The initial boom of antibiotic discovery led experts in the late 1940s to predict the end of bacterial infections as a threat (1). However, by 1965, the pipeline collapsed, leaving physicians desperate for new antibiotics “to overcome the problems of resistance … against gram-negative bacillary infections … and better ones against mycobacteria” (2). These threats from a halfcentury ago, which are eerily similar to those today, underscore that the crisis of antibiotic resistance is neither unprecedented nor unexpected. It is recurrent and foreseeable.
Years of advocacy to combat the latest crisis led to the establishment of economic incentives to support antibiotic research and development. Collectively, these incentives launched a boom of antibiotic development during the past decade (3). Unfortunately, incentive targeting has not been optimal. As a result, most of the approved, new antibiotics provide the same antimicrobial coverage as other recently incentivized and approved drugs (3). This inefficient use of public incentives reflects a lost opportunity cost and a failure to address the most critical patient care needs. As experts who have consulted for companies in this area, we propose a new approach to enable more precise targeting of public incentives to address critical unmet needs and improve patient outcomes (Figure).
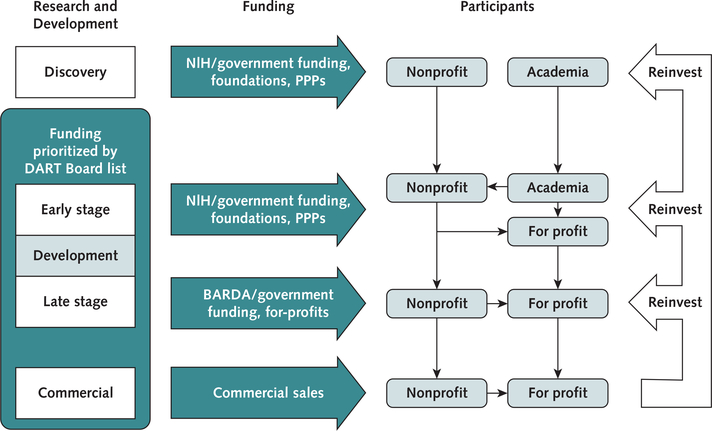
Figure.1. Schematic of a sustainable model for targeted discovery and development of new antibiotics.
Stages include Discovery (basic science work and lead-candidate identification), Early Development (lead optimization, manufacturing, preclinical toxicity, pharmacology, IND preparation, and phase I clinical trials), Late Development (phase II/III clinical trials and NDA filing), and Commercialization (postmarketing). Prioritization of funding to target clinical unmet needs would be set by the DART Board list from Early Development through Commercialization, with the ability to influence the Discovery phase. Academia will continue to conduct early disovery work using existing funding mechanisms. Nonprofits would participate at all stages. For-profit companies could continue to participate at all stages, particularly via outlicensing arrangements with academia and nonprofits. Revenue from commercial sales and licensing fees from for-profit companies would be fed back into nonprofits and/or government funding agencies whose mission is to discover and develop new antibiotics. BARDA = Biomedical Advanced Research and Development Authority; DART = Developing Antibiotics for Resistant Targets; IND = Investigational New Drug; NDA = New Drug Application; NIH = National Institutes of Health; PPPs = public private partnerships.
We propose the establishment of a new Developing Antibiotics for Resistant Targets (DART) Board to improve the targeting of incentives for antibiotic development. The DART Board, which would comprise clinical experts, patient advocates, and representatives from government, industry, and nonprofit organizations, would establish and regularly update an official list that restricts which pathogens can be targeted by publicly funded incentives. The list would focus on serious and life-threatening infections caused by pathogens that demonstrate, or are projected to develop, extreme drug resistance (XDR). This is defined as resistance to all antibiotics except for those more toxic or less effective than alternative options (4). It would also focus on other important clinical needs, such as oral antibiotics, to preclude the need for prolonged intravenous therapy. The list would be dynamic because the DART Board would remove XDR pathogens as new antibiotics become available to treat them and add new ones as they develop. Other countries could establish similar boards or harmonize with the U.S. DART Board.
Several pathogen threat lists exist but are not regularly updated. They also do not consider the severity of infection. For example, Qualified Infectious Disease Product (QIDP) criteria delineate pathogens eligible for incentives in the 2012 Generating Antibiotic Incentives Now Act (5). Unfortunately, this list includes many organisms with several effective available treatments (for example, methicillin-resistant Staphylococcus aureus). Because the QIDP list is contained in federal statute, new legislation is required for it to be updated. Alternate lists published by the Centers for Disease Control and Prevention in 2013 (6) and the World Health Organization in 2017 (7) also have no mechanism for regular updating and are not coupled to any authority to target incentives.
All 3 lists continue to include carbapenem-resistant Enterobacteriaceae as a priority pathogen despite approval of 5 new antibiotics targeting these organisms in the past 4 years. There are also several others in development. This illustrates the need for ongoing review and reassessment of priorities. Notably, all 5 antibiotics received QIDP designation, and 3 received substantial public funds for clinical development. However, these antibiotics primarily focused on relatively low-risk urinary or abdominal infections caused by non-XDR pathogens and thus failed to address the most important clinical unmet needs.
The shifting nature of resistance profiles creates risk to drug developers targeting pathogens that lose XDR status as new antibiotics are approved to treat them. The current approach is to cluster simultaneous antibiotic development efforts around a few pathogens, such as methicillin-resistant S aureus and carbapenem-resistant Enterobacteriaceae, while few drugs are being developed against others, such as mycobacteria and Acinetobacter. In contrast, the DART Board would influence early development decisions to focus on a broader array of target pathogens. As antibiotic resistance reemerges among certain pathogens, the DART Board would reinstate them on the list. This approach would also spread approvals out over time, helping to sustain the pipeline. As noted in 1965, “There is a possibility that not many new antibiotics remain to be discovered, and if so it is better that they should be introduced one by one at fairly long intervals” (2).
The DART Board’s list would help guide an evolving ecosystem for antibiotic development. Historical reliance on for-profit industry for antibiotic discovery has contributed to cyclical downturns when market conditions have been unfavorable. Expansion of discovery work in academic laboratories and by nonprofit organizations, funded by government and foundation grants, would help smooth boom-and-bust cycles. These entities can focus on drugs with smaller market niches, but potentially greater clinical impact, than for-profit companies (3, 8). The DART Board could help to inform these funding decisions. Nonetheless, research should focus on the most innovative science to provide diverse lead candidates for future development.
Facile regulatory mechanisms are needed to demonstrate clinical efficacy of antibiotics against XDR organisms causing severe infections. Lacking such pathways, trials predominantly enroll patients infected with nonresistant pathogens causing lower-risk infections. This has perversely led to approval of antibiotics with activity against XDR pathogens to treat nonresistant infections—a violation of fundamental antibiotic stewardship principles (9). Thus, unusually, the U.S. Food and Drug Administration cannot label antibiotics for appropriate use (that is, XDR pathogens) but approves labeling for inappropriate use (9). Innovative solutions include enabling XDR pathogen–specific, multibodysite clinical (4, 8) and platform trials (10) of sicker patients and requiring a stewardship justification on antibiotic labels (9).
In conclusion, the traditional entrepreneurial model of development has provided many effective antibiotics, but at the cost of repeated pipeline collapses despite increasing resistance. Unless we make fundamental changes in our current approach, the next collapse could be the last, leading to a postantibiotic era. This proposed model has the potential to create a targeted, sustainable solution to the unending challenge of antibiotic resistance.
Acknowledgments
Grant Support: By grants R01 AI130060, R01 AI117211, and R42 AI106375 from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health and by grant R01 HS025690 from the Agency for Healthcare Research and Quality.
Footnotes
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M19-1893.
Contributor Information
Brad Spellberg, Los Angeles County and University of Southern California Medical Center, Los Angeles, California.
Travis B. Nielsen, Stritch School of Medicine and Parkinson School of Health Sciences and Public Health, Loyola University Chicago, Maywood, Illinois.
David N. Gilbert, Providence Portland Medical Center and University of Oregon Health Sciences School of Medicine, Portland, Oregon.
Andrew F. Shorr, Pulmonary and Critical Care Medicine Service, MedStar Washington Hospital Center, Washington, DC.
Eric P. Brass, David Geffen School of Medicine at the University of California, Los Angeles, Los Angeles, California.
References
- 1.Johnson AS. Medicine’s responsibility in the propagation of poor protoplasm. N Engl J Med. 1948;238:755–8. [PMID: 18860758] [DOI] [PubMed] [Google Scholar]
- 2.Finland M, Kirby WM, Chabbert YA, et al. Round table: are new antibiotics needed? Antimicrob Agents Chemother (Bethesda). 1965;5:1107–14. [PMID: 5883409] [PubMed] [Google Scholar]
- 3.Nielsen TB, Brass EP, Gilbert DN, et al. Sustainable discovery and development of antibiotics—is a nonprofit approach the future? N Engl J Med. 2019;381:503–5. [PMID: 31216396] doi: 10.1056/NEJMp1905589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Infectious Diseases Society of America. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis. 2012;55:1031–46. [PMID: 22891041] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration. Generating Antibiotic Incentives Now. Accessed at www.fda.gov/media/110982/download on 1 August 2019.
- 6.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Antibiotic resistant threats in the United States, 2013. Accessed at www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf on 1 August 2019.
- 7.World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Accessed at www.who.int/medicines/publications /WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf on 1 August 2019.
- 8.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368:299–302. [PMID: 23343059] doi: 10.1056/NEJMp1215093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spellberg B, Srinivasan A, Chambers HF. New societal approaches to empowering antibiotic stewardship. JAMA. 2016;315: 1229–30. [PMID: 26914942] doi: 10.1001/jama.2016.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angus D, Alexander B, Berry S, et al. Adaptive Platform Trials Coalition. Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019. [PMID: 31462747] doi: 10.1038/s41573-019-0034-3 [DOI] [PubMed] [Google Scholar]