Sir,
In the recent article published by Brain, Eidhof and colleagues described a new autosomal recessive cerebellar ataxia subtype (ARCA27) caused by biallelic mutations in the ganglioside induced differentiation associated protein 2 (GDAP2) (Eidhof et al., 2018). The authors reported two unrelated cases with late-onset ataxia, progressive spasticity and dementia carrying biallelic loss-of-function mutations in GDAP2 (OMIM 618369), a gene previously not linked to any human diseases. In addition, studying its orthologous gene in the fruit fly, Drosophila melanogaster, showed that a ubiquitous knockdown of Gdap2 resulted in shortened lifespan and caused abnormal motor behaviour, including righting defects, uncoordinated walking, and poor wing movement with droopy wings. Gdap2 expression levels responded to stress treatments in control flies, and Gdap2 knockdown flies showed increased sensitivity to deleterious effects of stressors such as reactive oxygen species and nutrient deprivation. In brief, they provided evidence that Gdap2 knockdown in Drosophila and GDAP2 loss-of-function mutations in humans lead to locomotor phenotypes, which may be mediated by altered responses to cellular stress.
Here, we describe a third patient with a homozygous frameshift variant in GDAP2 and thus confirm the causality of this gene for the autosomal recessive spinocerebellar ataxia (Table 1). Immediately following publication of the report linking GDAP2 to ataxia, we identified the variant in a patient of Greek origin by reanalysing the exome data from our large cohort of patients with ataxia (consisting of 300 multi-ethnic ataxia index cases, with a wide range of ages of onset, recessive and dominant inheritance patterns, ranging from pure cerebellar ataxias to more complex ataxias). This study was approved by the ethics committee of University College London Hospital NHS Foundation Trust (UCLH) and the Eginition Hospital Ethics Committee. Written informed consent was obtained from the case according to the Declaration of Helsinki.
Table 1.
Clinical presentation of the previously reported cases of GDAP2 in Eidhof et al. (2018) as well as the newly identified case reported here
| Case | This study | Eidhof et al. (2018) | Eidhof et al. (2018) |
|---|---|---|---|
| GDAP2 mutation | Homozygous | Compound heterozygous | Homozygous |
| Nucleotide change | c.134del | c.946C>T; c.1305dup | c.1198_1199insG |
| Amino acid change | p.(Pro45Leufs*22) | p.(Gln316*); p.(Ser436fs*36) | p.(His400fs*15) |
| Mode of inheritance | Autosomal recessive | Autosomal recessive | Autosomal recessive |
| Ethnic origin | Greek | Dutch/Egyptian | Belgian |
| Gender | Male | Female | Female |
| Age at last examination | 58 | 44 | 52 |
| Presenting phenotype | Gait imbalance, limb ataxia, dysarthria | Gait difficulties, speech changes | Gait difficulties, speech changes |
| Age of onset, years | 33 | 34 | 38 |
| Course | Slowly progressive | Slowly progressive | Moderately progressive until death at 62 years |
| Ataxia milestone / disease duration | Gait and limb ataxia, brisk tendon reflexes (25 years) | Spastic ataxic gait, lower limb hypertonia, very brisk tendon reflexes, but downgoing plantar responses (12 years) | Severe gait ataxia, brisk reflexes (24 years) |
| Predominant clinical syndrome | Cerebellar ataxia, dementia | Cerebellar ataxia | Cerebellar ataxia, dementia |
| Other neurological features | Mild pyramidal features | Mild spastic paraplegia, cervical dystonia | Spasticity |
| Cognition | Dementia | Normal / mild bradyphrenia | Progressive decline, dementia at age 53 years |
| MRI brain (age) | Marked cerebellar atrophy (vermian and hemispheric), mild cortical atrophy, midbrain and pons atrophy (hummingbird sign), thinning of corpus callosum and symmetrical lentiform nucleus haemosiderin depositions | Mild cerebellar atrophy | Severe atrophy of the cerebellum, global supratentorial atrophy (cortical and central) |
| NCS/EMG | Normal | Normal | Normal |
| Ophthalmological evaluation | Normal | Normal | Normal |
NCS = nerve conduction study.
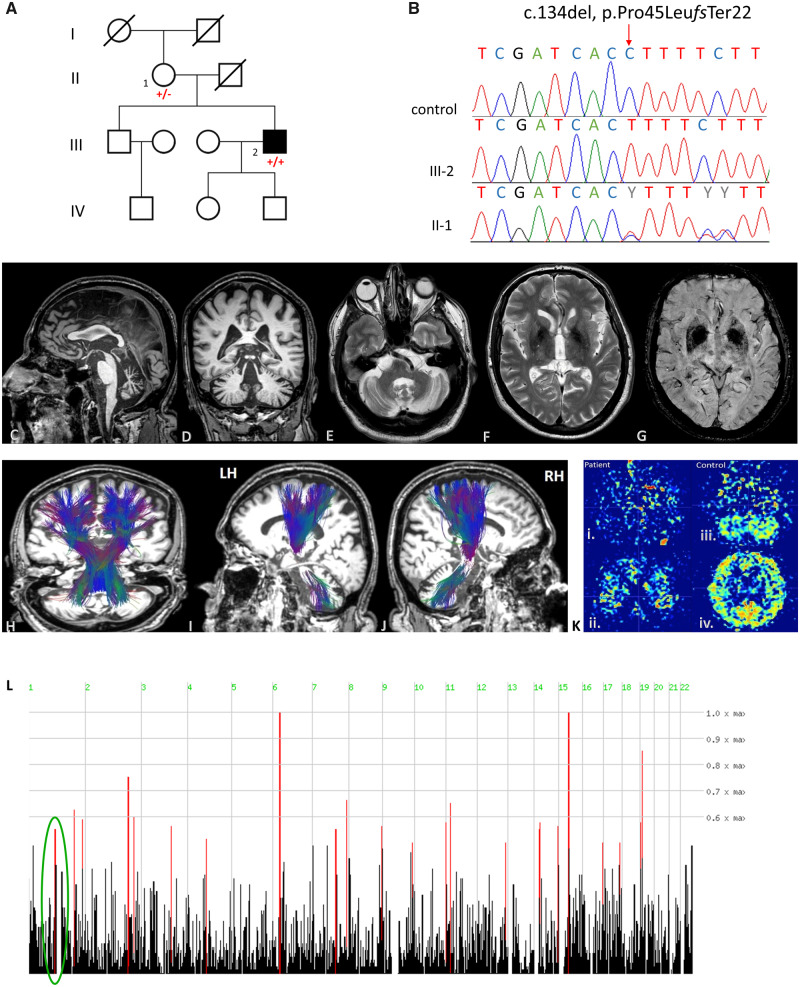
The proband is a 58-year-old male of Greek origin presenting with a progressive cerebellar syndrome starting at the age of 33 years with mild gait imbalance, ataxia and dysarthria. The patient had a slow progressive course (walking with unilateral support at 50 years of age, and with a stroller at 57 years of age). His relatives have reported personality changes with emotional lability, aggressive behaviour and depressive episodes. Family history is negative for neurological disorders. Both parents originated from the same small village but there was no known consanguinity in the family (Fig. 1A). Apart from dyslipidaemia, type 2 diabetes, and chronic diarrhoea presenting at 37 years of age and attributed to irritable bowel syndrome, the rest of the patient’s medical history is unremarkable.
Figure 1.
Genetic and neuroimaging findings of this case: (A) Family pedigree. (B) Validation of the variant identified (c.134del, p.Pro45Leufs*22) by Sanger sequencing as homozygous in the patient and heterozygous in the mother compared to a control reference. (C–J) Brain MRI findings of this case. (C) Sagittal T2-weighted sequence showing cerebellar atrophy, midbrain and pons atrophy (hummingbird sign), and thinning of corpus callosum. (D and E) Coronal and axial T1-weighted sequence. Cerebellar atrophy is noted particularly in upper and central cerebellar vermis, however cortical atrophy, and dilated fissures (Global Cortical Atrophy, GCA scale: 2) were also present. (F and G) Axial T2-weighted and SWI (susceptibility weighted imaging) sequences showing lentiform hemosiderin depositions. (H–J) Magnetic resonance tractography using diffusion tensor imaging shows no significant alterations in the reconstructed corticospinal and cerebellar tracts superimposed on anatomical 3D T1-weighted images in axial, coronal and sagittal planes. L = left; R = right; LH = left hemisphere; RH = right hemisphere. [K(i and ii)] Patient’s ASL (arterial spin labelling) representative colour-coded images at cerebellar and lateral ventricles level showing reduced perfusion in cerebellum and in cerebral cortex. [K(iii and iv)] Control ASL representative colour coded images at cerebellar and lateral ventricles level. (L) Homozygosity mapping showing homozygous segments on chromosome 1 where the variant identified resides within a 3.1 Mb homozygous chromosomal segment (ch1: 114 522 285–117 628 869).
Neurological examination at the age of 50, when the proband was first seen, showed severe gait ataxia with mild pyramidal features consisting of brisk reflexes, dysmetria, dysdiadochokinesia, cerebellar dysarthria, jerky pursuit and nystagmus, mild behavioural changes, and disturbances of short-term memory. Follow-up neurological examination at the age of 58, revealed severe gait disturbance, dysmetria, dysdiadochokinesia and brisk deep tendon reflexes with flexor plantar responses, as well as gaze-evoked nystagmus, jerky pursuit, hypermetric saccades, cerebellar dysarthria, and mild sensory loss in the lower limbs (Supplementary Video 1). Scale for the assessment and rating of ataxia (SARA) score at that time was 31/40 and Mini-Mental State Examination (MMSE) score was 24/30.
A detailed neuropsychological assessment was performed, revealing widespread deficits in encoding, learning, and consolidating new verbal information. Reading fluency was limited, but this could be attributed to the presence of dysarthria. Naming performance was marginal. Cognitive flexibility and processing speed were found to be impaired but inhibition was spared. Working memory for verbal stimuli was found to be impaired, but was relatively spared for visuospatial stimuli. Overall, the patient exhibited prominent deficits in episodic memory, verbal working memory, and executive functions.
A brain MRI scan, including susceptibility-weighted imaging (SWI), was performed and revealed marked cerebellar atrophy affecting the hemispheres and more significantly the vermis, as well as mild cortical atrophy, midbrain and pons atrophy (hummingbird sign) (Fig. 1C–J). Magnetic resonance tractography did not reveal any significant abnormalities in both reconstructed cortico-cerebellar bundles. Patient’s arterial spin labelling (ASL) representative colour coded images at cerebellar and lateral ventricles level showed reduced perfusion in cerebellum and cerebral cortex (Fig. 1K). Nerve conduction studies (NCS), electromyography (EMG) and ophthalmological evaluation were all normal. Laboratory testing of alpha-fetoprotein levels, vitamin E, copper, and folic acid were also normal. To exclude the possibility of an underlying autoimmune disease, the patient’s serum was tested for binding to various markers, including a panel of paraneoplastic markers of autoimmunity, GAD, NMDA, AMPA, GABAB, DPPX and VGKC, as well as monkey cerebellum frozen tissue. No binding to any of these markers was detected.
Blood samples from the proband and his mother were collected after written informed consent and DNA was extracted using standard procedures. Initial screening for Friedreich’s ataxia, as well as spinocerebellar ataxia (SCA) types 1, 2, 3, 6 and 7 in the proband was negative. To investigate the genetic cause of the disease, whole exome sequencing of the proband’s DNA was performed as previously described (Mencacci et al., 2016). In accordance with the recessive mode of inheritance, priority was given to rare biallelic functional variants with allele frequency <0.001% in public databases, including 1000 Genomes project, NHLBI Exome Variant Server, Complete Genomics 69, and gnomAD as well as our in-house database consisting of 12 000 exomes. After applying the above filtering criteria, no plausible compound heterozygous or homozygous variants were identified in genes previously associated with neurological phenotypes. However, following the publication of the article and by re-examining the exome data an ultra-rare homozygous 1-bp deletion in GDAP2 (NM_017686.4; c.134delC), resulting in a frameshift and premature termination (p.Pro45Leufs*22) was identified. The variant was residing within a 3.1Mb homozygous interval on chromosome 1 (ch1: 114 522 285–117 628 869) (Fig. 1L) and it was validated by Sanger sequencing. The mother was heterozygous carrier of the variant (Fig. 1B).
The GDAP2 protein contains two important functional domains: a macro and a CRAL-TRIO domain (Aken et al., 2016). In general, macro domain-containing proteins have been described as sensors of cellular metabolic states and are involved in cellular redox homeostasis, DNA damage and repair, the formation of cytosolic stress granules that can protect mRNAs from degradation, apoptosis, and necrosis (Karras et al., 2005; Han et al., 2011; Posavec et al., 2013; Gonzalez Esquivel et al., 2017). It is not yet known whether GDAP2 operates in these processes, but defects in these key pathways have been linked to many neurodegenerative disorders, including autosomal recessive cerebellar ataxias (ARCAs).
Eidhof et al. (2018) introduced ataxia as a clinical manifestation of biallelic mutations in the GDAP2 gene, describing a new ARCA subtype. Our patient is in line with the patients previously reported, presenting with a progressive cerebellar syndrome complicated by pyramidal features, dementia and dysexecutive syndrome. Our study provides a novel GDAP2 mutation in a Greek family affected by autosomal recessive cerebellar ataxia, increasing the total number of reported cases to three, expanding the spectrum of causative mutations and further confirming the pathogenicity of GDAP2 mutations in ARCA.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.
Supplementary Material
Acknowledgements
The family was recruited as part of the SYNaPS Study Group collaboration funded by The Wellcome Trust and strategic award (Synaptopathies) funding (WT093205 MA and WT104033AIA). This research was conducted as part of the Queen Square Genomics group at University College London, supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Funding
The MRC (MR/S01165X/1, MR/S005021/1, G0601943), The National Institute for Health Research University College London Hospitals Biomedical Research Centre, Rosetree Trust, Ataxia UK, MSA Trust, Brain Research UK, Sparks GOSH Charity, Muscular Dystrophy UK (MDUK), Muscular Dystrophy Association (MDA USA).
Competing interests
G.K. has received research grants from Genesis Pharma and Teva, consultation fees, advisory boards and honoraria from Genzyme, Genesis Pharma, Teva and Novartis. J.T. has shares in a diagnostic laboratory (Tzartos Neurodiagnostics) in Athens. M.B. has received travel sponsorship from Genesis Pharma, Teva, Pfizer, Sanofi-Genzyme. All other authors report no disclosures relevant to the manuscript.
References
- Aken BL, Ayling S, Barrell D, Clarke L, Curwen V, Fairley S, et al. The Ensembl gene annotation system. Database (Oxford) 2016; 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidhof I, Baets J, Kamsteeg EJ, Deconinck T, van Ninhuijs L, Martin JJ, et al. GDAP2 mutations implicate susceptibility to cellular stress in a new form of cerebellar ataxia. Brain 2018; 141: 2592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Esquivel D, Ramirez-Ortega D, Pineda B, Castro N, Rios C, Perez de la Cruz V.. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology 2017; 112: 331–45. [DOI] [PubMed] [Google Scholar]
- Han W, Li X, Fu X.. The macro domain protein family: structure, functions, and their potential therapeutic implications. Mutat Res 2011; 727: 86–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karras GI, Kustatscher G, Buhecha HR, Allen MD, Pugieux C, Sait F, et al. The macro domain is an ADP-ribose binding module. EMBO J 2005; 24: 1911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencacci NE, Kamsteeg EJ, Nakashima K, R’Bibo L, Lynch DS, Balint B, et al. De novo mutations in PDE10A cause childhood-onset chorea with bilateral striatal lesions. Am J Hum Genet 2016; 98: 763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posavec M, Timinszky G, Buschbeck M.. Macro domains as metabolite sensors on chromatin. Cell Mol Life Sci 2013; 70: 1509–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.