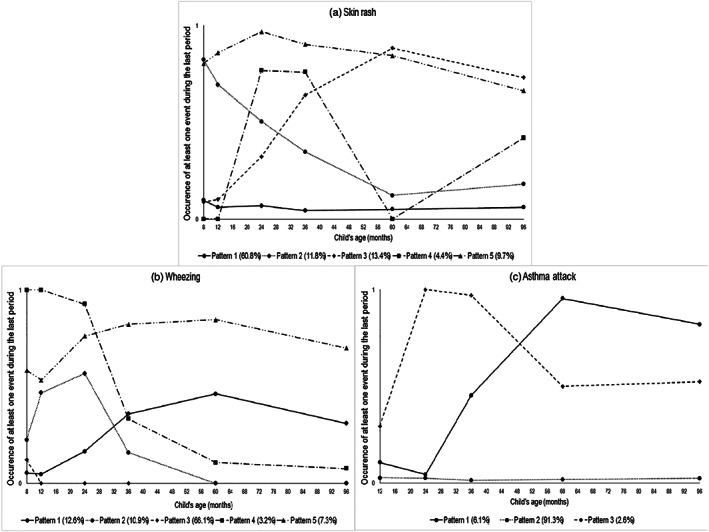
Longitudinal patterns of skin rash, wheezing, and asthma attack up to 8 years (n = 1,377). Pattern legend. (a) Skin rash: (1) “Never,” (2) “Decreasing throughout childhood,” (3) “Increasing throughout childhood,” (4) “Strong peak in early childhood,” and (5) “High throughout childhood”; (b) Wheezing: (1) “Low occurrence,” (2) “Peak in early childhood,” (3) “Never,” (4) “Decreasing throughout childhood,” and (5) “High throughout childhood”; (c) Asthma attack: (1) “Increasing throughout childhood,” (2) “Never,” and (3) “Strong peak in early childhood”
The optimal pattern model for describing wheezing patterns in childhood was a five‐group model with a square shape for each pattern (Figure 2a). The first pattern (13% of children) was characterized by a low occurrence of events throughout childhood and labelled “Low occurrence.” The second pattern (11% of children) was characterized by a small peak between 12 months and 2 years and labelled “Peak in early childhood.” The third pattern (66% of children) was characterized by no wheezing event throughout childhood and labelled “Never.” The fourth pattern (3% of children) was characterized by a decreasing occurrence of wheezing throughout childhood and labelled “Decreasing throughout childhood.” The last pattern (7% of children) was characterized by a high occurrence throughout childhood and labelled “High throughout childhood.”
The optimal pattern model for describing asthma attack patterns in childhood was a three‐group model with a square shape for all patterns (Figure 2b). The first pattern (6% of children) was characterized by an increasing occurrence of asthma attack throughout childhood and labelled “Increasing throughout childhood.” The second pattern (91% of children) was characterized by no asthma attack and labelled “Never.” The third pattern (3% of children) was characterized by a peak in asthma attacks between 2 and 3 years followed by a relatively steady frequency and labelled “Strong peak in early childhood.”
3.3. Breastfeeding and longitudinal patterns of infectious diseases up to 2 years
Both any and predominant breastfeeding were negatively associated with longitudinal patterns of early episodes (<4 months) of diarrhoea in the first year of life, whether these episodes persisted or not thereafter. Predominant breastfeeding duration, considered as a continuous variable, was also associated with a lower risk of late episodes of diarrhoea (Table 2).
Table 2.
Adjusted associations between breastfeeding status and longitudinal patterns of diarrhoea up to 1 year and otitis up to 2 years (n = 1,603)
| Diarrhoea (ref: never) | Otitis (ref: never) | |||||||
|---|---|---|---|---|---|---|---|---|
| Only early | High throughout infancy | Lagged occurrence | p | Lagged occurrence | Infrequent occurrence | Increasing throughout infancy | p | |
| Any breastfeeding | <1.10–4 | .3 | ||||||
| Never breastfed | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | ||
| Ever breastfed | 0.51 [0.33, 0.78] | 0.41 [0.27, 0.60] | 1.09 [0.82, 1.43] | 0.81 [0.58, 1.15] | 0.87 [0.65, 1.16] | 0.68 [0.45, 1.03] | ||
| Any breastfeeding duration (months) | 0.86 [0.80, 0.92] | 0.85 [0.80, 0.91] | 0.99 [0.96, 1.02] | <1.10–4 | 1.00 [0.96, 1.04] | 0.97 [0.94, 1.01] | 0.96 [0.91, 1.01] | .2 |
| Any breastfeeding duration | <1.10–4 | .5 | ||||||
| <1 month | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | ||
| 1 to <4 months | 0.52 [0.33, 0.81] | 0.42 [0.27, 0.65] | 0.90 [0.67, 1.20] | 0.93 [0.65, 1.34] | 0.96 [0.71, 1.30] | 0.77 [0.49, 1.20] | ||
| ≥4 months | 0.27 [0.16, 0.46] | 0.28 [0.17, 0.45] | 1.02 [0.76, 1.37] | 0.88 [0.61, 1.27] | 0.76 [0.55, 1.04] | 0.69 [0.43, 1.09] | ||
| Predominant breastfeeding | <1.10–4 | .2 | ||||||
| Never breastfed | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | ||
| Ever breastfed | 0.48 [0.33, 0.72] | 0.41 [0.28, 0.59] | 0.95 [0.74, 1.21] | 0.81 [0.59, 1.10] | 0.87 [0.67, 1.14] | 0.70 [0.48, 1.02] | ||
| Predominant breastfeeding duration (months) | 0.85 [0.77, 0.93] | 0.79 [0.72, 0.88] | 0.95 [0.91, 0.99] | <1.10–4 | 0.97 [0.92, 1.02] | 0.93 [0.88, 0.97] | 0.96 [0.89, 1.03] | .03 |
| Predominant breastfeeding duration | <1.10–4 | .08 | ||||||
| <1 month | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | ||
| 1 to <4 months | 0.42 [0.27, 0.65] | 0.43 [0.28, 0.64] | 0.96 [0.75, 1.23] | 0.87 [0.63, 1.19] | 0.85 [0.65, 1.11] | 0.75 [0.50, 1.11] | ||
| ≥4 months | 0.35 [0.19, 0.65] | 0.25 [0.13, 0.47] | 0.75 [0.54, 1.04] | 0.72 [0.47, 1.09] | 0.54 [0.37, 0.80] | 0.70 [0.42, 1.19] | ||
Note. Data are multinomial odds ratio [95% confidence interval], adjusted for centre, family history of allergy, parity, smoking status during pregnancy, maternal education level, maternal age at birth, family monthly income, sex, gestational age, caesarean section, age at first attendance to collective care arrangement, and age at introduction of solid food. Separate models were conducted for each breastfeeding exposure and for each outcome, diarrhoea, or otitis.
Any breastfeeding was not associated with otitis events in the first 2 years of life. Nonetheless, predominant breastfeeding duration, considered as a continuous variable, was associated with a lower risk of belonging to the otitis pattern “infrequent occurrence.” The same trend was observed for a long duration (≥4 months) of predominant breastfeeding (Table 2).
Breastfeeding was not associated with longitudinal trajectories of colds/nasopharyngitis (Table 3).
Table 3.
Adjusted associations between breastfeeding status and longitudinal patterns of respiratory infections in infancy (n = 1,603)
| Cold/nasopharyngitis (ref: moderate throughout infancy) | Bronchitis/bronchiolitis (ref: never) | |||||||
|---|---|---|---|---|---|---|---|---|
| Lagged occurrence | Increasing throughout infancy | High in early infancy | p | Infrequent occurrence | Peak in early infancy | Increasing throughout infancy | p | |
| Any breastfeeding | .3 | .1 | ||||||
| Never breastfed | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | ||
| Ever breastfed | 1.02 [0.77, 1.35] | 1.34 [0.94, 1.93] | 1.36 [0.71, 2.59] | 0.75 [0.58, 0.98] | 0.53 [0.27, 1.06] | 0.82 [0.52, 1.30] | ||
| Any breastfeeding duration (months) | 1.00 [0.97, 1.03] | 0.98 [0.94, 1.02] | 0.98 [0.90, 1.06] | .7 | 0.96 [0.93, 0.99] | 0.95 [0.87, 1.04] | 0.95 [0.90, 1.01] | .06 |
| Any breastfeeding duration | .9 | .2 | ||||||
| <1 month | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | ||
| 1 to <4 months | 1.14 [0.85, 1.53] | 1.02 [0.70, 1.47] | 1.21 [0.63, 2.32] | 0.71 [0.54, 0.94] | 0.83 [0.40, 1.73] | 0.81 [0.50, 1.29] | ||
| ≥4 months | 1.15 [0.85, 1.55] | 1.04 [0.71, 1.52] | 0.81 [0.39, 1.68] | 0.75 [0.56, 0.99] | 0.59 [0.27, 1.31] | 0.64 [0.38, 1.07] | ||
| Predominant breastfeeding | .8 | .04 | ||||||
| ever breastfed | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | ||
| Ever breastfed | 1.13 [0.88, 1.46] | 1.01 [0.74, 1.39] | 1.12 [0.62, 2.02] | 0.74 [0.58, 0.94] | 0.57 [0.30, 1.07] | 0.69 [0.45, 1.04] | ||
| Predominant breastfeeding duration (months) | 1.03 [0.99, 1.08] | 0.95 [0.89, 1.01] | 1.01 [0.90, 1.13] | .1 | 0.93 [0.90, 0.97] | 0.94 [0.84, 1.06] | 0.88 [0.80, 0.96] | .003 |
| Predominant breastfeeding duration | .6 | .07 | ||||||
| <1 month | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | ||
| 1 to <4 months | 1.12 [0.87, 1.46] | 0.80 [0.58, 1.11] | 1.04 [0.57, 1.89] | 0.89 [0.70, 1.14] | 0.98 [0.51, 1.88] | 0.73 [0.48, 1.12] | ||
| ≥4 months | 1.11 [0.79, 1.57] | 0.75 [0.48, 1.18] | 1.11 [0.48, 2.56] | 0.64 [0.46, 0.88] | 0.57 [0.23, 1.43] | 0.44 [0.23, 0.83] | ||
Note. Data are multinomial odds ratio [95% confidence interval], adjusted for centre, family history of allergy, parity, smoking status during pregnancy, maternal education level, maternal age at birth, family monthly income, sex, gestational age, caesarean section, age at first attendance to collective care arrangement, and age at introduction of solid food. Separate models were conducted for each breastfeeding exposure and for each outcome, cold/nasopharyngitis, or bronchitis/bronchiolitis.
Predominant breastfeeding and, to a lesser extent, any breastfeeding were both negatively associated with the risk of infrequent occurrence of bronchitis/bronchiolitis in the first 2 years of life. Long duration of predominant breastfeeding (≥4 months) was also associated with a lower risk of belonging to the trajectory “increasing throughout infancy” of bronchitis/bronchiolitis. The association was not significant when any breastfeeding duration was considered (Table 3).
3.4. Breastfeeding and longitudinal patterns of skin rash and respiratory symptoms up to 8 years
Ever breastfeeding and breastfeeding duration were not related to the longitudinal patterns of skin rash and respiratory symptoms up to 8 years (Tables 4 and 5).
Table 4.
Adjusted associations between breastfeeding status and longitudinal patterns of skin rash in childhood (n = 1,377)
| Skin rash | |||||
|---|---|---|---|---|---|
| (ref: never) | |||||
| Decreasing throughout childhood | Increasing throughout childhood | Strong peak in early childhood | High throughout childhood | p | |
| Any breastfeeding | .5 | ||||
| Never breastfed | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | |
| Ever breastfed | 0.81 [0.54, 1.20] | 0.96 [0.65, 1.42] | 1.70 [0.81, 3.55] | 1.04 [0.66, 1.64] | |
| Any breastfeeding duration (months) | 1.00 [0.95, 1.05] | 0.99 [0.94, 1.04] | 1.03 [0.96, 1.11] | 0.97 [0.91, 1.02] | .7 |
| Any breastfeeding duration | .5 | ||||
| <1 month | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | |
| 1 to <4 months | 0.99 [0.65, 1.53] | 0.96 [0.63, 1.47] | 2.22 [1.06, 4.65] | 1.24 [0.78, 1.98] | |
| ≥4 months | 0.95 [0.61, 1.49] | 0.98 [0.64, 1.50] | 1.76 [0.81, 3.80] | 0.82 [0.49, 1.36] | |
| Predominant breastfeeding | .8 | ||||
| Never breastfed | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | |
| Ever breastfed | 0.89 [0.62, 1.29] | 0.91 [0.64, 1.30] | 1.37 [0.73, 2.58] | 1.07 [0.70, 1.63] | |
| Predominant breastfeeding duration (months) | 0.96 [0.89, 1.03] | 1.00 [0.94, 1.07] | 0.85 [0.71, 1.01] | 0.99 [0.91, 1.07] | .3 |
| Predominant breastfeeding duration | .8 | ||||
| <1 month | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | |
| 1 to <4 months | 1.03 [0.70, 1.51] | 0.89 [0.61, 1.28] | 1.31 [0.71, 2.41] | 1.21 [0.80, 1.84] | |
| ≥4 months | 1.13 [0.66, 1.92] | 1.23 [0.77, 1.99] | 1.38 [0.63, 3.01] | 0.91 [0.50, 1.66] | |
Note. Data are multinomial odds ratio [95% confidence interval], adjusted for centre, family history of allergy, parity, smoking status during pregnancy, maternal education level, maternal age at birth, family monthly income, sex, gestational age, caesarean section, age at first attendance to collective care arrangement, and age at introduction of solid food. Separate models were conducted for each breastfeeding exposure.
Table 5.
Adjusted associations between breastfeeding status and longitudinal patterns of respiratory allergic symptoms in childhood (n = 1,377)
| Wheezing (ref: never) | p | Asthma attack (ref: never) | p | |||||
|---|---|---|---|---|---|---|---|---|
| Low occurrence | Peak in early childhood | Decreasing throughout childhood | High throughout childhood | Increasing throughout childhood | Strong peak in early childhood | |||
| Any breastfeeding | .9 | .4 | ||||||
| Never breastfed | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | ||
| Ever breastfed | 1.04 [0.70, 1.55] | 0.94 [0.61, 1.44] | 0.74 [0.36, 1.52] | 0.94 [0.57, 1.55] | 1.30 [0.76, 2.24] | 0.69 [0.32, 1.47] | ||
| Any breastfeeding duration (months) | 0.97 [0.92, 1.02] | 1.00 [0.95, 1.05] | 0.91 [0.82, 1.02] | 1.01 [0.95, 1.08] | .3 | 1.00 [0.94, 1.07] | 1.01 [0.91, 1.12] | 1 |
| Any breastfeeding duration | .6 | .7 | ||||||
| <1 month | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | ||
| 1 to <4 months | 1.27 [0.84, 1.93] | 0.89 [0.57, 1.41] | 0.70 [0.32, 1.50] | 0.77 [0.44, 1.34] | 1.01 [0.56, 1.82] | 0.65 [0.27, 1.56] | ||
| ≥4 months | 0.93 [0.60, 1.46] | 0.95 [0.60, 1.51] | 0.56 [0.24, 1.27] | 1.03 [0.60, 1.77] | 1.34 [0.75, 2.40] | 1.04 [0.44, 2.46] | ||
| Predominant breastfeeding | 1 | |||||||
| Never breastfed | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | .4 | 1 [Ref] | 1 [Ref] | |
| Ever breastfed | 1.01 [0.70, 1.46] | 0.81 [0.55, 1.20] | 0.56 [0.29, 1.09] | 0.96 [0.60, 1.53] | 1.05 [0.64, 1.71] | 1.03 [0.50, 2.14] | ||
| Predominant breastfeeding duration (months) | 1.02 [0.96, 1.09] | 0.99 [0.93, 1.06] | 1.04 [0.95, 1.15] | 0.96 [0.89, 1.04] | .6 | 1.01 [0.92, 1.10] | 1.08 [0.94, 1.24] | .6 |
| Predominant breastfeeding duration | .2 | 1 | ||||||
| <1 month | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | 1 [Ref] | ||
| 1 to <4 months | 1.23 [0.85, 1.77] | 0.89 [0.60, 1.32] | 0.55 [0.27, 1.10] | 0.98 [0.62, 1.56] | 1.03 [0.62, 1.72] | 0.75 [0.35, 1.62] | ||
| ≥4 months | 0.71 [0.41, 1.22] | 0.8 [0.47, 1.35] | 0.40 [0.14, 1.16] | 0.60 [0.30, 1.22] | 0.97 [0.48, 1.97] | 0.97 [0.33, 2.89] | ||
Note. Data are multinomial odds ratio [95% confidence interval], adjusted for centre, family history of allergy, parity, smoking status during pregnancy, maternal education level, maternal age at birth, family monthly income, sex, gestational age, caesarean section, age at first attendance to collective care arrangement, and age at introduction of solid food. Separate models were conducted for each breastfeeding exposure and for each outcome, wheezing, or asthma attack.
4. DISCUSSION
In the EDEN mother–child cohort, we confirmed that breastfeeding was related to a lower risk of diarrhoea events in early infancy and infrequent occurrence of bronchitis/bronchiolitis up to 2 years. Moreover, predominant breastfeeding duration was negatively related to the risk of diarrhoea events in late infancy, of infrequent otitis occurrence, and of repeated bronchitis/bronchiolitis events throughout infancy. However, we were not able to highlight association between breastfeeding and longitudinal patterns of cold/nasopharyngitis, skin rash, or respiratory symptoms.
Most of the studies regarding the association between breastfeeding and diarrhoea or respiratory infections were conducted in low‐ and middle‐income countries (Horta & Victora, 2013), but even in high‐income countries, where hygienic conditions do not benefit the development of germs, breastmilk has a protective role. However, the protective role of breastfeeding on gastrointestinal infections may last only when the infant is breastfed and shortly after (Kramer et al., 2003; Quigley, Kelly, & Sacker, 2007). Consistent with these findings, in the EDEN mother–child cohort, ever‐breastfed infants were less likely to show longitudinal patterns of diarrhoea characterized by an increased number of diarrhoeas in early infancy.
Concerning respiratory infections, the last meta‐analysis concluded a clear protective effect of breastfeeding (Horta & Victora, 2013). The latter finding was also highlighted in a systematic review of data from high‐income countries (Duijts, Ramadhani, & Moll, 2009). In this study, we did not find such a protective effect on cold/nasopharyngitis. Breastfed children seemed less likely to have infrequent occurrence of bronchitis/bronchiolitis (as compared with never occurrence), whatever the definition used for breastfeeding, whereas only predominantly breastfed infants, especially those breastfed for ≥4 months, seemed less likely to have frequent occurrence of bronchitis/bronchiolitis. Our results suggest that breastfeeding may be related not only to the incidence of respiratory symptoms but also to the reoccurrence of these symptoms throughout infancy. Frequent episodes of bronchiolitis are known to predispose to asthma in the early years of life, so low‐frequency bronchitis/bronchiolitis may rely more on infectious origins, whereas high‐frequency bronchitis/bronchiolitis may be related to the allergic background of the child. Thus, our results would suggest a protective effect of breastfeeding on respiratory infections. Using other statistical methods, the Pollution and Asthma Risk: an Infant Study (PARIS) cohort highlighted that children who were breastfed for at least 6 months were less likely to have the cough/rhinitis phenotype in the first 4 years of life (Ranciere, Nikasinovic, Bousquet, & Momas, 2013).
Concerning ear infections, a recent meta‐analysis of studies from the United States and Europe found consistent evidence of a protective effect of breastfeeding on acute otitis media occurrence during the first 2 years of life (Bowatte et al., 2015). In this meta‐analysis, the protective effect was clearer when considering exclusive breastfeeding for the first 6 months (odds ratio = 0.57 [95% confidence interval 0.44, 0.75]) than when considering any breastfeeding for >3 to 4 months (0.71 [0.42, 1.20]). In line with these findings, we did not find any association between any breastfeeding and longitudinal trajectories of otitis, but predominant breastfeeding duration was negatively related to the risk of infrequent occurrence of otitis events.
The protective effect of breastfeeding on the development of allergic symptoms remains controversial (Victora et al., 2016). Exclusive breastfeeding was found associated with reduced eczema prevalence at age 1 year in the cluster‐randomized Promotion of Breastfeeding Intervention Trial (Kramer et al., 2001), but recent meta‐analyses found no evidence of an association with eczema incidence and inconclusive evidence for an association with asthma or wheezing (Kramer & Kakuma, 2012; Lodge et al., 2015). In these meta‐analyses, asthma was not considered for children under age 5 in order to avoid misclassification of infants with transient wheezing. Our results agree with these findings despite some noticeable differences in the definition of allergic symptoms. In our study, we used skin rash instead of eczema, which widened the definition of this outcome. Moreover, eczema, wheezing, and asthma attacks do not always have an allergic origin. Finally, the EDEN mother–child cohort recorded a wide range of confounding factors such as family history of allergy or age at introduction of solid foods, which did not change the results when adjusted for in the analyses.
Beyond nutrients, breast milk transmits immunomodulatory components such as secretory immunoglobulin A, lactoferrin, food antigens or oligosaccharides, and microorganisms (Berdi et al., 2018; Hanson et al., 2003; Hoppu, Kalliomaki, Laiho, & Isolauri, 2001; Petherick, 2010). These components may influence gut microbiota as human milk oligosaccharides are substrates for the development of certain beneficial bacterial strains (Coppa, Bruni, Morelli, Soldi, & Gabrielli, 2004) and microorganisms may colonize the infant's digestive tract and prevent the development of other potentially harmful strains (Petherick, 2010).
To our knowledge, few studies have used GBTM to assess infection and allergic development. The method allows for longitudinal classification of infants and discrimination between transient and regular outbreaks, which can reflect the infant's susceptibility to infections and allergic profile. As any statistical method, the GBTM method is not perfect. It is not always easy to find the optimal number of groups or the right shape for each pattern. Criteria such as BIC, Average Posterior Probability, or Odds of Correct Classification are useful objective tools for decision making regarding the choice of number of groups, but the consistency of these groups with real life must not be neglected. Nonetheless, when assessing the links between breastfeeding and these patterns, the method brings additional information such as whether a potential association is found only when the infant is still breastfed or even after breastfeeding cessation or whether breastfeeding is associated with the temporal evolution of a symptom. Comparison of the same patterns from larger and foreign cohorts would give good information on the development of these outcomes and may lead to targeted interventions to prevent them.
The EDEN mother–child cohort is a French ongoing regional observational study. Because of the sample selection and attrition issues, these results cannot be generalized to the whole population. In high‐income countries, wealthy families are more likely to breastfed their infants, and these infants are at a lower risk of infections, which can lead to a probable overestimation of the associations between breastfeeding and a lower risk of infection. Therefore, further studies need to be conducted, especially in disadvantaged families. However, the insights and long‐term follow‐up of our results represent a major asset. Recent data from a French nationwide birth cohort reported 70% breastfeeding initiation and 22% breastfeeding rates at 4 months (Wagner et al., 2015), whereas in the EDEN mother–child cohort, 74% of children were ever breastfed and 36% were breastfed for at least 4 months. Breastfeeding rates in the EDEN cohort are higher than national ones, but still below guidelines.
5. CONCLUSION
Despite a context of low rate and duration of breastfeeding and high hygienic conditions, we found, using a longitudinal approach, a beneficial association between breastfeeding and diarrhoea, bronchitis/bronchiolitis, and to a lesser extent, otitis during infancy. The use of longitudinal patterns of infections allowed us to confirm that the potential protective effect of breastfeeding on diarrhoea events seems to be maximized when breastfeeding is still ongoing. However, we were not able to highlight any association between breastfeeding and skin rash or respiratory symptoms. These results and particularly the use of GBTM need to be replicated in larger and representative cohorts. Nonetheless, the promotion and facilitation of breastfeeding initiation and duration are part of prevention of the occurrence of infections and hence reduce their economic cost due to health care system usage (hospitalization, medication, etc.) and parental leave from work.
CONTRIBUTIONS
All authors have read and approved the final manuscript. CD‐P conceptualized and designed the study, conducted the statistical analyses, interpreted the results, and drafted the initial manuscript. KA‐P and SL contributed to the interpretation of the results and critically reviewed the manuscript. AF conducted the data collection and data management and critically reviewed the manuscript. IA‐M supervised the data collection and critically reviewed the manuscript. M‐AC and BH designed the data collection instruments, supervised data collection and data management, contributed to the interpretation of the results, and critically reviewed the manuscript. BdL‐G conceptualized and designed the study, contributed to the interpretation of the results, and reviewed and revised the manuscript
Supporting information
Table S1: Comparison of included and excluded families (Chi2 and Student t‐tests)
Table S2: Model criteria for longitudinal patterns of infection
Table S3: Model criteria for longitudinal patterns of allergic symptoms
Table S4: Non‐adjusted association between breastfeeding, any or predominant, and diarrhea and otitis in infancy (n = 1,603, chi‐square test)
Table S5: Non‐adjusted association between breastfeeding, any or predominant, and cold/nasopharyngitis and bronchitis/bronchiolitis in infancy (n = 1,603, chi‐square test)
Table S6: Non‐adjusted association between breastfeeding, any or predominant, and skin rash in infancy (n = 1,377, chi‐square test)
Table S7: Non‐adjusted association between breastfeeding, any or predominant, and wheezing and asthma in infancy (n = 1,377, chi‐square test)
ACKNOWLEDGMENTS
We thank the members of the EDEN Mother–Child Cohort Study Group: I. Annesi‐Maesano, J. Y. Bernard, J. Botton, M. A. Charles, P. Dargent‐Molina, B. de Lauzon‐Guillain, P. Ducimetière, M. de Agostini, B. Foliguet, A. Forhan, X. Fritel, A. Germa, V. Goua, R. Hankard, B. Heude, M. Kaminski, B. Larroque, N. Lelong, J. Lepeule, G. Magnin, L. Marchand, C. Nabet, F. Pierre, R. Slama, M. J. Saurel‐Cubizolles, M. Schweitzer, and O. Thiebaugeorges. The EDEN study is supported by the Fondation pour la Recherche Médicale (FRM), French Ministry of Research: Federative Research Institutes and Cohort Program, INSERM Human Nutrition National Research Program, and Diabetes National Research Program (by a collaboration with the French Association of Diabetic Patients [AFD]), French Ministry of Health, French Agency for Environment Security (AFSSET), French National Institute for Population Health Surveillance (InVS), Paris–Sud University, French National Institute for Health Education (INPES), Nestlé, Mutuelle Générale de l'Education Nationale (MGEN), French speaking association for the study of diabetes and metabolism (ALFEDIAM), National Agency for Research (ANR non thematic program), National Institute for Research in Public health (IRESP: TGIR 2008 cohort in health program). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors received no specific funding for this work.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
Davisse‐Paturet C, Adel‐Patient K, Forhan A, et al. Breastfeeding initiation or duration and longitudinal patterns of infections up to 2 years and skin rash and respiratory symptoms up to 8 years in the EDEN mother–child cohort. Matern Child Nutr. 2020;16:e12935 10.1111/mcn.12935
Contributor Information
Camille Davisse‐Paturet, Email: camille.davisse-paturet@inserm.fr.
Blandine de Lauzon‐Guillain, Email: blandine.delauzon@inserm.fr.
REFERENCES
- Berdi, M. , de Lauzon‐Guillain, B. , Forhan, A. , Castelli, F. A. , Fenaille, F. , Charles, M. A. , … on behalf of the EDEN Mother–Child Cohort Study Group (2018). Immune components of early breastmilk: Association with maternal factors and with reported food allergy in childhood. Pediatric Allergy and Immunology, 30, 107–116. 10.1111/pai.12998 [DOI] [PubMed] [Google Scholar]
- Betoko, A. , Charles, M. A. , Hankard, R. , Forhan, A. , Bonet, M. , Saurel‐Cubizolles, M. J. , … EDEN mother–child cohort study group (2013). Infant feeding patterns over the first year of life: Influence of family characteristics. European Journal of Clinical Nutrition, 67(6), 631–637. 10.1038/ejcn.2012.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bion, V. , Lockett, G. A. , Soto‐Ramirez, N. , Zhang, H. , Venter, C. , Karmaus, W. , … Arshad, S. H. (2016). Evaluating the efficacy of breastfeeding guidelines on long‐term outcomes for allergic disease. Allergy, 71(5), 661–670. 10.1111/all.12833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowatte, G. , Tham, R. , Allen, K. J. , Tan, D. J. , Lau, M. , Dai, X. , & Lodge, C. J. (2015). Breastfeeding and childhood acute otitis media: A systematic review and meta‐analysis. Acta Paediatrica, 104(467), 85–95. 10.1111/apa.13151 [DOI] [PubMed] [Google Scholar]
- Chiu, C.‐Y. , Liao, S.‐L. , Su, K.‐W. , Tsai, M.‐H. , Hua, M.‐C. , Lai, S.‐H. , … Huang, J.‐L. (2016). Exclusive or Partial Breastfeeding for 6 Months Is Associated With Reduced Milk Sensitization and Risk of Eczema in Early Childhood: The PATCH Birth Cohort Study. Medicine, 95(15), e3391 10.1097/md.0000000000003391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppa, G. V. , Bruni, S. , Morelli, L. , Soldi, S. , & Gabrielli, O. (2004). The first prebiotics in humans: Human milk oligosaccharides. Journal of Clinical Gastroenterology, 38(6 Suppl), S80–S83. 10.1097/01.mcg.0000128926.14285.25 [DOI] [PubMed] [Google Scholar]
- Duijts, L. , Ramadhani, M. K. , & Moll, H. A. (2009). Breastfeeding protects against infectious diseases during infancy in industrialized countries. A systematic review. Maternal & Child Nutrition, 5(3), 199–210. 10.1111/j.1740-8709.2008.00176.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, C. J. (2006). The immunological components of human milk and their effect on immune development in infants. The Journal of Nutrition, 135(1), 1–4. [DOI] [PubMed] [Google Scholar]
- Hanson, L. A. , Korotkova, M. , Lundin, S. , Haversen, L. , Silfverdal, S. A. , Mattsby‐Baltzer, I. , … Telemo, E. (2003). The transfer of immunity from mother to child. Annals of the New York Academy of Sciences, 987, 199–206. 10.1111/j.1749-6632.2003.tb06049.x [DOI] [PubMed] [Google Scholar]
- Heude, B. , Forhan, A. , Slama, R. , Douhaud, L. , Bedel, S. , Saurel‐Cubizolles, M. J. , … EDEN mother–child cohort study group (2016). Cohort profile: The EDEN mother–child cohort on the prenatal and early postnatal determinants of child health and development. International Journal of Epidemiology, 45(2), 353–363. 10.1093/ije/dyv151 [DOI] [PubMed] [Google Scholar]
- Hoppu, U. , Kalliomaki, M. , Laiho, K. , & Isolauri, E. (2001). Breast milk—immunomodulatory signals against allergic diseases. Allergy, 56(Suppl 67), 23–26. 10.1034/j.1398-9995.2001.00908.x [DOI] [PubMed] [Google Scholar]
- Horta, B. L. , & Victora, C. G. (2013). Short‐term effects of breastfeeding: A systematic review of the benefits of breastfeeding on diarrhoea and pneumonia mortality (W. H. Organization Ed.): World Health Organization.
- Kramer, M. S. , Chalmers, B. , Hodnett, E. D. , Sevkovskaya, Z. , Dzikovich, I. , Shapiro, S. , … for the, P. S. G (2001). Promotion of Breastfeeding Intervention Trial (PROBIT). JAMA, 285(4), 413–420. 10.1001/jama.285.4.413 [DOI] [PubMed] [Google Scholar]
- Kramer, M. S. , Guo, T. , Platt, R. W. , Sevkovskaya, Z. , Dzikovich, I. , Collet, J. P. , … Bogdanovich, N. (2003). Infant growth and health outcomes associated with 3 compared with 6 mo of exclusive breastfeeding. The American Journal of Clinical Nutrition, 78(2), 291–295. 10.1093/ajcn/78.2.291 [DOI] [PubMed] [Google Scholar]
- Kramer, M. S. , & Kakuma, R. (2012). Optimal duration of exclusive breastfeeding. The Cochrane Database of Systematic Reviews, 8(8), CD003517 10.1002/14651858.CD003517.pub2 [DOI] [PubMed] [Google Scholar]
- Lack, G. (2008). Epidemiologic risks for food allergy. The Journal of Allergy and Clinical Immunology, 121(6), 1331–1336. 10.1016/j.jaci.2008.04.032 [DOI] [PubMed] [Google Scholar]
- Lodge, C. J. , Tan, D. J. , Lau, M. X. , Dai, X. , Tham, R. , Lowe, A. J. , … Dharmage, S. C. (2015). Breastfeeding and asthma and allergies: A systematic review and meta‐analysis. Acta Paediatrica, 104(467), 38–53. 10.1111/apa.13132 [DOI] [PubMed] [Google Scholar]
- Nagin, D. (2005). Group‐Based Modeling of Development. Cambridge, MA: Harvard Univ. Press. [Google Scholar]
- Nagin, D. S. , & Odgers, C. L. (2010). Group‐based trajectory modeling in clinical research. Annual Review of Clinical Psychology, 6, 109–138. 10.1146/annurev.clinpsy.121208.131413 [DOI] [PubMed] [Google Scholar]
- Petherick, A. (2010). Development: Mother's milk: A rich opportunity. Nature, 468(7327), S5–S7. 10.1038/468S5a [DOI] [PubMed] [Google Scholar]
- Quigley, M. A. , Kelly, Y. J. , & Sacker, A. (2007). Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics, 119(4), e837–e842. 10.1542/peds.2006-2256 [DOI] [PubMed] [Google Scholar]
- Ranciere, F. , Nikasinovic, L. , Bousquet, J. , & Momas, I. (2013). Onset and persistence of respiratory/allergic symptoms in preschoolers: New insights from the PARIS birth cohort. Allergy, 68(9), 1158–1167. 10.1111/all.12208 [DOI] [PubMed] [Google Scholar]
- Victora, C. G. , Bahl, R. , Barros, A. J. , Franca, G. V. , Horton, S. , Krasevec, J. , … Lancet Breastfeeding Series, G (2016). Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet, 387(10017), 475–490. 10.1016/S0140-6736(15)01024-7 [DOI] [PubMed] [Google Scholar]
- Wagner, S. , Kersuzan, C. , Gojard, S. , Tichit, C. , Nicklaus, S. , Geay, B. , … de Lauzon‐Guillain, B. (2015). Breastfeeding duration in France according to parents and birth characteristics. Results from the ELFE longitudinal French Study, 2011. Bulletin Epidémiologique Hebdomadaire, 29, 522–532. [Google Scholar]
- World Health Organization . (2003). Feeding and nutrition of infants and young children, guidelines for the WHO European region, with emphasis on the former Soviet countries. Retrieved from Geneva:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Comparison of included and excluded families (Chi2 and Student t‐tests)
Table S2: Model criteria for longitudinal patterns of infection
Table S3: Model criteria for longitudinal patterns of allergic symptoms
Table S4: Non‐adjusted association between breastfeeding, any or predominant, and diarrhea and otitis in infancy (n = 1,603, chi‐square test)
Table S5: Non‐adjusted association between breastfeeding, any or predominant, and cold/nasopharyngitis and bronchitis/bronchiolitis in infancy (n = 1,603, chi‐square test)
Table S6: Non‐adjusted association between breastfeeding, any or predominant, and skin rash in infancy (n = 1,377, chi‐square test)
Table S7: Non‐adjusted association between breastfeeding, any or predominant, and wheezing and asthma in infancy (n = 1,377, chi‐square test)
Data Availability Statement
The data underlying the findings cannot be made freely available because of ethical and legal restrictions because this study includes an important number of variables that, together, could be used to reidentify the participants based on a few key characteristics and then be used to access other personal data. Therefore, the French ethical authority strictly forbids making such data freely available. However, they can be obtained upon request from the EDEN principal investigator. Readers may contact barbara.heude@inserm.fr to request the data.


