Abstract
Objective:
Few data about the development of infants born to women with bipolar disorder have been published. We hypothesized that infants of women with bipolar disorder (by DSM-IV criteria) treated with psychotropics (BD+) or untreated with psychotropics (BD−) would demonstrate poorer cognitive and behavioral development than infants of controls. On the basis of previous studies, we expected that psychotropic-exposed infants of women in the BD+ group would have poorer neuromotor performance during infancy.
Methods:
This longitudinal study included 197 mother-infant dyads recruited to participate between July 2006 and March 2011: 81 with prenatal maternal bipolar disorder without psychotropic treatment (BD–, n = 27) or bipolar disorder with psychotropic exposure (BD+, n = 54) and 116 in which infants were exposed to neither bipolar disorder nor psychotropics. Maternal psychopathology and pharmacotherapy exposure assessments were completed at 20, 30, and 36 prenatal weeks and 12, 26, and 52 weeks postpartum. Infants were evaluated with the Bayley Scales of Infant Development, Second Edition, which included the psychomotor (Psychomotor Development Index [PDI]), cognitive (Mental Development Index [MDI]), and behavioral (Behavioral Rating Scale [BRS]) components.
Results:
Neither prenatal exposure to BD− or BD+ significantly impacted overall PDI (P = .2449), MDI (P = .7886), or BRS (P = .6072) scores. However, we observed a significant effect of BD+ exposure-by-time interaction for the BRS Motor Quality index (F245 = 3.16, P = .0441), with BD+ exposed infants less likely to be above the 75th percentileat the 52-week assessment (mean = 11.5%) compared with BD− (mean = 40.0%) and nonexposed infants (mean = 48.4%).
Conclusions:
We found no significant impact of prenatal BD− or BD+ exposure on infant PDI, MDI, or overall BRS scores at 12, 26, or52 weeks of age, with most scores remaining within normal limits. Consistent with previous studies, we found a specific effect of prenatal BD+ exposure on quality of motor functioning at 1 year. However, the majority of infants were within normal limits on this developmental outcome.
Trial Registration:
ClinicalTrials.gov identifier: NCT00585702
Bipolar disorders are serious psychiatric conditions with a prevalence of nearly 4% in the United States.1 With typical illness onset in their late teens to early 20s, women are at increased risk for bipolar episodes during their reproductive years.2 Women with these disorders are more likely to have rapid cycling and depressive symptoms than their male counterparts.3 Although pharmacotherapy is the mainstay of treatment for bipolar disorder, some women choose to discontinue pharmacotherapy during pregnancy. The risks and benefits of medication must be carefully considered2 because maternal decompensation can result in psychosis, high-risk behavior, and suicide during pregnancy and the postpartum periods.
Although there are reports that bipolar disorder symptoms improve during pregnancy, the majority of women are at risk for recurrence.2 For women whose illness is responsive to lithium and who choose to discontinue the medication during pregnancy, the risk for relapse is high. Viguera et al4 reported that the recurrence risk was 2.3 times greater after discontinuation of mood stabilizer treatment (53 of 62, 85.5%) than with continued treatment (10 of 27, 37.0%). Women with bipolar disorder are also at high risk for symptom exacerbation during the immediate postpartum period, which may result in adverse neonatal outcomes and infant health. Although no studies have explored the risk of untreated maternal bipolar disorder on development of older infants, one investigator found that women with untreated bipolar disorder were at increased risk of having offspring with microcephaly and neonatal hypoglycemia.5 Bipolar symptom exacerbation during the postpartum period may have consequences for establishment of the developing attachment relationship.6
The risk of uncontrolled bipolar disorder must be balanced with consideration of the risks of pharmacologic treatment, the standard of care for bipolar disorder, during pregnancy.2 Although initial voluntary reports of first-trimester lithium exposure suggested a high risk for Ebstein’s anomaly, prospective studies have suggested it is associated with a much lower risk for morphological malformations.7 Other commonly prescribed antimanic agents include atypical antipsychotics and anticonvulsants. Studies of anticonvulsant use during pregnancy have been published in the neurology literature8 with increasing attention to valproic acid as a morphological and developmental teratogen. Lamotrigine has emerged as the anticonvulsant with a favorable reproductive risk profile.9 A number of studies that have specifically examined antipsychotic exposure during pregnancy have not demonstrated adverse neonatal outcomes.5,10,11 However, a cross-sectional study by Johnson et al12 demonstrated significantly lower neuromotor scores among 6-month-old infants with intrauterine antipsychotic exposure. Peng et al13 found that early differences in multiple domains of development on the Bayley Scales of Infant Development III did not persist through 12 months of age.
Women with bipolar disorder are more vulnerable to mood and psychotic episodes after childbirth than at any other time during their lives,14 and postpartum psychoses are most commonly manifestations of bipolar disorder.15,16 The massive, rapid gonadal steroid withdrawal after delivery contributes to destabilization in these neurobiologically17 and genetically18–20 vulnerable women. Sleep deprivation and disruption in circadian rhythms during late pregnancy, during labor, and for infant feeding also promote mood symptoms in women with bipolar disorder.2
Thus, prenatal exposure may be followed by a postpartum milieu of elevated stress, difficulty establishing routines, and poor self-care. An environment of unpredictability may in itself result in difficulties with behavioral and physiological regulation in infants of women with bipolar disorder. For example, Johnson et al21 found that 6-month-old infants of mothers with bipolar disorder showed an increase in respiratory sinus arrhythmia, a marker of physiological and affect regulation, in response to a stressor, the opposite pattern of vagal withdrawal that would be expected.21 Since our sample included women who were both treated and untreated with pharmacotherapy during pregnancy, we examined the potential direct effects of maternal bipolar disorder on infant developmental outcomes.
We examined group differences in infant developmental outcomes at 12, 26, and 52 weeks. Our hypothesis was that infants of women with bipolar disorder (BD+ and BD−) would demonstrate poorer motor, cognitive, and behavioral development than infants of controls. Based on the findings by Johnson et al,12 we expected that infants of women in the BD+ group would have poorer neuromotor performance during infancy.
METHODS
Participants
A total of 81 women with bipolar disorder were enrolled in this prospective observational study at or prior to 20 weeks gestation (Antimanic Use During Pregnancy: R01 MH 075921; K.L.W., principal investigator; ClinicalTrials.gov identifier: NCT00585702). Comparison data from 116 pregnant women whose infants had Bayley Scales of Infant Development, Second Edition (BSID-II) infant assessments were included from a parallel study (Antidepressant Use During Pregnancy: R01 MH60335) (Figure 1). The women were 18–44 years of age, English-speaking, and recruited in Pittsburgh, Pennsylvania, between July 2006 and March 2011. Recruitment was by self-referral, physician and community health center referral, and/or advertising. Approval was obtained from the University of Pittsburgh Institutional Review Board. All women provided written informed consent. To be included in this analysis, the women had a DSM-IV diagnosis of bipolar I, bipolar II, or bipolar not otherwise specified (NOS), as determined by evaluation with the full Structured Clinical Interview for DSM-IV.22 Decisions about treatment, including risk/benefit discussions, were made by the woman and her treatment provider in the community. The choice to accept or decline treatment or implement the consultation did not dictate entry or retention in the study.
Figure 1. CONSORT Diagram.
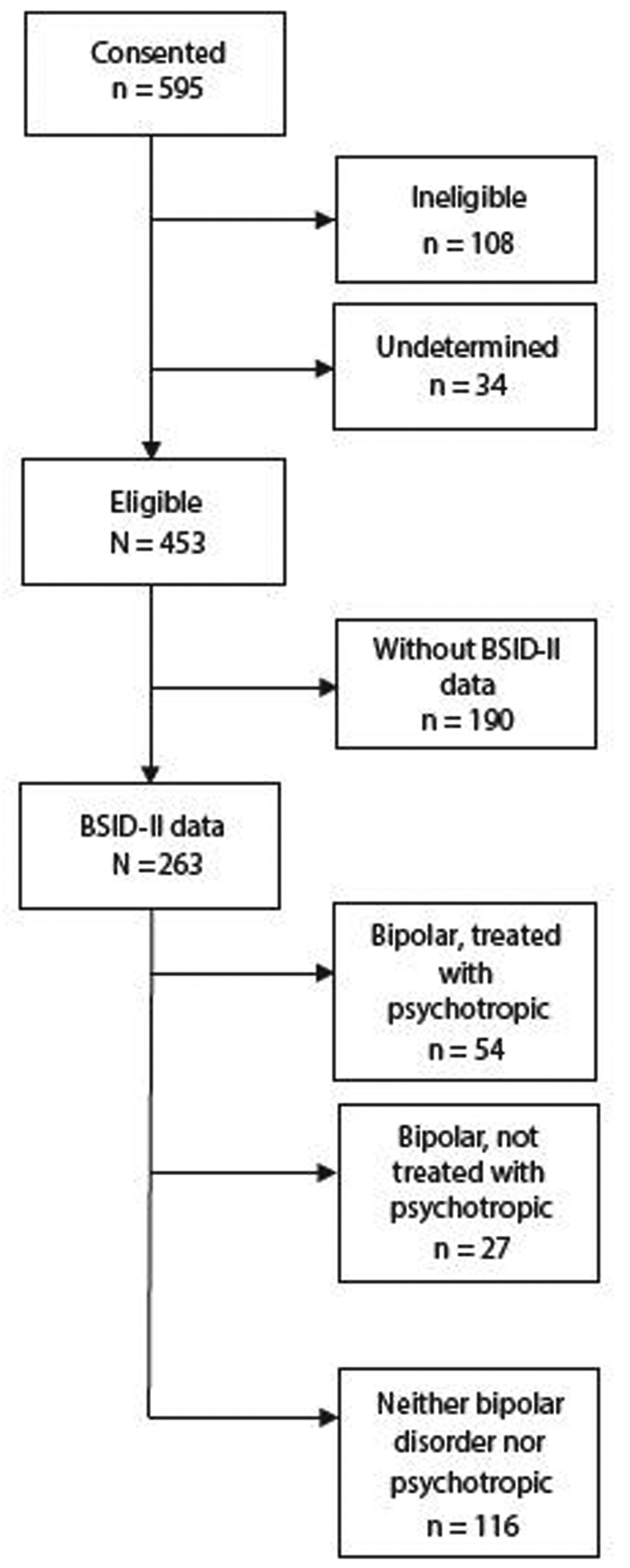
Abbreviation: BSID-II = Bayley Scales of Infant Development, Second Edition.
All methods are similar to those previously described in Santucci et al,23 which includes the women in this cohort who had unipolar depression. History and current use of alcohol, tobacco, and illicit substances were obtained. Women with alcohol or drug dependence during pregnancy or a positive drug screen for illicit drug use were excluded from the population with the exception of marijuana since use in this sample was common and would have impacted both generalizability and sample accrual. Women with exposure to US Food and Drug Administration pregnancy class D or X drugs, multiple births, or major medical disorders were excluded.
Maternal Assessments
Pregnancy assessments were completed as close to 20, 30, and 36 weeks gestation as possible. Postnatal assessments for mothers and infants, including BSID-II,24 were completed at approximately 12, 26, and 52 weeks. Maternal symptoms were assessed at each assessment point using the Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement (SIGH-ADS).25 Manic symptoms were evaluated with the Mania Rating Scale (MRS), which is derived from the Schedule for Affective Disorders and Schizophrenia Research Diagnostic Interview.26 A score of 10 or higher indicated significant symptomatology that warranted a diagnostic interview to assess for hypomania/ mania.
We evaluated 3 non-overlapping groups of subjects according to their pregnancy exposures:
No bipolar disorder, no pharmacotherapy (n = 116): no exposure to any psychotropic medication or bipolar disorder.
Bipolar disorder treated with pharmacotherapy (BD+) (n = 54): we used the general category of pharmacotherapy, which was operationalized as any psychotropic treatment because these patients were treated with a variety of drug types and polypharmacy was present in the majority (n = 35, 65%). Pharmacotherapy included treatmentwith anticonvulsants (12), antipsychotics (24), benzodiazepines (3), lithium (7), and serotonin reuptake inhibitor (SRI)/serotonin-norepinephrine reuptake inhibitor (SNRI) and non-SRI antidepressants (9). Of the 20 women taking only a single psychotropic medication, antipsychotics were the most common (n = 8), followed by SSRI/SNRIs (n = 6) and lithium (n = 5). The majority of women were treated continuously (n = 33; 61.1%) during gestation. Exposures included first and/or second trimester, but not the third (n = 11; 20.4%), and second and/or third trimester, but not the first (n = 9; 16.7%).
Bipolar disorder not treated with pharmacotherapy (BD−) (n = 27; 50%): presence of the diagnosis of bipolar disorder but with no psychotropic exposure.
Infant Assessments
At 12, 26, and 52 weeks of age (corrected for prematurity), infants were evaluated with the BSID-II. The 3 primary scales are the Mental Development Index (MDI), the Psychomotor Development Index (PDI), and the Behavioral Rating Scale (BRS). The MDI and PDI assess the infant’s cognitive, language, personal-social, and fine and gross motor development. The BRS assesses the infant’s behavior during testing. The MDI and PDI scales are age-adjusted and converted to a standardized value (index scores) with a mean of 100 and a standard deviation of 15, which were outcome variables in our analyses. The BRS total score is converted to a percentile score ranging from 1 to 100; percentiles above the 25th are within normal limits. Given the mixture of dimensions in the BRS percentage, the 4 factor scales (Attention/Arousal, Orientation/Engagement, Emotional Regulation, and Motor Quality) were also considered as primary outcomes. Duration of gestation, type of birth, neonatal intensive care unit admission (present or absent), infant sex, birth weight, and length were collected from hospital records by independent evaluators blind to the study hypotheses and design.
Analyses
Descriptive statistics are presented for continuous measures as means and standard deviations and for categorical measures as frequencies and proportions. Tests of association included analysis of variance for normally distributed continuous measures and Kruskal-Wallis otherwise. Tests of independence included χ2 when expected cell frequencies were of adequate size and Fisher exact otherwise. Probability values for all post hoc pairwise comparisons were adjusted using the Bonferroni correction.
We employed the same analytic strategy previously described in Santucci et al.23 The effect of exposure on the mental and physical indices was tested using repeated-measures mixed models with a random intercept and an unstructured covariance matrix. Percentile scores for the BRS and behavioral subscales were dichotomized at ≥ 75% because their distributions were heavily left-skewed. The effect of exposure on the dichotomized subscales was tested using repeated-measures mixed logistic models also with a random intercept and an unstructured covariance matrix. Due to the curvilinear relationship between BSID-II scores and time, a quadratic term (age2) was added to each model. Interactions between exposure and time and exposure and time squared were also added to each model to test for differential exposure effects across the postpartum period. The Attention/Arousal factor was not modeled by age since this assessment is made only at 12 weeks.
An approach to confounder selection that estimates effect sizes for each potential confounder on both exposure and each BSID-II index (MDI, PDI, BRS) and BRS subscale was used. Potential confounders were maternal age, race, education, current employment, relationship status, and use of tobacco or illicit drugs during pregnancy. An a priori rule was to retain a measure as a potential confounder if it had an effect on both exposure and BSID-II score that was in the “medium” range (ie, Cohen d ≥ 0.4). According to these criteria, age was included as a potential confounder in the MDI, Orientation/Engagement, and BRS total score models, and education and relationship status were also included in the MDI model.
RESULTS
Participants
Participants included 81 BD+/BD− and 116 control mother-infant pairs for a final sample of n = 197. Compared with the BD+ and BD− mother-infant pairs whose infants completed BSID-II assessments, the 190 mother-infant pairs (Figure 1) who did not participate in BSID-II assessments did not significantly differ on maternal race, education, employment, marital status, parity, alcohol or tobacco use, lifetime use of illicit drugs, or psychiatric hospitalization or infant outcomes including gestational age, infant sex, length, weight, or head circumference.
Maternal age, race, education, employment, married/ cohabiting status, parity, and tobacco and marijuana use were significantly related to exposure (Table 1). Post hoc analyses that remained significant after Bonferroni corrections revealed that women in the nonexposed group were older and more likely to have completed university-level education; in contrast, the untreated bipolar group (BD−) were the youngest and least likely to have completed university-level education of the 3 groups. BD+ and BD− women were less likely to be employed, married or cohabitating, and breastfeeding. They were more likely to smoke or have lifetime history of drug use than their nonexposed counterparts. BD− group mothers were also more likely to belong to a minority group than nonexposed women.
Table 1.
Mother’s Demographic, Clinical, and Behavioral Measures at 20 Weeks Gestation by Exposure During Pregnancy and Infant Demographic Measures at Birth
| Exposure During Pregnancy | Probability Values | |||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Total (N = 197) | None (n = 116) | BD+ (n = 54) | BD− (n = 27) | Overall | None vs BD+ | None vs BD− | BD+vs BD− |
| Maternal measures | ||||||||
| Age, mean ± SD, y | 29.0 ± 5.67 | 30.6 ± 5.06 | 28.0 ± 5.71 | 24.0 ± 4.84 | < .0001 | .004* | < .001* | .002* |
| Race, n (%) | .0480 | |||||||
| White | 146 (74.1) | 92 (79.3) | 40 (74.1) | 14 (51.9) | ||||
| Black | 42 (21.3) | 20 (17.2) | 12 (22.2) | 10 (37.0) | ||||
| Other | 9 (4.6) | 4 (3.4) | 2 (3.7) | 3 (11.1) | ||||
| White race | 146 (74.1) | 92 (79.3) | 40 (74.1) | 14 (51.9) | .0135 | .445 | .003* | .046 |
| Education level, n (%) | < .0001 | < .001* | < .001* | .027 | ||||
| < High school | 10 (5.1) | 2 (1.7) | 3 (5.6) | 5 (18.5) | ||||
| High school | 33 (16.8) | 10 (8.6) | 12 (22.2) | 11 (40.7) | ||||
| Some university | 52 (26.4) | 16 (13.8) | 29 (53.7) | 7 (25.9) | ||||
| University | 57 (28.9) | 47 (40.5) | 6 (11.1) | 4 (14.8) | ||||
| Graduate school | 45 (22.8) | 41 (35.3) | 4 (7.4) | 0 (0.0) | ||||
| Completed university | 102 (51.8) | 88 (75.9) | 10 (18.5) | 4 (14.8) | < .0001 | < .001* | < .001* | .765 |
| Employed, n (%) | 106 (55.2) | 83 (72.2) | 19 (36.5) | 4 (16.0) | < .0001 | < .001* | < .001* | .065 |
| Married/cohabiting, n (%) | 121 (61.4) | 92 (79.3) | 21 (38.9) | 8 (29.6) | < .0001 | < .001* | < .001* | .413 |
| Parity, n (%) | .0250 | .006* | .658 | .188 | ||||
| 1 | 76 (39.2) | 51 (44.3) | 16 (30.2) | 9 (34.6) | ||||
| 2 | 67 (34.5) | 42 (36.5) | 14 (26.4) | 11 (42.3) | ||||
| 3+ | 51 (26.3) | 22 (19.1) | 23 (43.4) | 6 (23.1) | ||||
| Pre-pregnancy BMI (kg/m2), mean ± SD | 26.0 ± 6.51 | 25.5 ± 6.22 | 27.6 ± 7.03 | 25.5 ± 6.59 | .1670 | |||
| Pre-pregnancy BMI 30+, n (%) | 42 (22.7) | 21 (18.9) | 14 (29.8) | 7 (25.9) | .2997 | |||
| Smoked tobacco, n (%) | 35 (17.9) | 7 (6.1) | 16 (29.6) | 12 (44.4) | < .0001 | < .001* | < .001* | .186 |
| Drank alcohol, n (%) | 49 (24.9) | 33 (28.4) | 9 (16.7) | 7 (25.9) | .2521 | |||
| Lifetime illicit drugs use, n (%) | 25 (12.7) | 2 (1.7) | 12 (22.2) | 11 (40.7) | < .0001 | < .001* | < .001* | .081 |
| Bipolar subtype, n (%) | .0576 | |||||||
| I | 47 (58.0) | 35 (64.8) | 12 (44.4) | |||||
| II | 25 (30.9) | 12 (22.2) | 13 (48.1) | |||||
| NOS | 9 (11.1) | 7 (13.0) | 2 (7.47) | |||||
| Any psychiatric hospitalization, n (%) | 4 (2.0) | 0 (0.0) | 4 (7.4) | 0 (0.0) | .0137 | .009* | 1.000 | .296 |
| MRS, mean ± SD | 0.84 ± 2.57 | 0.18 ± 0.66 | 1.98 ± 3.68 | 1.77 ± 4.19 | .0016 | < .001* | 0.012* | .810 |
| SIGH-ADS29, mean ± SD | 11.7 ± 8.17 | 7.33 ± 4.15 | 18.5 ± 8.82 | 18.8 ± 7.09 | < .0001 | < .001* | < .001* | .864 |
| Infant measures | ||||||||
| Gestational age, mean ± SD, wk | 39.0 ± 1.53 | 39.1 ± 1.49 | 38.8 ± 1.55 | 39.1 ± 1.64 | .2387 | |||
| Gestational age < 37 wk, n (%) | 16 (8.1) | 8 (6.9) | 6 (11.1) | 2 (7.4) | .6381 | |||
| Sex, n (%) | .0441 | .112 | .022* | .345 | ||||
| Male | 114 (57.9) | 75 (64.7) | 28 (51.9) | 11 (40.7) | ||||
| Female | 83 (42.1) | 41 (35.3) | 26 (48.1) | 16 (59.3) | ||||
| Weight, mean ± SD, g | 3,457 ± 545 | 3,528 ± 520 | 3,380 ± 550 | 3,248 ± 606 | .0483 | .117 | .026 | .329 |
| Length, mean ± SD, cm | 51.1 ± 2.84 | 51.3 ± 2.67 | 50.4 ± 3.10 | 50.9 ± 3.14 | .1835 | |||
| Head circumference, mean ± SD, cm | 34.6 ± 1.70 | 34.8 ± 1.56 | 34.6 ± 1.73 | 33.3 ± 2.01 | .0039 | .453 | < .001* | .030 |
| Ever breastfed, n (%) | 132 (69.5) | 90 (81.8) | 27 (50.0) | 15 (57.7) | < .0001 | < .001* | .008* | .519 |
Significant after Bonferroni correction.
Abbreviations: BD− = maternal bipolar disorder not treated with pharmacotherapy, BD+ = maternal bipolar disorder treated with pharmacotherapy, BMI = body mass index, MRS = Mania Rating Scale, NOS = not otherwise specified, SIGH-ADS = Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement.
As expected, the SIGH-ADS and MRS scores of mothers in both the BD+ (SIGH-ADS 18.5 ± 8.82, MRS 1.98 ± 3.68) and BD− (SIGH-ADS 18.8 ± 7.09, MRS 1.77 ± 4.19) groups were significantly higher than mothers in the nonexposure group (SIGH-ADS 7.33 ± 4.15, MRS 0.18 ± 0.66) (P values < .0001 and .0016) (Table 1). While not statistically significant at the α < .05 level, bipolar subtype was related to psychotropic exposure, with women in the BD+ more likely to be diagnosed as bipolar I or NOS and women in the BD− as bipolar II (P = .0576).
The characteristics of infants are shown in Table 1. BSID-II examinations were administered at 12 weeks (mean = 15.7 ± 3.4 weeks), 26 weeks (mean = 31.3 ± 4.5 weeks), and 52 weeks (mean = 56.2 ± 6.1 weeks). Infants of BD− mothers were more likely to have significantly smaller head circumference than BD+ (P = .030) and nonexposed infants (P < .001).
Missing Data
At each of the postpartum assessments, missed assessments of mother-infant pairs varied by exposure group (52% and 74% [BD+/BD−] vs 83% [nonexposed] retention at the 52-week assessment); therefore, we examined whether baseline characteristics were predictive of study completion. Women who were older (mean = 30.4 years vs mean = 27.2 years, P < .001), were employed (65.1% vs 35.9%, P = .0022), completed a university education (72,5% vs 27.5%, P < .001), were currently married/cohabitating (62.8% vs 37.2%, P = .0045), did not smoke tobacco (58.4% vs 41.6%, P = .0475), had no lifetime diagnosis of bipolar disorder (70.7% vs 29.3%, P < .001), and had no psychiatric hospitalizations (56.0% vs 44.0%, P = .0401) were more likely to have completed all 3 infant assessment time points in the study.
Models
Our models tested the main effects of and the interaction between exposure during pregnancy and weeks postpartum on BSID-II measures (Table 2). Model I (unadjusted) tested the main effects of infant age and exposure (BD+, BD−, nonexposed) on BSID-II measures at each of the 3 assessment time points (12, 26, and 52 weeks) and age-by-exposure interaction. A quadratic term (age2) was added to each model since there is a curvilinear relationship between BSID-II scores and time. There was a significant effect of age and age2 on MDI scores, with MDI scores showing an increase in early infancy across groups (P = .0103) and a subsequent slight decrease in MDI scores across the exposure groups (P = .0056) (Table 3). There were no significant effects of exposure, age, age2, or interactions on PDI, BRS, or BRS subscales (Attention Arousal, Orientation/Engagement, Emotional Regulation, or Motor Quality) except for an exposure-by-age2 effect on the Motor Quality (MQ). Infants of BD+ mothers were less likely to be at or above the 75th percentile than infants of nonexposed mothers on the MQ subscale (P = .0441) (Table 4). Distributions of MDI and PDI scores by week are shown in Figure 2.
Table 2.
Results of Repeated-Measures Mixed Models of BSID-II Scores
| Model I, unadjusted | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDI (dfDEN = 253) | PDI (dfDEN = 253) | AA (n = 160) | OE (dfDEN = 112) | ER (dfDEN = 112) | MQ (dfDEN = 245) | BRS (dfDEN = 232) | ||||||||
| Measure (dfNUM) | F | P | F | P | χ2 | P | F | P | F | P | F | P | F | P |
| Exposure (2) | 1.91 | .1508 | 2.77 | .0646 | 1.33 | .5131 | 0.76 | .4679 | 0.03 | .9711 | 2.30 | .1026 | 0.72 | .4857 |
| Age (1) | 7.89 | .0054 | 0.84 | .3597 | 0.44 | .5087 | 3.65 | .0587 | 0.61 | .4359 | 0.34 | .5626 | ||
| Exposure × age (2) | 0.24 | .7886 | 1.41 | .2449 | 0.64 | .5315 | 0.12 | .8831 | 2.56 | .0791 | 0.50 | .6072 | ||
| Age2 (1) | 9.91 | .0018 | 0.80 | .3707 | 0.89 | .3470 | 2.96 | .0879 | 0.09 | .7694 | 0.45 | .5012 | ||
| Exposure × age2 (2) | 0.15 | .8635 | 1.15 | .3181 | 0.44 | .6448 | 0.33 | .7189 | 3.16 | .0441 | 0.55 | .5792 | ||
| Model II, adjusted for MRS and SIGH-ADS29 | ||||||||||||||
| MDI (dfDEN = 234) | PDI (dfDEN = 234) | AA (n = 156) | OE (dfDEN = 98) | ER (dfDEN = 98) | MQ (dfDEN = 226) | BRS (dfDEN = 214) | ||||||||
| Measure (dfNUM) | F | P | F | P | χ2 | P | F | P | F | P | F | P | F | P |
| Exposure (2) | 1.95 | .1443 | 2.20 | .1131 | 1.75 | .4178 | 0.55 | .5808 | 0.05 | .9559 | 2.14 | .1198 | 0.69 | .5012 |
| Age (1) | 7.32 | .0073 | 0.49 | .4835 | 1.23 | .2702 | 2.77 | .0993 | 0.40 | .5298 | 0.78 | .3783 | ||
| Exposure × age (2) | 0.15 | .8648 | 1.02 | .3624 | 0.38 | .6832 | 0.10 | .9010 | 2.36 | .0963 | 0.61 | .5419 | ||
| Age2 (1) | 9.47 | .0023 | 0.51 | .4778 | 1.71 | .1937 | 2.38 | .1263 | 0.02 | .8827 | 0.83 | .3639 | ||
| Exposure × age2 (2) | 0.07 | .9353 | 0.83 | .4362 | 0.23 | .7922 | 0.30 | .7442 | 2.98 | .0529 | 0.74 | .4763 | ||
| MRS (1) | 0.42 | .5176 | 0.00 | .9775 | 4.18 | .0410 | 1.21 | .2739 | 1.92 | .1689 | 0.44 | .5084 | 0.06 | .8004 |
| SIGH-ADS29 (1) | 0.90 | .3432 | 0.15 | .7019 | 1.09 | .2961 | 0.38 | .5386 | 1.75 | .1886 | 1.19 | .2759 | 0.42 | .5166 |
| Model III, adjusted for MRS, SIGH-ADS29, mother’ s age, colleg e deg ree, and marital status (where necess ary) | ||||||||||||||
| MDI dfDEN = 233) | OE (dfDEN = 98) | BRS (dfDEN = 213) | ||||
|---|---|---|---|---|---|---|
| Measure (dfNUM) | F | P | F | P | F | P |
| Exposure (2) | 1.02 | .3607 | 0.77 | .4648 | 0.69 | .5022 |
| Age (1) | 6.62 | .0107 | 0.47 | .4950 | 0.74 | .3901 |
| Exposure × age (2) | 0.06 | .9464 | 0.62 | .5396 | 0.58 | .5594 |
| Age2 (1) | 8.54 | .0038 | 0.72 | .3996 | 0.78 | .3772 |
| Exposure × age2 (2) | 0.01 | .9860 | 0.45 | .6421 | 0.71 | .4926 |
| MRS (1) | 0.41 | .5228 | 1.67 | .1996 | 0.07 | .7879 |
| SIGH-ADS29 (1) | 1.47 | .2264 | 0.22 | .6437 | 0.37 | .5420 |
| Mother’s age (1) | 1.99 | .1596 | 9.67 | .0024 | 0.30 | .5844 |
| College degree (1) | 3.70 | .0555 | ||||
| Married (1) | 0.94 | .3341 | ||||
Table 3.
Mean BSID-II Mental Development Index (MDI) and Psychomotor Development Index (PDI) Scores
| No Exposure (n = 116) | BD+ (n = 54) | BD− (n = 27) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 wk (n = 99) | 26 wk (n = 106) | 52 wk (n = 96) | 12 wk (n = 44) | 26 wk (n = 31) | 52 wk (n = 28) | 12 wk (n = 17) | 26 wk (n = 16) | 52 wk (n = 20) | ||||||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| MDI | 104.7 | 8.2 | 107.8 | 6.4 | 104.4 | 12.6 | 97.2 | 7.9 | 103.3 | 7.1 | 99.7 | 11.8 | 102.9 | 6.0 | 104.1 | 6.1 | 100.1 | 12.2 |
| PDI | 100.5 | 9.4 | 100.9 | 4.1 | 101.7 | 16.4 | 93.8 | 10.7 | 100.3 | 12.5 | 98.1 | 18.9 | 99.8 | 8.4 | 99.3 | 8.6 | 96.5 | 15.5 |
Abbreviations: AA = Attention Arousal (BRS subscale), BRS = Behavioral Rating Scale, BSID-II = Bayley Scales of Infant Development, Second Edition, dfDEN = denominator degrees of freedom, dfNUM = numerator degrees of freedom, ER = Emotional Regulation (BRS subscale), MDI = Mental Development Index, MQ = Motor Quality (BRS subscale), MRS = Mania Rating Scale, OE = Orientation/Engagement (BRS subscale), PDI = Psychomotor Development Index, SIGH-ADS = Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement.
Abbreviations: BD− = maternal bipolar disorder not treated with pharmacotherapy, BD+ = maternal bipolar disorder treated with pharmacotherapy, BSID-II = Bayley Scales of Infant Development, Second Edition.
Table 4.
Proportion of Infants With a Percentile Score of 75 or Higher (total score, Orientation/ Engagement, Emotional Regulation, Motor Quality) and Mean Score (Attention/Arousal) on Components of the BSID-II Behavioral Rating Scale by Exposure During Pregnancy and Weeks Postpartum
| No Exposure (n=116) | BD+ (n=54) | BD− (n=27) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 wk (n = 105) | 52 wk (n = 91) | 26 wk (n = 30) | 52 wk (n = 26) | 26 wk (n = 16) | 52 wk (n = 19) | |||||||
| BRS Component | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE |
| Total score | 89.0 | 3.1 | 90.8 | 3.1 | 93.3 | 4.6 | 76.0 | 8.7 | 86.7 | 9.1 | 78.9 | 9.6 |
| OE | 87.6 | 3.2 | 89.0 | 3.3 | 90.0 | 5.6 | 80.8 | 7.9 | 86.7 | 9.1 | 78.9 | 9.6 |
| ER | 91.4 | 2.7 | 85.7 | 3.7 | 93.3 | 4.6 | 65.4 | 9.5 | 86.7 | 9.1 | 77.9 | 9.6 |
| MQ | 47.6 | 4.9 | 48.4 | 5.3 | 40.0 | 9.1 | 11.5 | 6.4 | 25.0 | 11.2 | 40.0 | 11.2 |
| None (n = 116) | BD+ (n = 55) | BD− (n = 26) | |||||
|---|---|---|---|---|---|---|---|
| 12 wk (n = 99) | 12 wk (n = 44) | 12 wk (n = 17) | |||||
| Mean | SE | Mean | SE | Mean | SE | ||
| AA | 83.7 | 37.2 | 86.4 | 34.7 | 94.1 | 24.3 | |
Abbreviations: AA = Attention/Arousal, BD− = maternal bipolar disorder not treated with pharmacotherapy, BD+ = maternal bipolar disorder treated with pharmacotherapy,BRS = Behavioral Rating Scale Total Score, BSID-II = Bayley Scales of Infant Development, Second Edition, ER = Emotional Regulation, MQ = Motor Quality, OE = Orientation/Engagement, SE = standard error.
Figure 2. Distribution of BSID-II Mental Development Index and Psychomotor Development Index Across Assessment Time Points (12, 26, and 52 Weeks).
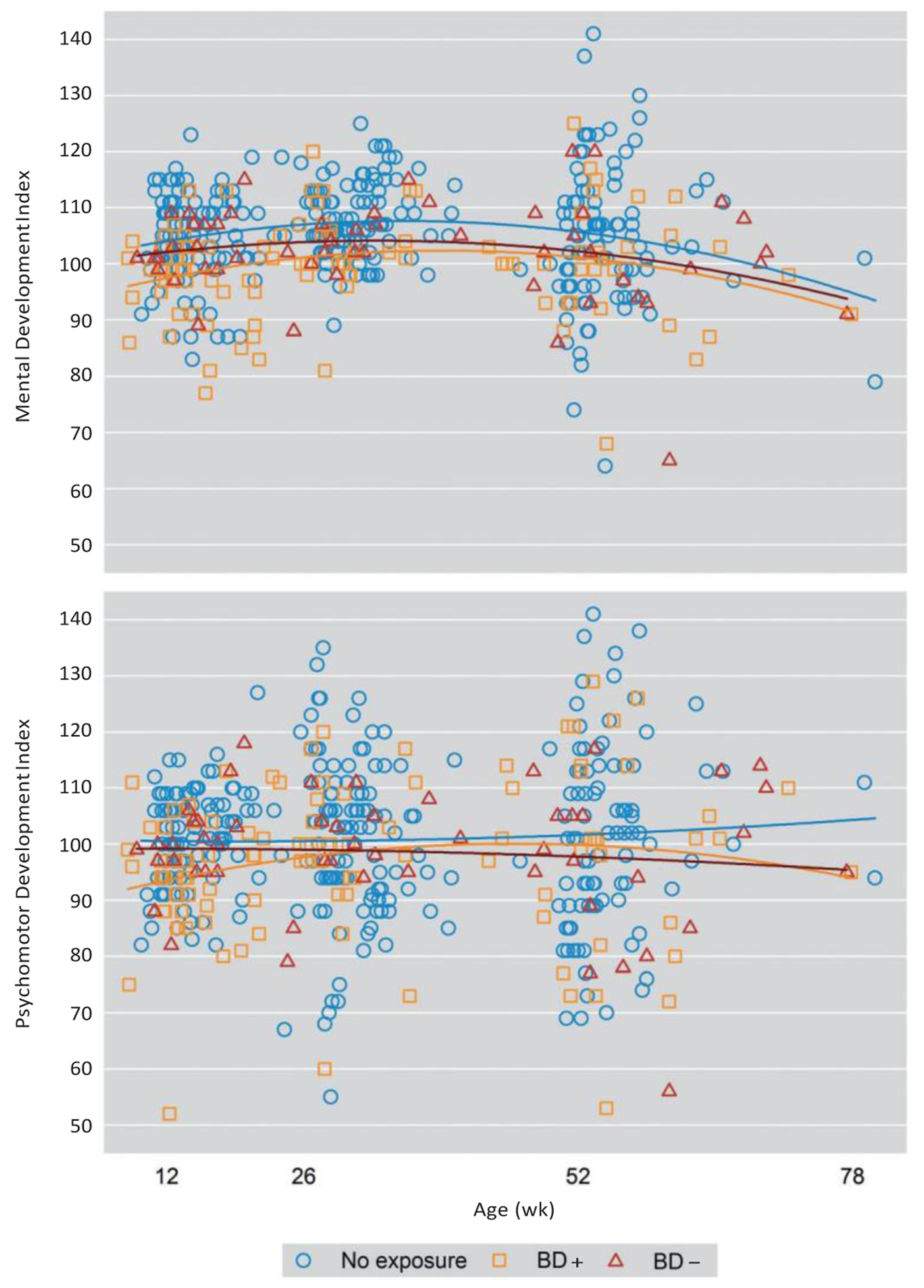
Abbreviations: BD+ = maternal bipolar disorder treated with pharmacotherapy, BD− = maternal bipolar disorder not treated with pharmacotherapy, BSID-II = Bayley Scales of Infant Development, Second Edition.
Model II added SIGH-ADS and MRS scores to the initial model to control for the effects of baseline symptomatology on Bayley scores. While the age and age2 effects remained significant on MDI scores (P = .0073 and .0023, respectively), the significant exposure-by-age2 interaction on the MQ became nonsignificant (P = .0529).
Model III (Table 2) tested significant confounders in addition to Model II where applicable. Model III was adjusted for maternal age and for postbirth SIGH-ADS and MRS scores to assess the impact of maternal mood on MDI, BRS total score, and Orientation/Engagement. Completion of university education and married/cohabiting status were added as predictors in the model for MDI only. The significant curvilinear relationship between infant age at assessment and MDI score (P values = .0107 and .0038, respectively) persisted with the added predictors. Maternal age made no difference in either model for BRS total score or Orientation/ Engagement.
DISCUSSION
In our investigation, infants whose mothers maintained their psychotropic bipolar disorder medication during pregnancy had poorer Motor Quality on the BSID-II. Contrary to our hypothesis, maternal mood disorder itself did not impact infant functioning as measured by the BSID-II in the domains of neuromotor, cognitive, and behavioral development.
The Motor Quality subscale is an examiner-rated evaluation of infant gross and fine motor movement required by tasks, overall control of movement, hypo/hypertonicity, tremulousness, slowed and delayed movement, and frenetic movement.24 Infants of BD+ mothers in our sample were more likely to be below the 75th percentile on this subscale, particularly at the 52-week developmental assessment (11.5% above the 75th percentile vs 40.0% in the BD− group and 48.4% in the nonexposed group). Our findings are consistent with those of Johnson et al,12 who found that infants with a history of intrauterine antipsychotic exposure (the majority were treated with multiple medications, as in our sample) had significantly lower scores on a standardized neuromotor examination. Another study found hypertonicity and other motor issues in newborn infants exposed to antipsychotics.27 Given the affinity of antipsychotic medications for the dopamine D2 receptor and, in some cases, the serotonin 5-HT1A receptor,28 early deficits in infant motor performance are biologically plausible since both neurotransmitters play a critical role in the development of motor skills and reinforcement of motor pathways.
We did not find an effect of maternal mood disorder on infant developmental outcomes as measured by the BSID-II; however, the BSID-II may not be sufficiently sensitive to early developmental consequences of prenatal or postnatal exposure to maternal mood disorders, or the most salient effects may manifest only in older children. Previous studies have shown that children and adolescents of women with bipolar disorder have higher rates of attentional and memory problems,29 impaired social functioning, and mood and behavioral dysfunction and may themselves go on to develop psychiatric disorders.30 An emerging body of literature has suggested that children of parents with bipolar disorder are more likely to show early deficits in executive functioning, higher cognitive functions that are essential for the control of information processing, planning, decision-making, and problem resolution, emerging from critical pathways within the prefrontal cortical regions.31,32 Infant and toddler assessments that target the developing prefrontal pathways, such as the A-not-B task,33 may be more sensitive to early differences in executive functioning.
Inclusion of a sample of both BD+ and BD− women allowed us to model the potential developmental outcomes related to maternal bipolar disorder during the prenatal period with and without exposure to pharmacotherapy, as well as multiple timepoints of both maternal and infant assessment. Neither postbirth maternal SIGH-ADS nor MRS scores significantly affected the BSID-II outcome scales.
A limitation of our sample was that we were unable to examine the effects of specific agents on infant outcomes due to the heterogeneity of treatment in the BD+ group. However, polypharmacy is a common treatment for bipolar disorder,34 and our sample reflects this, with two-thirds of the BD+ women treated with multiple medications. Although treatment heterogeneity and diagnostic variability (bipolar I, bipolar II, bipolar NOS) represent significant limitations to the study design, these sample characteristics can also be regarded as a strength as our sample represents a community sample of women treated for bipolar disorder.
A final limitation is the measure used to assess infant outcomes. Although a strength of the BSID-II is sensitivity to delayed development in a broad range of areas of functioning, a disadvantage is that this instrument cannot tell us the specificity of the delays.35 Although the infants in the BD+ maternal group appeared to have some minor deficits in motor quality (gross and fine motor movement required by tasks, overall control of movement, hypo/hypertonicity, tremulousness, slowed and delayed movement, and frenetic movement), it is difficult to determine what this might mean for everyday functioning and future mastery of specific motor skills. Future studies might compare exposed and nonexposed infants on fine and gross motor tasks requiring differing levels of attentional control.
CONCLUSIONS
Since many women with psychiatric illness require pharmacotherapy during pregnancy, our study provides an important contribution that informs patient and their physicians regarding the risks of treatment for bipolar disorder during pregnancy compared with the risks of untreated illness. Future studies that longitudinally follow mother-infant pairs well into childhood are needed to define developmental trajectories. Such studies must also evaluate the overall environmental stability in which the child develops in addition to the maternal-child relationship quality. It is noteworthy that women in our study without bipolar disorder were more likely to be in a committed relationship, to have present employment, and to have completed university-level education. Identification of supportive relationships and removal of barriers to care may have the best consequences for maternal and child outcomes in these vulnerable dyads.
■ Pharmacotherapy to treat bipolar disorder is the standard of care. During pregnancy, the risks and benefits of medication management must be weighed; however, few studies have explored the developmental outcomes of infants with these exposures.
■ Although we found an impact on quality of motor skills in infants whose mothers received pharmacologic treatment for bipolar disorder during pregnancy, the development of these infants was within normal limits.
Funding/support:
This research was supported by National Institute of Mental Health (NIMH) grant R01 MH075921 (principal investigator: Dr Wisner); all study data presented in this manuscript were collected and processed with the support of this funding. The corresponding author (Dr Santucci) was supported by the Clinical Research Training Program (T32 MH16804-22) at Western Psychiatric Institute and Clinic funded by NIMH while this research was conducted.
Footnotes
Drug names: lamotrigine (Lamictal and others), valproic acid (Depakene and others).
Potential conflicts of interest: The Department of Psychiatry at Northwestern University received contractual fees for Dr Wisner’s consultation to Quinn Emanuel Urquhart & Sullivan, LLP (New York City), who represent Pfizer Pharmaceutical Company, in 2015. Drs Santucci, Singer, Wisniewski, and Sit; Mr Luther; and Ms Eng have no conflicts to disclose.
Role of the sponsor: The funding sources had no role in the conduct or reporting of this study.
Additional information: This study was conducted at the Women’s Behavioral HealthCARE program, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center, Pittsburgh, PA.
REFERENCES
- 1.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. [DOI] [PubMed] [Google Scholar]
- 2.Yonkers KA, Wisner KL, Stowe Z, et al. Management of bipolar disorder during pregnancy and the postpartum period. Am J Psychiatry. 2004;161(4):608–620. [DOI] [PubMed] [Google Scholar]
- 3.Leibenluft E Women with bipolar illness: clinical and research issues. Am J Psychiatry. 1996;153(2):163–173. [DOI] [PubMed] [Google Scholar]
- 4.Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817–1824, quiz 1923. [DOI] [PubMed] [Google Scholar]
- 5.Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hipwell AE, Goossens FA, Melhuish EC, et al. Severe maternal psychopathology and infant-mother attachment. Dev Psychopathol. 2000;12(2):157–175. [DOI] [PubMed] [Google Scholar]
- 7.Diav-Citrin O, Shechtman S, Tahover E, et al. Pregnancy outcome following in utero exposure to lithium: a prospective, comparative, observational study. Am J Psychiatry. 2014;171(7):785–794. [DOI] [PubMed] [Google Scholar]
- 8.Meador K, Reynolds MW, Crean S, et al. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore JLA, Aggarwal P. Lamotrigine use in pregnancy. Expert Opin Pharmacother. 2012;13(8):1213–1216. [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni J, Worsley R, Gilbert H, et al. A prospective cohort study of antipsychotic medications in pregnancy: the first 147 pregnancies and 100 one year old babies. PLoS One. 2014;9(5):e94788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry. 2016;173(3):263–270. [DOI] [PubMed] [Google Scholar]
- 12.Johnson KC, LaPrairie JL, Brennan PA, et al. Prenatal antipsychotic exposure and neuromotor performance during infancy. Arch Gen Psychiatry. 2012;69(8):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng M, Gao K, Ding Y, et al. Effects of prenatal exposure to atypical antipsychotics on postnatal development and growth of infants: a case-controlled, prospective study. Psychopharmacology (Berl) 2013;228(4):577–584. [DOI] [PubMed] [Google Scholar]
- 14.Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150:662–673. [DOI] [PubMed] [Google Scholar]
- 15.Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munk-Olsen T, Laursen TM, Pedersen CB, et al. New parents and mental disorders: a population-based register study. JAMA. 2006;296(21):2582–2589. [DOI] [PubMed] [Google Scholar]
- 17.Bloch M, Schmidt PJ, Danaceau M, et al. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157(6):924–930. [DOI] [PubMed] [Google Scholar]
- 18.Binder EB, Newport DJ, Zach EB, et al. A serotonin transporter gene polymorphism predicts peripartum depressive symptoms in an at-risk psychiatric cohort. J Psychiatr Res. 2010;44(10):640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahon PB, Payne JL, MacKinnon DF, et al. ; NIMH Genetics Initiative Bipolar Disorder Consortium; BiGS Consortium. Genome-wide linkage and follow-up association study of postpartum mood symptoms. Am J Psychiatry. 2009;166(11):1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanjuan J, Martin-Santos R, Garcia-Esteve L, et al. Mood changes after delivery: role of the serotonin transporter gene. Br J Psychiatry. 2008;193(5):383–388. [DOI] [PubMed] [Google Scholar]
- 21.Johnson KC, Brennan PA, Stowe ZN, et al. Physiological regulation in infants of women with a mood disorder: examining associations with maternal symptoms and stress. J Child Psychol Psychiatry. 2014;55(2):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.First M, Spitzer R, Williams J, et al. Structured Clinical Interview for DSM-IV Disorders (SCID). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- 23.Santucci AK, Singer LT, Wisniewski SR, et al. Impact of prenatal exposure to serotonin reuptake inhibitors or maternal major depressive disorder on infant developmental outcomes. J Clin Psychiatry. 2014;75(10):1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayley N Bayley Scales of Infant Development. 2nd ed San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 25.Williams JB, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale With Atypical Depression Supplement (SIGH-ADS). New York, NY: NY State Psychiatric Institute, 2003. [Google Scholar]
- 26.Endicott J, Spitzer RL. A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry. 1978;35(7):837–844. [DOI] [PubMed] [Google Scholar]
- 27.Auerbach JG, Hans SL, Marcus J, et al. Maternal psychotropic medication and neonatal behavior. Neurotoxicol Teratol. 1992;14(6):399–406. [DOI] [PubMed] [Google Scholar]
- 28.Yatham LN, Goldstein JM, Vieta E, et al. Atypical antipsychotics in bipolar depression: potential mechanisms of action. J Clin Psychiatry. 2005;66(suppl 5):40–48. [PubMed] [Google Scholar]
- 29.Chang K, Steiner H, Ketter T. Studies of offspring of parents with bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C(1):26–35. [DOI] [PubMed] [Google Scholar]
- 30.Henin A, Biederman J, Mick E, et al. Psychopathology in the offspring of parents with bipolar disorder: a controlled study. Biol Psychiatry. 2005;58(7):554–561. [DOI] [PubMed] [Google Scholar]
- 31.David DP, Soeiro-de-Souza MG, Moreno RA, et al. Facial emotion recognition and its correlation with executive functions in bipolar I patients and healthy controls. J Affect Disord. 2014;152–154:288–294. [DOI] [PubMed] [Google Scholar]
- 32.Poletti S, Bollettini I, Mazza E, et al. Cognitive performances associate with measures of white matter integrity in bipolar disorder. J Affect Disord. 2015;174:342–352. [DOI] [PubMed] [Google Scholar]
- 33.Cuevas K, Bell MA. Developmental progression of looking and reaching performance on the A-not-B task. Dev Psychol. 2010;46(5):1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peindl KS, Masand P, Mannelli P, et al. Polypharmacy in pregnant women with major psychiatric illness: a pilot study. J Psychiatr Pract. 2007;13(6):385–392. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson J, Jacobson S. Methodological considerations in behavioral toxicology in infants and children. Dev Psychol. 1996;32:390–403. [Google Scholar]


