Abstract
Associations between plasma elements and chronic kidney disease (CKD) among the elderly are poorly understood. In this cross-sectional study, we explored the associations between exposure to four plasma elements and CKD in elderly people aged ≥ 90 years in longevity areas in China. We measured plasma selenium, manganese, iron, and zinc levels and used logistic regression models to investigate associations between CKD and these four plasma elements after adjusting for confounding factors among 461 participants aged ≥ 90 years in the fifth wave of the Chinese Longitudinal Healthy Longevity Study (CLHLS) conducted in 2009. The median plasma selenium, manganese, iron, and zinc levels were 120.51 μg/L, 26.64 μg/L, 2880.52 μg/L, and 1882.42 μg/L in the CKD group and 108.76 μg/L, 31.55 μg/L, 4512.00 μg/L, and 2294.24 μg/L in the non-CKD group, respectively. Single- and multiple-element multivariable models showed that plasma manganese, iron, and zinc were negatively associated with CKD. In the multiple-element multivariable models, the adjusted odds ratios for CKD were 0.48 (95% confidence interval [CI]: 0.27–0.86) for the second highest quartile of manganese, 0.37 (95% CI: 0.21–0.68) and 0.36 (95% CI: 0.19–0.65) for the third highest and highest quartiles of iron, respectively, and 0.53 (95% CI: 0.29–0.94) for the highest quartile of zinc, compared with the lowest quartiles of these three elements. Plasma manganese, iron, and zinc levels protect against CKD in elderly people aged ≥ 90 years in longevity areas.
Keywords: Chronic kidney disease, Elderly aged ≥ 90 years, Longevity areas, Iron, Zinc, Manganese
1. Introduction
Chronic kidney disease (CKD) is a leading public health problem that has received increased research attention because of its significantly increased prevalence over the last 10 years, especially in developing countries (Couser et al., 2011). The overall prevalence of CKD in China reached 10.8% in 2009–2010, which equates to approximately 119.5 million people (Zhang et al., 2012). The CKD burden has implications for renal replacement therapy, factors predicting cardiovascular risk, and causes of morbidity, mortality, and hospitalization (Best and Holmes, 2003; Tonelli et al., 2006).
CKD is a multifactorial disease, caused by genetic and environmental factors (Ingsathit et al., 2010) and is characterized by inflammation due to oxidative stress and deteriorated kidney function (Gonzalez Rico et al., 2006). Elements are ubiquitous, coexist in the natural environment, and mainly enter the body through the respiratory and digestive tracts. Many elements, such as selenium, iron, and zinc, are components of metalloenzymes and participate in reactive oxygen metabolism, free radical scavenging, and hormone activities (Tapiero and Tew, 2003; Shah, 2004; Zachara, 2015; Leaf and Swinkels, 2016). Several studies have estimated the inverse associations between CKD and elements exposure, but the results are inconsistent. A Mendelian randomization study found that serum iron exerted a causal protective effect on kidney function in adults (Del Greco et al., 2017). Patient-based studies have showed that serum zinc is negatively associated with CKD stages (Shih et al., 2012), and selenium and plasma glutathione peroxidases are significantly reduced in CKD patients (Zachara, 2015). A study conducted among 63 children in Brazil reported that blood manganese levels were significantly inversely associated with biomarkers of kidney function (Nascimento et al., 2016). However, a cross-sectional study conducted in China found no correlation between plasma selenium, manganese, iron, and zinc levels and renal function (Yang et al., 2019). Many studies have also showed that high manganese, iron, and zinc levels present potential poisoning risks to humans (Hingorani et al., 2009; Qin et al., 2013).
The population in China is aging rapidly, and CKD prevalence increases with age (Zhang et al., 2012). Environmental problems, such as air and water pollution, remain major concerns in China (Zhang et al., 2010). Several environmental factors, particularly protective elements for CKD, remain to be investigated. Population studies have mainly covered children, adults, and the elderly; however, studies on the extreme elderly are rare, especially in China. Thus, whether an interaction exists between the effects of protective plasma elements and CKD remains unknown. Here, we estimated the associations between plasma element levels and CKD in the extreme elderly population in longevity areas in China and investigated interactions among the plasma elements.
2. Methods
2.1. Study design and participants
Participants in the present study were from the fifth wave of the Chinese Longitudinal Healthy Longevity Study, an ongoing perspective survey conducted in longevity areas in China. The details of this survey were described in a previous work (Zeng, 2012). We interviewed all centenarians from Xiayi County of Henan Province, Sanshui District of Guangdong Province, Zhongxiang City of Hubei Province, Yongfu County of Guangxi Province, and Mayang County of Hunan Province who voluntarily agreed to participate in the study. We also interviewed one nearby nonagenarian of predefined age and sex for each centenarian interviewee in 2009. Four hundred ninety-six elderly people aged ≥90 years were enrolled, and 461 participants completed face-to-face questionnaires, underwent detailed health examinations, and provided blood and urine samples. The ethical review committees of Peking University and National University of Singapore approved the study, and all participants or their proxies provided written consent.
2.2. CKD case determination
Healthcare professionals collected fasting venous blood samples from all subjects in the morning. The 5 mL blood samples without anticoagulant were centrifuged at 3000×g for 10 min. Part of the serum was stored at −20 °C and delivered to the laboratory at Capital Medical University in Beijing for creatinine measurement. Urinary albumin was measured from fresh morning urine samples via the dipstick method, and albuminuria was defined as the presence of albumin in the urine. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation using data from the Chinese population (Ma et al., 2006). CKD events were defined as the presence of albuminuria or an eGFR less than 60 mL/min per 1.73 m2, calculated from the following equation:
where Scr is the serum creatinine concentration (mg/dL) and age is measured in years.
2.3. Plasma element measurement
Fasting venous blood samples were collected into 5 mL heparin anticoagulant vacuum tubes and centrifuged at 3000×g for 10 min. The plasma was isolated, stored at −20 °C, and shipped to the laboratory at Capital Medical University for elemental analysis. Prior to analysis, the plasma specimens were thawed at room temperature for 30 min. Exactly 0.5 mL of blood plasma and 4-mL of a mixture of HNO3 and HClO4 (4:1 by volume) were added to Teflon tubes with a cap and digested overnight using an electric hot plate. After digestion, the excess acids were evaporated, and the residuals were dissolved in 1% HNO3 to 4 mL. The plasma selenium and manganese concentrations were determined by inductively coupled plasma optical emission spectroscopy (ICPS-7000; Shimadzu Corporation). Three hundred microliters of blood plasma was directly subjected to atomic absorption spectroscopy (BH5300; Beijing Bohui Innovation Technology Co., Ltd.) to measure the iron and zinc concentrations.
We used internal and external standard methods to measure the plasma element levels. The ClinChek® control (no. 8882) was used as quality control reference during the. The elemental concentrations were determined in duplicate after measuring every 20 samples and the relative standard deviation of each element was <5% in the reproducibility test. We used a spiked pooled plasma sample to evaluate the method’s precision and accuracy. Each element was recovered at 90%–110%. The limits of detection (LOD) of the plasma selenium, manganese, iron, and zinc were 1.3 μg/L, 0.8 μg/L, 4.2 μg/L, and 15.1μg/L, respectively. If the plasma element concentrations fell below the LOD, we used the half of the detection limits.
2.4. Covariates
Face-to-face home-based interviews were performed along with a basic physical examination at each interviewee’s home. Trained interviewers collected the data using structured questionnaires, which included demographic and social characteristics (e.g., age, sex, education, marital status, and income), lifestyle (e.g., living arrangements, smoking and alcohol drinking status, and physical activities), medical history, and related risk factors for renal function. Medical personnel conducted the physical examinations, which included height, weight, and blood pressure (BP) measurements. Systolic BP (SBP) and diastolic BP (DBP) were measured twice, and the averages were calculated. Blood lipid and fasting glucose concentrations were also measured.
2.5. Statistical analyses
Characteristics of participants with and without CKD were compared using t-tests for continuous variables and chi-square tests for categorical variables. Because of the skewed data distribution, we used the Mann-Whitney U test to compare differences in plasma elements between participants with and without CKD. Spearman correlation was used to evaluate correlations among plasma element concentrations.
We used logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for CKD and plasma elements as categorical variables according to the tertile distribution of plasma element concentrations in all participants. All models were adjusted for age (continuous variable), sex (categorical variable), body mass index (BMI; continuous variable), education (categorical variable, <primary school, primary school, and >primary school), and county/city (categorical variable). Linear trend p-values were estimated by modeling the tertiles of the plasma elements as continuous variables in the adjusted models. Sensitivity analysis was conducted by adjusting for each participant’s physical activity (physical activity was defined as a response of “yes” to the question “Do you often do physical activities, including walking, playing ball, running, or Qigong?”), smoking and drinking status (current, former, or never smoker or drinker), hypertension (defined if the average BP value was ≥140 mmHg for SBP or ≥90 mmHg for DBP or if the participant was diagnosed with hypertension by a physician), diabetes (defined if the fasting glucose concentration was ≥7.0 mmol/L or if the participant was diagnosed with diabetes by a physician), or hyperlipidemia (defined if the total cholesterol level was ≥5.72 mmol/L or triglyceride level was ≥ 1.70 mmol/L or if the participant was diagnosed with hyperlipidemia by a physician).
We conducted a stratified analysis by including elements in the multiple-elements model and variables for subjects’ basic information, including age (100 years>age≥90 years, age≥100 years), sex (male or female), BMI (<19 or ≥19 kg/m2), and hypertension (yes or no). Joint associations among significant elements in the multiple-elements model were also evaluated. Elements that were significantly associated with CKD in the multiple-elements model (p<0.05) were included in the spline regression analysis. The reference value of each element was set at the 10th percentile.
Statistical analyses were conducted using R software (version 3.6.1; R Core Team), and p<0.05 (two-sided) was considered statistically significant.
3. Results
3.1. Characteristics of the study participants
Four hundred sixty-one elderly individuals aged ≥90 years, including 95 men and 366 women, were included in this study. Of these, 193 (41.9%) had CKD. The proportion of CKD was higher in woman than in men and in those in the low BMI group. Age, education, smoking and drinking status, physical activity, diabetes, hypertension, and hyperlipidemia did not significantly differ between participants with and without CKD. Plasma iron and zinc concentrations were significantly higher, and selenium was significantly lower in participants without CKD than in those with CKD (Table 1). Table S1 shows the spearman correlations among plasma elements. All plasma elements showed weak internal correlations (r<0.3).
Table 1.
Demographic characteristics of participants in the study
| Variables | Participant without CKD (n=268) | Participant with CKD (n=193) | p-Valuea |
|---|---|---|---|
| Age (years) | 97.7±5.0 | 98.2±5.3 | 0.24 |
| Male, n (%) | 67 (25.0) | 28 (14.5) | <0.01 |
| BMI (kg/m2) | 19.47±3.81 | 18.78±2.73 | 0.02 |
| Education, n (%) | |||
| Less than primary school | 234 (87.3) | 170 (88.1) | |
| Primary school | 20 (7.5) | 16 (8.3) | 0.69 |
| More than primary school | 14 (5.2) | 7 (3.6) | |
| Smoking status, n (%) | |||
| Current smoker | 36 (13.4) | 38 (19.7) | |
| Former smoker | 30 (11.2) | 23 (11.9) | 0.17 |
| Never smoker | 202 (75.4) | 132 (68.4) | |
| Drinking status, n (%) | |||
| Current drinker | 42 (15.7) | 27 (14.0) | |
| Former drinker | 23 (8.6) | 18 (9.3) | 0.86 |
| Never drinker | 203 (75.7) | 148 (76.7) | |
| Hypertension, n (%) | 154 (57.5) | 110 (57.0) | 0.92 |
| Hyperlipidemia, n (%) | 46 (17.2) | 23 (11.9) | 0.12 |
| Diabetes, n (%) | 31 (11.6) | 23 (11.9) | 0.91 |
| Physical activity, n (%) | 183 (68.3) | 123 (63.7) | 0.31 |
| Selenium (μg/L) | 108.76 (81.50–143.98) | 120.51 (84.98–166.97) | 0.02 |
| Manganese (μg/L) | 31.55 (15.99–65.78) | 26.64 (11.82–59.97) | 0.08 |
| Iron (μg/L) | 4512.00 (2429.65–8330.82) | 2880.52 (1587.20–6155.30) | <0.001 |
| Zinc(μg/L) | 2294.24 (1161.15–3590.73) | 1882.42 (969.96–2726.60) | 0.01 |
Note: Normally distributed variables were presented as mean ± SD. Categorical variables were presented as numbers (percentage). Skewed distributed variables were presented as median (P25, P75).
p-Values were derived from Student’s t-tests or Mann-Whitney U tests for continuous variables according to the data distributions, chi-square test for the category variables.
3.2. Plasma elements and CKD
The plasma manganese, iron, and zinc levels were significantly correlated with CKD risk in the single-element model after adjusting for age, sex, BMI, education, and county/city (Table S2). Selenium was not significantly correlated with CKD (p=0.08); thus, we excluded it from the multiple-element models. In the multiple-element models, plasma manganese, iron, and zinc levels retained their correlations with CKD risk, and the adjusted ORs for manganese, iron, and zinc were 0.48 (95% CI: 0.27–0.85 for the second vs. first quartiles), 0.34 (95% CI: 0.19–0.62 for the fourth vs. first quartiles), and 0.50 (95% CI: 0.28–0.89 for the fourth vs. first quartiles), respectively (Table 2). The sensitivity analysis results suggested that our findings were relatively robust (Table S3). ORs for manganese, iron, and zinc did not change considerably after adjusting for smoking and drinking status, physical activity, diabetes, hypertension, and hyperlipidemia in the multiple-element models.
Table 2.
Adjusted odds ratios (95% CI) for CKD according to quartiles of exposure for plasma elements in the logistic regression models.
| Plasma elements | Odds ratio (95% CI) | p-Trend | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Manganese(μg/L) | <13.63 | 13.63–28.80 | 28.80–63.87 | >63.87 | |
| Model1 | 1 | 0.52 (0.30–0.91) | 0.61 (0.35–1.03) | 0.68 (0.40–1.16) | 0.22 |
| Model2 | 1 | 0.48 (0.27–0.85) | 0.68 (0.39–1.20) | 0.95 (0.54–1.67) | 0.89 |
| Iron(μg/L) | <1964.42 | 1964.42–3818.90 | 3818.90–7608.24 | >7608.24 | |
| Model1 | 1 | 0.68 (0.40–1.16) | 0.34 (0.19–0.58) | 0.37 (0.21–0.65) | <0.001 |
| Model2 | 1 | 0.75 (0.44–1.31) | 0.34 (0.19–0.61) | 0.34 (0.19–0.62) | <0.001 |
| Zinc(μg/L) | <1078.14 | 1078.14–2071.06 | 2071.06–3201.89 | >3201.89 | |
| Model1 | 1 | 0.97 (0.57–1.65) | 0.64 (0.38–1.10) | 0.48 (0.28–0.83) | 0.003 |
| Model2 | 1 | 1.01 (0.58–1.75) | 0.73 (0.41–1.28) | 0.50 (0.28–0.89) | 0.009 |
Model1: plasma elements were included in the unconditional logistic regression models separately (single-element model) and adjusted for age, sex, BMI, education and county/city.
Model2: plasma elements were included in the logistic regression models simultaneously (multiple-elements model) and adjusted for the same variables as Model1.
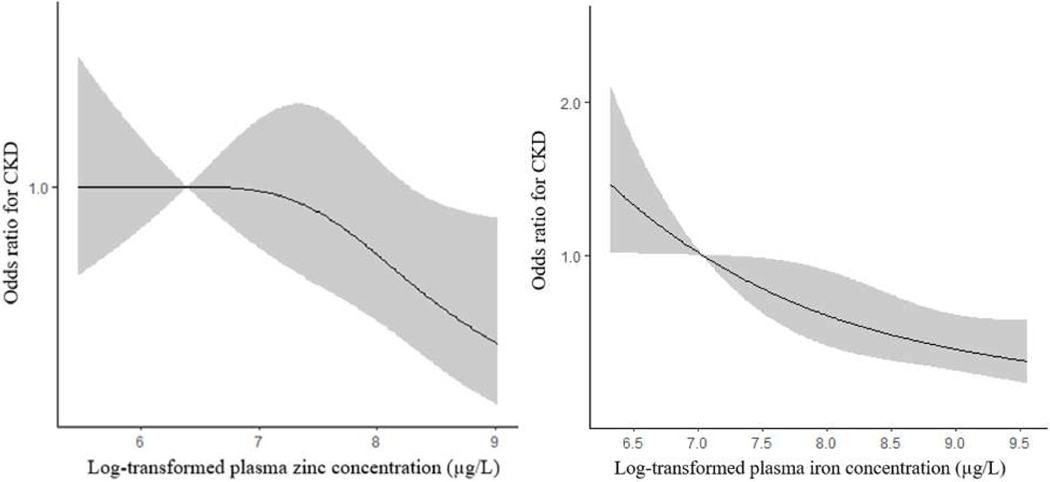
In the multiple-element models, trend tests for iron and zinc remained significant (p-trend<0.05). Therefore, spline regression analysis was limited to iron and zinc. Iron was significantly linearly correlated with CKD in the spline regression analysis (p<0.001), whereas, the correlation between zinc and CKD was nonlinear (p=0.06), and the slope for the zinc concentrations decreased sharply to >4337.3 μg/L (Figure 1).
Figure 1.
The restricted cubic spline for the associations of elements and CKD. The adjusted ORs were calculated by restricted cubic splines for the log-transformed concentrations of iron and zinc in the multi-element model and the reference value (OR=1) was set at the 10th percentage of each element. The variables adjusted were age, sex, BMI, education and county/city. Manganese was also included in the model.
3.3. Subgroup and element interaction analyses
Associations between plasma elements and CKD risk in the analyses stratified by age, BMI, and hypertension did not significantly differ (Table S4). The correlation was stronger among women than among men.
Table 3 shows the joint associations between plasma elements and CKD risk. Although high iron levels were associated with decreased CKD risk when the zinc levels were high (beyond the median), the p-values showed no significant interactions among the three elements.
Table 3.
The changes of odds ratios (95% CI) of different subgroups stratified by age, sex, BMI and hypertension
| Odds ratio (95% CI) | p-Trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Manganese(μg/L) | <13.63 | 13.63–28.80 | 28.80–63.87 | >63.87 | |
| 100>Age≥90 (n=248) | 1 | 0.41 (0.17–0.95) | 0.61 (0.29–1.29) | 1.11 (0.51–2.41) | 0.73 |
| Age≥100 (n=213) | 1 | 0.51 (0.22–1.19) | 0.86 (0.34–2.16) | 0.81 (0.33–1.98) | 0.90 |
| Male (n=95) | 1 | 0.64 (0.17–2.36) | 0.81 (0.23–2.79) | 1.51 (0.43–5.23) | 0.87 |
| Female (n=366) | 1 | 0.44 (0.23–0.86) | 0.62 (0.32–1.20) | 0.90 (0.46–1.75) | 0.97 |
| BMI<19 (n=245) | 1 | 0.53 (0.24–1.19) | 0.73 (0.33–1.64) | 0.75 (0.34–1.65) | 0.62 |
| BMI≥19 (n=216) | 1 | 0.40 (0.16–0.98) | 0.64 (0.28–1.44) | 1.40 (0.59–3.30) | 0.47 |
| Hypertension (n=264) | 1 | 0.56 (0.26–1.22) | 0.58 (0.27–1.25) | 0.86 (0.41–1.82) | 0.78 |
| No hypertension (n=197) | 1 | 0.37 (0.15–0.95) | 0.87 (0.36–2.13) | 1.32 (0.52–3.34) | 0.37 |
| Iron(μg/L) | <1964.42 | 1964.42–3818.90 | 3818.90–7608.24 | >7608.24 | |
| 100>Age≥90 (n=248) | 1 | 1.12 (0.53–2.33) | 0.51 (0.22–1.16) | 0.36 (0.16–0.84) | 0.01 |
| Age≥100 (n=213) | 1 | 0.41 (0.17–1.01) | 0.19 (0.08–0.46) | 0.26 (0.11–0.65) | 0.001 |
| Male (n=95) | 1 | 1.25 (0.33–4.73) | 0.31 (0.07–1.29) | 0.68 (0.18–2.54) | 0.81 |
| Female (n=366) | 1 | 0.70 (0.38–1.31) | 0.33 (0.17–0.64) | 0.27 (0.14–0.54) | <0.001 |
| BMI<19 (n=245) | 1 | 0.76 (0.34–1.69) | 0.31 (0.14–0.71) | 0.36 (0.17–0.78) | 0.004 |
| BMI≥19 (n=216) | 1 | 0.75 (0.33–1.68) | 0.38 (0.16–0.87) | 0.29 (0.11–0.76) | 0.01 |
| Hypertension (n=264) | 1 | 0.79 (0.37–1.69) | 0.52 (0.24–1.12) | 0.55 (0.26–1.19) | 0.13 |
| No hypertension (n=197) | 1 | 0.72 (0.31–1.69) | 0.19 (0.07–0.49) | 0.20 (0.07–0.55) | <0.001 |
| Zinc(μg/L) | <1078.14 | 1078.14–2071.06 | 2071.06–3201.89 | >3201.89 | |
| 100>Age≥90 (n=248) | 1 | 0.84 (0.38–1.82) | 0.74 (0.34–1.61) | 0.61 (0.28–1.33) | 0.26 |
| Age≥100 (n=213) | 1 | 0.99 (0.42–2.34) | 0.71 (0.29–1.71) | 0.37 (0.15–0.94) | 0.01 |
| Male (n=95) | 1 | 0.58 (0.14–2.33) | 0.50 (0.13–1.97) | 0.61 (0.15–2.58) | 0.55 |
| Female (n=366) | 1 | 1.21 (0.65–2.27) | 0.81 (0.43–1.54) | 0.42 (0.22–0.82) | 0.006 |
| BMI<19 (n=245) | 1 | 0.82 (0.39–1.73) | 0.65 (0.29–1.43) | 0.58 (0.26–1.30) | 0.15 |
| BMI≥19 (n=216) | 1 | 1.26 (0.53–2.99) | 0.80 (0.34–1.85) | 0.42 (0.17–1.01) | 0.02 |
| Hypertension (n=264) | 1 | 0.84 (0.40–1.75) | 0.54 (0.25–1.16) | 0.44 (0.21–0.93) | 0.02 |
| No hypertension (n=197) | 1 | 1.48 (0.58–3.74) | 1.22 (0.49–3.06) | 0.66 (0.26–1.72) | 0.28 |
Discussion
This work was the first to explore the associations between plasma elements and CKD risk in the elderly population aged ≥90 years in longevity areas in China. The primary results showed that plasma manganese, iron, and zinc levels were negatively associated with CKD risk after adjusting for covariates known to affect blood element levels and/or CKD. Moreover, the associations were stronger among women than among men.
Our study suggests that plasma iron helps protect against CKD in elderly people aged ≥90 years. Previous studies have yielded inconsistent results. For example, in a Mendelian randomization study, researchers found a causal protective effect of serum iron on kidney function in adults with mean iron concentrations ranging from 812 μg/L to 1243.2 μg/L across studies. The increase in eGFR per increase in standard deviation of iron was 1.3% (95% CI: 0.4%−2.1%). The results for ferritin, an iron storage biomarker, were consistent with those for iron (Del Greco et al., 2017). In a cross-sectional study conducted in China, researchers found no significant association between iron (median plasma iron concentrations of 1013.9 μg/L for controls and 828.6 μg/L for patients) and abnormal renal function in people aged >18 years (Yang et al., 2019). However, an age-matched case-control study of 79 CKD patients and matched controls in Sri Lanka reported that iron concentrations in hair and nails were higher in patients with CKD than in the controls (Diyabalanage et al., 2017). Iron accumulation in the kidneys can be nephrotoxic under iron overload conditions (Hingorani et al., 2009; Rostoker et al., 2012). Iron metabolism in the kidneys is an extremely complex process; an optimal window for iron levels may be present, and iron depletion or excess may be detrimental to the kidneys (Walter et al., 2002). In the present study, the median iron concentrations were 2880.5 μg/L in the non-CKD group and 4512.0 μg/L in the CKD group, which are much higher than those of previous studies (Del Greco et al., 2017; Yang et al., 2019). Spline analysis revealed that the correlation remained negative at high iron exposure levels. Our finding that higher plasma iron levels were negatively correlated with CKD risk should be confirmed, and the potential mechanisms should be evaluated in other populations.
Zinc, an important and essential protein and enzyme component, is involved in the oxidation–reduction process. Shih et al. (2012) reported that serum zinc was negatively associated with CKD stage, with a significant decreasing trend (Shih et al., 2012). Diyabalanage et al. (2017) found that zinc concentrations in the hair of patients with CKD were higher than those in controls (Diyabalanage et al., 2017). We identified a nonlinear correlation between zinc and CKD, with a significantly negative correlation between the fourth and first exposure quartiles (>3201.89 μg/L vs. <1078.14 μg/L). Spline regression analysis revealed a negative correlation at a plasma zinc concentration of >4337.3 μg/L. In a cross-sectional study conducted in China, plasma zinc was significantly inversely associated with abnormal eGFR. However, these associations were attenuated to null when adjusting for age, sex, BMI, education level, and smoking and drinking status (Yang et al., 2019). The median plasma zinc concentration of 894.6 μg/L was much lower than 4337.3 μg/L, which could explain the lack of a significant correlation. Our analysis revealed that zinc and CKD were negatively correlated at higher exposure levels. Because this study was a cross-sectional, our findings should be replicated in prospective cohort studies.
Manganese is an essential mineral that maintains homeostasis in human. In this study, plasma manganese was negatively associated with CKD, and this negative correlation was significant at the second versus first exposure quartiles (13.63–28.80 μg/L vs. <13.63 μg/L). An epidemiological study found no significant association between plasma manganese (median plasma manganese concentration, <10 μg/L) and abnormal eGFR (Yang et al., 2019). However, a study conducted among 63 children in Brazil reported a significant inverse association between blood manganese levels (32.8 μg/L in rural children and 19.0 μg/L in urban children) and biomarkers of kidney function (Nascimento et al., 2016). Manganese in both deficiency and excess can increase superoxide dismutase activity and lipid peroxidation (Zidenberg-Cherr et al., 1983). This finding indicates that the association between manganese and kidney function may reflect a U-shaped curve (i.e., a negative correlation at the median of the level and a positive correlation at both ends). Additional studies are needed to investigate the dose–response association between manganese and kidney function.
Interaction analyses for manganese, iron, and zinc showed that high zinc levels enhanced the negative association between iron and CKD risk. In patients with CKD, iron supplementation increases blood zinc levels (Mafra et al., 2004). Kim et al. (2017) reported that blood manganese is positively associated with hemoglobin levels (biomarker of blood iron) in patients with CKD (Kim et al., 2017). Additional research should investigate the underlying mechanism of the interactions among these elements.
This study had many strengths. First, we performed standardized sampling procedures for recruitment and blood sample collection and quality control during data collection and experimental assays to ensure data accuracy. Second, to our knowledge, this is the first study to investigate the associations between plasma elements and CKD among elderly individuals aged ≥90 years in China and could serve as preliminary evidence that multiple-elements exposure and kidney function are negatively correlated in this population. Third, we conducted subgroup analyses stratified by age, sex, BMI, and hypertension to determine their effects on the metal-CKD association.
This study also had some limitations. First, casual associations between plasma elements and CKD cannot be determined from cross-sectional studies. Further prospective cohort studies are required to verify our findings. Second, although plasma elements are the most commonly used biomarkers, they may be unsuitable for estimating internal exposures to all elements, especially elements with large fluctuations in plasma concentrations and short biological half-lives. Third, we only measured plasma selenium, manganese, iron, and zinc concentrations. Therefore, we could not determine how toxic metals modify these associations owing to the lack of toxic metal data for the plasma.
Conclusions
Plasma manganese, iron, and zinc levels were significantly associated with CKD in elderly people aged ≥90 years in longevity areas in China. Our findings should be confirmed in prospective cohort studies because this study was cross-sectional and the population size was small.
Supplementary Material
Table 4.
Adjusted odds ratios (95% CI) for CKD according to the combined categories of plasma element levels
| Elements | Odds ratio (95% CI) a | p-Interaction |
|---|---|---|
| Manganese-Iron | ||
| Low Mn + Low Fe | 1 | 0.69 |
| High Mn + Low Fe | 1.19 (0.69–2.07) | |
| Low Mn + High Fe | 0.47 (0.26–0.82) | |
| High Mn + High Fe | 0.48 (0.28–0.80) | |
| Zinc-Iron | ||
| Low Zn + Low Fe | 1 | 0.87 |
| High Zn + Low Fe | 0.59 (0.34–1.01) | |
| Low Zn + High Fe | 0.39 (0.22–0.69) | |
| High Zn + High Fe | 0.24 (0.13–0.42) | |
| Zinc-Manganese | ||
| Low Zn + Low Mn | 1 | 0.65 |
| High Zn + Low Mn | 0.66 (0.37–1.17) | |
| Low Zn + High Mn | 1.24 (0.70–2.18) | |
| High Zn + High Mn | 0.68 (0.40–1.16) |
Note: Plasma elements which were significant in multiple-models were included in the combined effect analysis. Mn, Manganese; Fe, Iron; Zn, Zinc.
The combined categories of elements levels (Low Mn<28.80 μg/L, High Mn≥28.80 μg/L; Low Fe<3818.90 μg/L, High Fe≥3818.90 μg/L; Low Zn<2071.06 μg/L, High Zn≥2071.06 μg/L) and adjusted variables (age, sex, BMI, education and county/city) were included in the multivariable-adjusted model.
The participants in this cross-sectional study were from the fifth wave of the Chinese Longitudinal Healthy Longevity Study (CLHLS) conducted in 2009.
The associations between plasma elements and CKD were examined.
Plasma manganese, iron, and zinc levels were significantly and negatively associated with CKD.
Acknowledgments
The study was funded by the National Natural Science Foundation of China (81273160, 71233001, and 71490732), the United Nations Fund for Population Activities, and the National Institute of Aging (2P01AG031719).
Footnotes
We declare that we have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Best PJ, Holmes DR Jr., 2003. Chronic kidney disease as a cardiovascular risk factor. Am. Heart. J. 145, 383–386. [DOI] [PubMed] [Google Scholar]
- Couser WG, Remuzzi G, Mendis S, Tonelli M, 2011. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney. Int. 80, 1258–1270. [DOI] [PubMed] [Google Scholar]
- Del Greco MF, Foco L, Pichler I, Eller P, Eller K, Benyamin B, Whitfield JB, Genetics of Iron Status, C., Consortium CK, Pramstaller PP, Thompson JR, Pattaro C, Minelli C, 2017. Serum iron level and kidney function: a Mendelian randomization study. Nephrol. Dial. Transplant. 32, 273–278. [DOI] [PubMed] [Google Scholar]
- Diyabalanage S, Fonseka S, Dasanayake D, Chandrajith R, 2017. Environmental exposures of trace elements assessed using keratinized matrices from patients with chronic kidney diseases of uncertain etiology (CKDu) in Sri Lanka. J. Trace Elem. Med. Biol. 39, 62–70. [DOI] [PubMed] [Google Scholar]
- Gonzalez Rico M, Puchades MJ, Garcia Ramon R, Saez G, Tormos MC, Miguel A, 2006. [Effect of oxidative stress in patients with chronic renal failure]. Nefrologia. 26, 218–225. [PubMed] [Google Scholar]
- Hingorani S, Molitoris BA, Himmelfarb J, 2009. Ironing out the pathogenesis of acute kidney injury. Am. J. Kidney Dis. 53, 569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingsathit A, Thakkinstian A, Chaiprasert A, Sangthawan P, Gojaseni P, Kiattisunthorn K, Ongaiyooth L, Vanavanan S, Sirivongs D, Thirakhupt P, Mittal B, Singh AK, Thai SG, 2010. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol. Dial. Transplant. 25, 1567–1575. [DOI] [PubMed] [Google Scholar]
- Kim M, Koh ES, Chung S, Chang YS, Shin SJ, 2017. Altered Metabolism of Blood Manganese Is Associated with Low Levels of Hemoglobin in Patients with Chronic Kidney Disease. Nutrients 91177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaf DE, Swinkels DW, 2016. Catalytic iron and acute kidney injury. Am. J. Physiol. Renal Physiol. 311, F871–F876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY, 2006. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J. Am. Soc. Nephrol. 17, 2937–2944. [DOI] [PubMed] [Google Scholar]
- Mafra D, Cuppari L, Favaro DI, Cozzolino SM, 2004. Zinc levels after iron supplementation in patients with chronic kidney disease. J. Ren Nutr. 14, 164–169. [DOI] [PubMed] [Google Scholar]
- Nascimento S, Baierle M, Goethel G, Barth A, Brucker N, Charao M, Sauer E, Gauer B, Arbo MD, Altknecht L, Jager M, Dias AC, de Salles JF, Saint’ Pierre T, Gioda A, Moresco R, Garcia SC, 2016. Associations among environmental exposure to manganese, neuropsychological performance, oxidative damage and kidney biomarkers in children. Environ. Res. 147, 32–43. [DOI] [PubMed] [Google Scholar]
- Qin HB, Zhu JM, Liang L, Wang MS, Su H, 2013. The bioavailability of selenium and risk assessment for human selenium poisoning in high-Se areas, China. Environ. Int. 52, 66–74. [DOI] [PubMed] [Google Scholar]
- Rostoker G, Griuncelli M, Loridon C, Couprie R, Benmaadi A, Bounhiol C, Roy M, Machado G, Janklewicz P, Drahi G, Dahan H, Cohen Y, 2012. Hemodialysis-associated hemosiderosis in the era of erythropoiesis-stimulating agents: a MRI study. Am. J. Med. 125, 991–999. [DOI] [PubMed] [Google Scholar]
- Shah SV, 2004. Oxidants and iron in chronic kidney disease. Kidney. Int. S91, S50–S55. [DOI] [PubMed] [Google Scholar]
- Shih C-T, Shiu Y-L, Chen C-A, Lin H-Y, Huang Y-L, Lin C-C, 2012. Changes in levels of copper, iron, zinc, and selenium in patients at different stages of chronic kidney disease. Genomic Medicine, Biomarkers, and Health Sciences. 4, 128–130. [Google Scholar]
- Tapiero H, Tew KD, 2003. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed. Pharmacother. 57, 399–411. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX, 2006. Chronic kidney disease and mortality risk: a systematic review. J. Am. Soc. Nephrol. 17, 2034–2047. [DOI] [PubMed] [Google Scholar]
- Walter PB, Knutson MD, Paler-Martinez A, Lee S, Xu Y, Viteri FE, Ames BN, 2002. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc. Natl. Acad. Sci. U S A. 99, 2264–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Yi X, Guo J, Xu S, Xiao Y, Huang X, Duan Y, Luo D, Xiao S, Huang Z, Yuan H, He M, Shen M, Chen X, 2019. Association of plasma and urine metals levels with kidney function: A population-based cross-sectional study in China. Chemosphere. 226, 321–328. [DOI] [PubMed] [Google Scholar]
- Zachara BA, 2015. Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv. Clin Chem. 68, 131–151. [DOI] [PubMed] [Google Scholar]
- Zeng Y, 2012. Towards Deeper Research and Better Policy for Healthy Aging --Using the Unique Data of Chinese Longitudinal Healthy Longevity Survey. China. Economic. J. 5, 131–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Mauzerall DL, Zhu T, Liang S, Ezzati M, Remais JV, 2010. Environmental health in China: progress towards clean air and safe water. Lancet. 375, 1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H, 2012. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 379, 815–822. [DOI] [PubMed] [Google Scholar]
- Zidenberg-Cherr S, Keen CL, Lonnerdal B, Hurley LS, 1983. Superoxide dismutase activity and lipid peroxidation in the rat: developmental correlations affected by manganese deficiency. J. Nutr. 113, 2498–2504 [DOI] [PubMed] [Google Scholar]
- Best PJ, Holmes DR Jr., 2003. Chronic kidney disease as a cardiovascular risk factor. Am Heart J 145, 383–386. [DOI] [PubMed] [Google Scholar]
- Couser WG, Remuzzi G, Mendis S, Tonelli M, 2011. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80, 1258–1270. [DOI] [PubMed] [Google Scholar]
- Del Greco MF, Foco L, Pichler I, Eller P, Eller K, Benyamin B, Whitfield JB, Genetics of Iron Status, C., Consortium CK, Pramstaller PP, Thompson JR, Pattaro C, Minelli C, 2017. Serum iron level and kidney function: a Mendelian randomization study. Nephrol Dial Transplant 32, 273–278. [DOI] [PubMed] [Google Scholar]
- Diyabalanage S, Fonseka S, Dasanayake D, Chandrajith R, 2017. Environmental exposures of trace elements assessed using keratinized matrices from patients with chronic kidney diseases of uncertain etiology (CKDu) in Sri Lanka. J Trace Elem Med Biol 39, 62–70. [DOI] [PubMed] [Google Scholar]
- Gonzalez Rico M, Puchades MJ, Garcia Ramon R, Saez G, Tormos MC, Miguel A, 2006. [Effect of oxidative stress in patients with chronic renal failure]. Nefrologia 26, 218–225. [PubMed] [Google Scholar]
- Hingorani S, Molitoris BA, Himmelfarb J, 2009. Ironing out the pathogenesis of acute kidney injury. Am J Kidney Dis 53, 569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingsathit A, Thakkinstian A, Chaiprasert A, Sangthawan P, Gojaseni P, Kiattisunthorn K, Ongaiyooth L, Vanavanan S, Sirivongs D, Thirakhupt P, Mittal B, Singh AK, Thai SG, 2010. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol Dial Transplant 25, 1567–1575. [DOI] [PubMed] [Google Scholar]
- Kim M, Koh ES, Chung S, Chang YS, Shin SJ, 2017. Altered Metabolism of Blood Manganese Is Associated with Low Levels of Hemoglobin in Patients with Chronic Kidney Disease. Nutrients 9,1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaf DE, Swinkels DW, 2016. Catalytic iron and acute kidney injury. Am J Physiol Renal Physiol 311, F871–F876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY, 2006. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17, 2937–2944. [DOI] [PubMed] [Google Scholar]
- Mafra D, Cuppari L, Favaro DI, Cozzolino SM, 2004. Zinc levels after iron supplementation in patients with chronic kidney disease. J Ren Nutr 14, 164–169. [DOI] [PubMed] [Google Scholar]
- Nascimento S, Baierle M, Goethel G, Barth A, Brucker N, Charao M, Sauer E, Gauer B, Arbo MD, Altknecht L, Jager M, Dias AC, de Salles JF, Saint’ Pierre T, Gioda A, Moresco R, Garcia SC, 2016. Associations among environmental exposure to manganese, neuropsychological performance, oxidative damage and kidney biomarkers in children. Environ Res 147, 32–43. [DOI] [PubMed] [Google Scholar]
- Qin HB, Zhu JM, Liang L, Wang MS, Su H, 2013. The bioavailability of selenium and risk assessment for human selenium poisoning in high-Se areas, China. Environ Int 52, 66–74. [DOI] [PubMed] [Google Scholar]
- Rostoker G, Griuncelli M, Loridon C, Couprie R, Benmaadi A, Bounhiol C, Roy M, Machado G, Janklewicz P, Drahi G, Dahan H, Cohen Y, 2012. Hemodialysis-associated hemosiderosis in the era of erythropoiesis-stimulating agents: a MRI study. Am J Med 125, 991–999. [DOI] [PubMed] [Google Scholar]
- Shah SV, 2004. Oxidants and iron in chronic kidney disease. Kidney Int Suppl, S50–55. [DOI] [PubMed] [Google Scholar]
- Shih C-T, Shiu Y-L, Chen C-A, Lin H-Y, Huang Y-L, Lin C-C, 2012. Changes in levels of copper, iron, zinc, and selenium in patients at different stages of chronic kidney disease. Genomic Medicine, Biomarkers, and Health Sciences 4, 128–130. [Google Scholar]
- Tapiero H, Tew KD, 2003. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother 57, 399–411. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX, 2006. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 17, 2034–2047. [DOI] [PubMed] [Google Scholar]
- Walter PB, Knutson MD, Paler-Martinez A, Lee S, Xu Y, Viteri FE, Ames BN, 2002. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc Natl Acad Sci U S A 99, 2264–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Yi X, Guo J, Xu S, Xiao Y, Huang X, Duan Y, Luo D, Xiao S, Huang Z, Yuan H, He M, Shen M, Chen X, 2019. Association of plasma and urine metals levels with kidney function: A population-based cross-sectional study in China. Chemosphere 226, 321–328. [DOI] [PubMed] [Google Scholar]
- Zachara BA, 2015. Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv Clin Chem 68, 131–151. [DOI] [PubMed] [Google Scholar]
- Zeng Y, 2012. Towards Deeper Research and Better Policy for Healthy Aging --Using the Unique Data of Chinese Longitudinal Healthy Longevity Survey. China Economic J 5, 131–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Mauzerall DL, Zhu T, Liang S, Ezzati M, Remais JV, 2010. Environmental health in China: progress towards clean air and safe water. Lancet 375, 1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H, 2012. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379, 815–822. [DOI] [PubMed] [Google Scholar]
- Zidenberg-Cherr S, Keen CL, Lonnerdal B, Hurley LS, 1983. Superoxide dismutase activity and lipid peroxidation in the rat: developmental correlations affected by manganese deficiency. J Nutr 113, 2498–2504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.