Abstract
Alcohol drinking is typically initiated in adolescence, with use sometimes escalating to problematic levels. Escalation of drinking is often associated with a shift in drinking motives, with goal-directed initial use later transitioning to more habitual behavior. This study assessed whether adolescents are more sensitive than adults to habit formation when indexed via insensitivity to reward devaluation in an operant task for food reward. Adolescent and adult Sprague-Dawley rats were trained on either a random ratio (RR) or random interval (RI) schedule before undergoing devaluation. Adolescent animals on both schedules increased the number of lever presses across all training days. In contrast, adults in the RR group increased the number of lever presses across days whereas RI adults remained relatively stable. In response to pellet devaluation, only adolescents exhibited reduced responding, suggestive of goal-directed behavior, whereas no age differences were evident following control (home cage chow) devaluation. Contrary to our hypothesis, adolescents (but not adults) displayed goal-directed responding indexed via sensitivity to reward devaluation. These findings suggest that adolescents are not necessarily more likely to develop habits than adults, and hence other factors may contribute to the greater propensity of adolescents to engage in and escalate alcohol use.
Keywords: adolescent, ethanol, habit formation, Sprague-Dawley, reward devaluation, goal-directed responding
Alcohol use is typically initiated during early-to-mid adolescence in the United States, with levels of per occasion use typically greater among adolescents than those observed in adulthood (e.g., Johnston et al, 2016). Enhanced consumption levels are likewise often seen among adolescents relative to adults in rodent studies (Brunell & Spear, 2005; Doremus et al, 2005; Garcia-Burgos et al, 2009; Hargreaves et al, 2009; Maldonado et al, 2008; Vetter, Doremus-Fitzwater, & Spear, 2007; Yoshimoto et al, 2002), suggesting that developmentally-enhanced alcohol intake during adolescence may be in part biologically-based. Some alcohol use among adolescents reaches problematic levels, with the incidence of individuals reaching DSM-IV criteria for alcohol abuse and dependence reaching the highest levels among late adolescents (18–23 year olds), followed by early adolescents (12–17 year olds), and the lowest incidence levels observed among individuals over 50 years of age (Harford et al, 2005). Age of onset of drinking during adolescence has been shown to be an important predictor of later alcohol problems and dependence, with individuals who began drinking early in adolescence being substantially more likely to develop alcohol use disorders and dependence than those who began drinking at 21 years of age or older (Chorlian et al, 2013; Guttmannova et al, 2011; Hingson et al, 2006; Morean, Corbin, & Fromme, 2012; Ohannessian et al, 2015). These associations, however, do not necessarily reflect causality and could reflect predisposing factors that increase both the propensity for early use as well as later problems with or dependence on alcohol.
A number of factors could contribute to the enhanced vulnerability of adolescents to develop alcohol abuse and dependence. One possibility is that adolescent use of alcohol may alter normal maturational changes occurring during adolescence in brain regions contributing to alcohol’s rewarding properties and other observed effects of alcohol and other drugs in a way that directs behavior towards these drugs and their cues and facilitates drug-seeking (e.g., see Geier, 2013, for review). For example, rodent studies have revealed that higher intakes of alcohol may be evident in adolescents than adults in part because the younger animals are more sensitive to alcohol’s reward and social facilitating effects while being relatively resistant to alcohol effects thought to serve as negative feedback cues to limit intake (e.g., sedation, aversion, motor impairment) (see Spear, 2016, for review).
It is also possible the developmental state of the adolescent brain may facilitate expression of processes thought to contribute to the development of dependence on alcohol and other drugs. These processes include the emergence of incentive sensitization to drug-predictive cues (Robinson & Berridge, 2008) as well as induction of negative affective states during withdrawal that can be countered by further use (Koob & Le Moal, 2005). Yet, there is some evidence that both the attribution of incentive salience and induction of negative affective states may be attenuated in adolescence. That is, adolescent rats have been shown to express less incentive sensitization than adults when indexed via “sign-tracking” (directing attention to a cue predicting a reward) (e.g., Anderson & Spear, 2011; Doremus-Fitzwater & Spear, 2011) and to exhibit less acute withdrawal (Brasser & Spear, 2002; Doremus et al, 2003; Doremus-Fitzwater & Spear, 2007; Varlinskaya & Spear, 2004) and display weaker aversive responses (see Doremus-Fitzwater & Spear, 2016, for review) to alcohol than adults. Other potential contributors to the propensity for the emergence of alcohol/drug abuse and dependence include high levels of impulsivity (Hammerslag et al, 2017; Winstanley et al, 2010) and low levels of self-control (Baler & Volkow, 2006), as well as the induction of compulsive, habitual drug-seeking (Belin et al, 2013). The former factors could potentially play significant roles in the emergence of alcohol use disorders in human youth given evidence derived from studies in both humans and laboratory animals that adolescents exhibit less inhibitory control (Andrzejewski et al, 2011; Geier, 2013; Hunt et al, 2016) and are more impulsive (Burton & Fletcher, 2012; Doremus-Fitzwater, Barreto, & Spear, 2012; Steinberg et al, 2009) than adults under some conditions. It is unclear, however, as to whether the emergence and persistence of alcohol/drug-taking behavior in adolescents might be related to a greater predisposition for habit formation, given the limited and mixed findings to date regarding the propensity of adolescents to develop habitual behaviors.
Instrumental behaviors induced by positive reinforcers (including natural reinforcers as well as alcohol and other drugs) reflect two different, albeit not completely separable processes: goal-directed behaviors and habits (Adams & Dickinson, 1981). Early in the acquisition of instrumental behavior, responding typically reflects intentional, goal-directed action related to the development of action-outcome associations. Such responding is highly sensitive to the value attributed to the goal and is disrupted by reinforcer devaluation induced via conditioning an aversion to the reinforcer or inducing pre-test satiation to the reinforcer (outcome degradation) or through removing the contingency between response and outcome (contingency degradation). After repeated instrumental training, strong associations often develop between the response and the contextual and specific conditioned stimuli (CSs) present at the time of reinforcement. As a result of these stimulus-response associations, habitual responding is thought to emerge, with this behavior being elicited “automatically” in response to the CSs and relatively resistant to devaluation of the reinforcer. Rate of emergence of habitual behavior can be promoted through the use of operant schedules where responses are linked relatively loosely to outcome (Dickinson et al, 1983). For example, habit formation has been reported to develop more rapidly with random/variable interval (RI; VI) schedules, where the time after the last reinforcer rather than the number of responses per se determines whether a given response leads to reinforcement, than with fixed ratio (FR) or random/variable ratio (RR; VR) schedules where amount of responding is directly related to the number of reinforcers received (see O’Tousa & Grahame, 2014, for discussion). Speed of habit formation is also influenced by the nature of the reinforcer, with habitual behavior emerging more rapidly with alcohol and other drugs of abuse than with natural reinforcers (see Everitt, 2014, for discussion and references).
Few studies have compared development of habit versus goal-directed behaviors among adolescents. In one of the few studies to compare adolescent and adult rats for development of habitual responding, adolescents but not adults trained on a RI schedule for sweetened ethanol showed decreases in responding after contingency degradation, a pattern of findings consistent with goal-directed responding in the adolescents and habit formation in the adults (Serlin & Torregrossa, 2015). Even with more extended training designed to promote stronger habit formation, the adolescents remained goal-directed. The opposite conclusion, however, was reached in two studies using a food reinforcer. In a study by Naneix and colleagues (2012), adolescent and adult Long-Evans rats were trained on a VR schedule for food and sucrose reward. Adults but not adolescents showed decreases in responding after contingency degradation, suggesting that it was the adults who were responding in a goal-directed manner, with the responding of the adolescents consistent with habit formation. Both adolescents and adults, however, markedly decreased responding after outcome degradation, suggesting evidence of goal-directed responding at both ages following this means of reinforcer devaluation. The study by Westbrook, Hankosky, Dwyer, and Gulley (2018) also identified a lack of age differences following outcome devaluation, with adults and adolescents both displaying goal-directed behavior. In contrast, evidence for adolescents to display more habitual responding after reward devaluation relative to adults was also reported in a Pavlovian approach task where a tone predicted automatic delivery of an upcoming food reward, and head dips in the pellet area during the tone were measured (Hammerslag & Gulley, 2014). In this study, adult males showed the greatest reduction in head dipping during tone delivery after devaluation, data suggesting that the behavior of adolescent Sprague-Dawley rats was less goal-directed in this task than that of their adult male counterparts. Clearly, more work is needed to determine the propensity of adolescents relative to adults to express habitual versus goal-directed responding.
The objective of the present study was to determine whether adolescent and adult male Sprague-Dawley rats differ in the expression of habitual behavior when assessed via reinforcer devaluation among animals trained on an instrumental conditioning task for food reward under RR and RI schedules of reinforcement. Our hypothesis was that the adolescents would be more likely to form habitual behavior than adults under these test circumstances, particularly under the RI schedule designed to promote stronger habit formation. Surprising, the results showed compelling evidence for the opposite – i.e., adolescents were observed to continue to show degradation-sensitive, goal-directed behavior at a time during training when adult responding had become habitual (degradation-insensitive).
General Method
Subjects
Male Sprague-Dawley rats derived from our breeding colony at Binghamton University were used. Animals were weaned, pair-housed with same-sexed littermates on postnatal day 21 (P21) and maintained in a temperature-controlled vivarium on a 12:12 h light-dark cycle (lights on at 07:00), with food (Purina rat chow, Lowell, MA), and water available ad libitum unless otherwise noted. Rats were reared, maintained and treated according to the Guide for the Care and Use of Laboratory Animals established by the National Institutes of Health, using protocols approved by the Binghamton University Institutional Animal Care and Use Committee. All animals were tested between 10:00 – 14:00 hours each day.
Apparatus
Animals were trained and tested in operant chambers (ENV-007 Med Associates INC, St. Albans, VT; dimensions: 30.5 cm x 24.1 cm x 21.0 cm) located within light- and sound-attenuating boxes (55.9 cm x 38 cm x 35.6 cm). Each chamber had two retractable levers that were positioned on either side of a food trough, with a house light located on the opposite wall. A pellet dispenser (ENV-203, MED Associates INC) delivered single 45 mg banana-flavored sucrose pellets (Bio-Serv, Frenchtown, NJ) into the food trough. The location of the rewarded lever was assigned randomly to each animal, with presses on the other lever also recorded, but not activating the pellet dispenser. On each training day, rats were placed individually into an operant chamber, with each session beginning with the activation of- and ending with the termination of the house-light. Rats were allowed five minutes to explore the chambers prior to session onset on the day of magazine training, with sessions each day thereafter beginning within one minute after placement into the operant chambers. Following each session, the operant chambers were wiped down with a 6% solution of hydrogen peroxide in tap water, and allowed to dry before the next group of animals was tested.
Pre-training (P22–27 or P63–68).
Prior to the onset of training, rats were re-housed with a same-aged, same-sexed non-littermate (on P22 or P63 for animals to be tested in adolescence and adulthood, respectively). Animals were handled for five minutes daily for the next five days. Beginning on P25 or P66, rats were gradually food restricted to reach 85–90 % of their free feeding body weight, and then were maintained on this trajectory throughout the remainder of the experiment based on age-weight charts derived previously in our laboratory (Vetter, Doremus-Fitzwater, & Spear, 2007). To accomplish this, rats were weighed each day and the amount of food provided adjusted daily as necessary to maintain animals on their target weight trajectory. The day prior to the onset of training (P27 or P68), the adolescent and adult rats, respectively, were given approximately five grams of sucrose pellets in their home cage to familiarize them with the reinforcer to be used in the operant sessions.
Experiment 1
Subjects.
Eight to ten rats were assigned to each of the four groups defined by the 2 Age (Adolescent; Adult) X 2 Schedule (RI; RR) factorial design of this study. Schedule assignment was counterbalanced across litters and testing squads.
Operant Training (P28–37 or P69–78).
Magazine training was conducted on P28 or P69. During this session, no levers were extended and only the house-light was illuminated. Sucrose pellets were dispensed into the food trough on a variable interval 60 second (VI60) schedule for a set amount of 30 pellets. Operant training on a fixed ratio schedule (FR) began the following day (P29 or P70), with each press of the assigned training lever (randomly assigned left or right lever) resulting in pellet delivery on an FR1 schedule. The reinforcer was a banana-flavored sucrose pellet (abbreviated “sucrose pellet” throughout). Activation of the house-light at the start of the session was accompanied by extension of the left and right levers, with retraction of the levers and deactivation of the house-light occurring when criterion (pressing 50 times on the training lever) was met, or at the conclusion of the 60 minute session, whichever came first. Rats received three days of FR1 training between P29–31 or P70-P72. Any animal that did not reach criterion by the end of the third session was excluded from the study. Following FR1 training, rats began training on a RR or RI schedule as determined by their group assignment. Each rat received two training sessions on their assigned RR10 or RI30 schedule followed by four days of training on the more demanding (RR20 or RI60) schedules, respectively. The criterion for successful completion of each these training sessions was receipt of 30 sucrose pellets within the 60 minute session. Animals failing to reach criterion on two consecutive scheduled training days were removed from analyses. Only one adult rat per schedule was removed after failing to meet FR1 criterion, with a final n of 8 for both adolescent groups and n of 9 for both adult groups.
Devaluation and Extinction Testing (P38–39 or P79–80).
Following the last day of RR20 or RI60 training, the rats underwent two days of devaluation and extinction testing. On the first devaluation/extinction day, animals received ad libitum access to either sucrose pellets or their standard chow pellets for a one-hour period prior to a five-minute extinction test during which correct and incorrect lever presses were recorded, but no reinforcements were delivered. On the subsequent day, the procedure was repeated, but with the alternative pre-test exposure provided to each animal. Order of exposure to the two pre-exposure conditions was counterbalanced across animals.
Data Analyses.
Acquisition of lever pressing (FR1) was analyzed using 2 age (adolescent; adult) x 3 day repeated measures analysis of variance (ANOVA). Training data on lever pressing/reward receipt during RR 10/20 and RI 30/60 were assessed using a 2 age (adolescent; adult) x 2 schedule (RR; RI) X 6 day repeated measures ANOVA. Lever pressing on the devaluation days was modified to represent a percent responding of the last day of training. To appropriately power a 3-way ANOVA to compare responding between devaluation types requires more animals than used in the current study and therefore responding following devaluation was analyzed using a 2 age (adolescent; adult) x 2 schedule (RR; RI) ANOVA for each devaluation type. Post-hoc analyses were conducted using Tukey’s tests.
Experiment 2
Subjects.
Animal source, husbandry, housing food restriction, pretraining protocol, and initial magazine training procedures were identical as in Experiment 1. Six to eight male rats were assigned to each group defined by the 2 (Age) X 2 (Schedule) X 2 (Devaluation Condition) factorial.
Operant Training (P29–52 or P70–93).
Four days of FR1 training (from P29–32 or P70–73) were conducted using procedures as outlined in Experiment 1. Any animal that did not reach training criteria for FR1 (failure to receive all 50 rewards on the final day of FR1 training) was removed from the study. The FR1 training period was followed by four days of training, i.e., from P33–36 or P74–77, on each animal’s assigned RR10 or RI30 schedule. The next day, the first devaluation and extinction test occurred. Rats were then advanced to their assigned RR20 or RI60 schedule for the next four days (P38–41 or P79–82), followed by a second devaluation and extinction test. This cycle of 4 days of training on the assigned RR20 or RI60 schedule followed by a devaluation/extinction test was repeated two more times for a total of 4 devaluation and extinction test sessions. The criterion for successful completion on each training day was receipt of 30 sucrose pellets within the 60 minute session. As in experiment 1, failure to meet this criterion on two consecutive days resulted in removal of the subject. However, following the first devaluation and extinction session, all exclusion criteria were removed given that animals had previously been tested under extinction conditions. All rats successfully completed FR1 training and the first set of training on RI30 and RR10, therefore no rats were removed from experiment 2.
Devaluation and Extinction Testing.
On each devaluation/extinction test day, animals received either 5 grams of sucrose pellets or standard chow pellets over a one-hour period prior to a five-minute extinction test during which correct and incorrect lever presses were recorded, but no reinforcements were delivered. Devaluation and extinction testing was conducted every fifth day – i.e. on P37, P42, P47 and P52 for adolescents; P78, P83, P88, P93 for adults. All animals received both types of food pre-exposure conditions, with half of the animals receiving sucrose pellet pre-exposure on the first extinction day and the remainder receiving pre-exposure to standard chow pellets, and order of exposure to the two pre-exposure conditions alternating across extinction sessions for each animal and counterbalanced across animals and groups.
Data Analyses.
Analysis focused on FR1 training was conducted using a 2 age (adolescent; adult) x 4 training day repeated measures ANOVA. For schedule training, lever presses per minute and rewards earned per minute were analyzed separately first using a 2 age (adolescent; adult) x 2 schedule type (RR; RI) x 4 training day repeated measures ANOVA for RR10 or RI30 training sessions. An identical ANOVA was then used to examine the following 12 days of training conducted under RR20 or RI60 schedules. As in experiment 1, we failed to identify schedule differences during devaluation and therefore lever presses during extinction were measured using a 2 age (adolescent; adult) x 2 devaluation type (standard chow pellet; sucrose pellet) ANOVA, for each extinction day separately. All post-hoc comparisons were conducted using Tukey’s post hocs.
Results
Experiment 1
Training:
Due to failure to meet criterion during FR1 training, one animal from each of the to-be scheduled adult groups was removed, resulting in final sample sizes of eight and nine rats per schedule condition in adolescent and adults respectively. When analyzing FR1 responding, significant effects of training day, F (2,64) = 104.4, p < .001, age, F (1,32) = 22.26, p < .001, and their interaction, F (2,64) = 7.2, p < .01, were evident. The number of lever presses increased across the three training days at both ages, suggesting acquisition of lever pressing. When comparing adolescent and adult responding, significant differences were evident on days 2 and 3 of FR1 training, with adults displaying more lever presses per minute on these days (see Fig. 1).
Fig. 1.
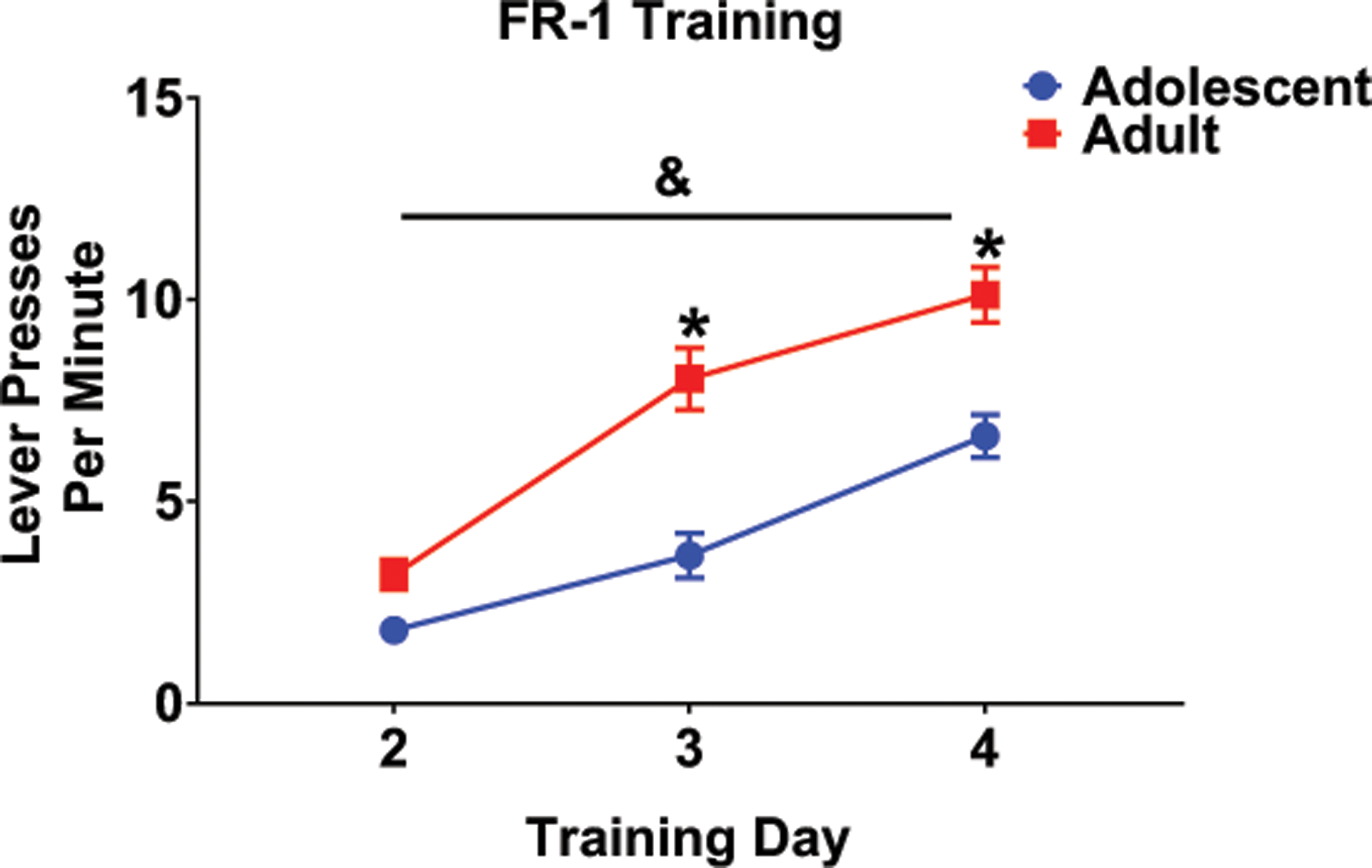
Regardless of age, all animals increased the number of lever presses per minute across the initial three days of FR1 training. On the two last days of FR1 training, adults significantly lever pressed more per minute in comparison to adolescents. Age differences indicated by * and main effect of day represented by &.
Training acquisition across the two schedules was examined via assessing lever presses per minute using a 2 schedule X 2 age X 6 day repeated measures ANOVA. This ANOVA revealed significant main effects of schedule, F (1,30) = 8.97, p = .005, age, F(1,30) = 38.04, p < .001, and training day, F (5,150) = 47.24, p < .001, along with a 3-way interaction of these variables, F (5,150) = 4.38, p < .001. Regardless of schedule type and changes to schedule difficulty, adolescents progressively increased the number of lever presses per minute (see Fig. 2a). For adults, however, whereas animals in the RR group steadily increased the number of lever presses per minute across training days, no change across days was seen for animals in the RI group. Tukey’s post hoc comparisons revealed significant differences between ages and schedules over the final three days of training, with adolescents lever pressing more in comparison to schedule-matched adults (Fig. 2a and Table 1).
Fig. 2.
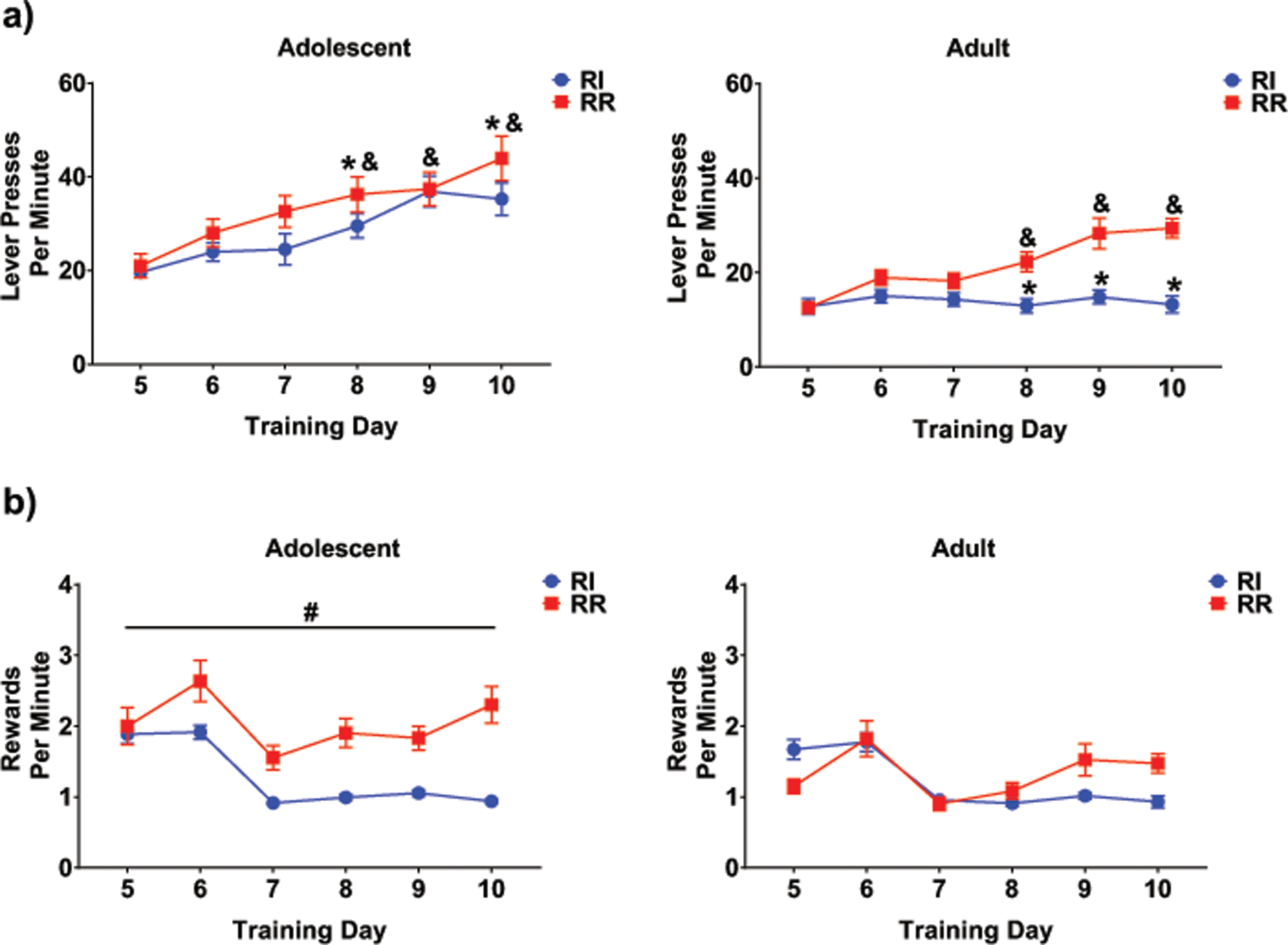
a) Adolescent animals increased lever presses per minute across days regardless of schedule, whereas this effect was only seen in RR scheduled adults (see &, comparing to day 1). Differences between ages within each schedule type were also found (see *). b) Across training days, adolescent animals in the RR group received significantly more rewards per minute in comparison to animals in the RI group (see #), an effect not found in adult animals.
Table 1.
Tukey’s Post Hoc Comparisons for Training Days in Experiment 1.
| Age Comparison | Schedule Comparison | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 |
|---|---|---|---|---|---|---|---|
| A vs B | RI vs RI | .955 | .671 | .411 | .005 | <.001 | <.001 |
| A vs A | RI vs RR | 1.000 | .999 | .855 | .973 | 1.000 | .767 |
| A vs B | RI vs RR | .939 | .999 | .981 | .921 | .736 | .991 |
| B vs A | RI vs RR | .798 | .079 | <.001 | <.001 | <.001 | <.001 |
| B vs B | RI vs RR | 1.000 | 1.000 | 1.000 | .540 | .040 | .004 |
| A vs B | RR vs RR | .759 | .638 | .027 | .038 | .640 | .024 |
Note: For age comparison, A denotes adolescent and B denotes adult. Bold text refers to a significant comparison and italicized text refers to a trend for a difference in the comparison.
A similar analysis was conducted analyzing rewards earned per minute (see Fig. 2b). This ANOVA revealed significant main effects of age, schedule type, and training day (F (1,30) = 13.88, p < .001, F(1,30) = 17.08, p < .001, F(5,150) = 32.27, p < .001, respectively). Interactions between schedule and age, F (1,30) = 9.14, p < .01, as well as schedule and training day, F (5,150) = 10.22, p < .001, were also evident. Tukey’s post hoc analyses revealed differences in the number of rewards received per minute between adolescents receiving training on the RI versus RR schedules (p < .001), with RR adolescents receiving more rewards than RI adolescents; however, no schedule differences emerged in adult animals (p = .850). Tukey’s post hoc comparisons assessing the schedule and training day interaction revealed significantly more rewards per min for animals on the RR schedule across the final three days of training (all ps < .05), an effect that appears driven primarily by adolescent RR animals.
Devaluation:
Consumption during the one-hour free access period preceding extinction can be seen in Figure 3a. Using a 2 (age) x 2 (devaluation type) repeated measures ANOVA, a main effect of age and devaluation type was evident (F (1,32) = 31.53, p < .001 and F (1,32) = 124.3, p < .001, respectively) as well as their interaction, F (1,32) = 9.48, p < .001. Overall, adolescents consumed more g/kg than adults and both ages typically consumed more when given access to sucrose pellets than standard chow pellets. Tukey’s post hoc analyses on the interaction revealed that the greater intake of adolescent than adult rats was driven by the high consumption levels of adolescents during the sucrose pellet access period.
Fig. 3.
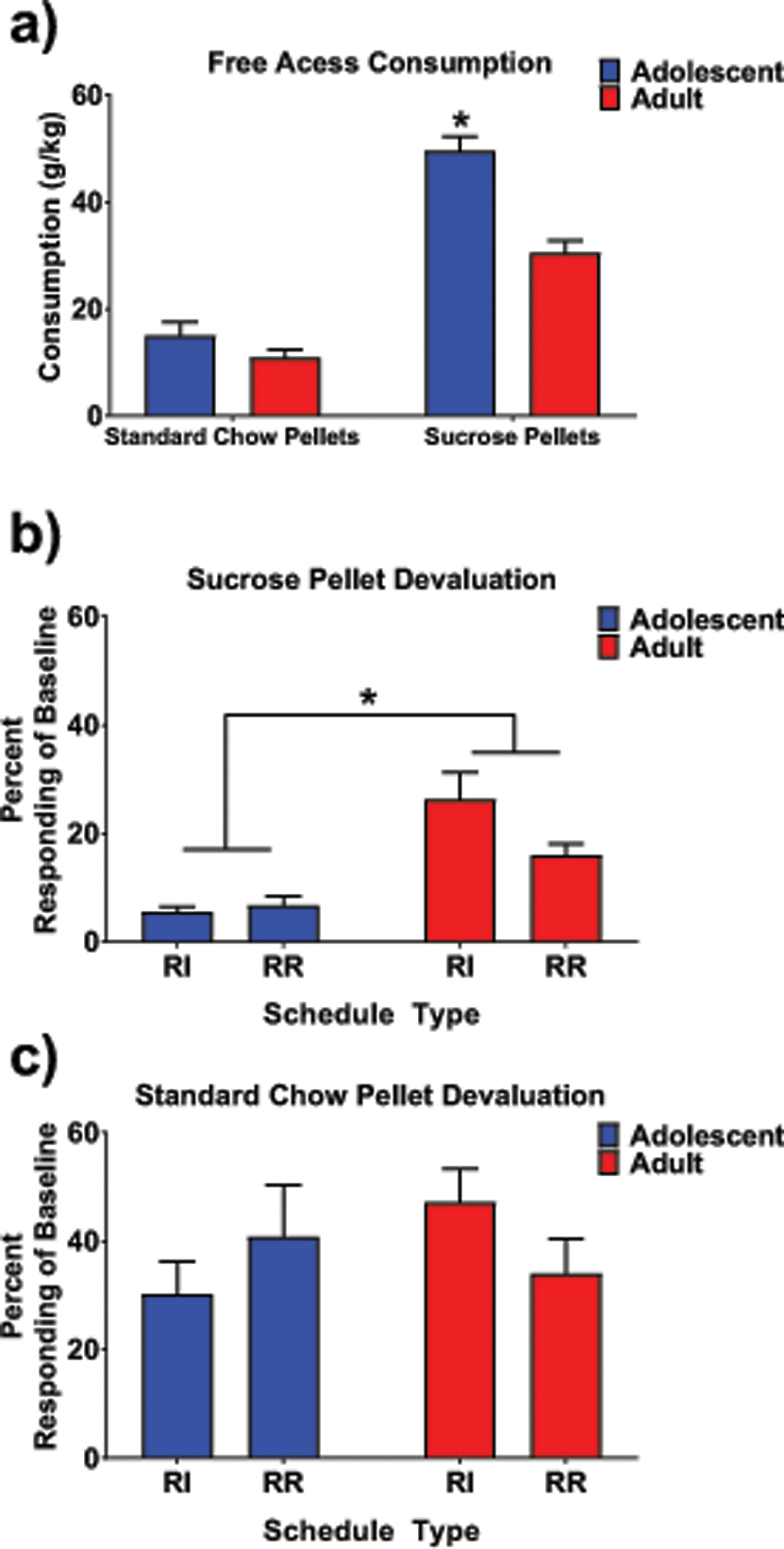
a) Adolescents consumed more g/kg than adults during devaluation procedures, an effect main due to greater intake during the sucrose pellet devaluation (* denotes effect of age). b) During an extinction trial following reinforcer devaluation, adolescents responded significantly less in comparison to adults however after devaluation using home cage chow (c), responding was not different between adolescents and adults. Main effect of age indicated by *.
To assess rate of responding on devaluation days relative to the last training day, lever presses per minute on the devaluation day were divided by lever presses per minute on the final day of training (day 9) and then multiplied by a 100 to create percent baseline responding. When analyzing the reinforcer devaluation data, only a main effect of age, F(1,30) = 25.01, p < .001, was evident, along with a trend towards an interaction between age and schedule, F(1,30) = 3.66, p = .065. Regardless of schedule, adolescent animals responded less following sucrose pellet devaluation than adults, suggesting a more goal-directed response pattern in adolescents relative to more habit-like responding for adults. These data are shown in Fig. 3b. For the standard chow pellet devaluation day, as would be expected, no significant differences were seen for schedule type or age (all ps > .05, Fig. 3c).
To address order effects, analyses were conducted comparing animals that received a devaluation of either sucrose pellets or standard chow pellets on the first devaluation day compared to animals receiving the same devaluation on the second day. For these analyses, we used 2 schedule x 2 day ANOVAs within each age and devaluation type. As can be seen in Supplementary Figure 1a, in most of these comparisons, responding was less on the second devaluation day than the first day (likely reflecting extinction). These differences across devaluation day reached statistical significance in both age groups for standard chow pellet devaluations (F(1,12) = 6.41, p < .05 and F(1,14) = 8.30, p <.05 respectively) and among adolescent RR animals only after sucrose pellet devaluation (the latter revealed by Tukey’s post hoc analyses of an interaction of day and schedule type, F(1,12) = 6.81, p< .05). To assess the possibility that the effects may have been driven by repeated devaluation and extinction testing, an analogous analysis was conducted comparing responding following devaluation only on the first day and similar results were obtained (see supplemental Fig. 2).
Experiment 2
Training:
All rats successfully completed FR1 training and none were removed due to failure to meet criterion during initial schedule training therefore sample sizes were 6–8 per group. When analyzing FR1 training for experiment 2 (that included an additional training day relative to experiment 1), a main effect of training day was evident, F (3,144) = 131.51, p < .001; the number of lever presses per minute steadily increased across days as can be seen in Figure 4. A main effect of age was also found, F (1,48) = 27.23, p = .05, with adults exhibiting more lever presses per minute than adolescents, an effect that seemed to be driven by responding on training days 3 and 4, although the interaction of training day and age did not reach significance.
Fig. 4.
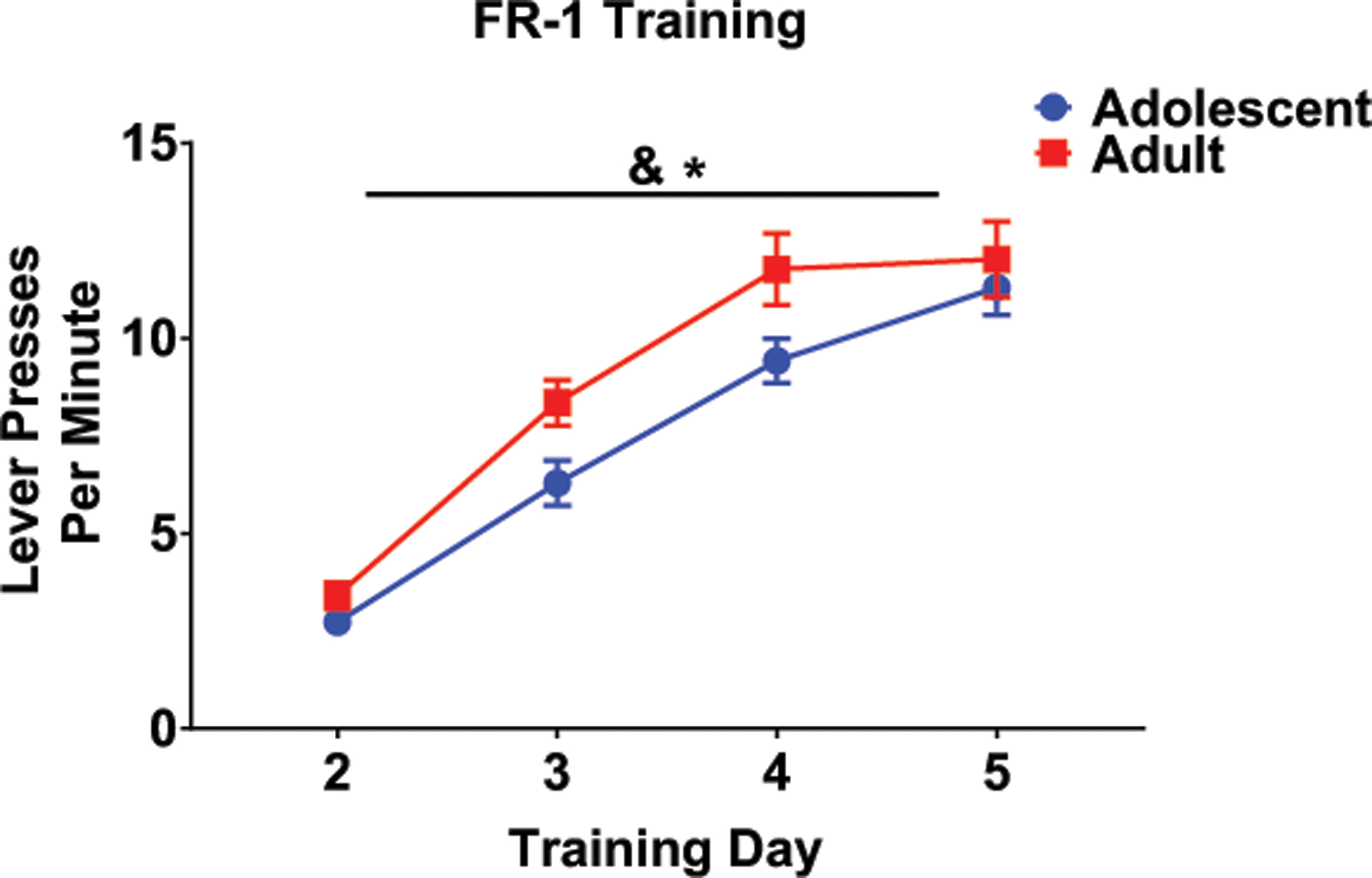
During the initial training on the FR1 schedule, both adolescents and adults increased lever presses per minute, representative of successful acquisition of the task (main effect of day indicated by &). Adults also lever pressed more per minute in comparison to adolescents (main effect of age denoted by *); although this effect appears to be driven by differences on days 3 and 4, the interaction of age and day did not reach significance.
Age and schedule differences potentially emerging during the schedule training were examined using a repeated measures ANOVA analyzing lever presses per minute. This ANOVA revealed significant main effects of age, F (1,46) = 7.24, p < .01, schedule, F (1,46) = 20.62, p < .001, and training day, F (15, 690) = 55.82, p < .001, tempered by significant interactions between training day and age, F (15,690) = 2.65, p < .001, and training day and schedule, F (15,690) = 14.29, p < .001, along with a trend for a significant interaction among all three factors, F (15,690) = 1.56, p = .08. Performance increased over days for both adolescent schedule types, although this effect was only seen in RR adults (Fig.5a). Adolescents typically exhibited more lever presses per minute than adults (Fig. 5b) and RR scheduled rats typically lever pressed more than RI animals, with the latter schedule differences becoming more pronounced over training days 11–24 (corresponding to the shift into RR20 and RI30 schedules and differences between schedules reaching significance over the last two blocks of training days.
Fig. 5.
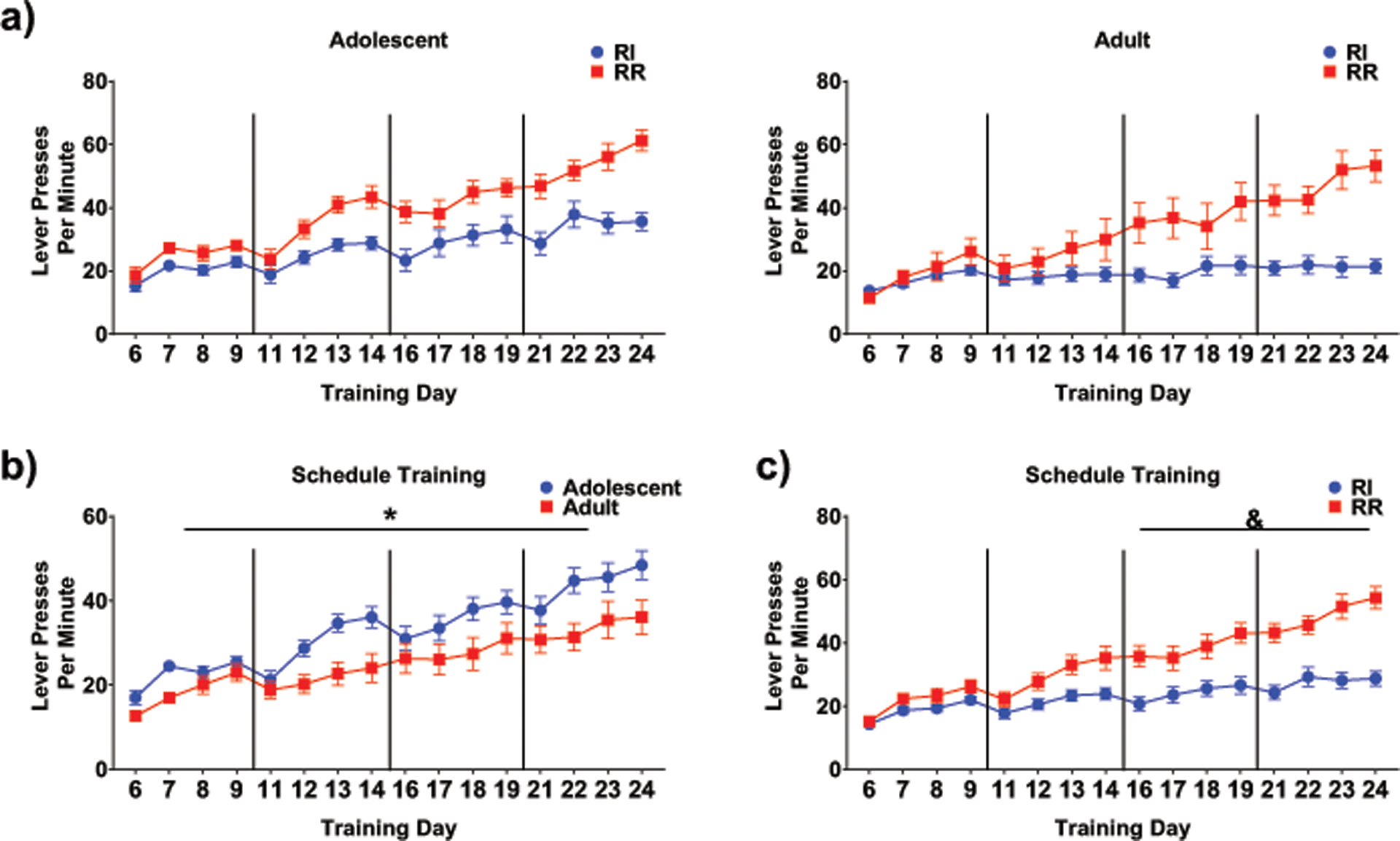
a) For adolescents, both schedule types increased the number of lever presses per minute across training days, an effect only seen in adult RR rats in the last few days (no significance noted due to only a trend for a 3-way interaction). b) Training collapsed by schedule type showing that adolescents generally had more lever presses per minute than adults with this difference reaching significance on day 22 (main effect of age indicated by *). c) When age groups were collapsed to compare the two different schedules, animals on the RR schedule lever pressed more per minute than rats on the RI schedule, an effect that emerged consistently across the second half of training (& denotes schedule difference).
Similar to the findings obtained in Experiment 1, the ANOVA of rewards earned per minute during RR and RI schedule training in this experiment revealed significant main effects of schedule, F (1,46) = 44.26, p < .001, and training day, F (15,690) = 26.47, p < .001, along with a significant interaction of training day and schedule, F (15,690) = 17.35, p < .001. Not surprisingly, rats in the RR20 group received more rewards per minute than those in the RI60 group. Animals on the RR20 schedule also continued to increase the number of rewards earned per minute across training whereas the number of rewards earned by rats on the RI60 remained constant throughout. A trend for an interaction between all three variables was also found, F (11,506) = 1.66, p = .078, with adolescents in the RR20 schedule tending to earn more rewards per minute than their RI60 peers across the majority of training whereas notable differences between RR and RI adults only emerged in the second half of training (data not shown).
Devaluation:
As found in experiment 1, analyses of devaluation including schedule as a factor failed to reveal any effects related to differences between RI and RR groups and therefore data were collapsed across schedules prior to further analyses. Each of the four devaluation days was analyzed separately using a 2 age x 2 devaluation type ANOVA. When analyzing devaluation 1, a main effect of devaluation type, F (1,46) = 8.24, p < .01, and an interaction between age and devaluation type, F (1,46) = 3.91, p = .05, were evident. Similar to the results of Experiment 1, adolescents responded less during extinction following sucrose pellet than standard chow pellet devaluation whereas adults did not (see Fig. 6a). For both devaluations 2 and 3, only main effects of age were evident (F (1,46) = 9.17, p < .01, F (1,46) = 5.92, p < .05, respectively) with adolescents showing lower baseline responding as a percentage of control in these extinction tests regardless of devaluation condition (see Figs. 6b and 6c). For the fourth devaluation, the ANOVA revealed no significant main effects or interactions (Fig. 6d).
Fig. 6.
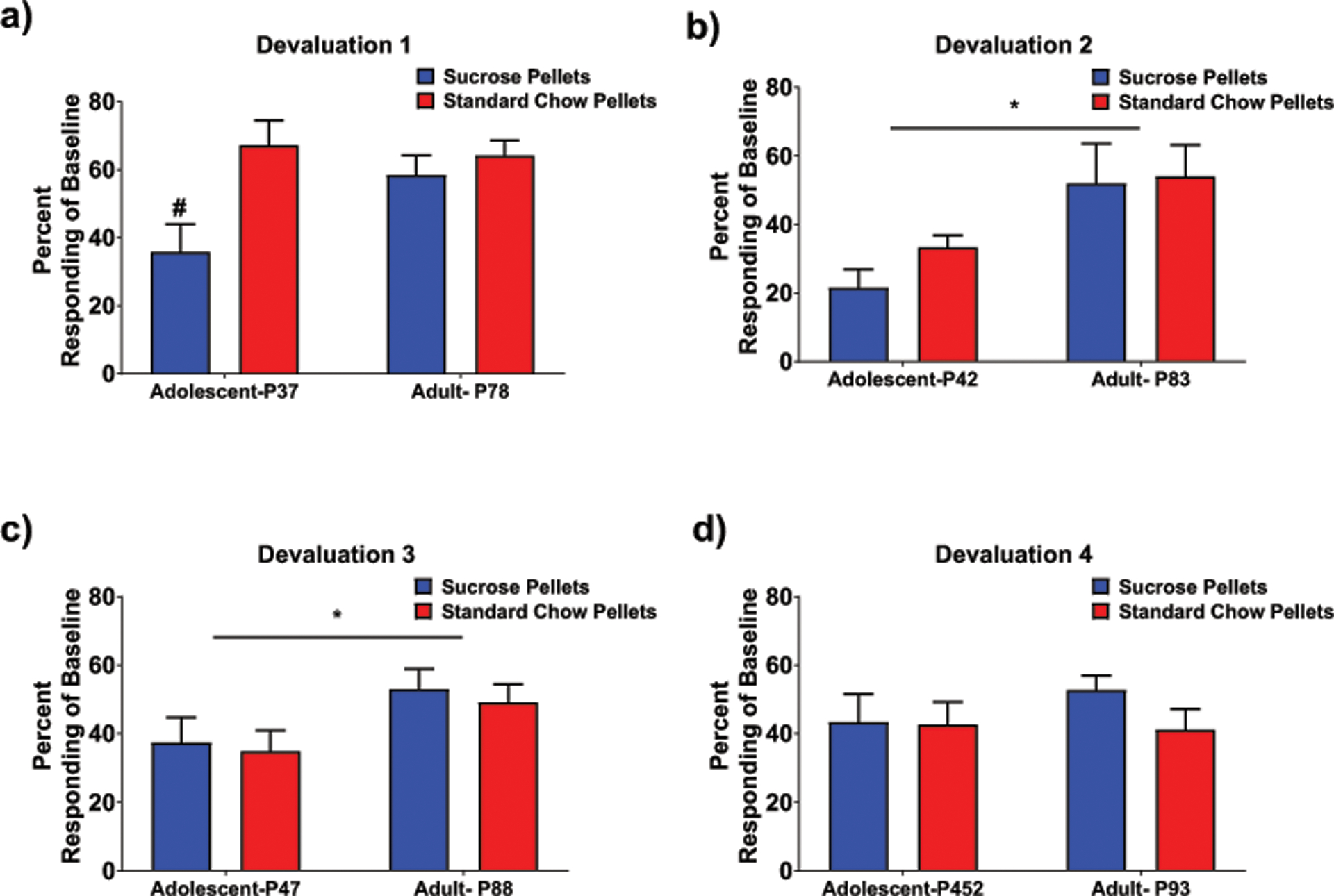
a) During the first devaluation and similar to the results of experiment 1, adolescents following sucrose pellet devaluation significantly reduced their lever pressing compared to baseline, an effect not seen in adults (devaluation effect denoted by #). b) and c) Lever pressing rates following devaluations 2 and 3 were lower for adolescents regardless of devaluation type (* indicates age effect). d) Responding during the extinction trial following the last devaluation type was not different between either age or devaluation group.
Discussion
In this study, we tested the hypothesis that adolescents would exhibit a greater propensity to develop habitual behavior under conditions where adults would display goal-directed behavior. The results indicated the opposite, with adolescent rats behaving more goal-directed after reinforcer devaluation in comparison to adults who responded in a manner more consistent with habitual behavior. More specifically, adolescents exhibited a significantly greater decrease in responding than adults when the reinforcement used on previous training days was given prior to extinction testing. In contrast, similar albeit more modest, decreases in responding were evident at both ages when access to standard chow pellets was given prior to the extinction trial, suggesting that the marked decrease in responding following sucrose pellet devaluation among the adolescents was not a result of general satiety but rather a specific response to sucrose pellet devaluation. The findings of experiment 2 further supported the finding that adolescents display more goal-directed behavior than adults, with this behavior becoming less pronounced with repeated testing and as the animals matured.
During the training phase of experiment 1, adolescent rats did not differ in the number of lever presses per minute as a function of schedule type, with both the RI and RR schedules showing a gradual increase in lever presses over the course of training. Similar results were found for experiment 2, in which adolescents did not display differences in lever pressing as a result of schedule until the last few training sessions. In contrast, the responding of adult rats increased later during training on the RR schedule whereas responding on the RI schedule remained consistent across the training days, an effect evident in both experiments. It is possible that the failure of adolescents to moderate their responding on the RI schedule could be a result of animals on this schedule failing to fully acquire the training pattern. However in experiment 2, where adolescents had extensive training (12 days) on the RI60 schedule, this pattern of responding still emerged. Previous work has also found age differences in responding under partial reinforcement, with adolescents responding more than adults under the same contingencies (Meyer & Bucci, 2016). Other studies have reported that adolescent rats struggle to inhibit behavioral responding as modeled by behaviors such as extinction, findings that further support the age differences in RI responding observed in the present experiments (Andrzejewski et al., 2011; Meyer & Bucci, 2014; Sturman, Mandell, & Moghaddam, 2010). The inability to find schedule differences within the adolescents may also be due to rats in this group not performing the task even though they have acquired the rule. Meyer and Bucci (2014) examined whether age differences in schedule training were due to failures in acquiring associated rules or the implementation of these rules. Through a series of experiments, Meyer and Bucci (2014) determined that adolescents had learned the ability to discriminate between rewarded and non-rewarded trials but failed to inhibit their responding until they reached late adolescence. In the current study, adolescent differences between schedules did emerge in the later portion of training in experiment 2, at a similar age as examined in Meyer and Bucci (2014), supporting the suggestion of a maturation effect occurring in inhibitory brain regions regulating the expression of the required behavior.
When evaluating rewards per minute, adolescents displayed a clear distinction in the number of rewards gained per minute between schedule types. Although adolescent RI animals lever pressed at similar levels to those in the RR group, adolescents in the RR schedule group received more rewards per minute than those in the RI schedule group across the majority of training. This difference between schedule types is most likely due to the inherent properties of an RI schedule, a schedule that requires time between reinforcements regardless of number of lever presses, thereby leading to fewer rewards per minute. Adults in the RR group likewise received more rewards/minute than the RI group, albeit only on the final two days of training in experiment 1 and during the later portion of training for experiment 2. This delay in the adults may be related to the emergence of schedule differences in lever presses/minute relatively late in the training phase, with enhanced responding in the RR adults leading to more rewards/minute on this schedule. As seen with the extended training protocol of experiment 2, these schedule differences in rewards per minute were exacerbated for both ages most likely due to further training.
The finding in the present study of enhanced goal-directed behavior in adolescents and not adults evident during the sucrose pellet devaluation phase in experiment 1 and the first devaluation in experiment 2 is similar to that reported by Serlin and Torregrossa (2015). When examining operant responding for ethanol, they likewise found that adolescents behaved in a goal-oriented pattern when using a contingency degradation procedure, whereas adults responded more habitually. The data, however, are mixed. Westbrook, Hankosky, Dwyer and Gulley (2018) found no age differences in responding following outcome devaluation whereas Naneix and colleagues (2012), when using a contingency degradation model, observed adolescents to exhibit more habitual responding. However, when they used a similar outcome devaluation protocol as used in the current study, both adolescents and adults displayed goal-like behavior, findings similar to that of Westbrook et al (2018). In contrast, Hammerslag and Gulley (2014) found adults to display more goal-directed behavior than adolescents when indexed via decreases in responding following reward devaluation in a Pavlovian instrumental task for food reward. Both contingency degradation and reward devaluation measure components of goal-directed and habitual behaviors, however there is evidence that these tasks are dependent upon different neural mechanisms. For example, dopamine (DA) innervation of prefrontal cortex is important for moderating contingency degradation but not outcome devaluation (Naneix et al, 2009). Further work is needed to address the relationship between contingency degradation and outcome devaluation for the evaluation of goal-directed behavior during ontogeny.
There are other differences that could contribute to variation in findings among studies examining age differences in habit formation. For example, differences in training/testing methodology may be of importance. As noted previously, training protocols varied across studies in the total number of days (e.g. 2 to 8 days) that animals were trained on a particular schedule as well as the number of devaluations used. Such variation in the length of training could contribute to observed differences in results, especially given that the time course for emergence of habit versus goal-directed behavior may vary with age, and hence age differences may be differentially expressed depending on the time during acquisition at which devaluation is assessed. As we found in experiment 2, age differences in responding during extinction changed across the different devaluation days, with adolescents shifting their responding from goal-directed to more habitual responding with subsequent training. Although it is not known whether these changes are related to repeated extinction testing or maturation from adolescence into adulthood, there is reasonable evidence that behavior following multiple devaluations and/or extended training is susceptible to change. It is also possible that the type of reinforcement administered may play a contributing role as well. For instance, Naneix et al. (2012) found adolescents to be resistant to contingency degradation when using sucrose as the reinforcer whereas Serlin and Torregrossa (2015) found the opposite when alcohol was the reinforcement. Although variation in reinforcers does not provide a simple explanation for the age differences in reinforcer degradation observed across studies, it is possible that the salience of the reinforcement may play a role in habit formation and its detection via degradation. Indeed, differences in the salience of rewards across age have been well characterized (see Doremus-Fitzwater, Varlinskaya, & Spear, 2010 for review). While it is unlikely that a single difference across studies accounts for observed age variations, a combination of variables such as training length, schedule properties and type of reinforcement may contribute to the nature of the findings obtained in each study. Further work directed toward reconciling factors leading to the enhancement versus resilience of adolescents in the formation of habits could yield important clues as to critical variables influencing how adolescents process information and direct their behavior.
In experiment 1, several order effects of devaluation day were evident for both adolescents and adults, with both schedules decreasing responding further on the second day of standard chow pellet devaluation, and some differences between RI and RR conditioned animals evident. These order effects associated with the counterbalancing procedures, however, emerged in analyses involving sample sizes of only 3–4 per group for each order, and hence the associated low power may have yielded spurious effects. To determine whether these shifts in responding between extinction testing may have been driving the observed effects, analyses were conducted comparing the first devaluation and extinction responding. These results supported our original findings; hence, this effect was robust enough to be maintained despite the use of low sample sizes for the comparison.
The current studies had a few limitations worth noting. First, only male rats were used in the experiments, and hence age differences in the habitual behaviors noted may only be applicable to adolescent and adult males. Evidence for a lack of age differences following devaluation in female rats has been noted elsewhere (Westbrook, Hankosky, Dwyer, & Gulley, 2018) in contrast to evidence supporting sex differences reported in Hammerslag and Gulley (2014). A second limitation in experiment 1 is that age differences in g/kg of sucrose pellets consumed during devaluation were evident and the greater g/kg consumption of adolescents could have contributed to the devaluation findings supporting more goal-directed behavior at this age. Unfortunately, the current studies did not utilize a post-extinction consumption test to examine how successful the devaluation procedures were in producing sensory-specific satiety and therefore we cannot make a conclusion about whether our procedure produced different levels of satiety between the ages tested. However, both ages had free access to the sucrose pellets with no restriction on consumption during the one hour access period, therefore adults had the ability to consume more and it is possible that the lower intake is correlated with satiety for these animals. That adolescents consumed more sweetened reinforcement when compared to adults is a not novel finding; for instance, Friemel, Spanagel, and Schneider (2010) found that the developmental period around puberty is marked by increased voluntary consumption of sweetened condensed milk relative to adult rats. Similar findings of enhanced consumption of sweetened condensed milk around puberty, specifically in males, were noted by Marshall et al. (2017). Most importantly, age differences in goal-directed behavior in experiment 1 were replicated in experiment 2 where both ages were restricted to 5 grams of intake during the devaluation period. If the age differences in sucrose pellet consumption and/or lack of sufficient devaluation among adult rats were driving the effects of experiment 1, the same age effect would not have been evident in experiment 2. Another limitation of the current study was that the majority of adolescent testing was done pre- and peri-pubertally which could have impacted the results. Early adolescence spans from P28-P45 (Spear, 2000) in rats, and our results measuring goal-directed behavior across the pubertal period could reflect in part ontogeny of those behaviors during the pre-, peri-, and post-pubertal period. Lastly, moderate food restriction as used in the current studies could have impacted the results, with adolescents requiring more food for growth therefore perhaps working harder to obtain that reward during schedule training as well as consuming more during the devaluation free access period in Experiment 1. Food restriction was carefully controlled however, to match the normal growth trajectory of adolescent rats (Vetter, Doremus-Fitzwater, & Spear, 2007), with animals at both ages maintained at roughly 90% of their normal expected weight throughout testing. It should be noted that previous work from our lab (Anderson, Bush, & Spear, 2013) found that food restriction during adolescence can promote goal-directed behavior which cannot be ruled out as a contributing factor in the current work.
The formation of goal-directed and habitual behaviors is of considerable relevance when considering age differences in the propensity for alcohol use and abuse. Previous studies are limited in number to date and have reached differing conclusions as to whether adolescents and adults differ in habit formation. The current study found that adolescents were more sensitive to incentive devaluation and hence appear more goal-oriented than adults, an effect that appears to change with repeated testing or as adolescents age into adulthood. In terms of acquisition, adults showed greater lever pressing later in acquisition on the schedule with greater response-outcome associations (RR) than the schedule with a looser association between response and outcome (RI). In contrast, adolescents showed no difference between schedule types until the final block of training in experiment 2. Although the current study supports the literature in suggesting that adolescents appear to express more goal-directed behavior following reward devaluation, it is not to say this behavior is any less important than habitual behaviors in its contribution to the vulnerability of adolescents to develop problems with alcohol use and abuse.
Supplementary Material
Supplemental Fig. 1. In most comparisons, animals responded less on the second day of devaluation suggestive of an influence of extinction during the prior trial. Main effect of day denoted by &.
Supplemental Fig. 2. To determine the influence of repeated devaluation and extinction testing, analyses were conducted comparing animals that received either sucrose pellet or standard chow pellets prior to extinction testing on the first devaluation day only. Similar results to analyses including both devaluation days were found, with age differences (indicated by *) evident for sucrose pellet devaluation but no differences for standard chow pellet devaluation.
Acknowledgements:
This work was funded by the National Institute of Alcoholism and Alcohol Abuse NADIA project (U01 AA019972), the Developmental Exposure Alcohol Research Center (P50 AA017823), and the Development and Neuroadaptation in Alcohol and Addictions training grant (T32 AA025606).
Footnotes
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Adams CD, & Dickinson A (1981). Actions and habits: Variations in associative representations during instrumental learning. Information Processing in Animals: Memory Mechanisms, 143–165. [Google Scholar]
- Anderson RI, & Spear LP (2011). Autoshaping in adolescence enhances sign-tracking behavior in adulthood: Impact on ethanol consumption. Pharmacology Biochemistry and Behavior, 98(2), 250–260. doi: 10.1016/j.pbb.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski ME, Schochet TL, Feit EC, Harris R, Mckee BL, & Kelley AE (2011). A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behavioral Neuroscience, 125(1), 93. doi: 10.1037/a0022038 [DOI] [PubMed] [Google Scholar]
- Baler RD, & Volkow ND (2006). Drug addiction: The neurobiology of disrupted self-control. Trends in Molecular Medicine, 12(12), 559–566. doi: 10.1016/j.molmed.2006.10.005 [DOI] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, & Everitt BJ (2013). Addiction: Failure of control over maladaptive incentive habits. Current Opinion in Neurobiology, 23(4), 564–572. doi: 10.1016/j.conb.2013.01.025 [DOI] [PubMed] [Google Scholar]
- Brasser SM, & Spear NE (2002). Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behavioral Neuroscience, 116(2), 305. doi: 10.1037//0735-7044.116.2.305 [DOI] [PubMed] [Google Scholar]
- Brunell SC, & Spear LP (2005). Effect of stress on the voluntary intake of a sweetened ethanol solution in pair housed adolescent and adult rats. Alcoholism: Clinical and Experimental Research, 29(9), 1641–1653. doi: 10.1097/01.alc.0000179382.64752.13 [DOI] [PubMed] [Google Scholar]
- Burton CL, & Fletcher PJ (2012). Age and sex differences in impulsive action in rats: The role of dopamine and glutamate. Behavioural Brain Research, 230(1), 21–33. doi: 10.1016/j.bbr.2012.01.046 [DOI] [PubMed] [Google Scholar]
- Chorlian DB, Rangaswamy M, Manz N, Wang JC, Dick D, Almasy L, … & Kang SJ (2013). Genetic and neurophysiological correlates of the age of onset of alcohol use disorders in adolescents and young adults. Behavior Genetics, 43(5), 386–401. doi: 10.1007/s10519-013-9604-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Nicholas DJ, & Adams CD (1983). The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. The Quarterly Journal of Experimental Psychology, 35(1), 35–51. [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P and Spear LP (2005). Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism: Clinical and Experimental Research, 29, 1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, & Spear LP (2003). Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacology Biochemistry and Behavior, 75(2), 411–418. doi: 10.1016/S0091-3057(03)00134-5 [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Barreto M, & Spear LP (2012). Age-related differences in impulsivity among adolescent and adult Sprague-Dawley rats. Behavioral Neuroscience, 126(5), 735. doi: 10.1037/a0029697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, & Spear LP (2007). Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcoholism: Clinical and Experimental Research, 31(9), 1516–1527. doi: 10.1111/j.1530-0277.2007.00457.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, & Spear LP (2011). Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behavioral Neuroscience, 125(4), 661. doi: 10.1037/a0023763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, & Spear LP (2016). Reward-centricity and attenuated aversions: An adolescent phenotype emerging from studies in laboratory animals. Neuroscience & Biobehavioral Reviews, 70, 121–134. doi: 10.1016/j.neubiorev.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, & Spear LP (2010). Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition, 72(1), 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ (2014). Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories – indications for novel treatments of addiction. European Journal of Neuroscience, 40, 2163–2182. doi: 10.1111/ejn.12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friemel CM, Spanagel R, & Schneider M (2010). Reward sensitivity for a palatable food reward peaks during pubertal developmental in rats. Frontiers in Behavioral Neuroscience, 4, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Burgos D, González F, Manrique T, and Gallo M (2009). Patterns of ethanol intake in preadolescent, adolescent, and adult wistar rats under acquisition, maintenance, and relapse like conditions. Alcoholism: Clinical and Experimental Research, 33, 722–728. doi: 10.1111/j.1530-0277.2008.00889.x [DOI] [PubMed] [Google Scholar]
- Geier CF (2013). Adolescent cognitive control and reward processing: Implications for risk taking and substance use. Hormones and Behavior, 64(2), 333–342. doi: 10.1016/j.yhbeh.2013.02.008 [DOI] [PubMed] [Google Scholar]
- Guttmannova K, Bailey JA, Hill KG, Lee JO, Hawkins JD, Woods ML, & Catalano RF (2011). Sensitive periods for adolescent alcohol use initiation: Predicting the lifetime occurrence and chronicity of alcohol problems in adulthood. Journal of Studies on Alcohol and Drugs, 72(2), 221–231. doi: 10.15288/jsad.2011.72.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerslag LR, Belagodu AP, Aladesuyi Arogundade OA, Karountzos AG, Guo Q, Galvez R, … & Gulley JM (2017). Adolescent impulsivity as a sex dependent and subtype dependent predictor of impulsivity, alcohol drinking and dopamine D 2 receptor expression in adult rats. Addiction Biology. doi: 10.1111/adb.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerslag LR and Gulley JM (2014). Age and sex differences in reward behavior in adolescent and adult rats. Developmental Psychobiology, 56, 611–621. doi: 10.1002/dev.21127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford TC, Grant BF, Yi H and Chen CM (2005). Patterns of DSM-IV alcohol abuse and dependence criteria among adolescents and adults: Results from the 2001 National Household Survey on Drug Abuse. Alcoholism: Clinical and Experimental Research, 29, 810–828. doi: 10.1097/01.ALC.0000164381.67723.76 [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR (2006). Age at drinking onset and alcohol dependence age at onset, duration, and severity. Archives of Pediatrics and Adolescent Medicine, 160(7), 739–746. doi: 10.1001/archpedi.160.7.739 [DOI] [PubMed] [Google Scholar]
- Hunt PS, Burk JA, & Barnet RC (2016). Adolescent transitions in reflexive and non-reflexive behavior: Review of fear conditioning and impulse control in rodent models. Neuroscience & Biobehavioral Reviews, 70, 33–45. doi: 10.1016/j.neubiorev.2016.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG & Schulenberg JE (2016). Monitoring the future: National results on adolescent drug use: Overview of key findings. Ann Arbor, MI. [Google Scholar]
- Koob GF, & Le Moal M (2005). Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature Neuroscience, 8(11), 1442. doi: 10.1038/nn1105-1442 [DOI] [PubMed] [Google Scholar]
- Maldonado AM, Finkbeiner LM, Alipour KK and Kirstein CL (2008). Voluntary ethanol consumption differs in adolescent and adult male rats using a modified sucrose fading paradigm. Alcoholism: Clinical and Experimental Research, 32, 1574–1582. doi: 10.1111/j.1530-0277.2008.00733.x [DOI] [PubMed] [Google Scholar]
- Marshall AT, Liu AT, Murphy NP, Maidment NT, & Ostlund SB (2017). Sex-specific enhancement of palatability-driven feeding in adolescent rats. PloS One, 12(7), e0180907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, & Bucci DJ (2014). The ontogeny of learned inhibition. Learning & Memory, 21(3), 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, & Bucci DJ (2016). Age differences in appetitive Pavlovian conditioning and extinction in rats. Physiology & Behavior, 167, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR and Fromme K (2012). Age of first use and delay to first intoxication in relation to trajectories of heavy drinking and alcohol-related problems during emerging adulthood. Alcoholism: Clinical and Experimental Research, 36, 1991–1999. doi: 10.1111/j.1530-0277.2012.01812.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Di Scala G, Pape JR, & Coutureau E (2009). A role for medial prefrontal dopaminergic innervation in instrumental conditioning. Journal of Neuroscience, 29(20), 6599–6606. doi: 10.1523/JNEUROSCI.1234-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Di Scala G, Pape JR, & Coutureau E (2012). Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. Journal of Neuroscience, 32(46), 16223–16232. doi: 10.1523/JNEUROSCI.3080-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohannessian CM, Finan LJ, Schulz J, & Hesselbrock V (2015). A long-term longitudinal examination of the effect of early onset of alcohol and drug use on later alcohol abuse. Substance Abuse, 36(4), 440–444. DOI: 10.1080/08897077.2014.989353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tousa D, & Grahame N (2014). Habit formation: Implications for alcoholism research. Alcohol, 48(4), 327–335. doi: 10.1016/j.alcohol.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2008). The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 363(1507), 3137–3146. DOI: 10.1098/rstb.2008.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serlin H, & Torregrossa MM (2015). Adolescent rats are resistant to forming ethanol seeking habits. Developmental Cognitive Neuroscience, 16, 183–190. doi: 10.1016/j.dcn.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews, 24(4), 417–463. [DOI] [PubMed] [Google Scholar]
- Spear LP (2016). Alcohol consumption in adolescence: A translational perspective. Current Addiction Reports, 3(1), 50–61. doi: 10.1007/s40429-016-0088-9 [DOI] [Google Scholar]
- Steinberg L , Graham S , O’Brien L , Woolard J , Cauffman E and Banich M (2009). Age differences in future orientation and delay discounting. Child Development, 80, 28–44. doi: 10.1111/j.1467-8624.2008.01244.x [DOI] [PubMed] [Google Scholar]
- Sturman DA, Mandell DR, & Moghaddam B (2010). Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behavioral Neuroscience, 124(1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2004). Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcoholism: Clinical and Experimental Research, 28(1), 40–50. doi: 10.1097/01.ALC.0000108655.51087.DF [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL and Spear LP (2007). Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcoholism: Clinical and Experimental Research, 31, 1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook SR, Hankosky ER, Dwyer MR, & Gulley JM (2018). Age and sex differences in behavioral flexibility, sensitivity to reward value, and risky decision-making. Behavioral Neuroscience, 132(2), 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, & Jentsch JD (2010). Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcoholism: Clinical and Experimental Research, 34(8), 1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto K , Hori M , Sorimachi Y , Watanabe T , Yano T and Yasuhara M (2002). Increase of rat alcohol drinking behavior depends on the age of drinking onset. Alcoholism: Clinical and Experimental Research, 26, 63s–65s. doi: 10.1111/j.1530-0277.2002.tb02704.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. In most comparisons, animals responded less on the second day of devaluation suggestive of an influence of extinction during the prior trial. Main effect of day denoted by &.
Supplemental Fig. 2. To determine the influence of repeated devaluation and extinction testing, analyses were conducted comparing animals that received either sucrose pellet or standard chow pellets prior to extinction testing on the first devaluation day only. Similar results to analyses including both devaluation days were found, with age differences (indicated by *) evident for sucrose pellet devaluation but no differences for standard chow pellet devaluation.


