Abstract
Ticks are obligate blood-feeding ectoparasites that transmit a wide variety of pathogens to animals and humans in many parts of the world. Currently, tick control methods primarily rely on the application of chemical acaricides, which results in the development of resistance among tick populations and environmental contamination. Therefore, an alternative tick control method, such as vaccines have been shown to be a feasible strategy that offers a sustainable, safe, effective, and environment-friendly solution. Nevertheless, novel control methods are hindered by a lack of understanding of tick biology, tick-pathogen-host interface, and identification of effective antigens in the development of vaccines. This review highlights the current knowledge and data on some of the tick-protective antigens that have been identified for the formulation of anti-tick vaccines along with the effects of these vaccines on the control of tick-borne diseases.
Keywords: anti-tick vaccines, Ixodes, Borrelia, transmission-blocking, blood, saliva
Introduction
Ticks are found in the class of Arachnida, and they are classified into two families, hard ticks (Ixodidae) and soft ticks (Argasidae), each containing different genera and species of ticks (1, 2). Ticks are vectors of the causative agents of viral, bacterial and protozoan diseases in both animals and humans, and there has been an increasing incidence of several tick-borne pathogens (TBPs) in many parts of the world. After mosquitoes, ticks are considered to be the second most common vectors of pathogens to humans (3). They are competent vectors of disease-causing pathogens including Borrelia burgdorferi sensu lato (s.l.), tick-borne encephalitis virus (TBEV), and Anaplasma phagocytophilum. The most common tick-borne diseases and their vectors with different transmission cycles are presented in Table 1 (3–7). As ticks can transmit a variety of pathogens, an alternative control approach is to target the tick vector itself, either interfering with tick blood feeding or with pathogen transmission.
Table 1.
Common tick-borne diseases and their vectors with different transmission cycles.
| Tick-borne pathogens (TBPs) | Main affected host | Tick-borne diseases (TBDs) | Time required for pathogen transmission | Main tick species | Transovarial transmission | References |
|---|---|---|---|---|---|---|
| Ehrlichia ruminantium | Farm animal | Heartwater | 48–96 h | Amblyomma hebraeum, A. variegatum | No | (3) |
| Anaplasma platys | Companion animal | Canine cyclic thrombocytopenia | 16–72 h | Rhipicephalus sanguineus | No | (3, 4) |
| Anaplasma phagocytophilum | Human and farm animal | Human granulocytic anaplasmosis, tick-borne fever | 24–48 h | Ixodes ricinus | No | (3, 4) |
| Rickettsia rickettsii | Human | Rocky Mountain spotted fever | 10 h | Dermacentor variabilis, D. andersoni, Rhipicephalus sanguineus, Amblyomma americanum | Yes | (3–7) |
| Anaplasma marginale | Farm animal | Bovine anaplasmosis | 24–48 h | Rhipicephalus microplus, Dermacentor andersoni | No | (3–7) |
| Borrelia burgdorferi (sensu lato) | Human and companion animal | Lyme disease | 16–72 h | Ixodes scapularis, I. pacificus | No | (3–7) |
| Crimean-Congo hemorrhagic fever virus | Human and farm animal | Crimean-Congo hemorrhagic fever | Immediate | Hyalomma impeltatum | Yes | (3–7) |
| Louping ill virus | Farm animal | Louping ill | Immediate | Ixodes ricinus | No | (3–7) |
| Tick-borne encephalitis virus | Human | Tick-borne encephalitis | Immediate | Ixodes ricinus, I. ovatus, I persulcatus | Yes | (6) |
| Theileria annulata | Farm animal | Tropical theileriosis | 48 h | Hyalomma anatolicum anatolicum, Hyalomma anatolicum excavatum, Rhipicephalus appendiculatus | No | (3) |
| Babesia divergens, B. microti | Human | Human babesiosis | 48–72 h | Ixodes persulcatus | Yes/ No | (6) |
| Babesia canis, B. vogeli | Companion animal | Canine babesiosis | 48 h | Rhipicephalus sanguineus sensu lato (s.l.), Dermacentor reticulatus, Haemaphysalis leachi | Yes | (3–7) |
| Babesia divergens, B. jakimovi, B. bovis, B. bigemina, B. ovata | Farm animal | Bovine babesiosis | 48–216 h | Rhipicephalus microplus, R. annulatus, Ixodes ricinus | No | (3–7) |
Globally, 1.3 billion cattle populations in the world are at risk of TBPs with an annual economic cost estimated to be US$22–30 billion in 1996 (8). This economic cost is going to be higher now in 2020 as it has been over 20 years. Currently, tick control depends mainly on the application of conventional chemicals or synthetic acaricides, resulting in acaricidal resistant tick populations in several developing countries and contamination of the environment and food products (9). Therefore, new approaches such as immunological control via vaccination are urgently required for effective control. Of which, vaccines seem to be a promising, safe, sustainable, cost-effective and environment-friendly solution. Only two ectoparasite vaccines were reported commercially in the early 1990s: The Australian TickGARD® (Hoechst Animal Health) and Latin American Gavac® (Heber Biotec). Both vaccines are derived from Rhipicephalus microplus midgut membrane-bound recombinant protein BM86 (10, 11). Despite the effectiveness of these commercial BM86-based vaccines for the control of cattle tick infestations, they show strain to strain variation in vaccine efficacy and are effective against Rhipicephalus tick species mainly (12). It has been nearly 30 years since the first anti-tick vaccines became commercially available. The identification of effective antigens remains to be the biggest hurdle in the development of further anti-tick vaccines. Thus, strategies to identify anti-tick vaccine antigen(s) should be based on expanding our knowledge of the biology of the ticks and its interaction with pathogens. This review will, therefore, focus on how the identification and functional characterization of selected tick proteins, with a particular focus on saliva, blood digestive and membrane-associated proteins, could help in the fight against tick-transmitted diseases and discuss the proteins suitability as anti-tick vaccine candidates.
Saliva-assisted Transmission Blocking Vaccine Candidates
Saliva-assisted transmission (SAT) is defined as “the indirect promotion of arthropod-borne pathogen transmission via the actions of arthropod saliva molecules on the mammalian host” (13). This characteristic has been reported for most hematophagous arthropods including ticks. In this section, we review work that focuses on tick saliva proteins (TSPs) which are critical to the success of ticks as vectors of TBPs, and thus might serve as targets in tick antigen-based vaccines to prevent TBP infections. Saliva-assisted transmission blocking anti-tick vaccine candidates are listed in Table 2 (14–21).
Table 2.
Saliva-assisted transmission blocking anti-tick vaccine candidates.
| Salivary molecule | Pathogen | Tick species | Target mechanism | Activity | References |
|---|---|---|---|---|---|
| TSLPI | Borrelia burgdorferi | Ixodes scapularis, I. ricinus | Tick salivary lectin pathway inhibitor | Facilitates transmission from ticks to mice and from mice to ticks | (14) |
| tHRF | B. burgdorferi | I. scapularis | Tick histamine release factor (vasodilation) | Promotes late stage feeding and thereby facilitates tick to host transmission | (15) |
| Salp15 | B. burgdorferi | I. scapularis | Secreted salivary protein (acquired immune responses) | Facilitates transmission from ticks to mice | (16) |
| Salp25 D | B. burgdorferi | I. scapularis | Salivary protein, antioxidant (acquired immune responses) | Facilitates transmission from mice to ticks | (17) |
| Salp16 | Anaplasma phagocytophilum | I. scapularis | Secreted salivary protein (acquired immune responses) | Facilitates transmission from ticks to mice | (18) |
| Subolesin (SUB) | B. burgdorferi | I. scapularis | A reduction in tick weight and/or oviposition | Tick protective antigen (innate immune response, reproduction, and development of ticks) | (19, 20) |
| Subolesin (SUB) | Tick-borne encephalitis virus | I. scapularis | Nuclear factor kappa B (NF-kB) | Tick protective antigen (innate immune response, reproduction, and development of ticks) | (19, 20) |
| 64P | Tick-borne encephalitis virus | I. scapularis | Dual-action (exposed and concealed) | Tick cement protein, secreted glycine-rich proteins | (21) |
| Sialo L2 | B. burgdorferi | I. scapularis | Secreted salivary protein (acquired immune responses) | Increases level of skin infection following syringe inoculation | (54, 55) |
| Sialo L2 | phagocytophilum | I. scapularis | Secreted salivary protein (acquired immune responses) | Protection by inhibiting inflammasome formation in mice | (54, 55) |
| Salp20 | Borrelia garinii | I. scapularis | Secreted salivary protein (acquired immune responses) | Protection by inhibiting the complement pathway | (27) |
Blocking attachment of a tick to the host is not only impairing blood feeding but also the transmission of tick-borne diseases (TBDs). The process of attachment starts with the intrusion of the tick's mouthparts into the host skin, followed by anchoring of the tick by a cement cone that mostly comprises secreted glycine-rich proteins. The molecular weight of 15-kDa tick cement protein, 64P, was first identified from the African brown ear tick Rhipicephalus appendiculatus that has been evaluated for its direct effect on TBEV transmission. Vaccination of mice with recombinant forms of 64P (64TRPs, expressed as truncated) significantly diminished TBEV transmission. In guinea pig, rabbit, mice and hamster models, these cement proteins act as a dual-action vaccine by targeting both “exposed” and “concealed” antigens, resulting in mortality of engorged ticks. Further, 64TPRs have been shown as a broad-spectrum vaccine candidate against adult and immature stages of several tick species, including Ixodes ricinus and Rhipicephalus sanguineus (22). The protective effect of anti-tick immunity against TBEV infection underpins the concept of transmission-blocking vaccines. Unlike 64TRP antigen, immunization with TickGARD did not provide protection against lethal infection with TBEV. Thus, 64TRPs antigens have the potential candidate for broad-spectrum transmission-blocking vaccines (21). Apart from 64TRPs, subolesin (SUB) is the second anti-tick vaccine candidate in the context of TBEV infection. It is an ortholog of vertebrate and tick akirins (AKR) that could be potentially considered as a universal vaccine against multiple blood-sucking arthropods including mosquitoes and ticks. Vaccination with recombinant subolesin protein showed a reduction of tick infestations and transmission of B. burgdorferi, Anaplasma marginale, A. phagocytophilum, and Babesia bigemina (19, 20). Havlíková et al. (23) found that SUB vaccines did not have any effect on TBEV infection and transmission. However, TBEV infection increases SUB mRNA transcript levels in tick tissues, thus supporting a role for this molecule in tick innate response to virus infection. Pilot studies have used Babesia and Anaplasma infection models to characterize SUB, TROSPA (identified in Ixodes scapularis as a receptor for B. burgdorferi) and SILK (a protein present in tick saliva) as potential antigens to control pathogen transmission in cattle. Rabbit polyclonal antibodies generated against SILK, TROSPA and SUB were added to infected or uninfected bovine blood to capillary feed R. microplus ticks. The results from capillary feeding showed a substantial effect on tick body weight, mortality, and oviposition rate, but infection levels were not changed in ticks treated with these antibodies (24). Because of the variations in infection levels from tick to tick, this experiment reported that artificial capillary blood feeding was not an ideal approach to characterize the efficacy of tick-pathogen interactions (24).
Salp15, a glycosylated salivary protein, was first identified from the black-legged tick I. scapularis. It is coded by 408 bases, and has a molecular weight of 14.7 kDa. The sequence analysis revealed weak similarities with two motifs of Inhibin A, a member of the TGF-ß super-family, and contained a signal peptide at N-terminal, suggesting that the protein may have immunosuppressive and secretory activity (21). It inhibits CD4+ coreceptor of mammalian T cells activation, dendritic cell-induced T cell activation through interaction with the C-type lectin and complement activity (25). Furthermore, the Salp15 protein binds to the outer membrane protein (OspC) of B. burgdorferi and protects the evasion of immune responses against spirochetes upon entry into the vertebrate host (16), facilitating long-lasting survival of the spirochetes, host infection and pathogen transmission. RNA interference-mediated (RNAi) silencing of Salp15 in infected I. scapularis significantly decreased the bacterial load in mice. RNAi studies provide the first direct evidence that Salp15 promotes TBPs transmission (16). Vaccination of mice with Salp15 showed substantial protection (60%) from B. burgdorferi infection (26), which provided further evidence. Following the discovery of Salp15-mediated transmission of Borrelia by I. scapularis, other salp15 homologs have been identified in Ixodes persulcatus, Ixodes ricinus, Ixodes affinis, and Ixodes sinensis ticks, they also bind Borrelia garinii and Borrelia afzelii OspC to facilitate spirochete transmission (26). Generally, high sequence similarity across various Ixodes species not only suggests an important role in blood feeding and tick–pathogen interactions but may also lead to the development of an anti-tick vaccine against TBDs.
Most salivary proteins are responsible for pathogen transmission from an infected tick to a host, but some saliva proteins are considered to aid in the acquisition of the pathogen by uninfected feeding ticks. One such protein is Salp25D, a 25-kDa tick salivary antioxidant protein expressed in both the salivary glands and midguts of I. scapularis. It has significant homology to glutathione peroxidase antioxidant that has been proven to play a critical role in protecting Borrelia from reactive oxygen produced by neutrophils and facilitating Borrelia acquisition by ticks (21). Knockdown of Salp25D significantly reduced acquisition of spirochetes by ticks engorging on B. burgdorferi-infected mice, but did not affect Borrelia transmission from infected nymphs to uninfected mice. Vaccination of mice with Salp25D also decreased the acquisition of Borrelia by ticks to three-fold in comparison to the control group (17). Hence, it seems that Salp25D facilitates the transmission of Borrelia from infected mice to uninfected ticks by protecting spirochetes from the toxic products of neutrophils activated by tick feeding (27).
Several other salivary proteins have been identified that play an important role in A. phagocytophilum-vector interactions. They include Salp16, alpha1-3-fucosyltransferase, P11 and an antifreeze glycoprotein (IAFGP) (21). Salp16, a 16-kDa protein, was identified in the salivary glands of I. scapularis and plays a role in A. phagocytophilum-vector interactions. Silencing of Salp16 induced significant (90%) reduction of A. phagocytophilum migration from gut to salivary glands through the haemolymph (18). Another study showed a significant increase in nuclear G-actin and phosphorylated actin upon A. phagocytophilum infection that subsequently influenced Salp16 gene expression (28). Two proteins, namely alpha 1-3-fucosyltransferase and IAFGP (I. scapularis tick antifreeze glycoprotein) were also found to be up-regulated upon the presence of A. phagocytophilum in ticks. RNAi-mediated knockdown of IAFGP and alpha 1-3-fucosyltransferase showed a significant reduction of A. phagocytophilum load in ticks when fed on infected mice (21). These results suggest the important role of these genes in tick-host interactions. P11, a secreted salivary protein, was up-regulated in A. phagocytophilum-infected ticks, which has been demonstrated in RNAi. The protein was shown to bind to A. phagocytophilum and facilitate the uptake of the pathogen by tick haemocytes, suggesting that haemocytes transport the pathogen from the midgut to the salivary glands (29).
Ticks have adopted a unique strategy to control the effects of host-derived histamine, and they secrete salivary molecules that neutralize the inflammatory effect of histamine (30). The tick histamine release factor (tHRF) was first characterized from I. scapularis using 2D fluorescence differential gel electrophoresis (DIGE). Real-time PCR analysis revealed that tHRF was expressed in both tick saliva and midgut and up-regulated in Borrelia-infected ticks. tHRF-silenced ticks or immunization of mice with recombinant tHRF significantly impaired the efficiency of tick feeding and spirochete transmission (15). As tHRF is critical for I. scapularis feeding irrespective of B. burgdorferi infection, and preferentially expressed between 48 and 72 h post-tick attachment (while B. burgdorferi s.s. transmission begins 36–48 h post-tick attachment), it appears that the effect on Borrelia transmission is due to the ability of tHRF to promote engorgement rather than to a specific interaction as with Salp15. Besides, increased vascular permeability effect of histamine may lead to the dissemination of Borrelia injected by the tick into the feeding site (15). Blocking tHRF might offer a viable strategy to develop vaccines that block tick feeding and therefore transmission of tick-borne pathogens. An I. ricinus ortholog of I. scapularis tHRF has been identified, but it had no effect on tick feeding upon vaccination. These results suggested that ticks have a multifaceted control mechanism for histamine and redundancy in the expression of histamine releasing factor proteins (31).
It has been shown that inhibition of the complement cascade at the tick bite site would not only be beneficial for the tick attachment but could also play a role in the survival and transmission of vector-borne pathogens in the mammalian host. Characterization of Salp15-assisted transmission of Lyme spirochetes demonstrates the importance of alternative and classical complement pathways in vertebrate host control of the spirochete. Unlike Salp 15, tick mannose-binding lectin inhibitor (TSLPI) does not bind to B. burgdorferi but instead interacts with the lectin-complement system, inhibiting complement cascade activation and reducing complement-mediated lysis of Borrelia. It is a glycosylated secretory salivary protein of 8 kD that was first identified from the salivary glands of I. scapularis. Expression of TSLPI mRNA levels is up-regulated in salivary glands 24 h after tick attachment compared to uninfected ticks, facilitating the survival of the spirochete and pathogen transmission. TSLPI-silenced ticks, or immunization of mice with anti-TSLPI antibodies, hampered B. burgdorferi transmission and acquisition by ticks (14). An ortholog from I. ricinus inhibits the lectin complement pathway and protects B. garinii and B. burgdorferi s.s. from complement-mediated killing in vitro, but TSLPI-assisted transmission of B. burgdorferi s.s. has not been identified. The saliva of Ornithodoros species inhibits both the alternative and classical pathways, while several Ixodes species (Ixodes uriae and Ixodes hexagonus) inhibit the alternative complement pathway, indicating that complement inhibitor in tick saliva is one of the important steps following tick attachment (13).
A recent study found that Amblyomma americanum ticks might utilize countervailing functions of pro- and anti-inflammatory proteins to regulate the evasion of host defenses. Both in vitro and in vivo data analyses demonstrated that the ticks first secreted pro-inflammatory proteins (insulin-like growth factor binding-related proteins: AamIGFBP-rP6L, AamIGFBP-rP6S, and AamIGFBP-rP-1) that activated macrophage into the pro-host defense phenotype and then secreted anti-inflammatory proteins (serpins, AAS41, and AAS27) to deactivate the activated immune cells including macrophages (32). Thus, the discovery of tick saliva immuno-modulatory proteins is highly sought after as these might serve as targets in tick-antigen based vaccines to prevent TBPs.
From Tick Biology To Anti-tick Vaccines
The tick biological processes are associated with host attachment, followed by feeding on the host, pathogen transmission to the host, intracellular blood digestion, nutrient extraction from the meal, as well as oviposition and egg-laying. Up to date, these approaches have offered several potential vulnerable targets for developing anti-tick vaccines (Figure 1). Successful vaccination against the tick has been previously achieved by targeting tick gut antigen, such as Bm86. In this section, we review work that has focused on saliva, transmembrane, and blood digestive proteins that are targeted to date and link the research findings to our current understanding and approaches for the development of anti-tick vaccines.
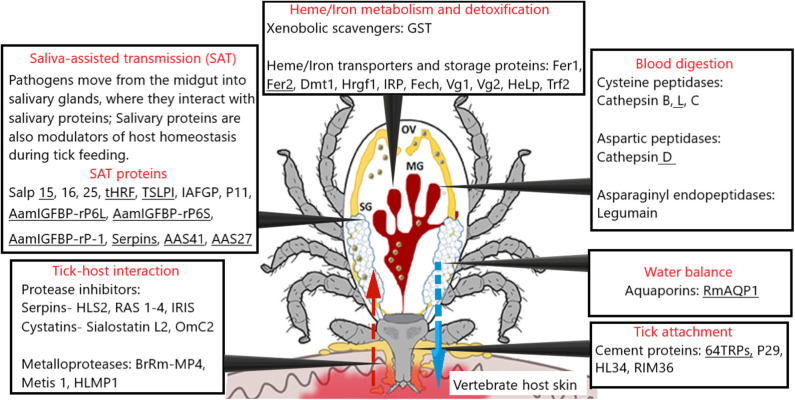
Figure 1.
A schematic representation of tick physiological processes and involved molecules tested as vaccine candidates [modified from (78, 103)]. The most promising vaccine candidates are underlined. The red arrow represents blood meal uptake, and the blue arrow represents saliva injection. Several tick protease inhibitor families have been reported in the salivary glands and implicated in both tick biology/physiology. Major blood digestive enzymes (cathepsin B, C, D, L, and Legumain), blood digestion, heme/iron metabolism, detoxification, and inter-tissue transport that may serve as rational targets for “anti-tick” intervention. SG, salivary glands; MG, midgut; OV, ovary; HLS2, Haemaphysalis longicornis serpin-2; RAS-1,2,3,4, Rhipicephalus appendiculatus; IRIS, Ixodes ricinus immunosuppressor; Sialostatin L, Ixodes scapularis; OmC2, Ornithodoros moubata; Metis-1, metalloproteases from Ixodes ricinus; BrRm-MP4, metalloproteases Rhipicephalus (Boophilus) microplus; HLMP1, Haemaphysalis longicornis metalloprotease.
Tick Salivary Protein Families With Anti-tick Vaccine Potential
Our knowledge of tick salivary glands and saliva components has substantially increased in recent years because of high-throughput transcriptomic, proteomic, and functional analysis (33). Hundreds or thousands of salivary proteins have been identified that are capable of modulating vertebrate host defense by impairing inflammatory reaction, hemostasis, complement cascade, and immunity. Some have been functionally annotated and can be grouped into several protein families, such as lipocalins, glycine-rich proteins, metalloproteases, anticoagulants of the madanin family, and mucins.
Metalloproteases
Tick metalloprotease proteins were identified in saliva, midgut and ovary and play a role in tick physiology, such as innate immunity, blood uptake, blood meal digestion, and vitellogenesis (34). Tick metalloproteases sequences from diverse tick species have been characterized, e.g., I. scapularis, I. ricinus, Ornithodoros savignyi, R. sanguineus, R. microplus, A. americanum, and Amblyomma maculatum (35). Putative metalloproteases (Metis 1 and Metis 2) were identified from the hard tick I. ricinus. RNAi-mediated gene silencing of Metis 1–2 induced a high mortality rate. Vaccination of rabbits with recombinant Metis 1 protein reduced both weight gain and oviposition rate of I. ricinus, but had no effect on mortality and feeding time of ticks (36). Immunization with another recombinant metalloprotease HLMP1 from the Haemaphysalis longicornis resulted in increased mortality up to 15% of ticks (37). The vaccine efficacy with recombinant metalloprotease BrRm-MP4 from the salivary glands of R. microplus was 60% (38). Due to these promising results, the therapeutic potential for metalloprotease proteins might be a challenging task due to their stability and the long-term maintenance of their activity (33).
Protease Inhibitor Families
Several tick protease inhibitor families have been reported in the salivary glands, which have an important role in tick–host interactions (39). There are four groups under serine protease inhibitors, namely Kunitz, trypsin inhibitor-like domain, Kazal-domain inhibitors, and serpins, while the cysteine protease inhibitors belong to the cystatin family (40). The therapeutic potential of Kunitz-, Kazal-type, and TIL-domain inhibitors are reviewed elsewhere (40). Here, we discuss the vaccine potential of the other two groups, serine protease inhibitors and cystatins.
Serine proteinase inhibitors (SERPINs) are one of the largest and most abundant super family of proteinase inhibitors that play essential roles in every living system, from animals to plants. This enzyme family is conserved throughout evolution, and these proteins are involved in normal tick physiology, including the production of antimicrobial peptides, melanization, innate immunity, and blood digestion (41, 42). A flexible reactive center loop (RCL) of SERPINs with a conserved domain structure of 350–400 amino acid residues makes their characteristic structure (42). It has been shown that SERPINs differ from Kunitz-domain protease inhibitors by a distinctive conformational change of the RCL during the inhibition of their target proteases (43). SERPINs also have the ability to bind various protease and non-protease ligands, for example kallistatin, nexin-1, headpin, maspin, C1-inhibitor, and antichymotrypsin (44). SERPINs sequences from diverse tick species have been identified, including Amblyomma hebraeum, Amblyomma variegatum, A. americanum, A. maculatum, Dermacentor variabilis, H. longicornis, R. appendiculatus, R. microplus, I. ricinus, and I. scapularis (45). Despite high numbers of SERPINs from different tick species being identified in both in vitro and in vivo experimental analyses, only a few of them have been characterized functionally. IRIS (I. ricinus immunosuppressor), an immunomodulatory serpin from the salivary glands of I. ricinus, has an effect on host defense mechanisms by interfering with the production of pro-inflammatory cytokines (46). IRIS, together with a serpin from H. longicornis (HLS1) and several other proteins, have methionine and cysteine in their reactive center loop (RCL) and lack a signaling peptide, suggesting intracellular rather than extracellular function. Immunization of rabbits with recombinant IRIS showed significant protection (up to 30% mortality of feeding nymphs and adults) against I. ricinus infestation, but had no effect on nymphs fed on vaccinated mice (47). HLS2 was identified from H. longicornis, and contains a signal sequence and RCL with high sequence similarities to both invertebrate and vertebrate serpins. HLS2 also demonstrated significant protective immunity against adults and nymphs fed on immunized rabbits (48). Four serpins (RAS-1,2,3,4) were identified from R. appendiculatus (40). They were tested in a vaccine trial either as a mix of recombinant RAS-1 and RAS-2 or as a cocktail composed of three antigens, RAS-3, RAS-4, and a 36-kDa immunodominant cement protein RIM36. Vaccination of cattle with recombinant RAS-1 and RAS-2 cocktail reduced the number of engorged R. appendiculatus nymphs by 61 and a 43% and 28 increase in mortality rate in adult males and females, respectively. Similarly, a combination of RAS-3, RAS-4, and RIM36 resulted in a 40% mortality rate for R. appendiculatus ticks and almost 50% for Theileria parva-infected female ticks. Nevertheless, no significant protective effect against infection with T. parva was reported (49, 50).
The second-largest group of tick protease inhibitors is cystatins that are ubiquitously distributed in a wide variety of organisms. Tick cystatins either modulate vertebrate biological processes during tick feedings, such as immunity, antigen processing and presentation, phagocytosis, cytokine expression, and apoptosis, or can regulate hemoglobin digestion in ticks, which is driven by cathepsins (39). There are four cystatin subgroups based on conserved sequence motifs and cystatin domains: type 1 cystatins (stefins), type 2 cystatins, type 3 cystatins (kininogens), and type 4 cystatins (histidine-rich proteins, fetuins) (51). Among them, type 1 and 2 cystatins have been reported in ticks. The type 1 cystatins are typically intracellular and non-secreted cysteine protease inhibitors, while type 2 cystatins are secreted proteins that contain signal peptides as well as single cystatin-like domain. The type 1 (stefins) was the first cystatin isolated from R. (Boophilus) microplus that can inhibit tick vitellin degrading cysteine endopeptidase (VDTCE) and human cathepsin L, suggesting an immunomodulatory role and interaction with the vertebrate pro-inflammatory immune system (52). Similarly to serpins, several cystatins from different tick species have been reported such as Bmcystatin from R. microplus, one cystatin from A. americanum, type 2 cystatin from H. longicornis, type 1 intracellular cystatin from H. longicornis, JpIocys2 from Ixodes ovatus, Om-cystatin 1 and 2 from Ornithodoros moubata soft tick and sialostatin L, L2 from I. scapularis (53). Sialostatin L2 and sialostatin L, novel type 2 secreted cysteine protease inhibitors, were biochemically and immunologically characterized from the salivary glands of I. scapularis ticks that are potentially implicated in the transmission of TBPs. Sialo L2 has been found to interfere with interferon-mediated immune responses in mouse dendritic cells (DC), resulting in enhanced replication of TBEV in bone marrow DC. Further, Sialo L2 promotes infection of mice by impairing inflammasome formation upon the presence of A. phagocytophilum in macrophages through targeting caspase-1 mediated inflammation, suggesting an important role in tick–pathogen interactions (54). Moreover, RNA silencing of sialo L2 resulted in increased mortality of ticks (up to 40%), as well as reduced tick size. Similar effects were reported when I. scapularis nymphs were exposed to guinea pigs immunized with sialo L2 (55). Several other cystatins from different tick species have been reported. Cystatin OmC2 was identified from the soft tick O. moubata. Immunization of mice with recombinant OmC2 significantly reduced the feeding ability and increased mortality (53). The vast majority of cystatin protease inhibitors identified in saliva have anti-inflammatory and immunosuppressive effects at the tick-host interface, suggesting cystatins might also be attractive anti-tick vaccine candidates. Targeting cysteine protease inhibitors does not only block tick attachment to the host and acquisition of the blood meal, but also impairs tick-borne pathogen transmission as demonstrated by vaccination of guinea pig against sialostatin L2 from I. scapularis (53, 55).
Transmembrane Proteins
Vaccine efficacy of transmembrane proteins is well-established since Bm86-based vaccines in cattle tick R. microplus have shown to be an effective tick control strategy. It has been reported that global vaccine efficacies vary from 45 to 100% because of sequence heterogeneity in the Bm86 gene among geographically separated R. microplus strains. A study on Thai cattle ticks found that Bm86 sequences were 91–93% similar to the amino acid reference sequences (56). Nevertheless, it is still the best option available for controlling ticks. Studies with Bm95 (homolog of Bm86 from R. microplus) demonstrated that it protects against a wide range of cattle ticks from different localities, suggesting that the Bm95-based vaccine could be a more universal choice (57, 58). Similarly, vaccination with recombinant Rhipicephalus annulatus Ba86 (an ortholog of Bm86 from R. microplus) was protective against R. annulatus and R. microplus infestations. The vaccine efficacy of Ba86 was slightly higher for R. annulatus than for R. microplus as expected (59). A recombinant Hyalomma anatolicum anatolicum Haa86 antigen is a Bm86 ortholog that is not only effective against tick infestations but also decreased Theileria annulata transmission (60, 61). ATAQ, a new member of promising antigen has been identified from metastriate ticks with high sequence similarity with Bm86 which is expressed in the tick gut and Malpighi tubes, unlike Bm86 orthologs that are expressed primarily in tick midgut (62). This novel protein contains specific epidermal growth factor-like domains (EGF-like) that could be considered as multi-antigen vaccine combination with carriers and adjuvants (63). Apart from Bm86 orthologs and homologs, the only other published antigen with a membrane association was aquaporin. Tick aquaporins belong to the membrane intrinsic protein (MIP) superfamily which is known to play a key role in the transportation of water, glycerol, and urea across the cell membrane. Osmoregulation and water balance functions of aquaporins were examined in I. ricinus by RNAi, which significantly reduced the body weight of semi-engorged females that fed for 5 days. A reported 75% vaccination trial efficacy has been reported against R. microplus infestations (64). Transcriptome studies of R. microplus showed that RmAQP1 as a potential vaccine antigen has significant amino acid sequence similarity to the human aquaporin 7 family and bovine AQP1 orthologs (64). This challenge can potentially be overcome by identifying highly conserved peptide motifs among tick AQP1 that differ from bovine and human AQP1 (65). It should be further tested against different tick species in different geographical regions.
Transmembrane proteins (TM) prediction using bioinformatic methods are reliable because of their transmembrane topology, simple structural features, signal peptide, and membrane-spanning regions (66). Richards et al. (67) have identified 878 putative TM from R. microplus which contribute to our understanding of membrane proteins as a whole and the possibility of using the identified proteins as anti-tick vaccines. TM (as a protein family) have not yet been fully exploited in vaccination studies, though their conserved molecular functions in both prokaryotic and eukaryotic organisms, such as chemical communication between the cell and its environment, cell-cell interactions, metabolism, transport of metabolites through ion channels, signal transduction, and recognition (68). More than 50% of the marketed drugs are targeting peptidergic G-protein-coupled receptors (GPCRs), highlighting their potential pharmacological and biological roles. GPCRs, also called seven-transmembrane receptors, are the most intensively studied drug targets in human medicine. Thus, the discovery of a tick neuropeptide GPCR may have commercial potential for tick control as lead molecules for drug/vaccine development. Moreover, there are no true orthologous mammalian receptors of the tick kinin receptor, which makes it an attractive potential (69, 70).
Blood Digestion Machinery in Ticks
The digestive tract in both ecto- and endoparasites is an excellent potential target for vaccine development, especially through the inhibition of blood digestion (71). It has been reasonably well-established that the tick digestive system is divided into three compartments, namely the foregut, midgut, and hindgut, and each part is further subdivided based on their function and location (72). Digestive cells of the tick midgut are playing an important role in blood digestion. Feeding and digestion of host blood are fundamental biological processes for ticks, which are essential for nutrition, development and transmission of pathogens. Unlike blood-feeding insects that digest blood in the neutral or alkaline pH of the gut lumen (73), ticks digest hemoglobin and other proteins intracellularly (within the endosomes of gut epithelium) in the acidic lysosome-like vesicles of digestive cells lining the midgut epithelium (74). There are notable differences in blood-feeding strategies between soft and hard ticks. Hard tick blood feeding lasts for several days or even longer followed by rapid engorgement (10–22 h), and feed only once in each active life-cycle stage. In contrast to hard ticks, soft ticks take their blood meal very rapidly (0.5–1 h), and oviposition and feeding of their females are repeated. Following blood feeding, midgut epithelium uses receptor-mediated endocytosis and fluid-phase endocytosis (RME) to take up blood meals from the gut lumen (75). Tick hemoglobinolytic systems are carried out by an acidic peptidase (optimum pH 3.5) that contains aspartic peptidases of the cathepsin D type, cysteine endo, and exopeptidases of the papain type (cathepsin C, B, and L) and asparaginyl endopeptidases of the legumain type (75). This multi-enzyme cascade and unique mechanism of activation and substrate binding have the potential for vaccine or drug discovery, as demonstrated by the results of RNAi-mediated silencing of individual peptidases in non-insect arthropods (75). For example, knockdown of cathepsin B (longipain) in H. longicornis by RNAi resulted in reduced blood digestion and transmission of Babesia spp. to the host. Similarly, the silencing of legumain gene (HlLgm1 and HlLgm2) in H. longicornis resulted in reduced oviposition rate, as well as reduced engorged-tick body weight (76).
The proposed hemoglobinolytic pathway was uncovered in the hard tick I. ricinus using proteomic analysis [(75); Figure 2]. Three endopeptidases are most likely responsible for gradual degradation of the hemoglobin substrate, namely cathepsin D (clan AA aspartic peptidases), cathepsin L (clan CA cysteine peptidases), and legumain (clan CD cysteine peptidases). Among these, aspartic cathepsin D is the most dynamic enzyme with high turnover efficiency. Subsequently, the cysteine proteases, namely cathepsin B (clan CA papain-family peptidase) and cathepsin C (clan CA dipeptidyl-peptidase) are the most abundant intestinal peptidases in the next phases of the hemoglobinolysis that cleave large hemoglobin fragments into dipeptides (75). Lists of cysteine and aspartic peptidases identified from different tick species are presented in Table 3.
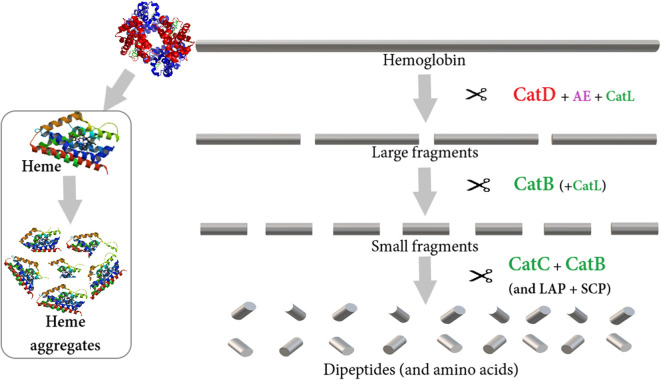
Figure 2.
A proposed model for the hemoglobinolytic pathway in I. ricinus [modified from (75)]. The enzymes are color-coded according to clan membership- AA clan aspartic peptidases (red), CD clan cysteine peptidases (purple), CA clan (papain family) cysteine peptidases (green), and serine and metallopeptidases (black). The endopeptidases, cathepsin D (CatD) supported by cathepsin L (CatL) and legumain (AE), are responsible for the primary cleavage of hemoglobin. The production of secondary small fragments is dominated by the endopeptidase activity of cathepsin B (CatB). Exopeptidases act on the peptides released by the action of the endopeptidases through the carboxy-dipeptidase activity of CatB and the amino-dipeptidase activity of cathepsin C (CatC). Monopeptidases, including leucine aminopeptidase (LAP) and serine carboxypeptidase (SCP), might participate in the liberation of free amino acids.
Table 3.
Lists of cysteine and aspartic peptidases identified from different tick species [modified from (75)].
| Tick species | Type of peptidases | Name of peptidase | Functional characterization | Tissue expression |
|---|---|---|---|---|
| Haemaphysalis longicornis | Serine proteases | HlSP, HlSP2, HlSP3 | RNAi and recombinant enzymes | Midgut and midgut lumen |
| Haemaphysalis longicornis | Legumain | H1Lgm, H1Lgm2 | RNAi and recombinant enzymes | Midgut |
| Ixodes ricinus | Cathepsin L | IrCL1 | RNAi and native or recombinant enzymes | Midgut, salivary glands, ovaries, and Malpighian tubules |
| Rhipicephalus microplus | Cathepsin L | BmCL1 | Native or recombinant enzymes | Midgut |
| Haemaphysalis longicornis | Cathepsin B | Longipain | RNAi and recombinant enzymes | Midgut |
| Ixodes ricinus | Cathepsin D | IrCD1 | RNAi and native or recombinant enzymes | Midgut |
| Haemaphysalis longicornis | Cathepsin D | Longepsin | RNAi and recombinant enzymes | Midgut and salivary glands |
| Haemaphysalis longicornis | Leucine amidopeptidase | HlLAP | RNAi and recombinant enzymes | Midgut, salivary glands, ovaries, and epidermis |
| Ixodes ricinus | Legumain | IrAE | RNAi and native or recombinant enzymes | Midgut |
| Haemaphysalis longicornis | Cathepsin L | H1CPL-A | Recombinant enzymes | Midgut |
| Ixodes ricinus | Cathepsin C | IrCC | Native enzyme | Midgut, salivary glands, ovaries, and Malpighian tubules |
| Ixodes ricinus | Cathepsin B | IrCB1 | Native enzyme | Midgut |
| Rhipicephalus microplus | Cathepsin D | BmAP | Native enzyme | Midgut |
A recent study showed that vaccination with cathepsin D has the potential to control poultry red mite infestations of chickens (77). However, vaccination trials on animals with an individual recombinant digestive enzyme, along with a cocktail of recombinant antigens, did not induce any significant level of protection against I. ricinus (78). Because of aspartic peptidases (APDs) redundancy in tick digestive apparatus, a multi-enzyme target strategy (a combination of aspartic and cysteine protease) might be necessary to exploit tick digestive hemoglobinolytic enzymes as new candidate antigens for the development of tick vaccines. It has been proposed that parasite vaccines should be based on at least two enzymes involved in different physiological processes, for example, blood digestive protein, together with proteins involved in essential physiological processes of ticks. As such, two combined recombinant hookworm antigens, glutathione S-transferases (GSTs) and cathepsin D-like aspartic protease haemoglobinase (APR-1), eventually became a leading vaccine candidate against the human hookworm Necator americanus (79). In this regard, a multi-antigenic vaccine against R. microplus was tested on cattle under field conditions. Vaccination studies with recombinant Boophilus yolk pro-cathepsin (BYC) and vitellogenin degrading cysteine endopeptidase (VTDCE) from R. microplus, and Hl-GST from H. longicornis affect tick physiology, decrease the number of semi-engorged female ticks in the host, resulting in an improved body weight gain of cattle (80). Similarly, cathepsin L and cathepsin D both have potential as vaccine antigens as part of a multi-component vaccine for controlling the poultry red mite Dermanyssus gallinae (81). Future work should be focused on identifying the appropriate synergistic combinations of these proteins to develop a cocktail vaccine for novel therapeutic interventions.
Iron Acquisition and Metabolism
During blood feeding, ticks have to protect themselves from the toxicity of heme and iron from the host blood. Free iron radicals are potentially toxic for all organisms that cause substantial damage to lipids, DNA and protein molecules (82). Unlike ticks, iron originated from heme degradation in hematophagous insects is catalyzed by heme oxygenase (HO) (83). This trait is, however, absent from other blood feeding non-insect arthropods like ticks and mites. Ticks possess a complex biochemical process via hemosome formation that efficiently utilizes non-heme iron within the tick body. Three proteins are involved in vertebrate iron metabolism, namely ferritin (Fer) and the iron regulatory proteins (IRP1) and IRP2 (84). Intracellular ferritin (Fer1) was identified in both soft and hard ticks, which shared sequence similarity with mammalian heavy-chain ferritins and conserved ferroxidase sites (85). Afterward, Fer2 and iron-responsive protein (IRP) were described in I. ricinus, which are exclusively expressed in tick gut, and are required for iron transport to the peripheral tissues for their normal function (84). It has been shown that Fer2 possesses all the important characteristics for the development of a vaccine, including: not being presented in the vertebrate host (concealed antigen property), non-redundancy gene, low homology to mammalian ferritins, and host antibodies can directly contact the tick gut while feeding on a vaccinated host (86). A study showed that vaccination with recombinant tick Fer2 has the potential to control tick infestations on rabbits against I. ricinus (87) and H. longicornis (88). Essentially, the vaccine efficacy of recombinant Fer2 from R. microplus was comparable with the commercial vaccine based on the Bm86 antigen (87). Furthermore, GSTs are present in ticks where they serve primarily in biological detoxification. This enzyme is capable of binding heme in blood-feeding arthropods including ticks, suggesting that GSTs can serve as detoxifying molecules. A vaccination trial on bovines with recombinant GST from H. longicornis showed a cross-protection immunity against infestation with R. microplus (89).
Reverse Vaccinology Approaches in Antigen Discovery
According to Louis Pasteur's principle, vaccine development was based on the “isolate–inactivate–inject” approach before the 1990s. The “first generation” vaccine included live, attenuated and killed types, while the “second generation” vaccine consisted of purified or recombinant components of the targeted antigen. Nevertheless, this approach is a long and laborious task even in successful projects. Besides, these approaches are often unsuccessful because of the complexity of tick's life cycle, complicated host-pathogen-tick interactions and pathogens not possessing clear immunogenic properties. Thus, next-generation vaccination approaches might overcome these challenges by using a combination of functional genomics, bioinformatics and systems biology methodologies (90). This method involves in silico screening of the whole genome of a tick to identify genes with important immunogenic properties followed by wet lab verification with vaccination trials. Tick antigens developed based on biological processes and vaccine approaches tested for vaccine development against ticks are listed in Table 4 (91–95).
Table 4.
Tick antigens developed based on biological process and vaccine approaches tested for vaccine development against ticks.
| Protein type | Species | Identity of protein | Antigen | Vaccination approach | Efficacy of vaccine | References |
|---|---|---|---|---|---|---|
| Secreted | Rhipicephalus appendiculatus, R. sanguineus | Putative cement protein | 64TRP | Recombinant protein, 2nd generation | 62 and 47% mortality | (21, 22) |
| Rhipicephalus appendiculatus | Serine protease inhibitor | RAS-3, RAS-4, RIM36 | Recombinant protein, 2nd generation | 39 and 48% mortality | (49, 50) | |
| Ixodes scapularis | Cystatin type 2 | Sialostatin L2 | Recombinant protein, 2nd generation | 40% mortality | (55) | |
| Ixodes scapularis | Cystatin type 2 | Sialostatin L | Recombinant protein, 2nd generation | Not reported | (55) | |
| Ixodes ricinus | Serine protease inhibitor | IRIS | Recombinant protein, 2nd generation | 30% mortality | (47) | |
| Rhipicephalus microplus, R. annulatus | Ferritin, iron transporter | RaFER2/RmFER2 | Recombinant protein, 2nd generation | 64 and 72% efficacy | (87) | |
| Membrane associated | Rhipicephalus microplus | Angiotensin converting enzyme | Bm91 | Recombinant protein, 2nd generation | 6% reduction of reproductive index | (91) |
| Rhipicephalus microplus, R. annulatus | Aquaporin | Aquaporin | Reverse vaccinology, 3rd generation | 75 and 68% efficacy | (64, 65) | |
| Rhipicephalus appendiculatus, R. decoloratus, R. microplus, Hyalomma anatolicum, H. dromedarii | Bm86 and orthologs | Bm86 and orthologs | Recombinant protein, 2nd generation | 45–100% efficacy | (57–61) | |
| Rhipicephalus microplus | 5′-nucleotidase | 4F8 | Recombinant protein, 2nd generation | No efficacy | (92) | |
| Rhipicephalus microplus | Mucin | BMA7 | Purified components, 2nd generation | 21% reduction in egg weights | (93) | |
| Intracellular | Rhipicephalus microplus | Elongation factor | Ef1a | Recombinant protein, 2nd generation | 31% efficacy | (94) |
| Rhipicephalus sanguineus, R. microplus | Acidic ribosomal protein P0 | pP0 | Synthetic peptide, 2nd generation | 96% efficacy | (95) | |
| Rhipicephalus microplus | Glutathione S transferase | GST-HI | Recombinant protein, 2nd generation | 57% efficacy | (89) | |
| Rhipicephalus annulatus, R. microplus | Regulator factor | Subolesin (4D8) | Recombinant protein, 2nd generation | 0–83% efficacy | (19, 20, 23) | |
| Rhipicephalus microplus, R. annulatus | Ubiquitin | UBE | Recombinant protein, 2nd generation | 15 and 55% efficacy | (94) |
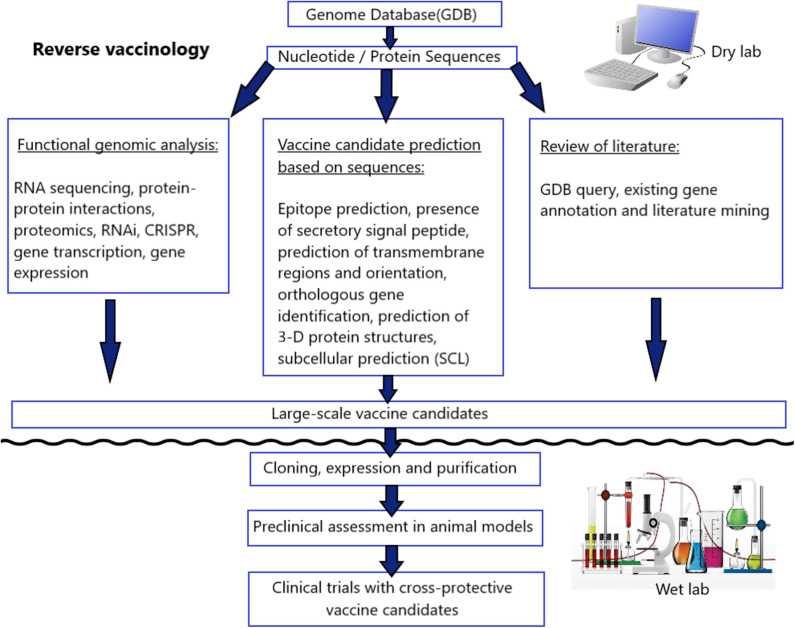
Reverse Vaccinology (RV) is a new methodology to design vaccines from systems biology and functional genomic information with the help of bioinformatics, which was first described in the year 2000 [(96); Figure 3]. Since approximately 2005, two types of RV approaches have been developed for the anti-tick vaccine study, one involving the use of gene knockdown study (RNAi) and the other involving the use of microarray analysis, EST library, subtraction libraries, and CattleTickBase approaches (97). RNAi has the potential to improve understanding of gene function where functional genomic data are limited. It has been reported in several tick species, namely Rhipicephalus haemaphysaloides, A. americanum, H. longicornis, I. scapularis, and D. variabilis. Another RV technique was developed based on a “systems biology” analysis of transcriptome datasets and RNAi screening of candidates, which identified aquaporin antigen from R. microplus (64, 98). Functional genomics has been applied to study the interaction of tick-pathogen-host for transmission blocking vaccine development. For example, molecular studies have been conducted to study the pathogen transmission at the tick-Anaplasma interface, either in vitro or in vivo (99). However, gene knockdown study is slow throughput, off-target effect, difficult to find an appropriate delivery system, the incompleteness of knockdowns and time-consuming approach (100). Hence, an alternative gene-manipulation approach such as CRISPR-based gene drive could be explored for the production of more efficient, stable, and safe parasite vaccines (101, 102).
Figure 3.
A schematic representation of the integrative reverse vaccinology approach toward vaccine development.
Conclusions
This review highlights the progress, opportunities, and challenges offered by recent research into vaccination, as well as provide a wealth of unprecedented information on the genes and proteins involved in the blood digestion process in the ticks, tick-host-pathogen interface, and proteins involved in pathogen transmissions, along with the discussion of potential vaccine targets in recent years. Based on our growing knowledge of tick genome complexity and protein superfamilies, tick vaccines are entering a new genomic era that will facilitate the discovery of novel vaccine antigens. It is becoming clear that the “One Health” approach is needed for developing anti-tick vaccines which means multidisciplinary sectors (biologists, immunologists, bioinformaticians, physicians, veterinarians and pharmaceutical industry, amongst others) communicate and work together to achieve better outcomes.
Author Contributions
QH and BB conceived and designed the study protocol and participated in the writing of the manuscript. BB was a major contributor in writing the manuscript. This manuscript was approved by BB and QH.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Kawser Ayon, Anindya Sen, Bijoy Sen, and Swarup Dhar for making figures and tables.
Footnotes
Funding. The National Key Research and Development Program of China (2017YFD0501200) and Hainan Provincial Key R & D Program (ZDYF2019073).
References
- 1.Bhowmick B, Zhao J, Øines Ø, Bi T, Liao C, Zhang L, et al. Molecular characterization and genetic diversity of Ornithonyssus sylviarum in chickens (Gallus gallus) from Hainan Island, China. Par Vect. (2019) 12:553. 10.1186/s13071-019-3809-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeyaprakash A, Hoy MA. First divergence time estimate of spiders, scorpions, mites and ticks (subphylum: Chelicerata) inferred from mitochondrial phylogeny. Exp Appl Acarol. (2009) 47:1–18. 10.1007/s10493-008-9203-5 [DOI] [PubMed] [Google Scholar]
- 3.de La Fuente J, Estrada-Peña A, Venzal J. Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci. (2008) 13:6938–46. 10.2741/3200 [DOI] [PubMed] [Google Scholar]
- 4.de la Fuente J, Contreras M, Estrada-Peña A, Cabezas-Cruz A. Targeting a global health problem: vaccine design and challenges for the control of tick-borne diseases. Vaccine. (2017) 35:5089–94. 10.1016/j.vaccine.2017.07.097 [DOI] [PubMed] [Google Scholar]
- 5.Kocan KM, de la Fuente J, Blouin EF, Coetzee JF, Ewing SA. The natural history of Anaplasma marginale. Vet Parasitol. (2010) 167:95–107. 10.1016/j.vetpar.2009.09.012 [DOI] [PubMed] [Google Scholar]
- 6.Baneth G. Tick-borne infections of animals and humans: a common ground. Int J Parasitol. (2014) 44:591–6. 10.1016/j.ijpara.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 7.de la Fuente J, Villar M, Contreras M, Moreno-Cid JA, Merino O, Pérez de la Lastra JM, et al. Prospects for vaccination against the ticks of pets and the potential impact on pathogen transmission. Vet Parasitol. (2015) 208:26–9. 10.1016/j.vetpar.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 8.de Castro JJ. Sustainable tick and tick borne disease control in livestock improvement in developing countries. Vet Parasitol. (1997) 71:77–97. 10.1016/s0304-4017(98)00096-x [DOI] [PubMed] [Google Scholar]
- 9.Abbas R, Zaman M, Colwell D. Acaricide resistance in cattle ticks and approaches to its management: the state of play. Vet Parasitol. (2014) 203:6–20. 10.1016/j.vetpar.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 10.de la Fuente J, Contreras M. Tick vaccines: current status and future directions. Expert Rev Vaccines. (2015) 14:1367–76. 10.1586/14760584.2015.1076339 [DOI] [PubMed] [Google Scholar]
- 11.de la Fuente J, Almazan C, Canales M, Perez de la Lastra JM, Kocan KM, Willadsen P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim Health Res Rev. (2007) 8:23–8. 10.1017/S1466252307001193 [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Garcia JC, Gonzalez IL, Gonzalez DM, Valdes M, Mendez L, Lamberti J, et al. Sequence variations in the Boophilus microplus Bm86 locus andimplications for immunoprotection in cattle vaccinated with this antigen. Exp Appl Acarol. (1999) 23:883–95. 10.1023/a:1006270615158 [DOI] [PubMed] [Google Scholar]
- 13.Nuttall PA, Labuda M. Saliva-assisted transmission of tick-borne pathogens. In: Bowman AS, Nuttall PA, editors. Ticks: Biology, Disease and Control. Cambridge: Cambridge University Press; (2008) p. 205–19. 10.1017/CBO9780511551802.011 [DOI] [Google Scholar]
- 14.Schuijt TJ, Coumou J, Narasimhan S, Dai J, Deponte K, Wouters D, et al. A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell Host Microbe. (2011) 10:136–46. 10.1016/j.chom.2011.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai J, Narasimhan S, Zhang L, Liu L, Wang P, Fikrig E. Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the Lyme disease agent. Plos Pathog. (2010) 6:e1001205. 10.1371/journal.ppat.1001205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. (2005) 436:573–7. 10.1038/nature03812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narasimhan S, Sukumaran B, Bozdogan U, Thomas V, Liang X, Deponte K, et al. A tick antioxidant facilitates the Lyme disease agent's successful migration from the mammalian host to the arthropod vector. Cell Host Microbe. (2007) 2:7–18. 10.1016/j.chom.2007.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sukumaran B, Narasimhan S, Anderson JF, DePonte K, Marcantonio N, Krishnan MN, et al. An Ixodes scapularis protein required for survival of Anaplasma phagocytophilum in tick salivary glands. J Exp Med. (2006) 203:1507–17. 10.1084/jem.20060208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bensaci M, Bhattacharya D, Clark R, Hu LT. Oral vaccination with vaccinia virus expressing the tick antigen subolesin inhibits tick feeding and transmission of Borrelia burgdorferi. Vaccine. (2012) 30:6040–6. 10.1016/j.vaccine.2012.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merino O, Almazán C, Canales M, Villar M, Moreno-Cid JA, Galindo RC, et al. Targeting the tick protective antigen subolesin reduces vector infestations and pathogen infection by Anaplasma marginale and Babesia bigemina. Vaccine. (2011) 29:8575–9. 10.1016/j.vaccine.2011.09.023 [DOI] [PubMed] [Google Scholar]
- 21.Rego ROM, Trentelman JJA, Anguita J, Nijhof AM, Sprong H, Klempa B, et al. Counterattacking the tick bite: towards a rational design of anti-tick vaccines targeting pathogen transmission. Parasit Vectors. (2019) 12:229. 10.1186/s13071-019-3468-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labuda M, Trimnell AR, Licková M, Kazimírová M, Davies GM, Lissina O, et al. An anti-vector vaccine protects against a lethal vector-borne pathogen. PLoS Pathog. (2006) 2:e27. 10.1371/journal.ppat.0020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havlíková S, Ličková M, Ayllón N, Roller L, Kazimírová M, Slovák M, et al. Immunization with recombinant subolesin does not reduce tick infection with tick-borne encephalitis virus nor protect mice against disease. Vaccine. (2013) 31:1582–9. 10.1016/j.vaccine.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 24.Antunes S, Merino O, Mosqueda J, Moreno-Cid JA, Bell-Sakyi L, Fragkoudis R, et al. Tick capillary feeding for the study of proteins involved in tick-pathogen interactions as potential antigens for the control of tick infestation and pathogen infection. Parasit Vectors. (2014) 7:42. 10.1186/1756-3305-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juncadella IJ, Anguita J. The immunosuppresive tick salivary protein, Salp15. Adv Exp Med Biol. (2009) 666:121–31. 10.1007/978-1-4419-1601-3_10 [DOI] [PubMed] [Google Scholar]
- 26.Dai J, Wang P, Adusumilli S, Booth CJ, Narasimhan S, Anguita J, et al. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host Microbe. (2009) 6:482–92. 10.1016/j.chom.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuttall PA. Tick saliva and its role in pathogen transmission. Wien Klin Wochenschr. (2019). 10.1007/s00508-019-1500-y [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sultana H, Neelakanta G, Kantor FS, Malawista SE, Fish D, Montgomery RR, et al. Anaplasma phagocytophilum induces actin phosphorylation to selectively regulate gene transcription in Ixodes scapularis ticks. J Exp Med. (2010) 207:1727–43. 10.1084/jem.20100276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Narasimhan S, Dai J, Zhang L, Cheng G, Fikrig E. Ixodes scapularis salivary gland protein P11 facilitates migration of Anaplasma phagocytophilum from the tick gut to salivary glands. EMBO Rep. (2011) 12:1196–203. 10.1038/embor.2011.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paesen GC, Adams PL, Harlos K, Nuttall PA, Stuart DI. Tick histamine-binding proteins: isolation, cloning, and three-dimensional structure. Molecular Cell. (1999) 3:661–71. 10.1016/s1097-2765(00)80359-7 [DOI] [PubMed] [Google Scholar]
- 31.Mulenga A, Macaluso KR, Simser JA, Azad AF. The American dog tick, Dermacentor variabilis, encodes a functional histamine release factor homolog. Insect Biochem Mol Biol. (2003) 33:911–9. 10.1016/s0965-1748(03)00097-3 [DOI] [PubMed] [Google Scholar]
- 32.Bakshi M, Kim TK, Porter L, Mwangi W, Mulenga A. Amblyomma americanum ticks utilizes countervailing pro and anti-inflammatory proteins to evade host defense. PLoS Pathog. (2019) 15:e1008128. 10.1371/journal.ppat.1008128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chmela?r J, Kotál J, Kova?ríková A, Kotsyfakis M. The use of tick salivary proteins as novel therapeutics. Front Physiol. (2019) 10:812 10.3389/fphys.2019.00812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francischetti IM, Mather TN, Ribeiro JM. Cloning of a salivary gland metalloprotease and characterization of gelatinase and fibrin(ogen)lytic activities in the saliva of the Lyme disease tick vector Ixodes scapularis. Biochem Biophys Res Commun. (2003) 305:869–75. 10.1016/s0006-291x(03)00857-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali A, khan S, Ali I, Karim S, da Silva Vaz I, Jr, Termignoni C. Probing the functional role of tick metalloproteases Physiol Entomol. (2015) 40:177–88. 10.1111/phen.12104 [DOI] [Google Scholar]
- 36.Decrem Y, Beaufays J, Blasioli V, Lahaye K, Brossard M, Vanhamme L, et al. A family of putative metalloproteases in the salivary glands of the tick Ixodes ricinus. FEBS J. (2008) 275:1485–99. 10.1111/j.1742-4658.2008.06308.x [DOI] [PubMed] [Google Scholar]
- 37.Imamura S, da Silva VI, Konnai S, Yamada S, Nakajima C, Onuma M, et al. Effect of vaccination with a recombinant metalloprotease from Haemaphysalis longicornis. Exp Appl Acarol. (2009) 48:345–58. 10.1007/s10493-009-9245-3 [DOI] [PubMed] [Google Scholar]
- 38.Ali A, Fernando Parizi L, Garcia Guizzo M, Tirloni L, Seixas A, da Silva Vaz I, Jr, et al. Immunoprotective potential of a Rhipicephalus (Boophilus) microplus metalloprotease. Vet Parasitol. (2015) 207:107–14. 10.1016/j.vetpar.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 39.Chmelar J, Kotál J, Karim S, Kopacek P, Francischetti IMB, Pedra JHF, et al. Sialomes and mialomes: a systems-biology view of tick tissues and tick–host interactions. Trends Parasitol. (2016) 32:242–54. 10.1016/j.pt.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parizi LF, Ali A, Tirloni L, Oldiges DP, Sabadin GA, Coutinho ML, et al. Peptidase inhibitors in tick physiology. Med Vet Entomol. (2018) 32:129–44. 10.1111/mve.12276 [DOI] [PubMed] [Google Scholar]
- 41.Meekins DA, Kanost MR, Michel K. Serpins in arthropod biology. Semin. Cell Dev Biol. (2017) 62:105–19. 10.1016/j.semcdb.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potempa J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem. (1994) 269:15957–60. [PubMed] [Google Scholar]
- 43.Rodriguez-Valle M, Xu T, Kurscheid S, Lew-Tabor AE. Rhipicephalus microplus serine protease inhibitor family: annotation, expression and functional characterisation assessment. Parasit Vectors. (2015) 8:7. 10.1186/s13071-014-0605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patston PA, Church FC, Olson ST. Serpin-ligand interactions. Methods. (2004) 32:93–109. 10.1016/S1046-2023(03)00201 [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro JM, Anderson JM, Manoukis NC, Meng Z, Francischetti IM. A further insight into the sialome of the tropical bont tick, Amblyomma variegatum. BMC Genomics. (2011) 12:136. 10.1186/1471-2164-12-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leboulle G, Crippa M, Decrem Y, Mejri N, Brossard M, Bollen A, et al. Characterization of a novel salivary immunosuppressive protein from Ixodes ricinus ticks. J. Biol. Chem. (2002) 277:10083–9. 10.1074/jbc.M111391200 [DOI] [PubMed] [Google Scholar]
- 47.Prevot PP, Couvreur B, Denis V, Brossard M, Vanhamme L, Godfroid E. Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine. (2007) 25:3284–92 10.1016/j.vaccine.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 48.Imamura S, Da Silva Vaz Junior I, Sugino M, Ohashi K, Onuma M. A serine protease inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine. (2005) 23:1301–11. 10.1016/j.vaccine.2004.08.041 [DOI] [PubMed] [Google Scholar]
- 49.Imamura S, Namangala B, Tajima T, Tembo ME, Yasuda J, Ohashi K, et al. Two serine protease inhibitors (serpins) that induce a bovine protective immune response against Rhipicephalus appendiculatus ticks. Vaccine. (2006) 24:2230–7. 10.1016/j.vaccine.2005.10.055 [DOI] [PubMed] [Google Scholar]
- 50.Imamura S, Konnai S, Vaz Ida S, Yamada S, Nakajima C, Ito Y, et al. Effects of anti-tick cocktail vaccine against Rhipicephalus appendiculatus. Jpn J Vet Res. (2008) 56:85–98. 10.14943/jjvr.56.2.85 [DOI] [PubMed] [Google Scholar]
- 51.Rawlings ND, Barrett AJ. Evolution of proteins of the cystatin superfamily. J Mol Evol. (1990) 30:60–71. 10.1007/bf02102453 [DOI] [PubMed] [Google Scholar]
- 52.Schwarz A, Valdés JJ, Kotsyfakis M. The role of cystatins in tick physiology and blood feeding. Ticks Tick Borne Dis. (2012) 3:117–27. 10.1016/j.ttbdis.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chmelar J, Kotál J, Langhansová H, Kotsyfakis M. Protease inhibitors in tick saliva: the role of serpins and cystatins in tick-host-pathogen interaction. Front Cell Infect Microbiol. (2017) 7:216. 10.3389/fcimb.2017.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen G, Wang X, Severo MS, Sakhon OS, Sohail M, Brown LJ, et al. The tick salivary protein sialostatin L2 inhibits caspase-1-mediated inflammation during Anaplasma phagocytophilum infection. Infect Immun. (2014) 82:2553–64. 10.1128/IAI.01679-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kotsyfakis M, Anderson JM, Andersen JF, Calvo E, Francischetti IMB, Mather TN, et al. Cutting edge: immunity against a “silent” salivary antigen of the Lyme vector Ixodes scapularis impairs its ability to feed. J Immunol. (2008) 181:5209–12. 10.4049/jimmunol.181.8.5209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaewmongkol S, Kaewmongkol G, Inthong N, Lakkitjaroen N, Sirinarumitr T, Berry CM, et al. Variation among Bm86 sequences in Rhipicephalus (Boophilus) microplus ticks collected from cattle across Thailand. Exp Appl Acarol. (2015) 66:247–56. 10.1007/s10493-015-9897-0 [DOI] [PubMed] [Google Scholar]
- 57.García-García JC, Montero C, Redondo M, Vargas M, Canales M, Boué O, et al. Control of ticks resistant to immunization with Bm86 in cattle vaccinated with the recombinant antigen Bm95 isolated from the cattle tick, Boophilus microplus. Vaccine. (2000) 18:2275–87. 10.1016/S0264-410X(99)00548-4 [DOI] [PubMed] [Google Scholar]
- 58.de la Fuente J, Kocan KM. Advances in the identification and characterization of protective antigens for development of recombinant vaccines against tick infestations. Exp Rev Vaccines. (2003) 2:583–93. 10.1586/14760584.2.4.583 [DOI] [PubMed] [Google Scholar]
- 59.Canales M, Almazán C, Naranjo V, Jongejan F, delaFuente J. Vaccination with recombinant Boophilus annulatus Bm86 ortholog protein, Ba86, protects cattle against B. annulatus and B. microplus infestations. BMC Biotechnol. (2009) 9:29. 10.1186/1472-6750-9-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeyabal L, Azhahianambi P, Susitha K, Ray DD, Chaudhuri P, Hmuaka V, et al. Efficacy of rHaa86, an orthologue of Bm86, against challenge infestations of Hyalomma anatolicum anatolicum. Transbound. Emerg. Dis. (2010) 57:96–102. 10.1111/j.1865-1682.2010.01107.x [DOI] [PubMed] [Google Scholar]
- 61.Azhahianambi P, delaFuente J, Suryanarayana VV, Ghosh S. Cloning, expression and immuno protective efficacy of rHaa86, the homologue of the Bm86 tick vaccine antigen, from Hyalomma anatolicum anatolicum. Parasite Immunol. (2009) 31:111–22. 10.1111/j.1365-3024.2008.01082.x [DOI] [PubMed] [Google Scholar]
- 62.Nijhof AM, Balk JA, Postigo M, Rhebergen AM, Taoufik A, Jongejan F. Bm86 homologues and novel ATAQ proteins with multiple epidermal growth factor (EGF)-like domains from hard and soft ticks. Int J Parasitol. (2010) 40:1587–97. 10.1016/j.ijpara.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aguirre AAR, Lobo FP, Cunha RC, Garcia MV, Andreotti R. Design of the ATAQ peptide and its evaluation as an immunogen to develop a Rhipicephalus vaccine. Vet Parasitol. (2016) 221:30–8. 10.1016/j.vetpar.2016.02.032 [DOI] [PubMed] [Google Scholar]
- 64.Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors. (2014) 7:475 10.1186/s13071-014-0475-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ndekezi C, Nkamwesiga J, Ochwo S, Kimuda MP, Mwiine FN, Tweyongyere R, et al. Identification of Ixodid tick-specific aquaporin-1 potential anti-tick vaccine epitopes: an in-silico analysis. Front. Bioeng. Biotechnol. (2019) 7:236. 10.3389/fbioe.2019.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen YK, Li KB. Predicting membrane protein types by incorporating protein topology, domains, signal peptides, and physicochemical properties into the general form of Chou's pseudo amino acid composition. J Theor Biol. (2013) 318:1–12. 10.1016/j.jtbi.2012.10.033 [DOI] [PubMed] [Google Scholar]
- 67.Richards SA, Stutzer C, Anna-Mari Bosman AM, Maritz-Olivier C. Transmembrane proteins – mining the cattle tick transcriptome. Ticks Tick Borne Dis. (2015) 6:695–710. 10.1016/j.ttbdis.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 68.Hubert P, Sawma P, Duneau JP, Khao J, Hénin J, Bagnard D, et al. Single-spanning transmembrane domains in cell growth and cell-cell interactions. Cell Adh Migr. (2010) 4:313–24. 10.4161/cam.4.2.12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhowmick B, Tang Y, Lin F, Øines Ø, Zhao J, Liao C, et al. Comparative morphological and transcriptomic analyses reveal novel chemosensory genes in the poultry red mite, Dermanyssus gallinae and knockdown by RNA interference. bioRxiv [preprint]. (2020). 10.1101/2020.04.09.034587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiong C, Baker D, Pietrantonio PV. The cattle tick, Rhipicephalus microplus, as a model for forward pharmacology to elucidate Kinin GPCR function in the Acari. Front Physiol. (2019) 10:1008. 10.3389/fphys.2019.01008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Willadsen P. Novel vaccines for ectoparasites. Vet Parasitol. (1997) 71:209–22. 10.1016/S0304-4017(97)00028-9 [DOI] [PubMed] [Google Scholar]
- 72.Pritchard J, Kuster T, Sparagano O, Tomley F. Understanding the biology and control of the poultry red mite Dermanyssus gallinae: a review. Avian Pathol. (2015) 44:143–53. 10.1080/03079457.2015.1030589 [DOI] [PubMed] [Google Scholar]
- 73.Briegel H, Lea AO. Relationship between protein and proteolytic activity in the midgut of mosquitoes. J Insect Physiol. (1975) 21:1597–604. 10.1016/0022-1910(75)90197-3 [DOI] [PubMed] [Google Scholar]
- 74.Grandjean O. Aeschlimann A. Contribution to the study of digestion in ticks: histology and fine structure of the midgut ephithelium of Ornithodorus moubata, Murray (Ixodoidea, Argasidae). Acta Trop. (1973) 30:193–212 [PubMed] [Google Scholar]
- 75.Sojka D, Franta Z, Horn M, Caffrey CR, Mares M, Kopacek P. New insights into the machinery of blood digestion by ticks. Trends Parasitol. (2013) 29:276–85. 10.1016/j.pt.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 76.Tsuji N, Miyoshi T, Battsetseg B, Matsuo T, Xuan X, Fujisaki K. A cysteine protease is critical for Babesia spp. transmission in Haemaphysalis ticks. PLoS Pathog. (2008) 4:e1000062. 10.1371/journal.ppat.1000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Price DRG, Küster T, Øines Ø, Margaret Oliver E, Bartley K, Nunn F, et al. Evaluation of vaccine delivery systems for inducing long-lived antibody responses to Dermanyssus gallinae antigen in laying hens. Avian Pathol. (2019) 48:S60–74. 10.1080/03079457.2019.1612514 [DOI] [PubMed] [Google Scholar]
- 78.de la Fuente J, Kopáček P, Lew-Tabor A, Maritz-Olivier C. Strategies for new and improved vaccines against ticks and tick-borne diseases. Parasite Immunol. (2016) 38:754–69. 10.1111/pim.12339 [DOI] [PubMed] [Google Scholar]
- 79.Hotez PJ, Diemert D, Bacon KM, Beaumier C, Bethony JM, Bottazzi ME, et al. The human hookworm vaccine. Vaccine. (2013) 31:B227–32. 10.1016/j.vaccine.2012.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seixas A, Oliveira P, Termignoni C, Logullo C, Masuda A. Rhipicephalus (Boophilus) microplus embryo proteins as target for tick vaccine. Vet Immunol Immunopathol. (2012) 148:149–56. 10.1016/j.vetimm.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 81.Bartley K, Huntley JF, Wright HW, Nath M, Nisbet AJ. Assessment of cathepsin D and L-like proteinases of poultry red mite, Dermanyssus gallinae (De Geer), as potential vaccine antigens. Parasitol. (2012) 139:755–65. 10.1017/S0031182011002356 [DOI] [PubMed] [Google Scholar]
- 82.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. (2004) 117:285–97. 10.1016/s0092-8674(04)00343-5 [DOI] [PubMed] [Google Scholar]
- 83.Zhou G, Kohlhepp P, Geiser D, Frasquillo Mdel C, Vazquez-Moreno L, Winzerling JJ. Fate of blood meal iron in mosquitoes. J Insect Physiol. (2007) 53:1169–78. 10.1016/j.jinsphys.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hajdusek O, Sojka D, Kopacek P, Buresova V, Franta Z, Sauman I, et al. Knockdown of proteins involved in iron metabolism limits tick reproduction and development. Proc Natl Acad Sci USA. (2009) 106:1033–8. 10.1073/pnas.0807961106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kopácek P, Zdychová J, Yoshiga T, Weise C, Rudenko N, Law JH. Molecular cloning, expression and isolation of ferritins from two tick species-Ornithodoros moubata and Ixodes ricinus. Insect Biochem Mol Biol. (2003) 33:103–113. 10.1016/s0965-1748(02)00181-9 [DOI] [PubMed] [Google Scholar]
- 86.Vaughan JA, Sonenshine DE, Azad AF. Kinetics of ingested host immunoglobulin G in hemolymph and whole body homogenates during nymphal development of Dermacentor variabilis and Ixodes scapularis ticks (Acari: Ixodidae). Exp Appl Acarol. (2002) 27:329-40. 10.1023/a:1023347930746 [DOI] [PubMed] [Google Scholar]
- 87.Hajdusek O, Almazan C, Loosova G, Villar M, Canales M, Grubhoffer L, et al. Characterization of ferritin 2 for the control of tick infestations. Vaccine. (2010) 28:2993–8. 10.1016/j.vaccine.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 88.Galay RL, Miyata T, Umemiya-Shirafuji R, Maeda H, Kusakisako K, Tsuji N, et al. Evaluation and comparison of the potential of two ferritins as anti-tick vaccines against Haemaphysalis longicornis. Parasit Vectors. (2014) 7:482. 10.1186/s13071-014-0482-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parizi LF, Utiumi KU, Imamura S, Onuma M, Ohashi K, Masuda A, et al. Cross immunity with Haemaphysalis longicornis glutathione S-transferase reduces an experimental Rhipicephalus (Boophilus) microplus infestation. Exp Parasitol. (2011) 127:113–8. 10.1016/j.exppara.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 90.Valle MR, Guerrero FD. Anti-tick vaccines in the omics era. Front Biosci. (2018) 10:122–36. 10.2741/e812 [DOI] [PubMed] [Google Scholar]
- 91.Lambertz C, Chongkasikit N, Jittapalapong S, Gauly M. Immune response of Bos indicus cattle against the anti-tick antigen Bm91 derived from local Rhipicephalus (Boophilus) microplus ticks and its effect on tick reproduction under natural infestation. J Parasitol Res. (2012) 2012:907607. 10.1155/2012/907607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hope M, Jiang X, Gough J, Cadogan L, Josh P, Jonsson N, et al. Experimental vaccination of sheep and cattle against tick infestation using recombinant 5'-nucleotidase. Parasite Immunol. (2010) 32:135–42. 10.1111/j.1365-3024.2009.01168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McKenna RV, Riding GA, Jarmey JM, Pearson RD, Willadsen P. Vaccination of cattle against the Boophilus microplus using a mucin-like membrane glycoprotein. Parasite Immunol. (1998) 20:325–36. 10.1046/j.1365-3024.1998.00149.x [DOI] [PubMed] [Google Scholar]
- 94.Almazán C, Moreno-Cantú O, Moreno-Cid JA, Galindo RC, Canales M, Villar M, et al. Control of tick infestations in cattle vaccinated with bacterial membranes containing surface-exposed tick protective antigens. Vaccine. (2012) 30:265–72. 10.1016/j.vaccine.2011.10.102 [DOI] [PubMed] [Google Scholar]
- 95.Rodríguez-Mallon A, Encinosa PE, Méndez-Pérez L, Bello Y, Rodriguez Fernandez R, Garay H, et al. High efficacy of a 20 amino acid peptide of the acidic ribosomal protein P0 against the cattle tick, Rhipicephalus microplus. Ticks Tick Borne Dis. (2015) 6:530–7. 10.1016/j.ttbdis.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 96.He Y, Rappuoli R, De Groot AS, Chen RT. Emerging vaccine informatics. J Biomed Biotechnol. (2010) 2010:218590. 10.1155/2010/218590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de la Fuente J, Almazán C, Blouin EF, Naranjo V, Kocan KM. RNA interference screening in ticks for identification of protective antigens. Parasitol Res. (2005) 96:137–41. 10.1007/s00436-005-1351-5 [DOI] [PubMed] [Google Scholar]
- 98.Guerrero FD, Miller RJ, Pérez de León AA. Cattle tick vaccines: many candidate antigens, but will a commercially viable product emerge? Int J Parasitol. (2012) 42:421-7. 10.1016/j.ijpara.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 99.de la Fuente J, Blouin EF, Manzano-Roman R, Naranjo V, Almazan C, Perez de la Lastra JM, et al. Differential expression of the tick protective antigen subolesin in Anaplasma marginale- and A. phagocytophilum-infected host cells. Ann N Y Acad Sci. (2008) 1149:27–35. 10.1196/annals.1428.056 [DOI] [PubMed] [Google Scholar]
- 100.Tuckow AP, Temeyer KB. Discovery, adaptation and transcriptional activity of two tick promoters: construction of a dual luciferase reporter system for optimization of RNA interference in Rhipicephalus (Boophilus) microplus cell lines. Insect Mol Biol. (2015) 24:454–66. 10.1111/imb.12172 [DOI] [PubMed] [Google Scholar]
- 101.Buchthal J, Evans SW, Lunshof J, Telford SR, Esvelt KM. Mice against ticks: an experimental community-guided effort to prevent tick-borne disease by altering the shared environment. Philos Trans R Soc Lond B Biol Sci. (2019) 374:20180105. 10.1098/rstb.2018.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suarez CE, Bishop RP, Alzan HF, Poole WA, Cooke BM. Advances in the application of genetic manipulation methods to apicomplexan parasites. Int J Parasitol. (2017) 47:701–10. 10.1016/j.ijpara.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 103.de la Fuente J, Antunes S, Bonnet S, Cabezas-Cruz A, Domingos AG, Estrada-Peña A, et al. Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front Cell Infect Microbiol. (2017) 7:114. 10.3389/fcimb.2017.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]