Abstract
Background
Human endothelin-1 (ET-1) gene polymorphism is closely associated with coronary artery disease (CAD). This study aimed to investigate the association of 2 single-nucleotide polymorphisms (SNPs), +138 I/D and Lys198Asn) of the ET-1 gene,with early onset of CAD in Han Chinese patients. We investigated the effects of Lys198Asn polymorphism on ET-1 protein expression upon stimulation with pro-inflammatory factors.
Material/Methods
Genotyping of the 2 SNPs +138 I/D and Lys198Asn was performed in 88 early-onset CAD patients (≤55 years for males; ≤60 years for females) and 52 healthy control participants using a polymerase chain reaction direct sequencing method. The association of the 2 SNPs was analyzed with SPSS 17.0 software. Western blotting was performed to assess the effects of ET-1 polymorphisms on ET-1 protein expression upon tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and lipopolysaccharide (LPS) stimulation in HEK-293T cells.
Results
Fisher’s exact test showed that the T allele (odds ratio [OR]=3.38, P=0.02) and GT/TT genotype (OR=3.76, P=0.02) of the ET-1 gene Lys198Asn were associated with increased early-onset CAD risk. Multivariate logistic regression analysis showed smoking was the single independent variable related to early-onset CAD (P<0.05). An increase of ET-1 protein levels in cells transfected with Asn198 plasmid was seen upon TNF-α or IL-6 stimulation.
Conclusions
T allele frequency in Lys198Asn loci might be associated with the pathogenesis of early-onset CAD. T-variant might contribute to early-onset CAD by upregulating ET-1 expression upon inflammatory cytokines stimulation, and smokers who have the T allele might be vulnerable to CAD in the Chinese population.
MeSH Keywords: Coronary Artery Disease; Cytokines; Endothelin-1; Polymorphism, Single Nucleotide
Background
Coronary artery disease (CAD) is one of the main causes of death and morbidity globally. It is a complex disorder stemming from interactions between multiple genetic and environmental risk factors. A series of studies have demonstrated that risk factors known to increase the likelihood of developing CAD include hypertension, diabetes, obesity, hypercholesterolemia, smoking, and diet [1]. The World Health Organization reported that about 17.9 million people succumbed to cardiovascular diseases in 2016, representing 31% of all deaths worldwide, out of which, 85% are ascribed to heart attack and stroke [2]. In the USA, 15.5 million people suffer from coronary heart disease according to the 2016 Heart Disease and Stroke Statistics update of the American Heart Association, with prevalence increasing with age for both women and men [3]. In China, the age-standardized death rate due to ischemic heart disease per 100 000 people increased by 13.3% from 1990 to 2015, and 95.3% of the disease burden in China has been reported to be due to environmental, behavioral, and metabolic risk factors [4].
Clinical and population-based studies have reported that genetic factors have an important role in CAD and myocardial infarction (MI). Family and parental history of CAD and MI have been recorded as risk factors for occurrence of the disease in offspring; the relative risk of the developing CAD was 8.1% for monozygotic and 3.8% for dizygotic twins [5]. Genome-wide association studies, genomes imputation, expression quantitative trait locus analyses, and interrogation of Encyclopedia of DNA Elements, Roadmap, and other data sets have identified about 60 common single-nucleotide polymorphisms (SNPs) as risk factors; the predicted heritability of CAD is 28% [6]. Many factors associated with early onset of CAD have been identified such as smoking, hypercholesterolemia, type 2 diabetes, hypertension, polymorphisms at the 9p21 locus, and gene variants in the NF-κB pathway such as NFKBIZ [7]. In Chinese patients, APOC4 rs1132899 polymorphism [8], variants in promoter of ADTRP [9], and MIF gene polymorphism [10] have been identified as risk factors for early onset of CAD.
Endothelin (ET) is an important vasoconstrictor mainly produced by vascular endothelial cells. Among the known 3 isoforms, ET-1 has been proven to be instrumental in the pathogenesis of atherosclerosis. ET-1 causes endothelial dysfunction, inflammation, contributes to atherosclerotic plaque formation during CAD, and increases myocardial necrosis and arrhythmogenesis during MI, but could aid in infarct healing and early ventricular remodeling [11]. In Korean patients, ET-1 gene polymorphisms, such as +138delA, G8002A, and Lys198Asn, were found to be related to variant angina [12]. In the Chinese population, polymorphism of TaqI of the ET-1 gene could be related to CAD and C allele might be a marker for susceptibility of CAD [13]. Out of the 5 tagSNPs (rs6458155, rs4145451, rs9369217, rs3087459, and rs2070699) within the ET-1 gene, rs6458155 was shown to be connected to CAD risk in the Chinese Han population [14].
Several variants of the ET-1 gene, such as insertion, transversion, repeated nucleotide polymorphism, and transition, could impact the hereditary risk of CAD have been located, genotyped, and examined. Ten polymorphisms, including insertion/delete +138 (+138/ex1ins/delA) (rs1800997); transition +3660 (Glu106Glu) (rs5369), G(8002)A (rs2071942), rs1476046 polymorphism, rs2071943 polymorphism, and rs9296345 polymorphism; and transversion −1370 (T-1370G) (rs1800541), +5665 (Lys198Asn) (rs5370), G2288T polymorphisms (rs2070699), and −974 C>A (rs3087459) polymorphism have been phenotyped [15]. Although ET-1 has a functional role in the pathogenesis of CAD, have not been many reports on the role of ET-1 gene variants on CAD risk in early onset of CAD in the Chinese population. Therefore, the purpose of this study was to explore the association of 2 SNPs (+138 I/D and Lys198Asn) of the ET-1 gene with early onset of CAD in Han Chinese patients, which could increase the predictability of a coronary event in this population. The effects of Lys198Asn polymorphism on ET-1 gene expression in transfected HEK-293T cells on stimulation with inflammatory markers, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), or lipopolysaccharide (LPS), were also scrutinized.
Material and Methods
Ethics statement
The Ethics Committee of the Fourth Affiliated Hospital of Nanchang University approved this study. All the participants gave written informed consent before the commencement of the study.
Study population
In this case-controlled study comprising of ethnic Han Chinese population of Jiangxi province in southeastern China, a total of 88 early-onset CAD patients (disease onset ≤55 years for males and ≤60 years for females) were consecutively recruited from The Fourth Affiliated Hospital of Nanchang University (Nanchang, China) from May 2013 to May 2017. Fifty-two age-and gender-matched volunteers were recruited simultaneously as the control group. Coronary angiography was initial performed in all participants after enrollment in this study. The diagnostic criteria of CAD were confirmed by coronary angiography and defined as more than 50% luminal stenosis in at least one segment of 3 main vessels of coronary artery. Two cardiologists who were responsible for the assessment of the study angiograms and both cardiologists underwent strict training and complied with the same diagnostic criteria. Participants with a history of heart failure, anemia, neoplasm, liver disease, renal disease, or thyroid disease were excluded.
Clinical data and survey of life habits
A standard mercury sphygmomanometer was used to measure blood pressure (BP) by the same nurse 2 times successively in a gap of 5 minutes in seated participants who had relaxed for ≥5 minutes. Phase I and Phase V Korotkov sounds were recorded as systolic BP (SPB) and diastolic BP (DBP), respectively. Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥90 mmHg and/or by the use of anti-hypertensive medication. Blood samples from a peripheral vein were drawn into disposable plastic vacuum tube after a requested 12-hour fast. Serum total cholesterol (TC) and low-density lipoprotein-cholesterol (LDL-C), as well as fasting blood sugar (FBS), were measured using commercially available kits and analyzed in an ADVIA2400 (Siemens, Germany). Body mass index (BMI), body weight in kilogram divided by squared height in meter, was used as means of determining presence or absence of obesity. Obesity was defined as BMI ≥28 kg/m2. Questionnaire on lifestyle factors was used to assess smoking and alcohol consumption habits.
DNA extraction
Genomic DNA from blood samples was extracted using a DNeasy Blood & Tissue Kits (Qiagen) as per the manufacturer’s instructions. All DNA samples were stored at −20°C until use.
SNPs selection and genotyping
Two SNPs (+138 I/D and Lys198Asn) were genotyped by polymerase chain reaction (PCR) direct sequencing using the ABI-3100 sequencer (Thermo Fisher Scientific). Specific primers were designed based on genomic sequences containing these SNPs obtained from the GenBank DNA database (NCBI) and were commercially synthesized by Sangon Biotech (Shanghai) Co., Ltd. PCR primer sequences are shown in Table 1. The PCR reaction reagents were purchased from MBI Fermentas; 200 ng of genomic DNA was used for the reaction with the following conditions: initial denaturation at 94°C for 9 minutes, followed by denaturation at 94°C at 30 seconds for 35 cycles, annealing at 56°C for 30 seconds and 72°C for 30 seconds, final extension at 72°C for 8 minutes. Before sequencing, amplification products were incubated with shrimp alkaline phosphatase (0.5 units; Amersham-Pharmacia Biotech, Uppsala, Sweden) and exonuclease I (5 units; Amersham-Pharmacia Biotech, Uppsala, Sweden) at 37°C for 30 minutes, followed by heat inactivation at 80°C for 15 minutes. To the PCR-amplified DNA samples, GeneScan™ 120 LIZ® Size Standard and Hi-Di™ Formamide (Applied Biosystems, Foster City, USA) were added as per manufacturer’s instructions, the mixture was incubated at 95°C for 5 minutes, followed by 5 minutes on ice, and then electrophoresis was performed. The results were analyzed using the ABI Prism GeneScan and Genotyper program (Applied Biosystems, Foster City, USA).
Table 1.
Primer sequences used for amplification of the two SNPs of the ET-1 gene.
| Name of the primer | Nucleotide sequences | |
|---|---|---|
| −138insA/delA (rs1800997) | Forward | 5′>TTCTCTCCTGGCAGG<3′ |
| Reverse | 5′> ATCTCAAAGCGATCCTTC<3′ | |
| G198T (rs5370) | Forward | 5′> TCTTTTGCCAAAGGGTGATT <3′ |
| Reverse | 5′> CAGGGTGGAGAGTGCAGAG <3′ |
+138insA/delA (rs1800997): 138 I/D; G198T (rs5370): Lys198Asn.
Plasmid construction
Human expression plasmids for FLAG-tagged ET-1 and Lys198Asn polymorphism were cloned between MluI and BglII sites in the firefly luciferase reporter vector pGL3.0 Basic (Promega) using standard molecular biology techniques. Briefly, 1×105 HEK-293T cells per well were added to 0.5 mL of RPMI 1640 supplemented with 100 U/mL penicillin, 100 ug/mL streptomycin, 2 mM glutamine, 5 uM 2-ME (Sigma-Aldrich), and 10% fetal bovine serum. Cell density was 50–80% confluent on the day of transfection. We added 0.75–1.75 μL of Lipofectamine LTX Reagent (ThermoFisher Scientific) to the DNA sample (0.5 μg of DNA diluted in 100 μL of Reduced Serum Media without serum), mixed gently and incubated for 30 minutes at room temperature. Then 100 μL of the DNA-Lipofectamine complex was added to each well containing cells and mixed gently. The cells were incubated at 37oC in a humidified CO2 incubator for 18–24 hours post-transfection before assaying for transgene expression. Cells were incubated in the presence of 10 ng/mL and 20 ng/mL of each of TNF-α, IL-6, or LPS for an additional 24 hours. Cell lysates were prepared for western blot analysis.
Western blot analysis
Total proteins from cells were lysed using RIPA (radioimmunoprecipitation assay) lysis buffer and concentrations were determined by BCA (bicinchoninic acid) assay method; 20 μg of proteins were separated on 10% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and transferred to the PVDF (polyvinylidene fluoride) membrane followed by blocking with 5% nonfat milk at room temperature. The membrane was incubated overnight with specific human monoclonal FLAG antibody (Sigma, 1: 1000) at 4°C. After washing with PBST (phosphate-buffered saline and Tween), the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (HRP-CST, Beverly, MA, USA) and detected using a SuperSignal™ West Pico PLUS Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA).
Statistical analysis
As quantitative variables, the values of clinical characteristics were expressed as means ± standard deviation (SD) and analyzed using unpaired Student’s t-test. In both patients and controls, the distribution of genotypes for each SNP were tested for deviations from Hardy-Weinberg equilibrium by a chi-square test and analysis was done using Fisher’s exact test. P<0.05 was considered statistically significant. To estimate the risk of association with genotypes, odds ratios (OR) and 95% confidence interval (CI) were used. Chi-square test for association was used for testing difference of the genotype frequencies between normal controls and early-onset CAD patients. Multivariate logistic regression analysis was performed to determine the independent risk factors of CAD. All the statistical analyses were performed using SPSS software 17.0 version (SPSS Inc., Chicago, IL, USA).
Results
The clinico-demographic characteristics of the patients are presented in Table 2. The values of all variables, except for age and sex, had significant differences between the 2 groups (P<0.05). The mean values of BMI, TC, LDL-C, SBP, DBP, and FBS were higher in patients than in controls. Incidence of cigarette smoking was higher in early onset of CAD patients than in healthy controls (P<0.05).
Table 2.
Clinical and demographic characteristics of the study subjects.
| Patients | Control | P-value | |
|---|---|---|---|
| No. of Subject | 88 | 52 | |
| Age (years) | 52.4±7.4 | 51.7±6.9 | 0.554 |
| Sex (Male) | 48 (54.5%) | 29 (55.8%) | 0.875 |
| BMI, kg/m2 | 23.2±3.0 | 21.1±2.5 | <0.001* |
| TC, mmol/L | 5.36±0.77 | 4.91±0.55 | <0.001* |
| LDL-C, mmol/L | 3.25±0.76 | 2.83±0.45 | <0.001* |
| FBS, mmol/L | 5.57±1.73 | 4.95±1.92 | 0.040* |
| Smoking | 53 (60.2%) | 22 (42.3%) | 0.045* |
| SBP, mmHg | 138.5±19.4 | 130.2±10.7 | 0.005* |
| DBP, mmHg | 83.5±9.7 | 78.6±7.8 | 0.003* |
BMI – body mass index; TC – total cholesterol; LDL-C – low-density lipoprotein-cholesterol; FBS – fasting blood sugar; SBP – systolic blood pressure; DBP – diastolic blood pressure.
P<0.05 was considered statistically significant.
Genetic equilibrium testing of the allele frequency distributions at the SNPs +138delA and Lys198Asn of the ET-1 gene were performed. The results were in line with the Hardy-Weinberg equilibrium (P>0.05) between the patients and the control group (Table 3). The genotype frequencies of the 2 SNPs in patients and controls are outlined in Table 4. The genotype frequencies of Lys198Asn in the patients were 76.1% (G/G), 21.6% (G/T), 2.3% (T/T), and in the T allele the frequency was 12.1%. On the contrary, in controls, the G/G, G/T, and T/T genotype frequencies were 92.3%, 7.7%, and 0% respectively with the T allele frequency of 3.9%. When compared between the 2 groups, carrying T allele (OR=3.38, P=0.02) and GT/TT genotype frequencies (OR=3.76, P=0.02) increased in early-onset CAD, which suggested the rise of T allele frequency may be strongly associated with the pathogenesis of early-onset CAD. No significant differences were observed between the groups for frequencies of both genotype and allele in the +l38delA loci. Multivariate logistic regression analysis was performed on parameters such as obesity, smoking, LDL-C, and hypertension to find the association of risk factors with rs5370 polymorphism (T=1, G=2) and revealed that smoking was the single independent variable related to early-onset CAD (P<0.05) (Table 5).
Table 3.
Hardy-Weinberg equilibrium test for the two SNPs.
| Patients (n=88) | Control (n=52) | |||||
|---|---|---|---|---|---|---|
| Genotype (rs5370) | G/G | G/T | T/T | G/G | G/T | T/T |
| Observed value | 67 | 19 | 2 | 47 | 5 | 0 |
| Theoretical value | 68 | 19 | 1 | 47 | 5 | 0 |
| χ2 value | 1.015 | 0.000 | ||||
| P-value | 0.63 | 0.97 | ||||
| Genotype (rs1800997) | 3A/3A | 3A/4A | 4A/4A | 3A/3A | 3A/4A | 4A/4A |
| Observed value | 42 | 37 | 9 | 28 | 19 | 5 |
| Theoretical value | 42 | 37 | 9 | 27 | 21 | 4 |
| χ2 value | 0.000 | 0.916 | ||||
| P-value | 0.91 | 0.72 | ||||
Table 4.
Analyses of SNPs of ET-1 in all subjects.
| SNP1 | n2 | Genotype frequency | Allele frequency | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | P-value3 | OR (95% CI)4 | Allele | P-value5 | OR (95% CI)4 | |||||
| +138insA/delA | 3A/3A | 3A/4A | 4A/4A | 1.28 (0.643–2.540) | 3A | 1.18 (0.69–2.01) | ||||
| Patients | 88 | 0.476 | 0.424 | 0.100 | 0.484 | 0.688 | 0.554 | |||
| Control | 52 | 0.543 | 0.364 | 0.093 | 0.721 | |||||
| G198T | G/G | G/T | T/T | 3.76 (1.21–11.66) | T | 3.38 (1.13–10.16) | ||||
| Patients | 88 | 0.761 | 0.216 | 0.023 | 0.02 | 0.121 | 0.02 | |||
| Control | 52 | 0.923 | 0.077 | 0.000 | 0.039 | |||||
SNP name for genotype in patients and controls.
Number of valid subjects who were successfully genotyped for each of SNP.
Analysis performed by a 2×2 table for each SNP using major homozygotes vs. others in patients and controls.
Reference group (controls) designated with an OR of 1.00.
Analysis performed by a 2×2 table for the number of each allele in cases and controls.
Table 5.
Logistic regression analysis of rs5370 polymorphism and risk factors of coronary heart disease.
| Risk factors | B | SE | Wald | P | QR | 95% CI |
|---|---|---|---|---|---|---|
| Allele rs5370 | 0.608 | 0.267 | 5.237 | 0.021 | 1.838 | 1.090~3.098 |
| Obesity | 0.274 | 0.175 | 2.435 | 0.117 | 1.272 | 0.922~1.834 |
| Smoking | 0.605 | 0.187 | 9.847 | 0.001* | 1.934 | 1.292~3.155 |
| LDL-C | 0.287 | 0.252 | 1.374 | 0.248 | 1.388 | 0.821~2.137 |
| Hypertension | 0.238 | 0.224 | 0.957 | 0.314 | 1.147 | 0.789~2.025 |
Multivariate logistic regression analysis was performed to analyze the study parameters as a whole and establish a regression model. rs5370 allele (T=1, G=2), obesity, smoking, low-density lipoprotein (LDL-C) and hypertension were taken as independent variables, and the dependent variable was the presence or absence of coronary heart disease (yes =1, no =0).
P<0.05 was considered statistically significant.
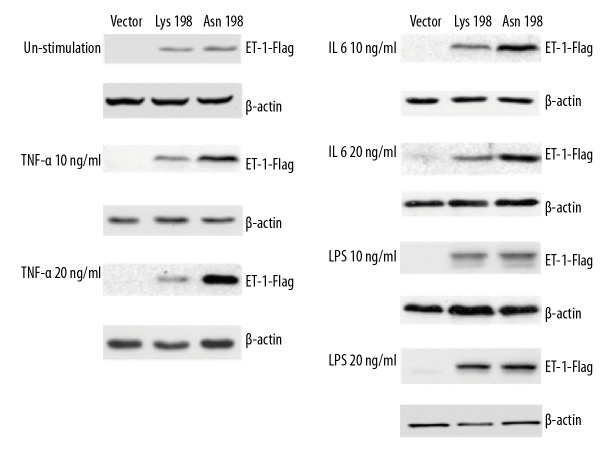
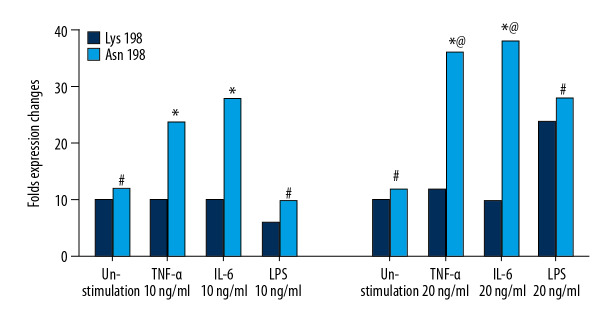
To further investigate the possible mechanisms by which Lys198Asn affects the genetic susceptibility to CAD, protein expression levels of ET-1 were measured in HEK-293T cells transfected either with Lys198 plasmid or Asn198 plasmid (Figures 1, 2). Higher levels of ET-1 were detected in the cells transfected with the plasmids as compared to the cells without ET-1 plasmid transfected vector. No difference in ET-1 protein levels was observed between Lys198 plasmid and Asn198 plasmid transfected cells in the unstimulated state. The expression level of ET-1 protein was higher in cells transfected with Asn198 plasmid compared to that of Lys198 plasmid at both concentrations (10 ng/mL and 20 ng/mL) of TNF-α or IL-6, while such differences were not observed in LPS at either concentration of stimulation. These results suggest that the polymorphism Lys198Lys on ET-1 gene might be functional and have an impact on ET-1 expression upon inflammatory cytokines stimulation, not affected by LPS stimulation.
Figure 1.
Effect of Lys198Asn polymorphism on ET-1 gene expression in transfected HEK-293T cells stimulated with TNF-α, IL-6, or LPS. The values shown below each gel indicate folds of expression changes of ET-1-FLAG normalized against loading controls (β-actin). ET-1 – endothelin-1; TNF – tumor necrosis factor; IL – interleukin; LPS – lipopolysaccharide.
Figure 2.
Relative protein level of Lys198Asn polymorphism on ET-1 gene expression in transfected HEK-293T cells stimulated with TNF-α, IL-6, or LPS. * P<0.05; # P>0.05: Asn198 group versus Lys198 group; @ P<0.05: TNF-α 20 ng/mL group versus TNF-α 10 ng/mL group; IL-6 20 ng/mL group versus IL-6 10 ng/mL group. All values are expressed as the mean±SD, n=3. ET-1 – endothelin-1; TNF – tumor necrosis factor; IL – interleukin; LPS – lipopolysaccharide; SD – standard deviation.
Discussion
In the current study, genetic analysis of the variants of 2 SNPs in the candidate gene ET-1 were evaluated in 2 groups: controls and patients with early-onset of CAD. We found that BMI, TC, LDL-C, SBP, DBP, and FBS were elevated in patients compared to controls and the prevalence of early onset of CAD was higher in smokers than in non-smokers. Also, the T allele frequency of Lys198Asn in patients was increased and smoking was the single independent variable related to early-onset CAD. Furthermore, following stimulation with TNF-α or IL-6, the expression level of ET-1 in HEK-293T cells transfected with Asn198 plasmid was significantly higher than that in cells transfected with Lys198 plasmid.
In our study, one variant was a G-to-T transition in exon 5 that causes the Lys-to-Asn substitution at codon 198(rs5370), which is designated as Lys198Lys, Asn198Asn; another variant was an ‘A’ insertion(I)/deletion(D) in exon-1 at position +138 (rs1800997), which is designated as 3A/3A (wild type/wild type), 3A/4A(wild type/deletion), 4A/4A (mutation/insertion). Both Lys198Asn and +138delA have been examined in earlier studies where Lys198Asn and 3A/4A polymorphisms have been reported to be in linkage disequilibrium [16,17].
Various studies on different ethnic groups have shown that ET-1 Lys198Asn polymorphism and homozygous TT carrier contribute to a higher prevalence of CAD [12,18,19]. On the other hand, some studies surmised that variants in 6 loci in the ET-1 gene did not contribute to early-onset MI in the Spanish population [20]; and ET-1 Lys198Asn genotypes were not related to either ET-1 levels or prevalence of early-onset acute MI in Egyptians [21]. In the present study, it was observed that the T allele frequency in Lys198Asn loci increased in early-onset CAD, however, no significant differences between CAD patients and the control group were observed with respect to the frequencies of both genotype and allele in +l38delA loci. From these results, we may deduce that the increase in T allele frequency in Lys198Asn loci might be associated with the pathogenesis of early-onset CAD corresponding to a previous report [18].
Diabetes mellitus, hypertension, smoking, hyperlipidemia, obesity, homocystinuria, and psychosocial stress are some of the risk factors for CAD [1]. In our study, clinical parameters such as BP, BMI, TC, LDL-C, and FBS, as well as lifestyle factor of smoking showed significant differences among the control group and the patient groups. A recent study showed that a genetic variant associated with CAD and hypertension was a distal regulator of ET-1 gene expression [22]. EDN1 Lys198Asn polymorphism was shown to be related to obesity and blood pressure, and T allele carriers were more sensitive to gain weight than GG homozygotes in association with hypertension [23,24]. Lys198Asn in the ET-1 gene was found to be related to HDL cholesterol and minor allele T (Asn) was associated with lower HDL cholesterol values [25]. In contrast, our results were not in accordance with the aforementioned previous findings. This difference in findings might be because of the lower sample size and the ethnicity of our study group. It is known that interaction of multiple agents from the genome via physiological and biochemical pathways with exposure to environmental factors contributes to the clinical phenotype of an individual and thus causes heterogeneity in causation and prognosis of CVD among various subsets of the population [26]. Moreover, it has been shown that ET-1/Lys198Asn polymorphism is influenced by race and ethnicity related to variations in blood pressure [27]. Hence, it is reasonable for such differences to be noted between our study and previous studies because of different population sets and living and environmental conditions.
As a crucial risk factor for cardiovascular diseases, tobacco-smoking is partially determined by genetic background and is associated with altered epigenetic patterns [28]. Interestingly, we observed that the subset of smokers in early-onset CAD patients carrying the T allele (Asn) genotype was significantly higher than G/G homozygous patients, hinting that smokers containing T allele might be associated with early-onset CAD, which is in line with a previous study on the Chinese population [29].
Since inflammation may engender pathogenesis of CAD [30], we sought to find the expression levels of pro-inflammatory cytokines that control inflammatory cascades. TNF-α and IL-6 participate in several physiological processes and play a key role in inflammation in atherosclerosis [30,31]. To our knowledge, the association during pathogenesis of CAD between the SNP rs5370 and cytokines has not been reported to date. Our data showed that the Asn-variant contributed to an upregulation of ET-1 protein expression upon both TNF-α and IL-6 stimulation, which indicated that the polymorphism Lys198Asn on the ET-1 gene might be functional and have an impact on ET-1 expression with inflammatory cytokines stimulation. The higher expression and secretion of ET-1 might result in higher hereditary susceptibility to CAD in the Chinese population. ET-1 might cause endothelial dysfunction and inflammation leading to atherosclerotic plaque formation [11] since inflammatory factors, such as TNF-α, TGF-β, ILs, insulin, and angiotensin II, upregulate ET-1 mRNA levels [32].
Some limitations of our study were that the sample size was modest, consisting of only one ethnic group. Moreover, we did not explore mechanistic insights in-depth to reach a definitive conclusion related to the effect of cytokines on ET-1 regulated inflammation. Therefore, future studies with large sample size, along with pathway-based studies both in vitro and with patient samples, are warranted to investigate the genotypic effects of ET-1 polymorphism on CAD.
Conclusions
In conclusion, our results showed that the rise of T allele frequency in Lys198Asn loci might be associated with the pathogenesis of early-onset CAD, and T-variant might upregulate ET-1 expression upon the stimulation of both TNF-α and IL-6. In addition, smokers with T allele might be more susceptible to CAD in the Chinese population.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Malakar AK, Choudhury D, Halder B, et al. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234:16812–23. doi: 10.1002/jcp.28350. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Cardiovascular Diseases. https://www.who.int/health-topics/cardiovascular-diseases/
- 3.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4:256. doi: 10.21037/atm.2016.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang G, Yu C, Zhou M, et al. Burden of ischaemic heart disease and attributable risk factors in China from 1990 to 2015: findings from the global burden of disease 2015 study. BMC Cardiovasc Disord. 2018;18:18. doi: 10.1186/s12872-018-0761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai X, Wiernek S, Evans JP, Runge MS. Genetics of coronary artery disease and myocardial infarction. World J Cardiol. 2016;8:1–23. doi: 10.4330/wjc.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPherson R, Tybjaerg-Hansen A. Genetics of coronary artery disease. Circ Res. 2016;118:564–78. doi: 10.1161/CIRCRESAHA.115.306566. [DOI] [PubMed] [Google Scholar]
- 7.Coto E, Reguero JR, Avanzas P, et al. Gene variants in the NF-κB pathway (NFKB1, NFKBIA, NFKBIZ) and risk for early-onset coronary artery disease. Immunol Lett. 2019;208:39–43. doi: 10.1016/j.imlet.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Xu S, Cheng J, Li NH, et al. The association of APOC4 polymorphisms with premature coronary artery disease in a Chinese Han population. Lipids Health Dis. 2015;14:63. doi: 10.1186/s12944-015-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang EW, Peng LY, Zheng JX, et al. Common variants in promoter of ADTRP associate with early-onset coronary artery disease in a southern Han Chinese Population. PLoS One. 2015;10:e0137547. doi: 10.1371/journal.pone.0137547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo JY, Xu R, Li XM, et al. MIF gene polymorphism rs755622 is associated with coronary artery disease and severity of coronary lesions in a Chinese Kazakh population: A case-control study. Medicine (Baltimore) 2016;95:e2617. doi: 10.1097/MD.0000000000002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolettis TM, Barton M, Langleben D, Matsumura Y. Endothelin in coronary artery disease and myocardial infarction. Cardiol Rev. 2013;21:249–56. doi: 10.1097/CRD.0b013e318283f65a. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Cheong SS, Kim J. Association of endothelin-1 gene polymorphisms with variant angina in Korean patients. Clin Chem Lab Med. 2008;46:1575–80. doi: 10.1515/CCLM.2008.313. [DOI] [PubMed] [Google Scholar]
- 13.Jin S, Guo X, Wang M, Ji X. [Association between polymorphism of TaqI of ET-1 gene and coronary heart disease]. Molecular Cardiology of China. 2007:208–13. [in Chinese] [Google Scholar]
- 14.Liang LL, Chen L, Zhou MY, et al. Genetic susceptibility of five tagSNPs in the endothelin-1 (EDN1) gene to coronary artery disease in a Chinese Han population. Biosci Rep. 2018;38(5) doi: 10.1042/BSR20171320. pii: BSR20171320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed M, Rghigh A. Polymorphism in endothelin-1 gene: An overview. Curr Clin Pharmacol. 2016;11:191–210. doi: 10.2174/1574884711666160701000900. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka C, Kamide K, Takiuchi S, et al. Evaluation of the Lys198Asn and -134delA genetic polymorphisms of the endothelin-1 gene. Hypertens Res. 2004;27:367–71. doi: 10.1291/hypres.27.367. [DOI] [PubMed] [Google Scholar]
- 17.Fang Z, Li M, Ma Z, Tu G. Association of endothelin-1 gene polymorphisms with essential hypertension in a Chinese population. Genet Mol Res. 2017;16(3) doi: 10.4238/gmr16037446. [DOI] [PubMed] [Google Scholar]
- 18.Popov AF, Schulz EG, Hind J, et al. Impact of endothelin-1 Lys198Asn polymorphism on coronary artery disease and endogean damage in hypertensives. Cordon Artery Dis. 2008;19:429–34. doi: 10.1097/MCA.0b013e32830936e5. [DOI] [PubMed] [Google Scholar]
- 19.Vargas-Alarcon G, Vallejo M, Posadas-Romero C, et al. The −974C>A (rs3087459) gene polymorphism in the endothelin gene (EDN1) is associated with risk of developing acute coronary syndrome in Mexican patients. Gene. 2014;542:258–62. doi: 10.1016/j.gene.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Palacin M, Rodriguez-Pascual F, Reguero JR, et al. Lack of association between endothelin-1 gene variants and myocardial infarction. J Atheroscler Thromb. 2009;16:388–95. doi: 10.5551/jat.no1149. [DOI] [PubMed] [Google Scholar]
- 21.Abdel Rahman MF, Hashad IM, Abou-Aisha K, et al. Assessment of the link between endothelin K198n Snp, endothelin concentration and acute myocardial infarction in Egyptians. Clin Exp Pharmacol Physiol. 2017;44:132–34. doi: 10.1111/1440-1681.12684. [DOI] [PubMed] [Google Scholar]
- 22.Asai T, Ohkubo T, Katsuya T, et al. Endothelin-1 gene variant associates with blood pressure in obese Japanese subjects: The Ohasama Study. Hypertension. 2001;38:1321–24. doi: 10.1161/hy1101.095333. [DOI] [PubMed] [Google Scholar]
- 23.Gupta RM, Hadaya J, Trehan A, et al. A genetic variant associated with five vascular diseases is a distal regulator of endothelin-1 gene expression. Cell. 2017;170:522–33.e15. doi: 10.1016/j.cell.2017.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin JJ, Nakura J, Wu Z, et al. Association of endothelin-1 gene variant with hypertension. Hypertension. 2003;41:163–67. doi: 10.1161/01.hyp.0000043680.75107.cf. [DOI] [PubMed] [Google Scholar]
- 25.Pare G, Serre D, Brisson D, et al. Genetic analysis of 103 candidate genes for coronary artery disease and associated phenotypes in a founder population reveals a new association between endothelin-1 and high-density lipoprotein cholesterol. Am J Hum Genet. 2007;80:673–82. doi: 10.1086/513286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sing CF, Stengard JH, Kardia SL. Genes, environment, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:1190–96. doi: 10.1161/01.ATV.0000075081.51227.86. [DOI] [PubMed] [Google Scholar]
- 27.Gregoski MJ, Buxbaum SG, Kapuku G, et al. Interactive influences of ethnicity, endothelin-1 gene, and everyday discrimination upon nocturnal ambulatory blood pressure. Ann Behav Med. 2013;45:377–86. doi: 10.1007/s12160-013-9472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breitling LP. Current genetics and epigenetics of smoking/tobacco-related cardiovascular disease. Arterioscler Thromb Vasc Biol. 2013;33:1468–72. doi: 10.1161/ATVBAHA.112.300157. [DOI] [PubMed] [Google Scholar]
- 29.Chai XH, Li B, Wang JP, et al. [Association of coronary heart disease and endothelin-1 Lys198Asn polymorphism in smoking]. Molecular Cardiology of China. 2013;13:526–28. [in Chinese] [Google Scholar]
- 30.Kaptoge S, Seshasai SR, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35:578–89. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fatkhullina AR, Peshkova IO, Koltsova EK. The role of cytokines in the development of atherosclerosis. Biochemistry (Mosc) 2016;81:1358–70. doi: 10.1134/S0006297916110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorin E, Webb DJ. Endothelium-derived endothelin-1. Pflugers Arch. 2010;459:951–58. doi: 10.1007/s00424-009-0763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]