Abstract
Domoic acid (DA) is a neuroexcitotoxic amino acid that is naturally produced by some species of marine diatoms during harmful algal blooms (HABs). The toxin is transferred through the food web from plantivorous fish and shellfish to marine mammals resulting in significant morbidity and mortality. Due to the timing and location of DA producing HABs, it is well documented that pregnant female California sea lions (CSL) are regularly exposed to DA through their diet thereby posing exposure risks to a neuroteratogen in developing fetuses. In the present study, fluids from 36 fetuses sampled from naturally exposed pregnant CSLs were examined for DA. Domoic acid was detected in 79% of amniotic fluid (n=24), 67% of allantoic fluid (n=9), 75% of urine (n=4), 41% of meconium (n=17) and 29% of stomach content (n=21) samples opportunistically collected from CSL fetuses. The distribution of DA in fetal samples indicates an increased prenatal exposure risk due to recirculation of DA in fetal fluids and continuous exposure to the developing brain.
Introduction:
Domoic acid (DA) is a potent glutaminergic excitatory neurotoxin that is naturally produced by some species of marine diatoms in the genus Pseudonitzschia (Bates, 2000; Berman and Murray, 1997). The toxin accumulates in planktivorous finfish and shellfish such as anchovies, sardines, clams, mussels and scallops resulting in trophic transfer to marine mammals and humans (Andjelkovic et al., 2012; Bejarano et al., 2008b; James et al., 2005; Lefebvre et al., 2002). Domoic acid toxicosis was first characterized in humans in 1987 when over 100 people became ill after consuming DA-contaminated mussels (Perl et al., 1990; Wright et al., 1989). Since then, increasingly frequent Pseudonitzschia blooms along the west coast of the United States have resulted in repeated exposure of marine mammals to DA, causing widespread mortality, especially of California sea lions (CSL, Zalophus californianus) (Bejarano et al., 2008a; Scholin et al., 2000). These blooms have resulted in closures of many seafood fisheries to protect human health, with levels of 20 ppm set as the regulatory limit in seafood destined for human consumption (McCabe et al., 2016).
Domoic acid has also been implicated in intrauterine fetal death, abortions, and neonatal death in sea lions when DA exposure occurred during pregnancy (Brodie et al., 2006). In these cases, DA was detected in fetal sea lion fluids including amniotic fluid (Brodie et al., 2006). Domoic acid in amniotic fluid raises concern that fetal sea lions could be exposed during development by ingestion of contaminated amniotic fluid. Such exposure is of concern as in utero DA exposure in rodent models has been shown to reduce the number of live fetuses brought to term and is associated with post natal hippocampal damage, and lasting behavioral effects (Dakshinamurti et al., 1993; Doucette and Tasker, 2016). Domoic acid toxicity models in rodents have been extrapolated to predict similar toxicity from in utero exposure in CSLs (Ramsdell and Zabka, 2008). In a rat exposure model, it was suggested that recirculation of DA from the amniotic fluid may result in greater fetal sensitivity to low doses of DA as a result of repetitive exposure during gestation (Maucher Fuquay et al., 2012).
In human seafood consumers, low dose exposures are more likely to occur than high exposures due to regulatory measures that protect against high-level exposures that are known to induce excitotoxic symptoms such as seizures (Lefebvre and Robertson, 2010). A recent shellfish consumption study reports that some razor clam consumers (including women of childbearing age) in the Pacific Northwest (USA) are chronically exposed to low levels of DA for multiple consecutive months and/or sporadically exposed to levels above the currently established tolerable daily intake limit (0.075 mg/kg) due to high razor clam consumption and DA levels retained in clams (Ferriss et al., 2017; Marien, 1996). Additionally, a recent long term exposure study in mice revealed significant cognitive deficits after six months of weekly exposures to low levels of DA (doses below those that elicit visible signs of excitotoxicity) (Lefebvre et al., 2017). Accordingly, public health advisories have recently included warnings for pregnant women consuming legally harvested shellfish at a rate of more than 15 clams per month per year (Washington State Department of Health October 7, 2016 https://www.doh.wa.gov/Portals/1/Documents/1600/NewsReleases/2016/16-116-RazorClamsAdvisoryNewsRelease.pdf).
In this study, DA levels were quantified in fetal fluids collected opportunistically from naturally exposed pregnant sea lions to characterize fetal DA exposure risks in environmentally relevant conditions. Pathways for DA recirculation and fetal exposure are proposed based on these findings of DA distribution in fetal fluids.
Methods:
Sample Collection
A combination of amniotic fluid, allantoic fluid, urine, meconium and/or stomach content samples were opportunistically collected from 36 California sea lion fetuses. The fetus with intact placenta and fetal membranes was removed from the gravid uterine horn of adult female sea lions that stranded along the central California coast and either died or were euthanized due to poor prognosis based on their clinical signs of DA toxicosis as defined in Goldstein et al (Goldstein et al., 2008). In some cases, fetuses were also collected when adult females aborted. Amniotic and allantoic fluids were collected by sterile hypodermic syringe from the appropriate fetal membrane compartment while urine was collected from the fetal urinary bladder, and meconium from the rectum upon dissection of the fetus.
Quantification of Domoic Acid
Domoic acid was extracted from amniotic fluid, allantoic fluid, urine, meconium and/or stomach content samples in 50% methanol in a 1:4 wt:wt ratio, followed by homogenization for 1 minute using a LabGEN 700 110V homogenizer with a stainless steel 10 mm rotor-stator generator probe tip. Homogenized samples were then centrifuged at 5,000 rpm for 20 minutes in a DuPont Sorvall RC-5C Plus, and filtered through a 0.22 membrane microcentrifuge tube filter (Millipore Ultra-Free MC-GV centrifugal filters). Samples were then diluted in 10% methanol in PBS-T at ratios of 1:10 for amniotic fluid, allantoic fluid and urine samples, and at ratios of 1:100 and 1:50 for meconium and stomach content samples, respectively. Domoic acid levels were quantified using commercially available Biosense (ASP) enzyme-linked immunosorbent assay (ELISA) kits (www.abraxiskits.com).
Selected fetal samples (n = 1 urine; n = 5 allantoic fluid; n = 5 amniotic fluid) were also analyzed via LC/MS-MS methods for confirmation. For LC/MS-MS quantification, the above samples were extracted with 100% methanol at 1:1 v/v ratio. Samples were centrifuged at 16,100 g for 15 minutes to precipitate protein. Clear supernatant was collected for analysis. Standard curves were prepared in naïve human urine with spiked domoic acid concentration ranging between 0.3 – 40 ng/mL. Domoic acid was quantified in extracted samples using AB Sciex 6500 qTrap Q-LIT mass spectrometer (AB Sciex, Foster City, CA) in line with a Shimadzu UFLC XR DGU-20A5 (Shimadzu Scientific Instruments, Columbia, MD). Analytes were separated using Synergi™ Hydro-RP 100 Å LC column (2.5 μm, 50 × 2 mm; Phenomenex) with a guard cartridge (2 × 2.1mm, sub 2μm; Phenomenex). A gradient elution at 0.5 mL/min initiated with 95% A (A = water with 0.1 formic acid) and 5% B (B= 95:1 (v:v) acetonitrile and water with 0.1% formic acid) for 1 minute, increased to 100% B by 4 minutes, returned to 5% B by 5 minutes and continued to run at initial conditions until 9 minutes. Domoic acid was detected using positive ion electrospray ionization at the mass transitions (m/z): 312 → 266. The method was validated according to the FDA guidance for bioanalytical method validation and had <20% CV% at all concentrations quantified. The Limit of quantification for the method was 2.5ng/mL.
Results:
Table 1 summarizes the DA levels quantified in all fetal samples analyzed. As samples were obtained opportunistically not all matrices were available for each fetus (amniotic fluid n = 24; allantoic fluid n = 9; urine n = 4; meconium n = 17; and stomach contents n = 21). Domoic acid was detected in 79% of amniotic fluid samples, 67% of allantoic fluid samples, 75% of urine samples, 41% of meconium samples, and 29% of stomach content samples examined (Figure 1). In six fetuses from which at least four matrix types were available for testing, the highest levels of DA were detected in allantoic fluid (Table 1). A comparison of DA levels quantified in four fetuses that had detectable levels of DA in both allantoic and corresponding amniotic fluids revealed consistently higher toxin levels in allantoic fluids (Figure 2).
Table 1:
Domoic acid (DA) levels quantified in samples from 36 California sea lion fetuses analyzed via Enzyme-Linked Immunosorbent Assays (ELISA). Ten samples were also analyzed via Liquid Chromatography Mass Spectrometry (LC/MS). DA concentrations are in ng/ml. LC/MS confirmation values are shown in parentheses. ND = not detected. +BLQ = DA detected but below the level of quantification.
| Fetus ID # | Amniotic fluid | Allantoic fluid | Urine | Meconium | Stomach Content |
|---|---|---|---|---|---|
| 10514-F | 1.2 | ND (ND) | 0.5 | ND | ND |
| 10487-F | ND (ND) | 0.4 | 0.4 | 10.9 | |
| 13183-F | 6.8 (4.2) | 26.5 (21.2) | 8.8 | 8.9 | |
| 13305-F | 0.8 | 1.3 | 4.6 | 3.1 | |
| 12006-F | 0.6 | ND | ND | ND | |
| 10837-F | 2.1 | 9.9 (22.6) | ND | 2.3 | |
| 12817-F | 0.8 | ND | ND | ||
| 10787-F | ND | ND | ND | ||
| 13020-F | ND (ND) | ND | ND | ||
| 10532-F | 5.9 | 8.6 | ND | ||
| 12510-F | 2.3 | 3.1 (ND) | |||
| 7159-F | ND | ND | |||
| 6855-F | 4.1 (+BLQ) | ND | |||
| 6856-F | 1.9 | 3.3 | |||
| 11478-F | 0.5 | 2.3 | |||
| 7167-F | ND | ND | |||
| 10840-F | ND | ND | |||
| 10889-F | ND | ND | |||
| 11114-F | 6.0 | 2.8 | |||
| 12846-F | ND | ND | |||
| 11131-F | 8.9 | ND | |||
| 10909-F | ND | ND | |||
| 6451-F | ND | ||||
| 10708 | 7.8 | ||||
| 10879-F | 0.8 | ||||
| 12793-F | 0.6 | ||||
| 12806-F | 0.6 | ||||
| 6857-F | 3.8 | ||||
| 6860-F | 0.5 | ||||
| 7147-F | 5.1 (+BLQ) | ||||
| 7160-F | 0.5 | ||||
| 7616-F | 0.4 | ||||
| 10832-F | 2.5 (ND) | ||||
| 11143-F | 57.5 | ||||
| 11003-F | ND | ||||
| 10503-F | ND |
Figure 1:
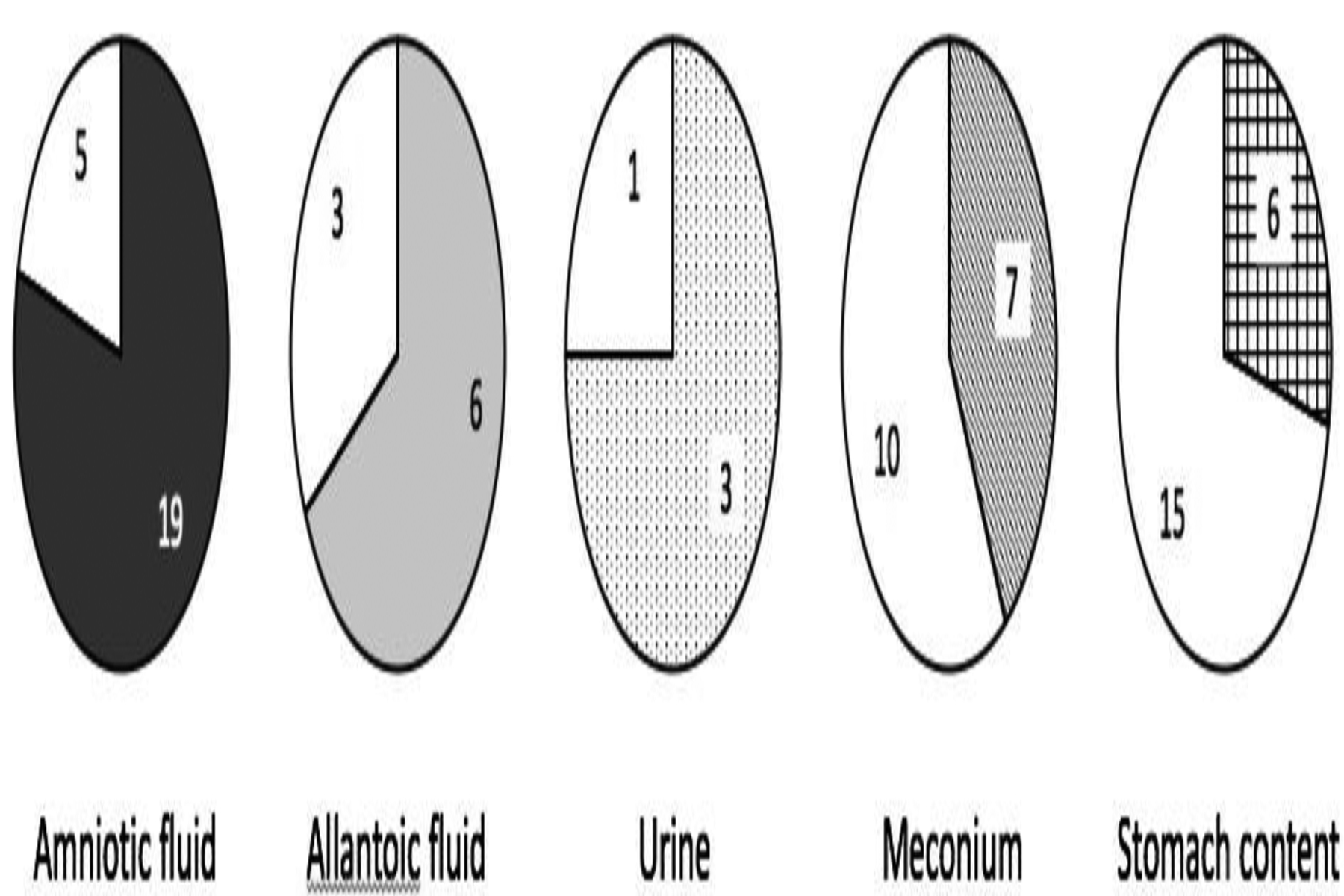
Pie graphs showing the proportion of samples containing quantifiable levels of domoic acid (gray shaded area) for each of the five sample matrices examined; amniotic fluid (n=24), allantoic fluid (n=9), urine (n=4), meconium (n=17), and stomach contents (n=21).
Figure 2:
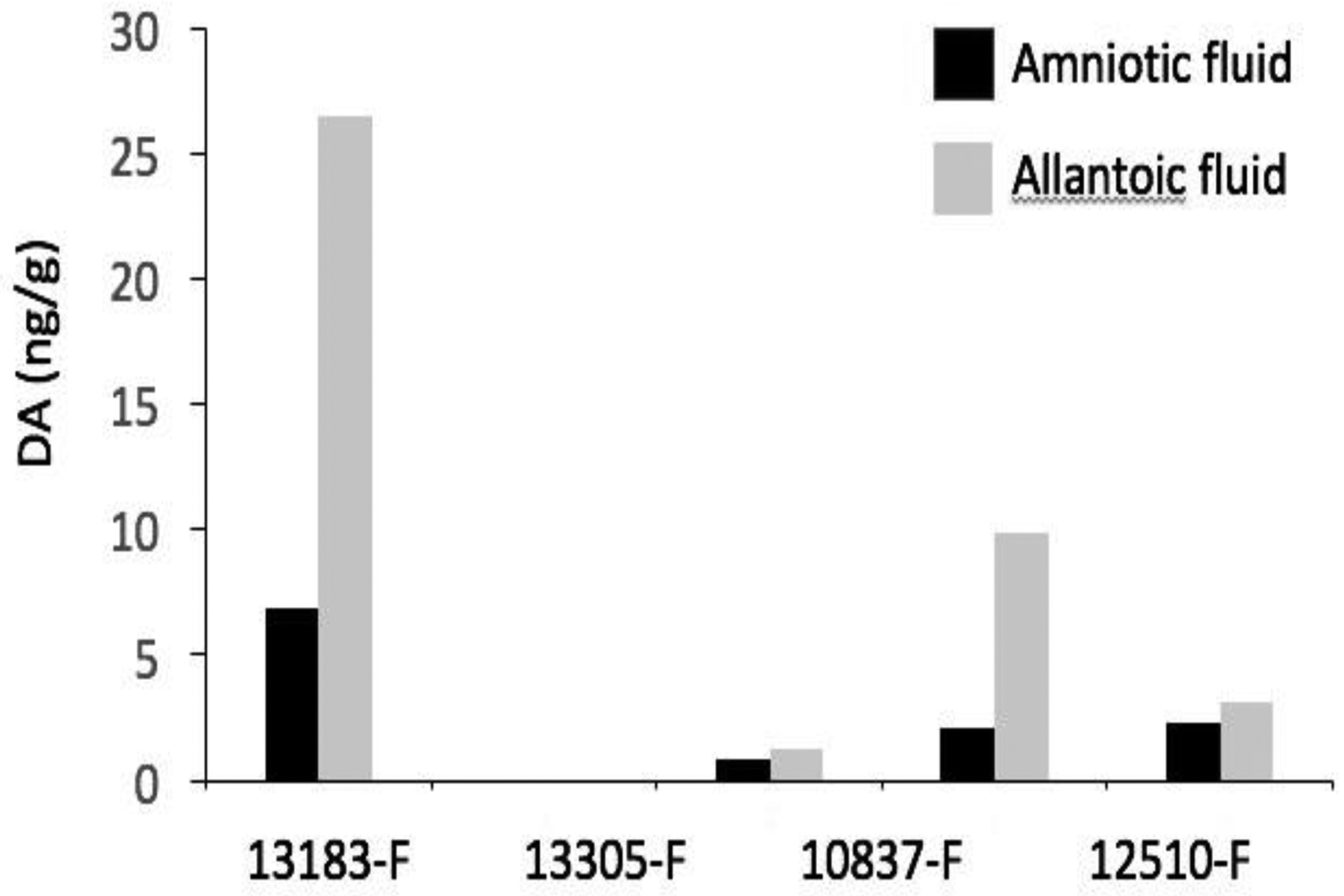
Domoic acid (DA) levels detected in allantoic and amniotic fluid samples from the four California sea lion (CSL) fetuses that had quantifiable levels of toxin in both corresponding fluids. Domoic acid levels were consistently higher in allantoic fluids.
Domoic acid levels in ten fetal fluid samples selected for validation via LC/MS-MS analyses are shown in parenthesis in Table 1. Limits of detection were lower for ELISA than LC/MS-MS, but linear regression for ELISA and LC/MS-MS values confirmed a significant relationship (p = 0.003; R square = 0.69; Figure 3) and provided confirmation of DA positive samples. Five of the seven fluid samples positive for DA via ELISA were also positive via LC/MS-MS although two of those were below the limit of quantification (BLQ) by LC/MS-MS (Table 1). In all but one case, ELISA values were higher than corresponding LC/MS-MS values and the fold-change for DA positive samples by ELISA and LC/MS-MS was 2.3 ± 1.9 (mean ± sd). Two samples (12510-F and 10832-F) with the lowest DA values reported via ELISA, were below detection limits (ND=not detected) of LC/MS-MS. All samples positive via LC/MS-MS were also quantifiable via ELISA.
Figure 3:
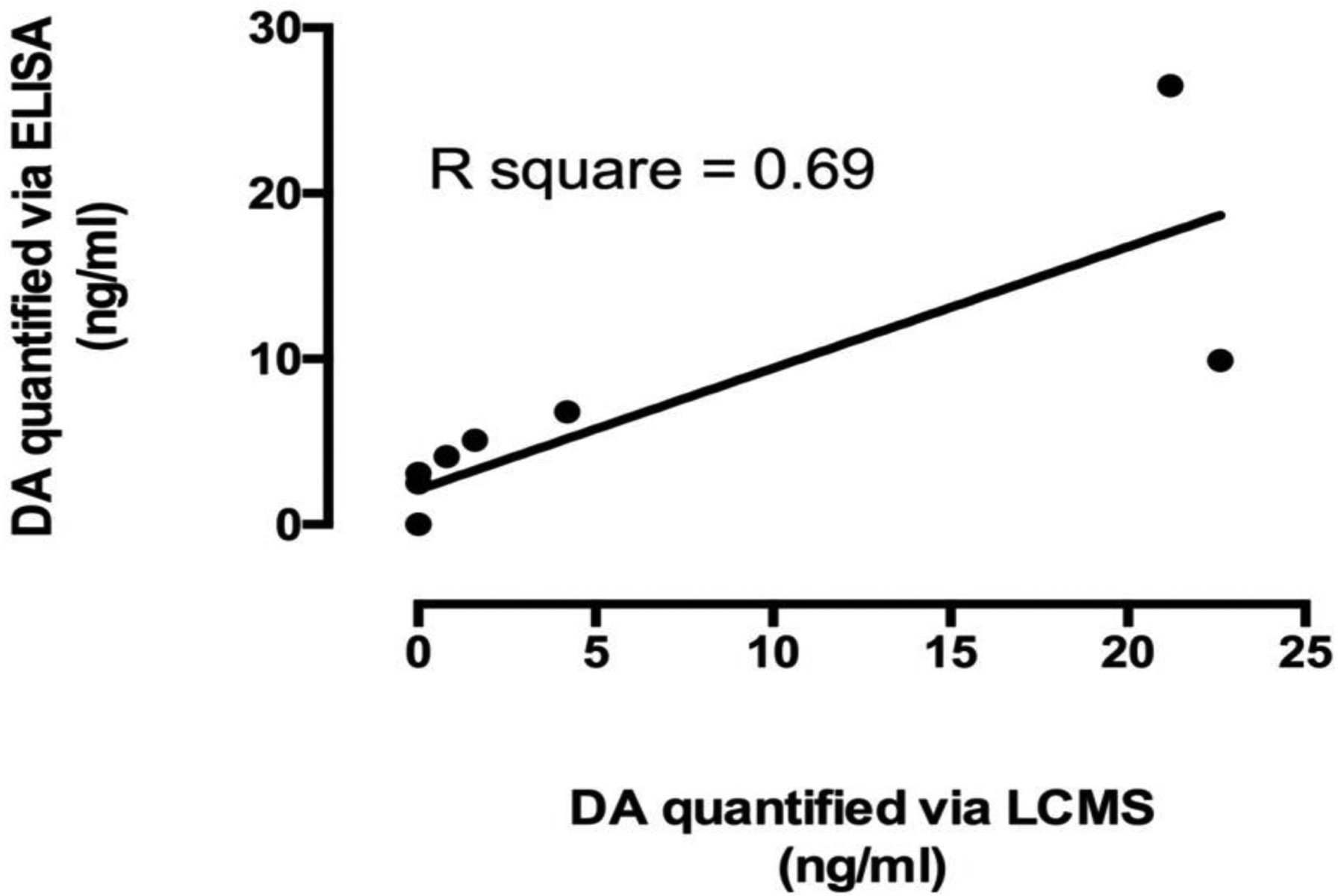
Linear regression of domoic acid (DA) levels quantified via Enzyme-Linked Immunosorbent Assays (ELISA) and Liquid Chromatography Mass Spectrometry (LC/MS-MS) for ten California sea lion fetal fluid samples (p = 0.003; R square = 0.69).
Discussion:
Initial exposure of fetal sea lions to DA presumably results from placental transfer of DA in blood from mother (post ingestion) to fetus. Once in the fetal blood stream, the dynamics of DA metabolism are unknown but recirculation of DA can be inferred from the levels reported here and the anatomy of fetal structures. The sea lion has a zonary endotheliochorial placenta, with an allantoic sac surrounding the amnion (Talent and Talent, 1975). The detection of DA in fetal meconium and urine indicate that fetal sea lions are excreting DA through the kidneys and gastrointestinal tract, as is expected from distribution studies in laboratory rodents and primates (Iverson et al., 1989; Suzuki and Hierlihy, 1993). When excreted in fetal urine, DA accumulates in the allantois, and when excreted in meconium during fetal stress, DA could reach the amniotic fluid (Figure 4). The presence of DA in allantoic and amniotic fluids suggest there is transfer of DA between these two compartments. Once in amniotic fluid, sea lion fetuses can be re-exposed to DA following swallowing of amniotic fluid during gestation. Thus DA can be recirculated through the gastrointestinal tract, allowing for continuous exposure of the developing fetus (Figure 4).
Figure 4:
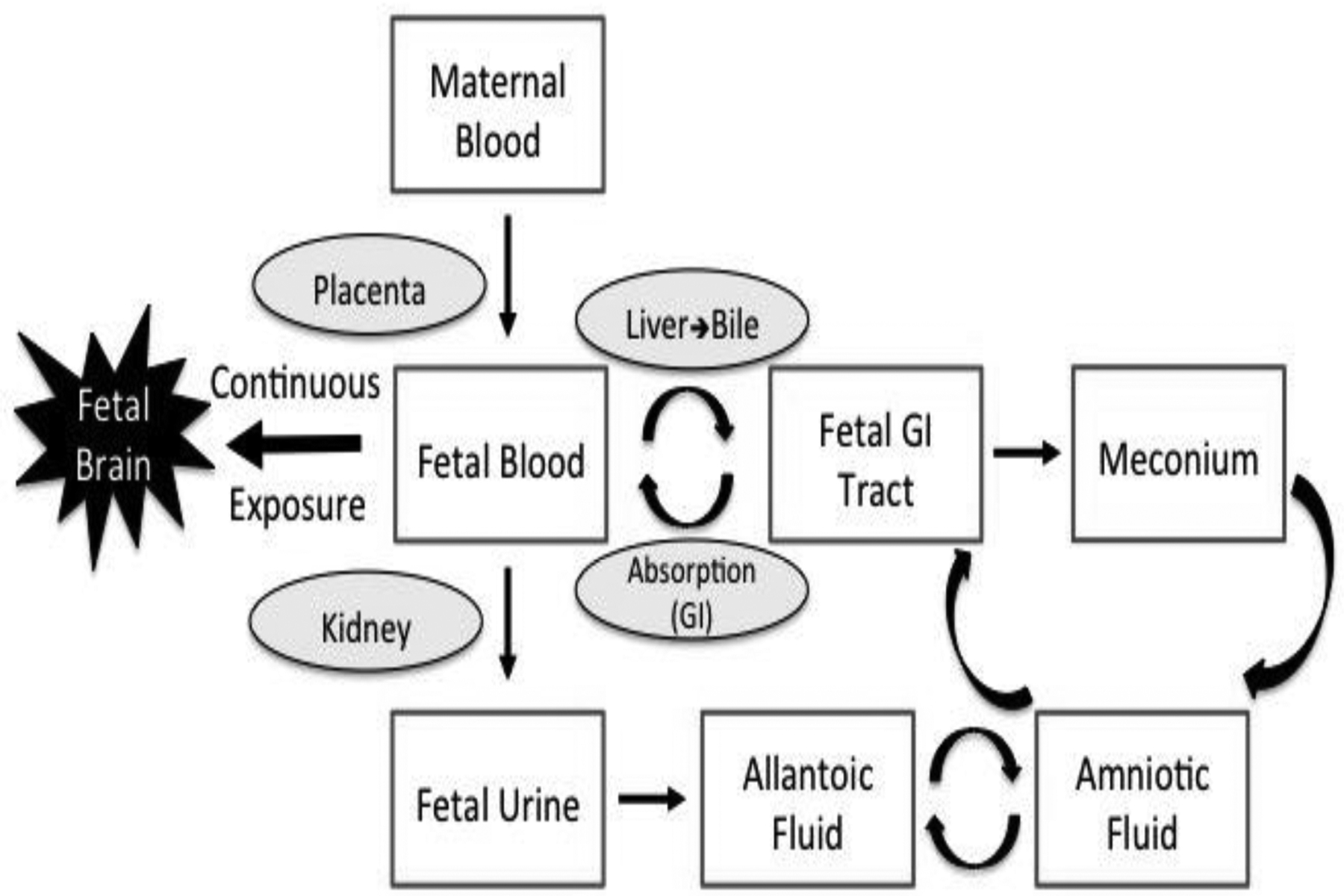
Schematic of domoic acid (DA) transfer from maternal blood to the developing fetus followed by recirculation of DA through fetal fluids and continuous exposure to the fetal brain. Curved arrows represent potential pathways of recirculation. Recirculation via meconium would only occur under fetal stress.
These data indicating recirculation of DA in fetal fluid compartments suggest that any ingestion of even low levels of DA by pregnant females poses a risk of DA exposure to the developing fetal brain (Figure 4). This is consistent with previous laboratory studies in rodent models that have documented maternal transfer of DA to the developing fetus and postnatal neurologic effects of intrauterine exposure including progressive hippocampal injury (Dakshinamurti et al., 1993), impairments in locomotor and cognitive domains (Levin et al., 2005), severe learning and memory impairment (Tanemura et al., 2009), and diminished social investigation and altered sensorimotor gating (Zuloaga et al., 2016). These effects of intrauterine exposure occurred after intravenous (IV), intraperitoneal (IP) or subcutaneous (SC) injection of DA in pregnant females at doses that did not elicit visible signs of toxicosis in the dams, emphasizing the likelihood that fetal sea lions are affected by DA even when acute excitotoxic DA symptoms are not obvious in adult pregnant sea lions.
Further evidence supporting the hypothesis that low level DA exposure in pregnant females can have persistent effects in offspring was observed in a recent oral gavage study by Shiotani et al (2017). Pregnant mice were orally exposed to DA at doses that did not induce visible signs of excitotoxicity in the dams (1 and 3 mg/kg). The offspring were then tested at multiple time points (early development, adolescence, and adulthood) for lasting neurobehavioral consequences after exposure during gestation. Both dose and sex related differences where observed in motor coordination, circadian activity patterns, and exploratory behavior (Shiotani et al., 2017). This study was particularly valuable in that the oral exposure doses in pregnant mice were very low at 1 and 3 mg/kg and comparable to predicted human exposure levels of 0.045 (Costa et al., 2010)and 0.075 mg/kg (Ferriss et al., 2017) especially in light of the fact that humans exhibit excitotoxic symptoms at oral DA doses at least ten times lower than rodents (Iverson et al., 1989; Lefebvre and Robertson, 2010; Perl et al., 1990; Teitelbaum et al., 1990; Todd, 1993). Persistent neurobehavioral effects observed at these low maternal doses not only emphasizes that sea lion offspring are likely impacted due to the prevalence of DA in sea lion prey, but also suggests risks of fetal exposure in human seafood consumers (Ferriss et al., 2017; Grattan et al., 2016).
Conclusion:
California sea lion fetuses are exposed to DA during harmful algal bloom events. The varying levels of DA in fetal fluids with highest levels in allantoic fluid suggest that DA is excreted by the fetal kidney to the urine and then to the allantoic sac. Domoic acid is also detected in amniotic fluid after passage through the fetal gastro-intestinal tract. Re-ingestion of amniotic fluid by the fetus results in sustained exposure of the fetus throughout gestation (Figure 4). Sustained DA exposure in utero presents an increased exposure risk to developing fetuses when pregnant animals are exposed to DA in their diet that likely exceeds the risk to postnatal animals. It also indicates that even sub-clinical exposures to pregnant females could have lasting health impacts on offspring. These findings in naturally exposed CSLs have implications for health risks in other marine mammal species as well as for human seafood consumers.
Acknowledgements:
This research was supported by the National Institutes of Health (NIH) R01 ES021930 (to DJM and KAL), the National Science Foundation (NSF) OCE-1314088 (to DJM and KAL) and the NIH R01 ES023043 (to NI). California sea lion samples were obtained from the Marine Mammal Center in Sausalito, CA and analyzed in Seattle, WA at the Wildlife Algal Toxins Research and Response Network (WARRN-West/Northwest Fisheries Science Center) and at the Department of Pharmaceutics (University of Washington).
References:
- Andjelkovic M, Vandevijvere S, Van Klaveren J, Van Oyen H, Van Loco J, 2012. Exposure to domoic acid through shellfish consumption in Belgium. Environment International 49, 115–119. [DOI] [PubMed] [Google Scholar]
- Bates SS, 2000. Domoic acid-producing diatoms: Another genus added! Journal of Phycology 36(6), 978–983. [Google Scholar]
- Bejarano AC, Gulland FM, Goldstein T, St Leger J, Hunter M, Schwacke LH, VanDolah FM, Rowles TK, 2008a. Demographics and spatio-temporal signature of the biotoxin domoic acid in California sea lion (Zalophus californianus) stranding records. Mar Mammal Sci 24(4), 899–912. [Google Scholar]
- Bejarano AC, VanDola FM, Gulland FM, Rowles TK, Schwacke LH, 2008b. Production and toxicity of the marine biotoxin domoic acid and its effects on wildlife: A review. Human and Ecological Risk Assessment 14, 544–567. [Google Scholar]
- Berman FW, Murray TF, 1997. Domoic acid neurotoxicity in cultured cerebellar granule neurons is mediated predominantly by NMDA receptors that are activated as a consequence of excitatory amino acid release. J Neurochem 69(2), 693–703. [DOI] [PubMed] [Google Scholar]
- Brodie EC, Gulland FMD, Greig DJ, Hunter M, Jaakola J, St Leger J, Leighfield TA, Van Dolah FM, 2006. Domoic acid causes reproductive failure in california sea lions (Zalophus californianus). Mar Mammal Sci 22(3), 700–707. [Google Scholar]
- Costa LG, Giordano G, Faustman EM, 2010. Domoic acid as a developmental neurotoxin. Neurotoxicology 31(5), 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamurti K, Sharma SK, Sundaram M, Watanabe T, 1993. Hippocampal changes in developing postnatal mice following intrauterine exposure to domoic acid. J Neurosci 13(10), 4486–4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette TA, Tasker RA, 2016. Perinatal domoic acid as a neuroteratogen. Current Topics in Behavorial Neuroscience 29, 87–110. [DOI] [PubMed] [Google Scholar]
- Ferriss BE, Lefebvre KA, Ayres D, Borchert J, Marcinek DJ, 2017. Acute and chronic dietary exposure to domoic acid in recreational harvesters: a survey of shellfish consumption behavior. Environment International 101, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein T, Mazet JA, Zabka TS, Langlois G, Colegrove KM, Silver M, Bargu S, Van Dolah F, Leighfield T, Conrad PA, Barakos J, Williams DC, Dennison S, Haulena M, Gulland FM, 2008. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. Proc Biol Sci 275(1632), 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan LM, Boushey C, Tracy K, Trainer VL, Roberts SM, Schluterman N, Morris JG Jr., 2016. The association between razor clam consumption and memory in the CoASTAL cohort. Harmful Algae 57(Part B, Sp. Iss. SI), 20–25. [DOI] [PubMed] [Google Scholar]
- Iverson F, Truelove J, Nera E, Tryphonas L, Campbell J, Lok E, 1989. Domoic acid poisoning and mussel-associated intoxication: preliminary investigations into the response of mice and rats to toxic mussel extract. Food Chem Toxicol 27(6), 377–384. [DOI] [PubMed] [Google Scholar]
- James KJ, Gillman M, Amandi MF, Lopez-Rivera A, Puente PF, Lehane M, Mitrovic S, Furey A, 2005. Amnesic shellfish poisoning toxins in bivalve molluscs in Ireland. Toxicon 46(8), 852–858. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Bargu S, Kieckhefer T, Silver MW, 2002. From sanddabs to blue whales: the pervasiveness of domoic acid. Toxicon 40(7), 971–977. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Kendrick PS, Ladiges WC, Hiolski EM, Ferriss BE, Smith DR, Marcinek DJ, 2017. Chronic low-level exposure to the common seafood toxin domoic acid causes cognitive deficits in mice. Harmful Algae 64, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre KA, Robertson A, 2010. Domoic acid and human exposure risks: a review. Toxicon 56(2), 218–230. [DOI] [PubMed] [Google Scholar]
- Levin ED, Pizarro K, Pang WG, Harrison J, Ramsdell JS, 2005. Persisting behavioral consequences of prenatal domoic acid exposure in rats. Neurotoxicology and teratology 27(5), 719–725. [DOI] [PubMed] [Google Scholar]
- Marien K, 1996. Establishing tolerable dungeness crab (Cancer magister) and razor clam (Siliqua patula) domoic acid contaminant levels. Environmental Health Perspectives 104(11), 1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maucher Fuquay J, Muha N, Wang Z, Ramsdell JS, 2012. Toxicokinetics of domoic acid in the fetal rat. Toxicology 294(1), 36–41. [DOI] [PubMed] [Google Scholar]
- McCabe RM, Hickey BM, Kudela RM, Lefebvre KA, Adams NG, Bill BD, Gulland FDM, Thomson RE, Cochlan WP, Trainer VL, 2016. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophysical Research Letters 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd EC, Remis RS, 1990. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med 322(25), 1775–1780. [DOI] [PubMed] [Google Scholar]
- Ramsdell JS, Zabka TS, 2008. In utero domoic acid toxicity: A fetal basis to adult disease in the california sea lion (Zalophus californianus). Marine Drugs 6(2), 262–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholin CA, Gulland F, Doucette GJ, Benson S, Busman M, Chavez FP, Cordaro J, DeLong R, De Vogelaere A, Harvey J, Haulena M, Lefebvre K, Lipscomb T, Loscutoff S, Lowenstine LJ, Marin R 3rd, Miller PE, McLellan WA, Moeller PD, Powell CL, Rowles T, Silvagni P, Silver M, Spraker T, Trainer V, Van Dolah FM, 2000. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 403(6765), 80–84. [DOI] [PubMed] [Google Scholar]
- Shiotani M, Cole TB, Hong S, Park JJY, Griffith WC, Burbacher TM, Workman T, Costa LG, Faustman EM, 2017. Neurobehavioral assessment of mice following repeated oral exposures to domoic acid during prenatal development. Neurotoxicology and teratology. [DOI] [PubMed] [Google Scholar]
- Suzuki CA, Hierlihy SL, 1993. Renal clearance of domoic acid in the rat. Food Chem Toxicol 31(10), 701–706. [DOI] [PubMed] [Google Scholar]
- Talent LG, Talent CL, 1975. An extrauterine fetus in a Steller sea lion, Eumetopias jubata. California Fish and Game 61, 233–234. [Google Scholar]
- Tanemura K, Igarashi K, Matsugami TR, Aisaki K, Kitajima S, Kanno J, 2009. Intrauterine environment-genome interaction and children’s development (2): Brain structure impairment and behavioral disturbance induced in male mice offspring by a single intraperitoneal administration of domoic acid (DA) to their dams. The Journal of toxicological sciences 34 Suppl 2, Sp279–286. [DOI] [PubMed] [Google Scholar]
- Teitelbaum JS, Zatorre RJ, Carpenter S, Gendron D, Evans AC, Gjedde A, Cashman NR, 1990. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N Engl J Med 322(25), 1781–1787. [DOI] [PubMed] [Google Scholar]
- Todd ECD, 1993. Domoic acid and amnesic shellfish poisoning: A review. Journal of Food Protection 56(1), 69–83. [DOI] [PubMed] [Google Scholar]
- Wright JLC, Boyd RK, Freitas A, Falk M, Foxall RA, Jamieson WD, Laycock MV, McCulloch AW, McInnes AG, et al. , 1989. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. Can. J. Chem 67(3), 481–490. [Google Scholar]
- Zuloaga DG, Lahvis GP, Mills B, Pearce HL, Turner J, Raber J, 2016. Fetal domoic acid exposure affects lateral amygdala neurons, diminishes social investigation and alters sensory-motor gating. Neurotoxicology 53, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]


