Abstract
Background
COPD is a well-known risk factor for lung cancer, independent of smoking behavior. By investigating the retrospective National Health Insurance Service-National Sample Cohort (NHIS-NSC) in Korea, this study attempted to prove the hypothesis that COPD is a risk factor for major cancers developing outside of the lungs. We also aimed to investigate the environmental factors associated with the development of lung cancer in COPD patients.
Methods
This study analyzed data from the NHIS-NSC over a 12-year period. Among the 514,795 subjects in the NHIS-NSC, 16,757 patients who were diagnosed with any cancer from 2002 to 2003 were excluded. This cohort enrolled six arms consisting of never-smokers without COPD (N = 313,553), former smokers without COPD (N = 41,359), smokers without COPD (N = 112,627), never-smokers with COPD (N = 7789), former smokers with COPD (N = 1085), and smokers with COPD (N = 2677).
Results
Incident rate of lung cancer per 100,000 person-year was higher according to smoking and COPD (216 in non-COPD and 757 in COPD among never-smokers, 271 in non-COPD and 1266 in COPD among former smokers, 394 in non-COPD and 1560 in COPD among smokers, p < 0.01). Old age, male sex, lower BMI, low exercise level, history of diabetes mellitus, smoking, and COPD were independent factors associated with the development of lung cancer (p < 0.01). Multi-variable analyses showed that COPD, regardless of smoking status, contributed to the development of lung cancer, and colorectal cancer and liver cancer among other major cancers (p < 0.01).
Conclusion
Our data suggested that COPD was an independent risk factor for the development of lung cancer, and colorectal cancer and liver cancer among other major cancers in the Korean population, regardless of smoking status.
Keywords: COPD, Cancer, Smoking
Background
Chronic obstructive pulmonary disease (COPD) outpaces other major diseases as a cause of mortality throughout the entire world and is expected to rank third among all causes of death by 2020 [1–3].
Many co-morbidities accompanying COPD influence the major outcomes of COPD [2, 4, 5]. Interestingly, COPD is a well-known risk factor for the development of lung cancer, independent of smoking behavior [6–8]. Therefore, many studies have evaluated the relationship between COPD and lung cancer [6–9]. The pathologic mechanism contributing to the development of lung cancer in COPD has been explained by telomere shortening, chronic inflammation, the increased expression of growth factors in COPD lungs, and oxidant-induced DNA damage resulting in mutations and carcinogenesis [8, 10, 11]. However, it has not been reported yet whether COPD can be a risk factor for cancers developing outside of the lungs despite the evidence that systemic inflammation is the characteristic feature of COPD [2, 5]. In addition to smoking, radiation, exposure to carcinogens, such as asbestos and radon, air pollution, etc., are risk factors for the development of lung cancer [12, 13]. Nevertheless, most environmental risk factors for lung cancer, except for smoking exposure, require further validation.
Therefore, the authors hypothesized that the process of tumorigenesis in COPD may not be limited to the lungs but can also affect the body systemically, leading to the development of major cancers outside the lungs. Hence, this study attempted to prove the hypothesis that COPD is a risk factor for, not only lung cancer but also other major cancers by investigating a cohort in the National Health Insurance Service–National Sample Cohort (NHIS-NSC). The current study also further sought to examine the environmental factors associated with the development of lung cancer in COPD patients.
Methods
Study population
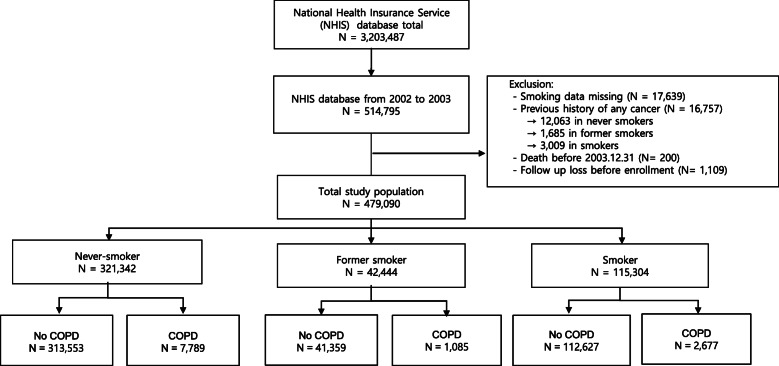
This study analyzed data from the National Health Insurance Service-National Sample Cohort (NHIS-NSC). The NHIS-NSC is a population-based cohort established by the National Health Insurance Service (NHIS) in South Korea [14]. The NHIS is a single-payer health insurance system in South Korea that covers the entire South Korean population (approximately 48 million in 2003). The NHIS provides biennial health screening to all insured adults aged 40 years or older. The NHIS-NCS database consists of 514,795 participants who were aged between 40 and 79 in 2002 and underwent health screening programs in 2002 or 2003; 2002 for participants born in an even year and 2003 for participants born in an odd year (Fig. 1).
Fig. 1.
Flow diagram of this study
After excluding 16,757 patients (12,063 never smokers, 1685 former smokers, and 3009 smokers) with previous histories of cancer diagnoses before January 1, 2004, classified by the International Classification of Diseases 10th revision (ICD-10) codes for cancer diagnoses or questionnaires on previous medical history, 321,342 never-smokers, 42,444 former smokers, and 115,304 smokers were included in the final study cohort (Fig. 1). Finally, the cohort consisted of six arms, never-smokers without COPD (N = 313,553), former smokers without COPD (N = 41,359), smokers without COPD (N = 112,627), never-smokers with COPD (N = 7789), former smokers with COPD (N = 1085), and smokers with COPD (N = 2677) (Fig. 1). We investigated the development of new cancers, including lung cancer, stomach cancer, colorectal cancer, liver cancer, acute myeloid leukemia (AML), esophageal cancer, bladder cancer, and kidney cancer for a 12-year period from January 1, 2004, to December 31, 2015 (Figs. 1 and 2).
Fig. 2.
Study design
Definition and covariates
COPD was identified by the combination of J43-J44 ICD-10 codes for COPD and use of the following medications for COPD (≥ 4 times in 2 years): long-acting muscarinic antagonists (LAMA), long-acting beta-2 agonists (LABA), LAMA + LABA, inhaled corticosteroids (ICS) + LABA, triple therapy (LAMA + LABA + ICS), short-acting muscarinic antagonists (SAMA), short-acting beta-2 agonists (SABA), phosphodiesterase-4 (PDE-4) inhibitors, mucolytics, or theophylline [15, 16].
Detailed history of smoking (smoking amount, duration, and non-smoking period) and exercise status (intensity and frequency per week) were evaluated by self-administered questionnaires at baseline in 2002 or 2003. Former smokers were defined as smokers whose smoking cessation period was 1 year or more at enrollment [17]. Data on body mass index (body weight in kilograms divided by height in meters squared; kg/m2), systolic and diastolic blood pressure, fasting serum glucose, and fasting total cholesterol level measured at baseline were obtained.
Main outcome measures
The primary outcome was the incidence of lung cancer. The secondary outcomes were the incidence of stomach cancer, colorectal cancer, liver cancer, AML, esophageal cancer, bladder cancer, and kidney cancer.
The outcome measures were ascertained by health insurance claims data in the NHIS from January 1, 2004, to December 31, 2015. The first incident event was only used in the analyses for participants with more than one event. ICD-10 codes were used to identify outcomes as follows: lung cancer (C33, C34), stomach cancer (C16), colorectal cancer (C18 - C20), liver cancer (C22), AML (C92.0), esophageal cancer (C15), bladder cancer (C67), and kidney cancer (C64 - C66).
Statistical analyses
All values were expressed as mean ± standard deviation. Continuous and categorical variables were analyzed with one-way analyses of variance (ANOVA) and chi-square tests, respectively. Univariate Cox proportional hazards regression analyses were used to identify significant variables predicting the occurrence of an event individually (p < 0.01). Then, multivariable Cox proportional hazard models were performed to evaluate the independent effects of COPD on the development of cancer, adjusting for age, gender, hypertension, diabetes, body mass index, and exercise variables. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated for the risk of lung cancer, stomach cancer, colorectal cancer, liver cancer, AML, esophageal cancer, bladder cancer, and kidney cancer.
A conservative threshold of p < 0.01 was determined to be significant, considering a large sample size of this study [18]. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Ethics statement
Informed written consent was obtained from all participants and the present study was approved by the Institutional Review Board of Ajou University Hospital (No. AJIRB-MED-EXP-17-167).
Results
Baseline characteristics of this cohort
The baseline characteristics of the NHIS-NSC participants are shown in Table 1.
Table 1.
Baseline characteristics of the cohort
| Never smoker | Former smoker | Smoker | |||||
|---|---|---|---|---|---|---|---|
| No COPD | COPD | No COPD | COPD | No COPD | COPD | P | |
| Number | 313,553 (65.45) | 7789 (1.63) | 41,359 (8.63) | 1085 (0.23) | 112,627 (23.51) | 2677 (0.56) | |
| Age (years) | 53.64 ± 9.62 | 62.23 ± 9.87 | 51.65 ± 9.12 | 62.43 ± 10.16 | 50.92 ± 8.74 | 61.29 ± 9.83 | < 0.001 |
| Gender (male), N (%) | 107,393 (34.25) | 2799 (35.94) | 39,292 (95.00) | 1005 (92.63) | 106,695 (94.73) | 2368 (88.46) | < 0.001 |
| BMI (kg/m2) | 24.08 ± 3.01 | 24.02 ± 3.39 | 24.31 ± 2.73 | 23.18 ± 3.05 | 23.73 ± 2.94 | 22.97 ± 3.28 | < 0.001 |
| < 20 | 23,181 (7.39) | 847 (10.87) | 2205 (5.33) | 157 (14.47) | 11,102 (9.86) | 514 (19.20) | < 0.001 |
| 20 ≤ < 25 | 178,681 (56.99) | 4071 (52.27) | 22,958 (55.51) | 642 (59.17) | 65,174 (57.87) | 1466 (54.76) | < 0.001 |
| 25 ≤ < 30 | 101,536 (32.38) | 2556 (32.82) | 15,247 (36.87) | 265 (24.42) | 34,045 (30.23) | 633 (23.65) | < 0.001 |
| 30 ≤ | 10,155 (3.24) | 315 (4.04) | 949 (2.29) | 21 (1.94) | 2306 (2.05) | 64 (2.39) | < 0.001 |
| Systolic blood pressure (mmHg) | 126.71 ± 18.55 | 130.61 ± 19.21 | 128.56 ± 17.16 | 130.07 ± 18.70 | 127.71 ± 17.55 | 128.88 ± 18.59 | < 0.001 |
| Diastolic blood pressure (mmHg) | 97.28 ± 33.29 | 100.50 ± 38.18 | 99.56 ± 32.21 | 101.26 ± 44.08 | 100.40 ± 37.72 | 101.86 ± 34.78 | < 0.001 |
| Fasting serum glucose (mg/dL) | 200.88 ± 38.50 | 201.56 ± 39.47 | 201.17 ± 37.52 | 196.79 ± 42.59 | 199.10 ± 38.39 | 195.80 ± 42.63 | < 0.001 |
| Total cholesterol (mg/dL) | 313,553 (65.45) | 7789 (1.63) | 41,359 (8.63) | 1085 (0.23) | 112,627 (23.51) | 2677 (0.56) | < 0.001 |
| Exercise level | |||||||
| Non exercise | 187,422(60.81) | 5387 (70.08) | 16,159 (39.74) | 619 (58.18) | 59,485 (52.97) | 1848 (69.42) | < 0.001 |
| Once or twice a week | 63,023 (20.45) | 1078 (14.02) | 13,986 (34.40) | 239 (22.46) | 33,756 (30.06) | 450 (16.90) | |
| At least three times a week | 57,778 (18.75) | 1225 (15.90) | 10,517 (25.86) | 206 (19.36) | 19,063 (16.97) | 364 (13.67) | |
| Incident rate of lung cancer per 100,000 person year: average of six arms = 278 | 216 | 757 | 271 | 1266 | 394 | 1560 | < 0.001 |
| Prevalence of cancer for 12 years, N (%) | |||||||
| Lung cancer (C33-C34) | 7669 (2.44) | 609 (7.82) | 1263 (3.05) | 129 (11.89) | 4899 (4.35) | 398 (14.87) | < 0.001 |
| Stomach cancer (C16) | 7979 (2.54) | 310 (3.98) | 1464 (3.54) | 64 (5.90) | 4170 (3.70) | 143 (5.34) | < 0.001 |
| Colorectal cancer (C18-C20) | 12,126 (3.87) | 505 (6.48) | 1847 (4.47) | 65 (5.99) | 4783 (4.25) | 203 (7.58) | < 0.001 |
| Liver cancer (C22) | 12,681 (4.04) | 446 (5.73) | 2091 (5.06) | 81 (7.47) | 6131 (5.44) | 188 (7.02) | < 0.001 |
| AML (C92.0) | 181 (0.06) | 5 (0.06) | 19 (0.05) | 1 (0.09) | 74 (0.07) | 3 (0.11) | 0.600 |
| Esophageal cancer (C15) | 463 (0.15) | 29 (0.37) | 124 (0.30) | 3 (0.28) | 474 (0.42) | 15 (0.56) | < 0.001 |
| Bladder cancer (C67) | 2367 (0.75) | 100 (1.28) | 426 (1.03) | 18 (1.66) | 1083 (0.96) | 39 (1.46) | < 0.001 |
| Kidney cancer (C64-C66) | 1740 (0.55) | 58 (0.74) | 276 (0.67) | 12 (1.11) | 729 (0.65) | 24 (0.90) | < 0.001 |
| Past medical history, N (%) | |||||||
| Hypertension | 29,436 (9.39) | 1257 (16.14) | 3334 (8.06) | 143 (13.18) | 5974 (5.30) | 282 (10.53) | < 0.001 |
| Diabetes mellitus | 12,904 (4.12) | 539 (6.92) | 1949 (4.71) | 65 (5.99) | 4559 (4.05) | 173 (6.46) | < 0.001 |
Definition of abbreviations: AML Acute myeloid leukemia, BMI Body mass index, COPD Chronic obstructive pulmonary disease
Among 514,795 subjects in the NHIS-NSC database, this study enrolled six arms consisting of never-smokers without COPD (N = 313,553, males = 34.25%), former smokers without COPD (N = 41,359, males = 95.00%), smokers without COPD (N = 112,627, males = 94.73%), never-smokers with COPD (N = 7789, males = 35.94%), former smokers with COPD (N = 1085, males = 92.63%), and smokers with COPD (N = 2677, males = 88.46%) selected by COPD ICD 10 codes, history of COPD medications, and excluding patients with previous histories of any cancer (Fig. 1).
During the 12-year period, lung cancer developed much higher in never-smokers with COPD (7.82%), former smokers with COPD (11.89%), and smokers with COPD (14.87%) than in never-smokers without COPD (2.44%) (p < 0.001). Incident rate of lung cancer per 100,000 person-year was higher according to smoking and COPD (216 in non-COPD and 757 in COPD among never-smokers, 271 in non-COPD and 1266 in COPD among former smokers, 394 in non-COPD and 1560 in COPD among smokers, p < 0.001).
The prevalence of lung cancer, stomach cancer, colorectal cancer, liver cancer, esophageal cancer, bladder cancer, and kidney cancer was higher according to smoking history and COPD diagnosis (p < 0.001).
Risk factors for the development of lung cancer
Univariate cox regression analysis found that older age, male sex, lower BMI, history of hypertension, history of diabetes mellitus, exercise level, COPD diagnosis, and smoking history were associated with the development of lung cancer (p < 0.001) (Table 2). Multi-variable Cox regression analyses showed that older age, male sex, lower BMI, exercise level, COPD diagnosis, and smoking history were independently associated with the development of lung cancer (p < 0.01) (Table 2).
Table 2.
Risk factors for the development of lung cancer
| Cox regression analysis | ||||
|---|---|---|---|---|
| Univariate Hazard ratio (95% CI) | p-value | Multivariate Hazard ratio (95% CI) | p-value | |
| Age (years) | 1.072 (1.070–1.074) | < 0.001 | 1.074 (1.072–1.076) | < 0.001 |
| Male (vs female) | 1.866 (1.803–1.931) | < 0.001 | 1.789 (1.717–1.864) | < 0.001 |
| BMI (kg/m2) | < 0.001 | < 0.001 | ||
| < 20 | 1.661(1.582–1.744) | 1.323 (1.256–1.393) | ||
| 20 ≤ < 25 | Reference | Reference | ||
| 25 ≤ < 30 | 0.852 (0.769–0.945) | 0.885 (0.795–0.985) | ||
| 30 ≤ | 0.446 (0.396–0.501) | 0.837 (0.705–0.994) | ||
| History of hypertension | 1.414(1.344–1.488) | < 0.001 | 0.990(0.939–1.044) | 0.718 |
| History of diabetes mellitus | 1.579(1.477–1.688) | < 0.001 | 1.186(1.108–1.269) | < 0.001 |
| COPD diagnosis | 3.752(3.530–3.988) | < 0.001 | 2.046(1.922–2.177) | < 0.001 |
| Exercise level | < 0.001 | < 0.001 | ||
| Never Exercise | 1.178 (1.129–1.229) | 1.145 (1.095–1.198) | ||
| 1–2 times a week | 0.888 (0.816–0.966) | 1.010 (0.925–1.103) | ||
| ≥ 3 times a week | Reference | Reference | ||
| Smoking status | < 0.001 | < 0.001 | ||
| Never smoker | Reference | Reference | ||
| Former smoker | 1.277(1.205–1.352) | 1.108(1.042–1.178) | ||
| Current smoker | 1.860(1.797–1.926) | 1.689(1.622–1.759) | ||
Definition of abbreviations: BMI Body mass index, COPD Chronic obstructive pulmonary disease, * = statistically significant hazard ratio (p-value < 0.01)
Risk factors for the development of other major cancers including stomach cancer, colorectal cancer, liver cancer, esophageal cancer, and bladder cancer
Smoking was independently associated with the development of stomach cancer, colorectal cancer, liver cancer, esophageal cancer, and bladder cancer, and exercise level was independently associated with the development of stomach cancer and liver cancer (p < 0.01) (Supplement table 1, 2, 3, 4 and 5).
COPD as a risk factor for the development of major cancers
Multi-variable cox regression analyses were performed in three models: Model 1 (adjusted for age and sex), Model 2 (Model 1 + an additional adjustment for history of hypertension and history of diabetes mellitus), Model 3 (Model 2 + an additional adjustment for BMI and exercise). The multi-variable Cox regression analyses demonstrated that COPD in never-smokers, former smokers, and smokers contributed to the development of lung cancer in all three models (Model 3: COPD in never-smokers, HR = 2.046; COPD in former smokers, HR = 2.267; COPD in smokers, HR = 3.456 (all p-values < 0.001) (Table 3). In addition, COPD in never-smokers, former smokers, and current smokers contributed to the development of colorectal cancer and liver cancer among other major cancers (all p-values < 0.001) (Tables 3 and 4).
Table 3.
COPD as a risk factor for the development of major cancers by multi-variate cox regression analyses
| Hazard Ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Model 1 Hazard ratio (95% CI) | p-value | Model 2 Hazard ratio (95% CI) | p-value | Model 3 Hazard ratio (95% CI) | p-value | |
| Lung cancer | ||||||
| Never smoker without COPD | Reference | Reference | Reference | |||
| Former smoker without COPD | 1.100 (1.035–1.170) | 0.002 | 1.100 (1.035–1.169) | 0.002 | 1.108 (1.043–1.178) | < 0.001 |
| Smoker without COPD | 1.752 (1.683–1.823) | < 0.001 | 1.749 (1.681–1.821) | < 0.001 | 1.689 (1.622–1.759) | < 0.001 |
| Never smoker with COPD | 2.086 (1.960–2.220) | < 0.001 | 2.086 (1.960–2.220) | < 0.001 | 2.046 (1.922–2.177) | < 0.001 |
| Former smoker with COPD | 2.295 (2.105–2.503) | < 0.001 | 2.294 (2.104–2.502) | < 0.001 | 2.267 (2.079–2.472) | 0.001 |
| Current smoker with COPD | 3.654 (3.396–3.932) | < 0.001 | 3.650 (3.392–3.927) | < 0.001 | 3.456 (3.211–3.720) | < 0.001 |
| Stomach cancer | ||||||
| Never smoker without COPD | Reference | Reference | Reference | |||
| Former smoker without COPD | 1.105 (1.042–1.172) | 0.001 | 1.104 (1.041–1.171) | 0.001 | 1.111 (1.048–1.178) | < 0.001 |
| Smoker without COPD | 1.249 (1.198–1.302) | < 0.001 | 1.249 (1.197–1.302) | < 0.001 | 1.289 (1.184–1.289) | < 0.001 |
| Never smoker with COPD | 1.067 (0.976–1.167) | 0.154 | 1.067 (0.976–1.167) | 0.155 | 1.056 (0.966–1.155) | 0.231 |
| Former smoker with COPD | 1.179 (1.060–1.312) | 0.002 | 1.178 (1.060–1.311) | 0.003 | 1.174 (1.055–1.306) | 0.003 |
| Current smoker with COPD | 1.333 (1.208–1.470) | < 0.001 | 1.332 (1.208–1.470) | < 0.001 | 1.305 (1.183–1.440) | < 0.001 |
| Colorectal cancer | ||||||
| Never smoker without COPD | Reference | Reference | Reference | |||
| Former smoker without COPD | 1.045 (0.992–1.102) | 0.096 | 1.043 (0.990–1.099) | 0.113 | 1.046 (0.992–1.102) | 0.095 |
| Smoker without COPD | 1.086 (1.046–1.128) | < 0.001 | 1.089 (1.048–1.131) | < 0.001 | 1.088 (1.047–1.130) | < 0.001 |
| Never smoker with COPD | 1.277 (1.197–1.374) | < 0.001 | 1.277 (1.186–1.374) | < 0.001 | 1.273 (1.183–1.370) | < 0.001 |
| Former smoker with COPD | 1.335 (1.221–1.460) | < 0.001 | 1.332 (1.218–1.456) | < 0.001 | 1.331 (1.217–1.455) | < 0.001 |
| Current smoker with COPD | 1.387 (1.278–1.505) | < 0.001 | 1.390 (1.281–1.509) | < 0.001 | 1.385 (1.276–1.503) | < 0.001 |
| Liver cancer | ||||||
| Never smoker without COPD | Reference | Reference | Reference | |||
| Former smoker without COPD | 1.034 (0.985–1.086) | 0.181 | 1.031 (0.982–1.083) | 0.219 | 1.040 (0.990–1.093) | 0.116 |
| Smoker without COPD | 1.168 (1.128–1.209) | < 0.001 | 1.168 (1.128–1.210) | < 0.001 | 1.162 (1.122–1.204) | < 0.001 |
| Never smoker with COPD | 1.229 (1.139–1.322) | < 0.001 | 1.228 (1.138–1.325) | < 0.001 | 1.217 (1.128–1.313) | < 0.001 |
| Former smoker with COPD | 1.271 (1.162–1.391) | < 0.001 | 1.266 (1.157–1.386) | < 0.001 | 1.266 (1.157–1.386) | < 0.001 |
| Current smoker with COPD | 1.435 (1.321–1.560) | < 0.001 | 1.434 (1.320–1.559) | < 0.001 | 1.415 (1.302–1.537) | < 0.001 |
Model 1: adjusted for age and sex
Model 2: Model 1 + additional adjustment for history of hypertension, and history of diabetes mellitus
Model 3: Model 2 + additional adjustment for BMI and Exercise
Definition of abbreviations: BMI Body mass index, COPD Chronic obstructive pulmonary disease, * = statistically significant hazard ratio (p-value < 0.01)
Table 4.
COPD as a risk factor for the development of other cancers by multi-variate cox regression analyses
| Hazard Ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Model 1 Hazard ratio (95% CI) | p-value | Model 2 Hazard ratio (95% CI) | p-value | Model 3 Hazard ratio (95% CI) | p-value | |
| AML | ||||||
| Never smoker without COPD | Reference | Reference | Reference | |||
| Former smoker without COPD | 0.617 (0.382–0.998) | 0.049 | 0.616 (0.381–0.996) | 0.048 | 0.632 (0.391–1.022) | 0.061 |
| Smoker without COPD | 0.922 (0.684–1.242) | 0.593 | 0.925 (0.686–1.246) | 0.608 | 0.914 (0.678–1.233) | 0.556 |
| Never smoker with COPD | 1.064 (0.544–2.083) | 0.856 | 1.064 (0.544–2.082) | 0.857 | 1.047 (0.535–2.049) | 0.895 |
| Former smoker with COPD | 0.657 (0.289–1.494) | 0.316 | 0.655 (0.288–1.491) | 0.314 | 0.661 (0.291–1.505) | 0.324 |
| Current smoker with COPD | 0.981 (0.473–2.037) | 0.959 | 0.984 (0.474–2.043) | 0.965 | 0.957 (0.460–1.989) | 0.905 |
| Esphageal cancer | ||||||
| Never smoker without COPD | Reference | Reference | Reference | |||
| Former smoker without COPD | 1.303 (1.061–1.600) | 0.012 | 1.303 (1.061–1.599) | 0.012 | 1.304 (1.062–1.601) | 0.011 |
| Smoker without COPD | 2.082(1.810–2.395) | < 0.001 | 2.080 (1.808–2.394) | < 0.001 | 1.968 (1.709–2.267) | < 0.001 |
| Never smoker with COPD | 0.973 (0.722–1.312) | 0.858 | 0.973 (0.722–1.312) | 0.858 | 0.945 (0.700–1.274) | 0.708 |
| Former smoker with COPD | 1.268 (0.885–1.817) | 0.197 | 1.268 (0.884–1.817) | 0.197 | 1.231 (0.859–1.765) | 0.257 |
| Current smoker with COPD | 2.026 (1.460–2.810) | < 0.001 | 2.024 (1.459–2.808) | < 0.001 | 1.859 (1.338–2.583) | < 0.001 |
| Bladder cancer | ||||||
| Never smoker without COPD | Reference | Reference | Reference | |||
| Former smoker without COPD | 1.090 (0.977–1.216) | 0.121 | 1.088 (0.975–1.214) | 0.129 | 1.089 (0.976–1.215) | 0.127 |
| Smoker without COPD | 1.131 (1.044–1.225) | 0.003 | 1.136 (1.049–1.231) | 0.002 | 1.142 (1.053–1.237) | 0.001 |
| Never smoker with COPD | 1.145 (0.973–1.347) | 0.104 | 1.145 (0.973–1.347) | 0.103 | 1.146 (0.974–1.348) | 0.101 |
| Former smoker with COPD | 1.248 (1.027–1.516) | 0.026 | 1.246 (1.026–1.514) | 0.027 | 1.247 (1.027–1.516) | 0.026 |
| Current smoker with COPD | 1.295 (1.081–1.550) | 0.005 | 1.301(1.087–1.558) | 0.004 | 1.308 (1.092–1.566) | 0.004 |
| Kidney cancer | ||||||
| Never smoker without COPD | Reference | Reference | Reference | |||
| Former smoker without COPD | 1.002 (0.875–1.148) | 0.975 | 0.998 (0.871–1.143) | 0.974 | 0.999 (0.873–1.144) | 0.992 |
| Smoker without COPD | 1.054 (0.956–1.163) | 0.288 | 1.066 (0.967–1.176) | 0.198 | 1.075 (0.974–1.185) | 0.152 |
| Never smoker with COPD | 1.076 (0.870–1.331) | 0.499 | 1.076 (0.870–1.330) | 0.501 | 1.077 (0.871–1.332) | 0.493 |
| Former smoker with COPD | 1.079 (0.839–1.386) | 0.555 | 1.073 (0.835–1.379) | 0.581 | 1.076 (0.838–1.383) | 0.565 |
| Current smoker with COPD | 1.135 (0.899–1.432) | 0.287 | 1.147 (0.909–1.447) | 0.247 | 1.157 (0.917–1.461) | 0.219 |
Model 1: adjusted for age and sex
Model 2: Model 1 + additional adjustment for history of hypertension, and history of diabetes mellitus
Model 3: Model 2 + additional adjustment for BMI and Exercise
Definition of abbreviations: AML Acute myeloid leukemia, BMI Body mass index, COPD Chronic obstructive pulmonary disease, * = statistically significant hazard ratio (p-value < 0.01)
Discussion
Our study provides a comprehensive analysis of COPD as a risk factor for major cancers in the Korean population. Our data revealed that COPD in the Korean population was an independent risk factor contributing to the development of lung cancer, and colorectal cancer and liver cancer among other major cancers, irrespective of smoking status. In this analysis of a national cohort representative of the Korean population with up to 12 years of follow-up, multi-variable analysis demonstrated that male gender, lower BMI, history of diabetes mellitus, and exercise level, along with smoking status and COPD diagnosis, were independent risk factors for the development of lung cancer.
Our study presents several interesting findings.
First, our data showed that COPD diagnosis was independently associated with the occurrence of lung cancer by all models analyzed by Cox regression analyses, suggesting that COPD per se contributes to the development of lung cancer, irrespective of smoking behavior. Studies based on Korean National Health and Nutrition Examination Survey also showed that advanced age, male gender, low income, pulmonary tuberculosis, and asthma were independent risk factors for COPD in non-smokers [19, 20]. The incident rate of lung cancer per 100,000 person-year was higher than the previous report on general Korean population [21]. This higher rate of lung cancer could be explained by the average older age of this cohort (higher than 50 years old in all six arms), because of which this cohort cannot represent general Korean population.
The association between COPD and lung cancer has been explained by several mechanisms [22–24]. Repeated injury and repair by chronic inflammation and frequent exacerbations in COPD may result in tissue injury and DNA damage, leading to malignant cell transformation and the development of lung cancer [22]. Multiple genetic factors may explain the link between the development of COPD and lung carcinogenesis [23, 24]. However, there is an opposing perspective that the pathologic processes of COPD and lung cancer appear to be different, since features of COPD include destruction and apoptosis, whereas lung cancer is characterized by unrestrained proliferation and lack of apoptosis [9, 25].
Recent bodies of clinical evidence have suggested that emphysema and severe airflow obstruction increased the risk of lung cancer beyond the effect of smoking [26, 27]. Several pathological mechanisms, including premature aging, genetic predisposition, and epigenetic changes, have been proposed to explain the carcinogenesis in emphysematous lungs [10, 28]. In a clinical trial in a large Veterans Affairs patient cohort, statins were shown to be protective against the development of lung cancer, reducing the incidence of lung cancer over 50% [29].
Second, our data found that the prevalence of stomach cancer, colorectal cancer, and liver cancer was higher according to smoking and COPD diagnoses. Multi-variable analyses demonstrated an independent association between COPD, and colorectal cancer and liver cancer among other major cancers. Theoretically, the spillover of aberrant inflammation in COPD can lead to systemic consequences, such as carcinogenesis in other organs. Our study supports our original hypothesis that COPD is an independent risk factor for the development of some major cancers occurring outside the lungs. A recent study demonstrated the intimate relationship between malignant cells and their inflammatory microenvironment [30]. Inflammation is increased in COPD and experiments with anti-inflammatory treatments, such as Nrf2 activators and statins, showed the potential to inhibit the proliferation of cancer cells by reducing inflammation [31]. To our knowledge, no study has ever reported any link between COPD and major cancers occurring outside the lung. Hence, these findings should be further validated with other big cohorts.
Third, this cohort study identified environmental factors including low exercise level and lower BMI, as independent risk factors contributing to the development of lung cancer, indicating that multidisciplinary approaches are required for the prevention of lung cancer in COPD.
Our finding that a reduced BMI was independently linked to the development of lung cancer contradicts the conventional notion that obesity is pathogenically linked to carcinogenesis [32, 33]. Several studies highlighted an obesity paradox suggesting that obesity may be protective and associated with reduced lung cancer mortality after surgery or chemotherapy [32, 34, 35]. Although the mechanisms behind this paradoxical relationship are not fully understood, anti-tumor adipokines, anti-tumor energy reserve, metabolic fitness, relative lack of sarcopenia, etc., have been suggested as potential biological mechanisms to explain this obesity paradox [34]. Most studies, however, have focused on the mortality of patients with lung cancer after surgery or chemotherapy [32, 34, 35]. Our study is different from previous studies on the prognosis after a lung cancer diagnosis because we investigated the development of cancers in a cancer-free cohort by a longitudinal design [32, 34, 35]. Our findings are supported by previous reports that emphysema characterized by lower BMI was a risk factor for lung cancer, although this study did not examine the phenotype of COPD in our cohort [36, 37].
However, a recent cohort study comparing 433 COPD patients with 279 healthy controls reported that obesity in COPD patients predicted higher lung cancer risk [36]. Therefore, further studies are required to investigate the controversial relationship between obesity and lung cancer.
Our finding that lower exercise levels were an independent risk factor for the development of lung cancer is supported by previous studies [38–40]. Some studies reported an approximate 25% reduction in lung cancer risk by higher levels of physical activities [38, 40]. Good adherence to established nutrition and physical activity through cancer prevention guidelines is recommended since significant reductions in overall cancer incidence and mortality were reported by these methods [39]. Epidemiologic studies have suggested that healthy lifestyle choices should be encouraged since controlling environmental factors, such as diet, BMI, and physical activity, can effectively lower the prevalence of cancers [39, 41]. Further prospectively designed research on environmental factors contributing to cancer development should provide valid scientific data from which to develop appropriate public health strategies.
We acknowledge several limitations of this study. First, defining COPD by pulmonary function test was not possible due to the lack of information. Second, the pathologic type of each cancer was not investigated because of limited information in the NHIS-NSC. Third, disease-specific risk factors were not controlled in some cancers, including infections by hepatitis B and C viruses in liver cancer and emphysema in lung cancer. Fourth, medication history was not taken into consideration in our analysis because the current study was not a randomized controlled trial. Fifth, air pollution and occupation were not investigated as causal factors for cancer development in our analysis. Sixth, some major cancers such as breast cancer and prostate cancer were not included in the analysis because data access was not available due to technological problems. Seventh, selection bias could be inherent in this study because the ratio of non-smoker COPD, women, and never-smokers was higher in this cohort than in general Korean population. The NHIS-NSC includes only those who participated in the health screening program provided by NHIS in South Korea. Hence, the unscreened population of Korea cannot be represented by this cohort.
Conclusion
Our data demonstrated that a COPD diagnosis was an independent risk factor contributing to the development of lung cancer and, colorectal cancer and liver cancer among other major cancers, irrespective of smoking status in the Korean population. This study also suggested that old age, male gender, lower BMI, history of diabetes mellitus, and low exercise level, along with smoking status and COPD diagnosis were independent factors contributing to the development of lung cancer, suggesting that multidisciplinary approaches are required for the prevention of lung cancer in COPD patients.
Supplementary information
Additional file 1. Table 1. Risk factors for the development of stomach cancer. Table 2. Risk factors for the development of colorectal cancer. Table 3. Risk factors for the development of liver cancer. Table 4. Risk factors for the development of esophageal cancer. Table 5. Risk factors for the development of bladder cancer.
Acknowledgements
Not applicable.
Abbreviations
- AML
Acute myeloid leukemia
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- CI
Confidence intervals
- HR
Hazard ratio
- ICS
Inhaled corticosteroid
- NHIS-NSC
National Health Insurance Service–National Sample Cohort
- LABA
Long-acting beta-2 agonist
- LAMA
Long-acting muscarinic antagonist
- PDE-4 inhibitor
Phosphodiesterase-4 inhibitor
- SABA
Short-acting beta-2 agonist
- SAMA
Short-acting muscarinic antagonist
Authors’ contributions
SV Ahn, E Lee, and B Park helped the preparation of this manuscript and equally contributed to this paper as a first author. JH Park coordinated and designed this study, helped the preparation of this manuscript, and is responsible for the integrity of this paper as a corresponding author. B Park, E Lee, SV Ahn, SSoo Sheen, and JH Jung contributed to the analysis of our data. SC Hwang, KJ Park, JE Park, and HS Park contributed to the design of this study and critically reviewed this study. The author(s) read and approved the final manuscript.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C0992).
Availability of data and materials
NHIS-NSC is an open and public data to which any researcher can get access through the website (https://nhiss.nhis.or.kr).
Ethics approval and consent to participate
Informed written consent was obtained from all participants and the present study was approved by the Institutional Review Board of Ajou University Hospital (No. AJIRB-MED-EXP-17-167).
Consent for publication
Not applicable.
Competing interests
All the authors have nothing to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Song Vogue Ahn, Eunyoung Lee and Bumhee Park equally contributed as a first author.
Contributor Information
Song Vogue Ahn, Email: songvogue.ahn@gmail.com.
Eunyoung Lee, Email: eylee@aumc.ac.kr.
Bumhee Park, Email: bhpark@ajou.ac.kr.
Jin Hee Jung, Email: jh.choung91@gmail.com.
Ji Eun Park, Email: petitprince012@aumc.ac.kr.
Seung Soo Sheen, Email: sssheen@ajou.ac.kr.
Kwang Joo Park, Email: parkkj@ajou.ac.kr.
Sung Chul Hwang, Email: schwang@ajou.ac.kr.
Jae Bum Park, Email: jbpark@aumc.ac.kr.
Hae-Sim Park, Email: hspark@ajou.ac.kr.
Joo Hun Park, Email: jhpamc@naver.com, Email: jhpamc@hanmail.net.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12890-020-01194-8.
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global burden of disease study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 3.Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4:502–506. doi: 10.1513/pats.200701-001FM. [DOI] [PubMed] [Google Scholar]
- 4.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, Rutten EP, Op 't Roodt J, Wouters EF, Franssen FM. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:728–735. doi: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]
- 6.de Torres JP, Marin JM, Casanova C, Cote C, Carrizo S, Cordoba-Lanus E, Baz-Davila R, Zulueta JJ, Aguirre-Jaime A, Saetta M, et al. Lung cancer in patients with chronic obstructive pulmonary disease-- incidence and predicting factors. Am J Respir Crit Care Med. 2011;184:913–919. doi: 10.1164/rccm.201103-0430OC. [DOI] [PubMed] [Google Scholar]
- 7.Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34:380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 8.Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med. 2007;176:285–290. doi: 10.1164/rccm.200612-1792OC. [DOI] [PubMed] [Google Scholar]
- 9.Biswas A, Mehta HJ, Folch EE. Chronic obstructive pulmonary disease and lung cancer: inter-relationships. Curr Opin Pulm Med. 2018;24:152–160. [DOI] [PubMed]
- 10.Barnes PJ, Adcock IM. Chronic obstructive pulmonary disease and lung cancer: a lethal association. Am J Respir Crit Care Med. 2011;184:866–867. doi: 10.1164/rccm.201108-1436ED. [DOI] [PubMed] [Google Scholar]
- 11.Caramori G, Adcock IM, Casolari P, Ito K, Jazrawi E, Tsaprouni L, Villetti G, Civelli M, Carnini C, Chung KF, et al. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax. 2011;66:521–527. doi: 10.1136/thx.2010.156448. [DOI] [PubMed] [Google Scholar]
- 12.Tseng CH, Tsuang BJ, Chiang CJ, Ku KC, Tseng JS, Yang TY, Hsu KH, Chen KC, Yu SL, Lee WC, et al. The relationship between air pollution and lung Cancer in nonsmokers in Taiwan. J Thorac Oncol. 2019;14:784–792. doi: 10.1016/j.jtho.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Ruano-Ravina A, Kelsey KT, Fernandez-Villar A, Barros-Dios JM. Action levels for indoor radon: different risks for the same lung carcinogen? Eur Respir J. 2017;50:1701609. doi: 10.1183/13993003.01609-2017. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Lee JH, Kim JA, Rhee CK. Trend of cost and utilization of COPD medication in Korea. Int J Chron Obstruct Pulmon Dis. 2017;12:27–33. doi: 10.2147/COPD.S121687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CH, Kim K, Hyun MK, Jang EJ, Lee NR, Yim JJ. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax. 2013;68:1105–1113. doi: 10.1136/thoraxjnl-2012-203175. [DOI] [PubMed] [Google Scholar]
- 17.Sheen S, Sun JS, Park JH, Oh YM, Ki SK, Kim K, Park SB, Kim BT, Lee M, Jung YJ, et al. Unique features of non-obstructive emphysema and pure airway obstruction. Int J Tuberc Lung Dis. 2014;18:109–116. doi: 10.5588/ijtld.13.0258. [DOI] [PubMed] [Google Scholar]
- 18.Zhu W. P < 0.05, < 0.01, < 0.001, < 0.0001, < 0.00001, < 0.000001, or < 0.0000001. J Sport Health Sci. 2016;5:77–79. doi: 10.1016/j.jshs.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo KH, Kim YS, Sheen SS, Park JH, Hwang YI, Kim SH, Yoon HI, Lim SC, Park JY, Park SJ, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the fourth Korean National Health and nutrition examination survey, 2008. Respirology. 2011;16:659–665. doi: 10.1111/j.1440-1843.2011.01951.x. [DOI] [PubMed] [Google Scholar]
- 20.Myong JP, Yoon HK, Rhee CK, Kim HR, Koo JW. Risk factors for lung function impairment among the general non-smoking Korean population. Int J Tuberc Lung Dis. 2015;19:1019–1026. doi: 10.5588/ijtld.14.0929. [DOI] [PubMed] [Google Scholar]
- 21.Park JY, Jang SH. Epidemiology of lung Cancer in Korea: recent trends. Tuberc Respir Dis (Seoul) 2016;79:58–69. doi: 10.4046/trd.2016.79.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes M, Teixeira AL, Coelho A, Araujo A, Medeiros R. The role of inflammation in lung cancer. Adv Exp Med Biol. 2014;816:1–23. doi: 10.1007/978-3-0348-0837-8_1. [DOI] [PubMed] [Google Scholar]
- 23.Yang P, Sun Z, Krowka MJ, Aubry MC, Bamlet WR, Wampfler JA, Thibodeau SN, Katzmann JA, Allen MS, Midthun DE, et al. Alpha1-antitrypsin deficiency carriers, tobacco smoke, chronic obstructive pulmonary disease, and lung cancer risk. Arch Intern Med. 2008;168:1097–1103. doi: 10.1001/archinte.168.10.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Pottelberge GR, Mestdagh P, Bracke KR, Thas O, van Durme YM, Joos GF, Vandesompele J, Brusselle GG. MicroRNA expression in induced sputum of smokers and patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:898–906. doi: 10.1164/rccm.201002-0304OC. [DOI] [PubMed] [Google Scholar]
- 25.Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer. 2013;13:233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- 26.Maldonado F, Bartholmai BJ, Swensen SJ, Midthun DE, Decker PA, Jett JR. Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest. 2010;138:1295–1302. doi: 10.1378/chest.09-2567. [DOI] [PubMed] [Google Scholar]
- 27.Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data from the first National Health and nutrition examination survey follow-up. Arch Intern Med. 2003;163:1475–1480. doi: 10.1001/archinte.163.12.1475. [DOI] [PubMed] [Google Scholar]
- 28.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 29.Khurana V, Bejjanki HR, Caldito G, Owens MW. Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest. 2007;131:1282–1288. doi: 10.1378/chest.06-0931. [DOI] [PubMed] [Google Scholar]
- 30.Peek RM, Jr, Mohla S, DuBois RN. Inflammation in the genesis and perpetuation of cancer: summary and recommendations from a national cancer institute-sponsored meeting. Cancer Res. 2005;65:8583–8586. doi: 10.1158/0008-5472.CAN-05-1777. [DOI] [PubMed] [Google Scholar]
- 31.Maksimova E, Yie TA, Rom WN. In vitro mechanisms of lovastatin on lung cancer cell lines as a potential chemopreventive agent. Lung. 2008;186:45–54. doi: 10.1007/s00408-007-9053-7. [DOI] [PubMed] [Google Scholar]
- 32.Leung CC, Lam TH, Yew WW, Chan WM, Law WS, Tam CM. Lower lung cancer mortality in obesity. Int J Epidemiol. 2011;40:174–182. doi: 10.1093/ije/dyq134. [DOI] [PubMed] [Google Scholar]
- 33.Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67:128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Liu Y, Shao H, Zheng X. Obesity paradox in lung Cancer prognosis: evolving biological insights and clinical implications. J Thorac Oncol. 2017;12:1478–1488. doi: 10.1016/j.jtho.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Gupta A, Majumder K, Arora N, Mayo HG, Singh PP, Beg MS, Hughes R, Singh S, Johnson DH. Premorbid body mass index and mortality in patients with lung cancer: a systematic review and meta-analysis. Lung Cancer. 2016;102:49–59. doi: 10.1016/j.lungcan.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Husebo GR, Nielsen R, Hardie J, Bakke PS, Lerner L, D'Alessandro-Gabazza C, Gyuris J, Gabazza E, Aukrust P, Eagan T. Risk factors for lung cancer in COPD - results from the Bergen COPD cohort study. Respir Med. 2019;152:81–88. doi: 10.1016/j.rmed.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Mouronte-Roibas C, Leiro-Fernandez V, Fernandez-Villar A, Botana-Rial M, Ramos-Hernandez C, Ruano-Ravina A. COPD, emphysema and the onset of lung cancer. A systematic review. Cancer Lett. 2016;382:240–244. doi: 10.1016/j.canlet.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, et al. Association of Leisure-Time Physical Activity with Risk of 26 types of Cancer in 1.44 million adults. JAMA Intern Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, Jacobs ET. Adherence to diet and physical activity Cancer prevention guidelines and Cancer outcomes: a systematic review. Cancer Epidemiol Biomark Prev. 2016;25:1018–1028. doi: 10.1158/1055-9965.EPI-16-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee IM, Sesso HD, Paffenbarger RS., Jr Physical activity and risk of lung cancer. Int J Epidemiol. 1999;28:620–625. doi: 10.1093/ije/28.4.620. [DOI] [PubMed] [Google Scholar]
- 41.Greenwald P, Dunn BK. Landmarks in the history of cancer epidemiology. Cancer Res. 2009;69:2151–2162. doi: 10.1158/0008-5472.CAN-09-0416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table 1. Risk factors for the development of stomach cancer. Table 2. Risk factors for the development of colorectal cancer. Table 3. Risk factors for the development of liver cancer. Table 4. Risk factors for the development of esophageal cancer. Table 5. Risk factors for the development of bladder cancer.
Data Availability Statement
NHIS-NSC is an open and public data to which any researcher can get access through the website (https://nhiss.nhis.or.kr).