Abstract
Background
Inflammatory bowel diseases (IBDs) are chronic idiopathic diseases with increased occurrence and recurrence rates. The aim of this study was to explore whether methane-rich saline (MRS) would be beneficial to IBD.
Material/Methods
Dextran sulfate sodium (DSS) was utilized to establish an IBD model. Male C57BL/6J mice were randomly grouped as follows: the control group, the DSS+NS group, the DSS+5-ASA group, the DSS+MRS (1) and DSS+MRS (10) groups. Seven days after model induction, blood and colon tissues were collected to assess the treatment effects.
Results
The DSS+MRS (10) group showed obviously reduced weight loss, disease activity index, and spleen index. The isolated colon samples had a notably longer length, less thickness and weight, and better macroscopic score with MRS treatment compared with the DSS+NS group. Additionally, assessment of morphological impairment revealed a milder and lower microscopic score in the DSS+MRS (10) group, consistent with the myeloperoxidase (MPO) results. The inflammation-related molecules levels were dramatically reduced by MRS. MRS also significantly reduced oxidative stress related proteins. In addition, apoptotic cells were visually decreased in the DSS+MRS (10) group, in which the pro-apoptotic molecules Bax and cleaved caspase-3 were reduced, whereas the level of Bcl-2 was increased. Furthermore, MRS markedly decreased the TLR4, MyD88, p-NF-κB p65, p-IKKαβ, and p-IκBα, and increased IL-10, p-JAK1, and p-STAT3 expression levels. Proteins involved in endoplasmic reticulum stress (ERS) were also notably reduced under MRS treatment.
Conclusions
MRS exerts protective effects on DSS-induced IBD via inhibiting inflammatory reaction, promoting anti-inflammatory capacity, suppressing oxidative stress, and ameliorating apoptosis.
MeSH Keywords: Endoplasmic Reticulum Stress, Inflammation, Inflammatory Bowel Diseases Toll-Like Receptor 4
Background
Crohn’s disease (CD) and ulcerative colitis (UC), which are referred to as inflammatory bowel diseases (IBD), are chronic idiopathic diseases leading to inflammation of the bowel with uncertain etiology, including environmental factors, pathogenic microorganism, genetics, etc. [1]. The main manifestations of IBD include abdominal pain, diarrhea, rectal bleeding, colonic inflammation, altered bowel motility, weight loss, and weakness [2]. Based on the therapeutic principles of IBD to control inflammation and alleviate symptoms, IBD treatments are classified into traditional and biological therapies. The traditional class of therapies includes antibiotics, immunosuppressor, antiphlogistic medicine, and intestinal probiotics [3,4]. Biological therapy includes antibodies against pivotal cytokines involved in IBD, of which the anti-TNFα agent is of the most popular [5].
Inflammation is one of the major components to impaired mucosal homeostasis contributing to the pathogenesis of IBD [6]. Increased pro-inflammatory cytokines, including interleukin (IL)-1 (IL-1), IL-6, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) play key roles in inflammatory-induced bowel injury, and inhibition or blockage of these cytokines is considered a novel therapeutic approach [7]. Toll-like receptor 4 (TLR4), a receptor on the surface of immune cells, plays an important role in initiating and exacerbating the progress of inflammation. It can activate myeloid differentiation factor 88 (MyD88)-dependent pathways, and result in the nuclear translocation of nuclear factor-κB (NF-κB), which is thought to be a central “switch” in the inflammatory cascade in IBD [8]. On the contrary, the anti-inflammatory signaling pathway, such as IL-10/JAK1/STAT3, which is in charge of controlling the degree and duration of inflammation, also plays a key role in the progression of gut inflammation [9]. Oxidative stress is also involved in the pathogenesis of IBD, and antioxidant therapy is useful in this scenario [10]. In addition to the impairment due to inflammation and oxidative stress, cell apoptosis was also observed in an IBD animal model, and cell arrest could lead to ineffective remodeling of injury sites [11]. Tremendous efforts have been made to explore the pathophysiological mechanisms of IBD; however, no complete and concrete explanations have been published to date.
Methane is the simplest alkane and is a component of the most abundant organic gases in nature. Methane also exists in human intestines as a result of chemical reactions, fermentation of methanogens therein and air swallowing. Researchers have indicated that methane exerts positive effects on multiple conditions, such as ischemia reperfusion organ damage, acute lung injury, sepsis, diabetic retinopathy, acute liver injury, and so on. The possible mechanisms of the protective role of methane are associated with the suppression of inflammation, oxidative stress, and apoptosis [12–14]. Methane-rich saline (MRS) tends to be a preferable option given the risk of methane gas explosions. Thus, we performed this study to ascertain the impact of MRS on mice with IBD and detect the probable mechanisms.
Material and Methods
Animals and MRS preparation
Male C57BL/6J mice (4 to 5 weeks old, 21 to 26 g) were purchased from Animal Feeding Center of Xi’an Jiaotong University Health Science Center
IACUC protocol number: XJTULAC2014-207. The animals were housed with restrained laboratory conditions including stationary temperature of 23°C, 12-hour light/dark cycle, 50% relative humidity, and standard animal diet and water ad libitum for 7 days before experiment. All the mice were housed (5 per cage) and cared under minimized discomfort. MRS was produced by dissolving of methane gas in normal saline under 0.4–0.6 MPa for 8 hours. Prepared MRS was stored in an aluminum bag at 4°C. We used γ-radiation for sterilization one day before usage. The concentration of MRS was determined as 1.2~1.5 mmol/L by gas chromatography.
Animal model establishment and experimental design
Dextran sulfate sodium (DSS) is widely used to mimic pathogenesis of IBD in mice due to the high reproducibility [15]. The experimental mice were administered with DSS (5% (wt./vol.), 35–50 kDa, Sigma-Aldrich, St. Louis, MO, USA) contained in daily drinking water during 0–5 days. On the sixth and seventh days, tap water was substituted for DSS-contained water. Correspondingly, the control group was given the equal equilibrium of tap water as DSS throughout the 7 days.
All the mice were randomly separated into 5 groups (n=6): 1) the blank control group; and 2) DSS+normal saline (NS) group as the negative control. Both the blank and negative control groups were intraperitoneal injected with normal saline (5 mL/kg) twice a day for another 7 days after IBD modeling. 3) DSS+5-aminosalicylic acid (5-ASA) group as the positive control, in which 5-ASA (75 mg/kg/day) was injected intraperitoneal as a traditional treatment; 4) DSS+MRS (1) group, in which MRS was injected intraperitoneal at 1 mL/kg/day; and 5) DSS+MRS (10), in which 10 mL/kg/day of MRS was utilized. All these treatments were executed for one week immediately following successful animal model. Seven days later, mice were sacrificed after anesthetized by isoflurane gas. Blood samples were collected by eyeball extirpating and the serum was obtained by centrifugation (4°C, 3000 g) for 15 minutes. Colon samples, the primary affected organ during IBD, were removed immediately and kept at −80°C for further detection.
Evaluation of tissue damage macroscopically and microscopically
The weight, presence of hematochezia, and stool customs of all mice were observed daily. After sacrifice, the length, weight as well as thickness of isolated colon samples were measured. The mean disease activity index and the macroscopic score were calculated and used to evaluate the degree of tissue damage [16]. Spleen index was calculated as the ratio of the weight of the spleen and the mouse. To evaluate the morphological change further, the distal colon sections were fixed in formalin solution (10%). Samples were sliced into consecutive 5-μm thickness sections, and hematoxylin and eosin (H&E) staining was executed. Microscopic scores depending on the representative fields were assessed according to the criteria [17]. Two researchers went through the examination in a blinded method.
Analysis of myeloperoxidase (MPO) activity
The myeloperoxidase (MPO) activity of colon sample was determined via kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the instruction.
Assay of inflammatory cytokines in serum
Serum TNF-α, IL-1β, IL-6, and IL-10 levels were detected by enzyme-linked immunosorbent assays (ELISA) (Beyotime Biotechnology, Beijing, China) in accordance with the instructions.
Measurements of oxidative stress parameters
The malondialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) levels of colon samples were measured by relative kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturers’ instructions.
Assay of apoptosis in colon
Colonic cell apoptosis was examined by Hoechst Staining Kit (Apoptosis-Hoechst staining kit, Beyotime Biotechnology, Beijing, China). The results were observed with a fluorescence microscope, and representative fields were chosen for assessment.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA from colon sample was extracted by the RNAfast200 kit. PrimeScript RT reagent kit was used for reverse transcription. The expression of mRNA was evaluated in triplicate with the expression of 18S mRNA standardized. The comparative-Ct method (ΔΔCt method) was performed to calculate the relative levels. The sequences of corresponding primers are listed in Table 1.
Table 1.
Primer sequences of RT-PCR in colon tissues.
| Gene | Sequence (5′-3′) |
|---|---|
| TNF-α | F: AAGCCTGTAGCCCACGTCGTA |
| R: AGGTACAACCCATCGGCTGG | |
| IL-1β | F: GGA GAC TTC ACA GAG GAT AC |
| R: CCA GTT TGG TAG CAT CCA TC | |
| IL-6 | F: TCC ATC CAG TTG CCT TCT TG |
| R: TTC CAC GAT TTC CCA GAG AAC | |
| CSF-1 | F: GGCTTGGCGGGATGATTCT |
| R: GAGGGTCTGGCAGGTACTC | |
| MCP-1 | F: GAGGACAGATGTGGTGGGTTT |
| R: AGGAGTCAACTCAGCTTTCTCTT | |
| IL-10 | F: GCT CTT ACT GAC TGG CAT GAG |
| R: CGC AGC TCT AGG AGC ATG TG |
RT-PCR – reverse transcription polymerase chain reaction; TNF – tumor necrosis factor; IL – interleukin; CSF-1 – colony stimulating factor 1; MCP-1 – monocyte chemoattractant protein-1.
Western blot analysis
All proteins from the colon tissues were prepared in accordance with the instruction of manufacturer. The concentration of protein extraction was determined by bicinchoninic acid (BCA) protein assay kit. The protein sample was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto poly-vinylidene difluoride (PVDF) membranes, which were blocked with skim milk (10%) for 1 hour and subsequently immunoblotted overnight at 4°C with specific primary antibodies obtained from Beijing Biosynthesis Biotechnology Co., Ltd. The membranes were washed for 3 times using phosphate-buffered saline (PBS) and incubated with horseradish peroxidase (HRP) conjugated secondary antibodies, after which the blots were washed again and observed using chemiluminescence (ECL) Kit (Pierce, Rockford, IL, USA). The levels of proteins were analyzed by ImageJ software with normalizing to β-actin.
Statistical analysis
All the data were presented as mean±standard deviation. GraphPad Prism 7.0 was used to compare among multiple groups, one-way analysis of variance followed by the Student-Newman-Keuls post hoc test was performed. P-values of less than 0.05 was considered as statistical significance.
Results
MRS alleviated colon damage macroscopically and microscopically
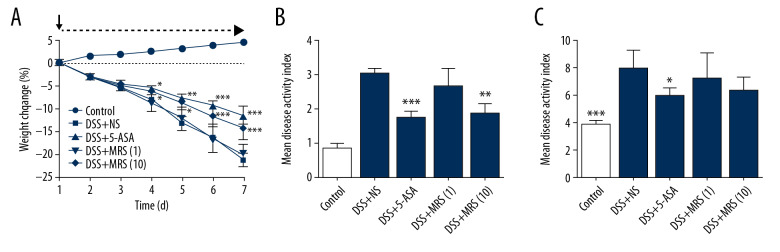
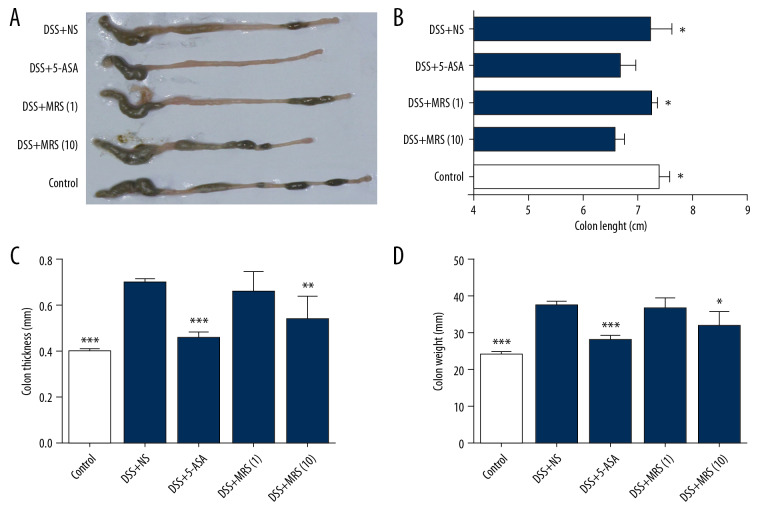
We monitored the weight change of the mice and observed greater weight reduction in the DSS+NS group when compared with the DSS+5-ASA group or the DSS+MRS (10) group with a significant difference since day 4 to day 7 as shown in Figure 1A. The disease activity index (DAI) was calculated to evaluate the severity of disease symptoms (Figure 1B). The result showed that the DAI in groups treated with 5-ASA or MRS (10 mL/kg) were significantly reduced compared with the DSS+NS group. Furthermore, spleen enlargement was evaluated based on the spleen index, and the result indicated that DSS administration caused an apparent increase in the spleen index versus the control group. By contrast, 5-ASA treatment reduce the spleen index (Figure 1C). The colon samples were isolated from the sacrificed mice, and representative samples are shown in Figure 2A. Length measurements revealed shortening in the DSS+NS group compared with the control group, and length was increased upon 5-ASA or MRS (10 mL/kg) treatment as shown in Figure 2B. Colon thickness increased with DSS establishment compared with the control group. Similarly, the conditions in the DSS+5-ASA and MRS (10) groups improved markedly (Figure 2C). The variation trend in colon weight was the same as aforementioned (Figure 2D). The macroscopic score was calculated to make an assessment of gross changes on the whole (Figure 3A), revealing that mice in the DSS group obtained higher scores than the control, but these values significantly decreased upon 5-ASA and MRS (10 mL/kg) treatment.
Figure 1.
Methane-rich saline improved the clinical manifestation of DSS-induced inflammatory bowel diseases: (A) weight loss of the mice; (B) mean disease activity index; (C) spleen index. (* P<0.05, ** P<0.01, *** P<0.001 as compared with the DSS+NS group). DSS – dextran sulfate sodium; NS – normal saline.
Figure 2.
Methane-rich saline improved the macroscopic impairment of DSS-induced inflammatory bowel diseases: (A) isolated colon tissues; (B) colon length; (C) colon thickness; (D) colon weight. (* P<0.05, ** P<0.01, *** P<0.001 as compared with the DSS+NS group). DSS – dextran sulfate sodium; NS – normal saline.
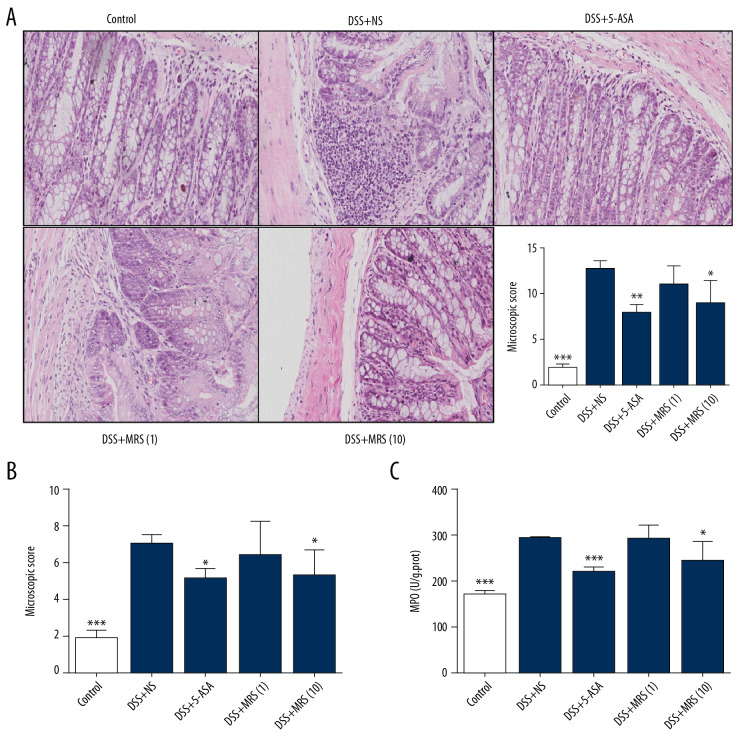
Figure 3.
Methane-rich saline improved the morphological impairment of DSS-induced inflammatory bowel diseases: (A) H&E staining of colon specimens (200×) and macroscopic score; (B) microscopic score; (C) Colonic MPO level. (* P<0.05, ** P<0.01, *** P<0.001 as compared with the DSS+NS group). DSS – dextran sulfate sodium; NS – normal saline; H&E – hematoxylin and eosin; MPO – myeloperoxidase.
When the tissue slices were observed by optical microscopy, severe inflammation was noted in the colons of the DSS group, presenting with mucosal hyperemia, crypt abscesses, inflammatory cell infiltration, destruction of mucosal structure and depletion of goblet cells (Figure 3A), which was consistent with an obvious increase in the microscopic score versus the control group (Figure 3B). The administration of 5-ASA or MRS (10 mL/kg) to mice with colitis alleviated the histological impairments as noted by decreased scores. MPO level is a crucial symbol for neutrophil infiltration [18], and the detection results showed that the MPO level increased notably with DSS treatment compared with the control (Figure 3C). Conditions improved with 5-ASA or MSA (10 mL/kg) administration. However, 1 mL/kg MRS failed to work in all the tested elements representative of colon damage. In general, the results indicated that 10 mL/kg of MRS exerted a similar protective effect as 5-ASA on colon impairment based on gross or histological aspects.
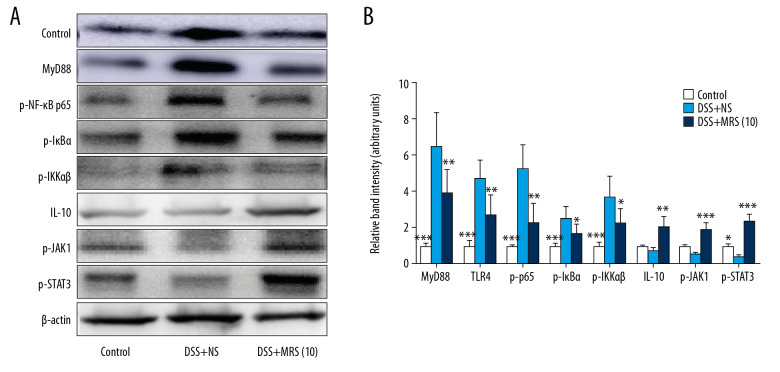
MRS inhibited DDS-induced inflammation via downregulating the TLR4/MyD88/NF-κB and upregulating the IL-10/JAK1/STAT3 signaling pathways
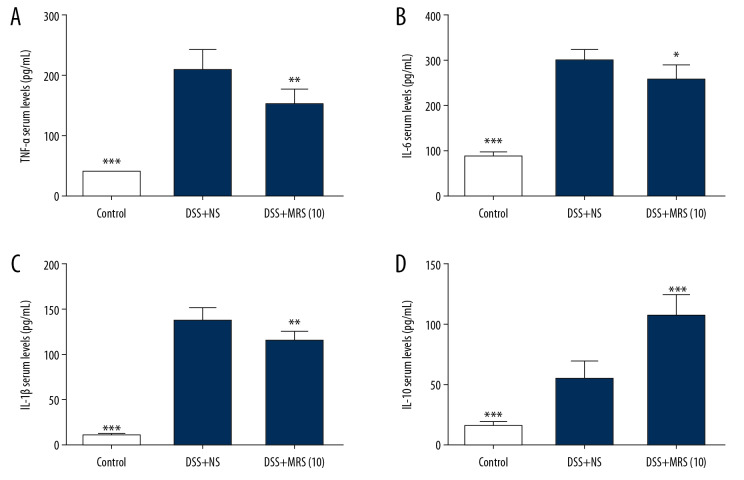
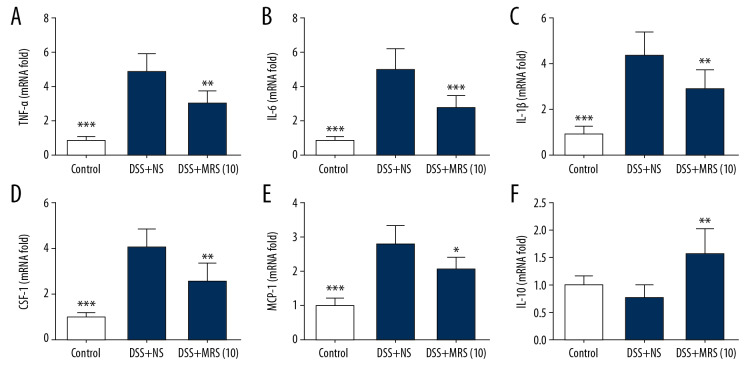
Serum TNF-α, IL-1β, and IL-6 levels were markedly increased in the DSS+NS group. By contrast, the DSS+MRS (10) group exhibited opposite results (Figure 4A–4C). Meanwhile, ELISA analysis indicated that MRS significantly increased the serum IL-10 level (Figure 4D). In addition, by examining their relative mRNA levels in the colon, we found that all the 3 cytokines (TNF-α, IL-1β, and IL-6) were expressed at increased levels in the DSS group compared with the control group. MRS (104mL/kg) treatment obviously reduced their transcriptional levels as shown in Figure 5A–5C. Moreover, crucial factors involved in inflammation caused by DSS, such as colony stimulating factor 1 (CSF-1) and monocyte chemoattractant protein-1 (MCP-1) were also detected. The results showed increased mRNA levels in the DSS group compared with the control group, and levels were significantly reduced in the DSS+MRS (10) group (Figure 5D, 5E). Likewise, RT-PCR result of IL-10 was consistent with the corresponding ELISA assay (Figure 5F). To further assess the influence of MRS on inflammation in IBD, the classical inflammation-related signaling pathway including TLR4, MyD88, and NF-κB was analyzed. As exhibited in Figure 6A and 6B, TLR4, MyD88, p-NF-κB p65, p-IκBα, and pIKKαβ expression levels were markedly increase in the DSS-induced IBD mouse model, while treatment with MRS (10 mL/kg) downregulated these levels. Additionally, we also found expression of IL-10, p-JAK1, and p-STAT3 were decreased notably after DSS treatment while MRS could significantly improve them. These results suggested that the administration of MRS (10 mL/kg) could downregulate the TLR4/MyD88/NF-κB signaling pathway and upregulate the IL-10/JAK1/STAT3 anti-inflammatory response.
Figure 4.
Methane-rich saline reduced the pro-inflammatory cytokine and increased the anti-inflammatory cytokine levels in DSS-induced inflammatory bowel diseases: serum levels (A) TNF-α; (B) IL-6; (C) IL-1β; and (D) IL-10. (* P<0.05, ** P<0.01, *** P<0.001 as compared with the DSS+NS group). DSS – dextran sulfate sodium; NS – normal saline; TNF – tumor necrosis factor; IL – interleukin.
Figure 5.
Methane-rich saline reduced the pro-inflammatory cytokines and increased the anti-inflammatory cytokine transcriptional levels in DSS-induced inflammatory bowel diseases: (A) TNF-α; (B) IL-6; (C) IL-1β; (D) CSF-1; (E) MCP-1 and (F) IL-10 mRNA levels in colon tissues. (* P<0.05, ** P<0.01, *** P<0.001 as compared with the DSS+NS group). DSS – dextran sulfate sodium; NS – normal saline; TNF – tumor necrosis factor; IL – interleukin; CSF-1 – colony stimulating factor 1; MCP-1 – monocyte chemoattractant protein-1.
Figure 6.
Methane-rich saline inhibited the TLR4/MyD88/NF-κB and promoted the IL-10/JAK1/STAT3 signaling pathways in DSS-induced inflammatory bowel diseases: (A) western blot analysis of MyD88, TLR4, p-NF-κB p65, p-IκBα, p-IKKαβ, IL-10, p-JAK1, and p-STAT3 in colon tissues and (B) relative band intensity. (* P<0.05, ** P<0.01, *** P<0.001 as compared with the DSS+NS group). DSS – dextran sulfate sodium.
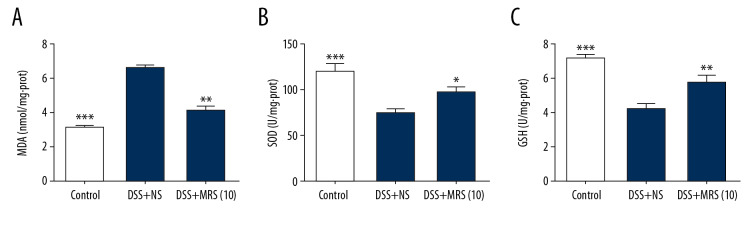
MRS suppressed oxidative stress in IBD
Levels of the antioxidants SOD and GSH were reduced in the DSS group compared with the control group. By contrast, the DSS+MRS (10) group exhibited the opposite results. MDA represents the level of oxidative stress and was notably decreased upon MRS (10 mL/kg) treatment (Figure 7).
Figure 7.
Methane-rich saline alleviated oxidative stress in the colon tissues in DSS-induced inflammatory bowel diseases: (A) MDA; (B) SOD and (C) GSH levels in colon tissues. (* P<0.05, ** P<0.01, *** P<0.001 as compared with the DSS+NS group). DSS – dextran sulfate sodium; MDA – malondialdehyde; SOD – superoxide dismutase; GSH – glutathione; NS – normal saline.
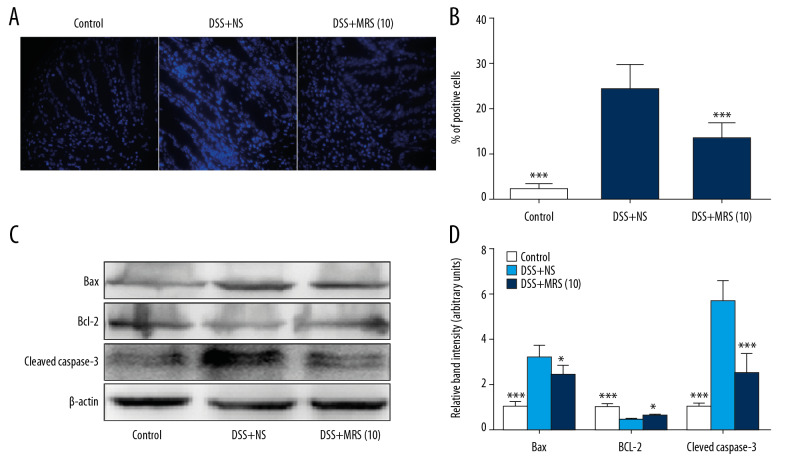
MRS mitigated colonic cell apoptosis via inhibiting endoplasmic reticulum stress (ERS)
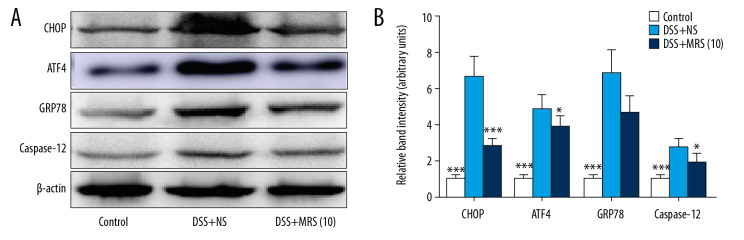
Hoechst Staining was performed to assess colonic apoptosis. More apoptotic cells were noted in the DSS group compared with the control group, and level was notably reduced in the DSS+MRS (10) group (Figure 8A, 8B). Western blot analysis of pro-apoptotic molecules, Bax and cleaved caspase-3, revealed higher levels in the context of DSS. The levels of the anti-apoptotic molecule, Bcl-2 were reduced with DSS establishment. However, opposite results were noted with MRS (10 mL/kg) administration (Figure 8C, 8D). Endoplasmic reticulum stress (ERS) was evaluated further to assess the molecular mechanism. Crucial molecules involved in ERS, including CHOP, ATF4, GRP78, and caspase-12, were expressed at higher levels in the DSS group compared with control group, whereas administration of MRS (10 mL/kg) reversed the results as shown in Figure 9A, 9B. These results demonstrated that apoptosis in IBD could be improved upon treatment with MRS due to ERS suppression.
Figure 8.
Methane-rich saline reduced apoptosis in the colon tissues in DSS-induced inflammatory bowel diseases: (A) Hoechst staining (400×), in which blue spots represented for apoptotic cells; (B) proportion of positive staining cells; (C) western blot of Bax, Bcl-2, and cleaved caspase-3 in colon tissues and (D) relative band density. (* P<0.05, *** P<0.001 as compared with the DSS+NS group). DSS – dextran sulfate sodium; NS – normal saline.
Figure 9.
Methane-rich saline inhibited the endoplasmic reticulum stress signaling pathway in DSS-induced inflammatory bowel diseases. (A) Western blot assay of CHOP, ATF4, GRP78, and caspase-12 in colon tissues and (B) relative band intensity. (* P<0.05, ** P<0.01, *** P<0.001 as compared with the DSS+NS group). DSS – dextran sulfate sodium; NS – normal saline.
Discussion
IBD is a group of inflammation-related disorders accompanied by symptoms of abdominal pain, diarrhea, rectal bleeding, and weight loss. The number of patients who suffer from such diseases with high recurrence rates and risks of colorectal cancer has increased in recent decades. Clinical treatments, such as 5-ASA, corticosteroids, or biological agents, are effective to some extent; nevertheless, the side effects and the complicated etiology of IBD make the disease troublesome for patients and the health-care system [19]. Here, we introduced a novel approach, namely, MRS to treat IBD. The DSS-induced IBD model was established in the study given its simplicity and excellent imitation of colitis as demonstrated in previous reports. After observing and analyzing the symptoms of all experimental mice, we found that MRS could alleviate the IBD, which manifested as reduced weight loss, disease activity index, spleen enlargement, colon length and thickness, compared to DSS establishment. Microscopic examination revealed that the colon specimens had less severe inflammatory cell infiltration, disruption of architecture, goblet cells loss and crypt abscesses upon MRS treatment. Consistently, the quantitative scores of the macroscopic and histological changes showed a reduction when mice were treated with MRS. MPO activity was suppressed with MRS, indicating the alleviation of neutrophil infiltration. Our results revealed that MRS played a beneficial role in DSS-induced colitis in a manner similar to 5-ASA. Of note, only the higher dose of 10 mL/kg of MRS yield statistically significant improvements.
To explain how MRS works in IBD, inflammation in mice was detected given that excessive inflammation contributes to the pathogenesis of IBD and is a potential target for more effective biological treatments [20,21]. The expression of pro-inflammatory cytokines is critical for mucosal damage in the bowel, and antibodies against these proteins have been used to improve IBD [22]. In our study, the TNF-α, IL-1β, and IL-6 mRNA and protein serum levels were dramatically reduced by 10 mL/kg of MRS. The CSF-1 and MCP-1 mRNA levels were decreased by MRS compared with the levels noted with DSS induction. Therein, CSF-1, a hemopoietic growth factor, is reported to intervene in inflammation and the cell cycle of monocytes and macrophages, and its blockade is helpful for the inhibition of DSS-induced colitis [23]. MCP-1 is also a significant chemokine involved in the pathological process in IBD, which could be another potential target for this disease [24]. The powerful anti-inflammation effect was almost the most important characteristic of methane. Methane could also decrease inflammatory cell infiltration and reduce other cytokines like IL-4, IL-5, IL-13, IFN-γ, and CXCL15 secretion [25]. NF-κB is a pivotal transcription factor that promotes the activation of TNF-α, IL-1β, and IL-6. Stimuli could be transferred from TLR4 to MyD88 to activate the IKK complex. Thereafter, IκB is destroyed by the proteasome, and the NF-κB p65 subunit is released from translocation into nuclei to activate downstream inflammatory factors [26]. Increased TLR4, MyD88, NF-κB p65, and IκBα expression levels were downregulated by MRS, indicating that the possible anti-inflammation mechanism of MRS was associated with inhibition of TLR4/MyD88/NF-κB signaling pathway. Moreover, we found MRS could not only inhibit the inflammatory reaction, but also improve the anti-inflammatory ability by promoting IL-10/JAK1/STAT3 signaling pathway. IL-10/JAK1/STAT3 is the most-studied anti-inflammatory response related signaling pathway which can control the degree and duration of inflammation [9]. Impairment of it could lead uncontrolled and augmentative inflammation. Previous studies showed that IL-10 was one key target of MRS, which were proved in different diseases such as cognitive dysfunction, sepsis, acute liver injury, pain, acetic acid induced colitis and so on [27–29]. In turn, the activation of STAT3 could suppress the expression of pro-inflammatory genes. Thus, MRS could not only inhibit inflammation through TLR4/MyD88/NF-κB signaling pathway, but also elevate the anti-inflammatory ability through IL-10/JAK1/STAT3 signaling pathway.
Oxidative stress is involved in IBD development [10]. Increased oxidants represent a burden for cells via impaired membrane permeability, lipid peroxidation, stimulation of inflammatory pathways, and eventually cell death. MDA is an end product of polyunsaturated fatty acids oxidation and was assessed as a marker of oxidative stress. On the contrary, GSH and SOD act as scavengers of oxygen radicals to exert anti-oxidative properties [30]. MRS treatment decreased MDA levels but increased GSH and SOD activities in our study. Oxidative stress is the key target of MRS which has been revealed by almost every previous study. Methane could also decrease expression of 8-hydroxyguanosine (8-OHdG) and 3-nitrotyrosine (3-NT), and increase catalase (CAT) capacity. Our own research found that MRS could exert anti-oxidative effect through the Nrf2/HO-1/NQO1 signaling pathway which might be the most key mechanism [13].
In addition to inflammation and oxidative stress-induced epithelial cell injury, apoptosis represents another form of injury [31]. The susceptibility of colorectal cancer has been shown to be increased when the imbalance of apoptosis and complementary proliferation is exacerbated [32]. We observed increased numbers of apoptosis cells in DSS-induced colitis, which was consistent with previous studies [33]. MRS treatment altered the situation. The Bcl-2 family proteins play an indispensable role in regulating apoptosis. Bcl-2 and Bax were analyzed in this study as representative members of the Bcl-2 family. The former molecule accounts for the anti-apoptotic effect and the latter has opposite effects [34]. Our results revealed that MRS increases the expression of Bcl-2 rather than Bax, and downstream activation of caspase-3 was inhibited. ERS was investigated to identify factors that further induced apoptosis given that the process of ERS is tightly connected with lethal activation of caspases, especially caspase-12 [35]. GRP78 is exclusively expressed in the ER and is a specific marker of activated ERS. Excessive expression of ERS leads to the activation of ATF4, a transcription factor that induces CHOP transcription to mediate cell arrest and death [36]. The over-expression CHOP, ATF4, GRP78, and caspase-12 by DSS was downregulated with MRS administration in this study, indicating that MRS could weaken ERS to alleviate apoptosis in DSS-induced IBD. In fact, not only in IBD, but our previous study had revealed the effect of MRS could also reduce sepsis-induced renal injury by targeting ER stress and apoptosis [37].
Our current study revealed the beneficial effect of MRS against DSS-induced colitis via controlling inflammation, suppressing oxidative stress, and ameliorating apoptosis. The biological properties verified here were consistent with previous studies reporting protective effects of methane on diseases such as IRF-related organ disorders, hepatitis, etc. [12]. Nevertheless, the physicochemical properties, biological mechanisms, and usage of methane remain imprecise and incomplete. Methane gas can penetrate the cell membrane easily. Thus, the interaction between methane and proteins embedded in the membrane represent a possible mechanism. Moreover, methane might also combine with specific oxygenase and be transformed to alcohols due to the reducibility of methane itself. However, no systematic and precise report has been published to date to persuade researchers. The other problem associated with MRS research is the determination of the appropriate dosage; our study indicated that 10 mL/kg MRS was more effective than 1 mL/kg. More efforts are needed to explore methane medicine in the future.
Conclusions
In this study, we hypothesized that MRS could effectively treat IBD for the first time, and a DSS-challenge mouse model was established to mimic IBD. The results indicated that MRS exerted protective effects on DSS-induced IBD, and the possible mechanisms were that MRS alleviated inflammation via inhibiting the TLR4/MyD88/NF-κB and promoting the IL-10/JAK1/STAT3 signaling pathways, suppressed oxidative stress, and ameliorated apoptosis via weakening ERS.
Acknowledgements
We are indebted to all individuals who participated in or helped with this research project.
Footnotes
Conflicts of interest
None.
Source of support: This study was supported by funding from “The National Nature Science Foundation of China” (Grant No. 81601672)
References
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2018;390(10114):2769–78. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 2.Butter M, Weiler S, Biedermann L, et al. Clinical manifestations, pathophysiology, treatment and outcome of inflammatory bowel diseases in older people. Maturitas. 2018;110:71–78. doi: 10.1016/j.maturitas.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185–210. doi: 10.2147/DDDT.S11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichele DD, Young R. Medical management of inflammatory bowel disease. Surg Clin North Am. 2019;99(6):1223–35. doi: 10.1016/j.suc.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Mitrev N, Leong RW. Therapeutic drug monitoring of anti-tumour necrosis factor-α agents in inflammatory bowel disease. Expert Opin Drug Saf. 2017;16(3):303–17. doi: 10.1080/14740338.2017.1269169. [DOI] [PubMed] [Google Scholar]
- 6.Ramos GP, Papadakis KA. Mechanisms of disease: Inflammatory bowel diseases. Mayo Clin Proc. 2019;94(1):155–65. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14(5):329–42. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: Recent developments and future challenges. Brief Funct Genomics. 2013;12(6):489–98. doi: 10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian T, Wang Z, Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/4535194. 4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen J, LaCasse EC, Seidelin JB, et al. Inhibitors of apoptosis (IAPs) regulate intestinal immunity and inflammatory bowel disease (IBD) inflammation. Trends Mol Med. 2014;20(11):652–65. doi: 10.1016/j.molmed.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Jia Y, Li Z, Liu C, Zhang J. Methane medicine: A rising star gas with powerful anti-inflammation, antioxidant, and anti-apoptosis properties. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/1912746. 1912746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y, Cui R, Li Z, et al. Methane alleviates acetaminophen-induced liver injury by inhibiting inflammation, oxidative stress, endoplasmic reticulum stress, and apoptosis through the Nrf2/HO-1/NQO1 signaling pathway. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/7067619. 7067619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen O, Ye Z, Cao Z, et al. Methane attenuates myocardial ischemia injury in rats through anti-oxidative, anti-apoptotic and anti-inflammatory actions. Free Radic Biol Med. 2016;90:1–11. doi: 10.1016/j.freeradbiomed.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol. 2017;23(33):6016–29. doi: 10.3748/wjg.v23.i33.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen NY, Bi JB, Zhang JY, et al. Hydrogen-rich water protects against inflammatory bowel disease in mice by inhibiting endoplasmic reticulum stress and promoting heme oxygenase-1 expression. World J Gastroenterol. 2017;23(8):1375–86. doi: 10.3748/wjg.v23.i8.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sałaga M, Polepally PR, Zakrzewski PK, et al. Novel orally available salvinorin A analog PR-38 protects against experimental colitis and reduces abdominal pain in mice by interaction with opioid and cannabinoid receptors. Biochem Pharmacol. 2014;92(4):618–26. doi: 10.1016/j.bcp.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Chami B, Martin NJJ, Dennis JM, Witting PK. Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease. Arch Biochem Biophys. 2018;645:61–71. doi: 10.1016/j.abb.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 19.de Lange KM, Barrett JC. Understanding inflammatory bowel disease via immunogenetics. J Autoimmun. 2015;64:91–100. doi: 10.1016/j.jaut.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Digby-Bell JL, Atreya R, Monteleone G, Powell N. Interrogating host immunity to predict treatment response in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(1):9–20. doi: 10.1038/s41575-019-0228-5. [DOI] [PubMed] [Google Scholar]
- 21.Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019 doi: 10.1155/2019/7247238. 7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Fierro ML, Garza-Veloz I, Rocha-Pizaña MR, et al. Serum cytokine, chemokine, and growth factor profiles and their modulation in inflammatory bowel disease. Medicine (Baltimore) 2019;98(38):e17208. doi: 10.1097/MD.0000000000017208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall D, Cameron J, Lightwood D, Lawson AD. Blockade of colony stimulating factor-1 (CSF-I) leads to inhibition of DSS-induced colitis. Inflamm Bowel Dis. 2007;13(2):219–24. doi: 10.1002/ibd.20055. [DOI] [PubMed] [Google Scholar]
- 24.Khan WI, Motomura Y, Wang H, et al. Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol. 2006;291(5):G803–11. doi: 10.1152/ajpgi.00069.2006. [DOI] [PubMed] [Google Scholar]
- 25.Meng Y, Jiang Z, Li N, et al. Protective effects of methane-rich saline on renal ischemic-reperfusion injury in a mouse model. Med Sci Monit. 2018;24:7794–801. doi: 10.12659/MSM.911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulero MC, Huxford T, Ghosh G. NF-κB, IκB, and IKK: Integral components of immune system signaling. Adv Exp Med Biol. 2019;1172:207–26. doi: 10.1007/978-981-13-9367-9_10. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D, Li N, Wang Y, et al. Methane ameliorates post-operative cognitive dysfunction by inhibiting microglia NF-κB/MAPKs pathway and promoting IL-10 expression in aged mice. Int Immunopharmacol. 2019;71:52–60. doi: 10.1016/j.intimp.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Li N, Shao H, et al. Methane limit LPS-induced NF-κB/MAPKs signal in macrophages and suppress immune response in mice by enhancing PI3K/AKT/GSK-3β-mediated IL-10 expression. Sci Rep. 2016;6:29359. doi: 10.1038/srep29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G, Xu B, Shi F, et al. Protective effect of methane-rich saline on acetic acid-induced ulcerative colitis via blocking the TLR4/NF-κB/MAPK pathway and promoting IL-10/JAK1/STAT3-mediated anti-inflammatory response. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/7850324. 7850324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Negroni A, Cucchiara S, Stronati L. Apoptosis, necrosis, and necroptosis in the gut and intestinal homeostasis. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/250762. 250762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoian M, State N, Stoica V, Radulian G. Apoptosis in colorectal cancer. J Med Life. 2014;7(2):160–64. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Wang Y, Shen W, et al. Grape seed polyphenols ameliorated dextran sulfate sodium-induced colitis via suppression of inflammation and apoptosis. Pharmacology. 2020;105(1–2):9–18. doi: 10.1159/000501897. [DOI] [PubMed] [Google Scholar]
- 34.Peña-Blanco A, García-Sáez AJ. Bax, Bak and beyond-mitochondrial performance in apoptosis. FEBS J. 2018;285(3):416–31. doi: 10.1111/febs.14186. [DOI] [PubMed] [Google Scholar]
- 35.Kaneko M, Imaizumi K, Saito A, et al. ER stress and disease: Toward prevention and treatment. Biol Pharm Bull. 2017;40(9):1337–43. doi: 10.1248/bpb.b17-00342. [DOI] [PubMed] [Google Scholar]
- 36.van Vliet AR, Agostinis P. Mitochondria-associated membranes and ER stress. Curr Top Microbiol Immunol. 2018;414:73–102. doi: 10.1007/82_2017_2. [DOI] [PubMed] [Google Scholar]
- 37.Jia Y, Li Z, Feng Y, et al. Methane-rich saline ameliorates sepsis-induced acute kidney injury through anti-Inflammation, antioxidative, and antiapoptosis effects by regulating endoplasmic reticulum stress. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/4756846. 4756846. [DOI] [PMC free article] [PubMed] [Google Scholar]