Abstract
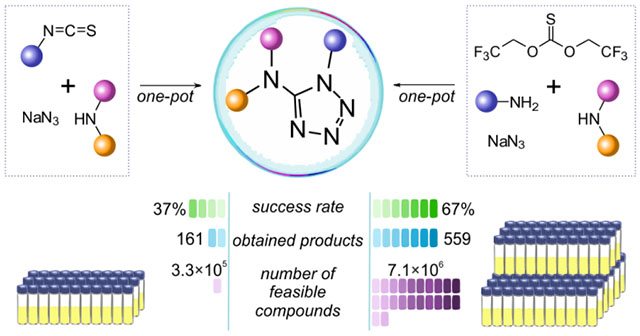
Two protocols for the combinatorial synthesis of 5-(dialkylamino)tetrazoles were developed. The best success rate (67%) was shown by the method which used primary and secondary amines, 2,2,2-trifluoroethylthiocarbamate and sodium azide as the starting reagents. The key steps included the formation of unsymmetrical thiourea, subsequent alkylation with 1,3-propane sultone and cyclization with azide anion. A 559-member aminotetrazole library was synthesized by this approach; the overall readily accessible (REAL) chemical space covered by the method exceeded 7 million feasible compounds.
Keywords: tetrazoles; 2,2,2-trifluoroethylthiocarbamate; thiourea; heterocyclization; REAL (readily accessible) compounds
Graphical Abstract
INTRODUCTION.
According to the recent reports, tetrazole is the third most frequently occurring azole system in the structures of FDA approved drugs.1,2 A broad range of biological activities of tetrazole derivatives, including antimicrobial, antiviral, antifungal, antitumor, anticonvulsant, antihypertensive properties are extensively reviewed in the literature.3–6 However, the availability of diversely substituted tetrazoles for their application in medicinal and other industries is highly dependent on the capability of the currently used synthetic methods for both multigram preparation and microgram-scale parallel synthesis.3,7,8 To date, the reported combinatorial approaches to tetrazole ring construction were limited to a small set of reactions. The most commonly utilized method involves Ugi–azide four-component reaction leading to (1H-tetrazol-5-yl)methanamines.9–16 Other less commonly used methods include azide cycloaddition with electron-poor nitriles17 and reaction of peptide N-terminal amino group with arylisothiocyanates.18
Whereas 5-substituted tetrazoles are well-known bioisosteres of carboxylic acid function,19 1,5-disubstituted derivatives can be used to mimic cis-configuration of the amide bond,4,8 which is higher in energy to the commonly found trans-configuration (Figure 1, A). Recently, 1-alkyl-5-(alkylamino)tetrazole motif was envisaged as a potential replacement of alkyl urea moiety,20 which is present in structures of dozens therapeutics.21,22 1,5-Disubstituted tetrazoles are represented by a series of approved cephalosporin antibiotics (e.g. Cefmetazole,23,24 Ceforanide25,26) and antiplatelet therapeutic Cilostazol27,28 (Figure 1, B). Therefore, access to the diverse library based on N-substituted 1-alkyl-5-aminotetrazole scaffold 1 became the prime objective of the current work.
Figure 1.
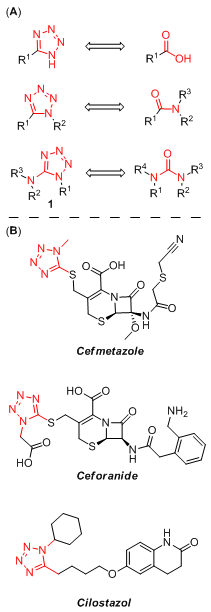
Tetrazoles as bioisosteres of carboxylic acid, amide and urea moieties (A). Representative drugs containing 1,5-disubstituted tetrazoles (B).
One of the options to obtain 1-substituted 5-aminotetrazoles is nucleophilic substitution at the carbon atom of tetrazole bearing a good leaving group, for example amination of 5-sulfonyltetrazoles 2 (Scheme 1, A).20 Alternative methods include the reaction of NaN3 with aminoiminomethanesulfonic acids 3a,29 α-chloroformamidines 3b,30 (benzotriazolyl)-carboximidamides 3c,31 thioureas 4,32 and substituted cyanamides 5,33,34 the reaction of primary amines with highly reactive cyanogen azide (6),35 and diazotization of aminoguanidines 7.36 Because the aforementioned reagents are not readily accessible and some of them are difficult to handle, they are not suitable for combinatorial synthesis. Recently described one-pot azide–isocyanide denitrogenative coupling followed by cyclization of the resulting carbodiimide with TMSN3 catalyzed by Pd(0) – Fe(III) (B) might be a better choice for the tetrazole parallel synthesis.37 However, poor availability of isocyanide reagents dramatically limits the scope of this method. One-pot three-component procedure developed by Ponnuswamy and colleagues, which used isothiocyanates, amines and NaN3 as the starting materials, might be a more plausible method (C).38 Nevertheless, utilization of HgCl2 for the desulfurization step is undesirable due to the toxicity of mercury contaminants.
Scheme 1.
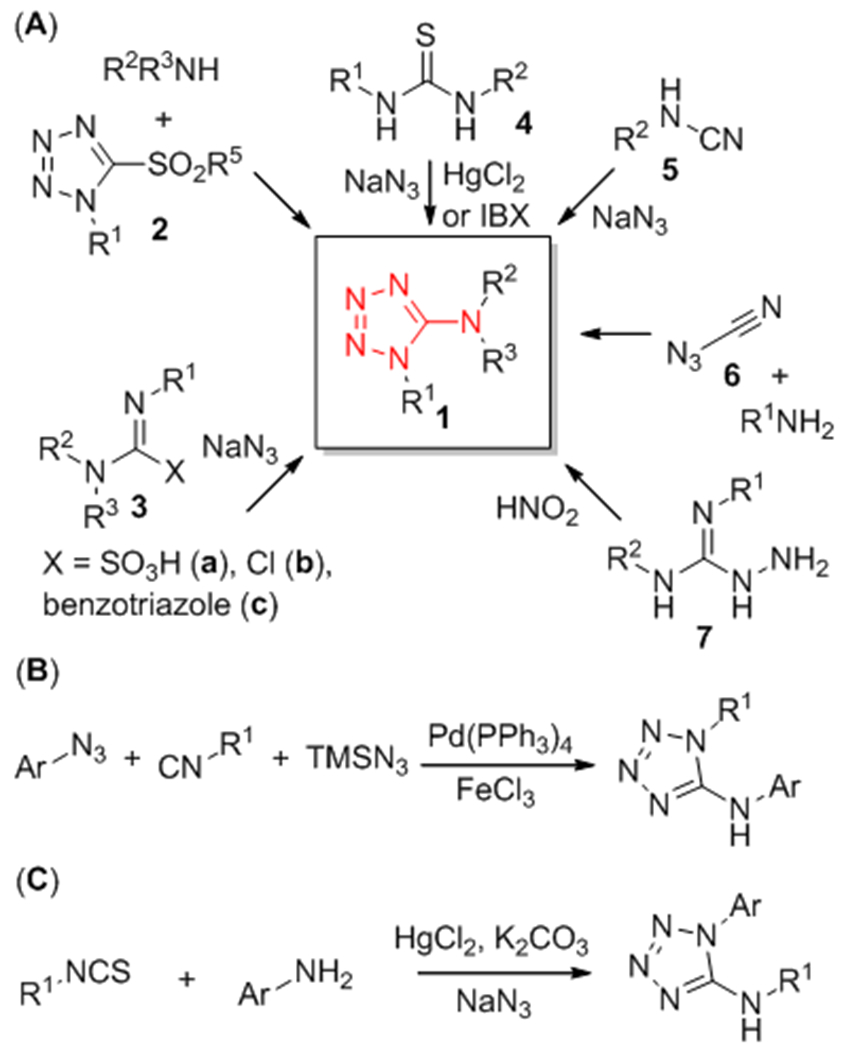
Reported approaches towards 1-substituted 5-aminotetrazoles 1.
In this work, two different combinatorial approaches towards synthesis of 1-substituted 5-aminotetrazole derivatives 1 were elaborated (Scheme 2). Utility and scope of these methods were tested on a wide range of commercially available substrates. In addition to the synthesis of two 5-aminotetrazole libraries 8 and 9, the capacity of the developed protocols for the generation of large collections for HTS was evaluated.
Scheme 2.
Retrosynthetic analysis of the scaffold 1.
RESULTS AND DISCUSSION.
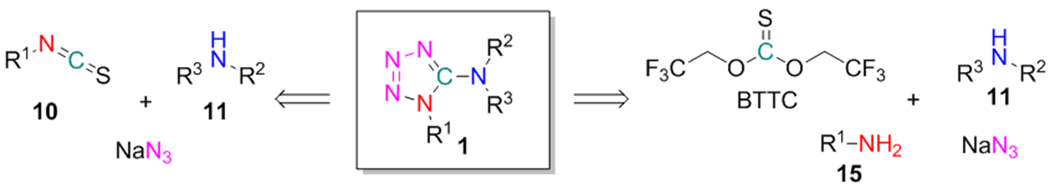
Our initial efforts were put to the synthesis of 1-substituted 5-aminotetrazoles 8 from isothiocyanates 10 and disubstituted amines 11 (isothiocyanate-based method, Scheme 3). The reaction sequence involved the formation of thioureas 12. In contrast to the previously mentioned work of Ponnuswamy and colleagues (Scheme 1, C),38 our protocol did not involve desulfurization of 12 but instead included their conversion to S-alkylisothiourea intermediates 13. After the alkylation of 12 with 1,3-propane sultone 14, excess of 14 and acidic sulfonate was quenched by adding NEt3. Finally, the resulting S-alkylisothioureas 13 underwent cyclization with NaN3 leading to the target products 8, which were further purified by reverse-phase HPLC on the C18 column. This reaction sequence can be considered as a modification of our previous method for the combinatorial synthesis of 3-amino-1,2,4-triazoles, where hydrazides were used as the nucleophilic reagents instead of NaN3.39.
Scheme 3.
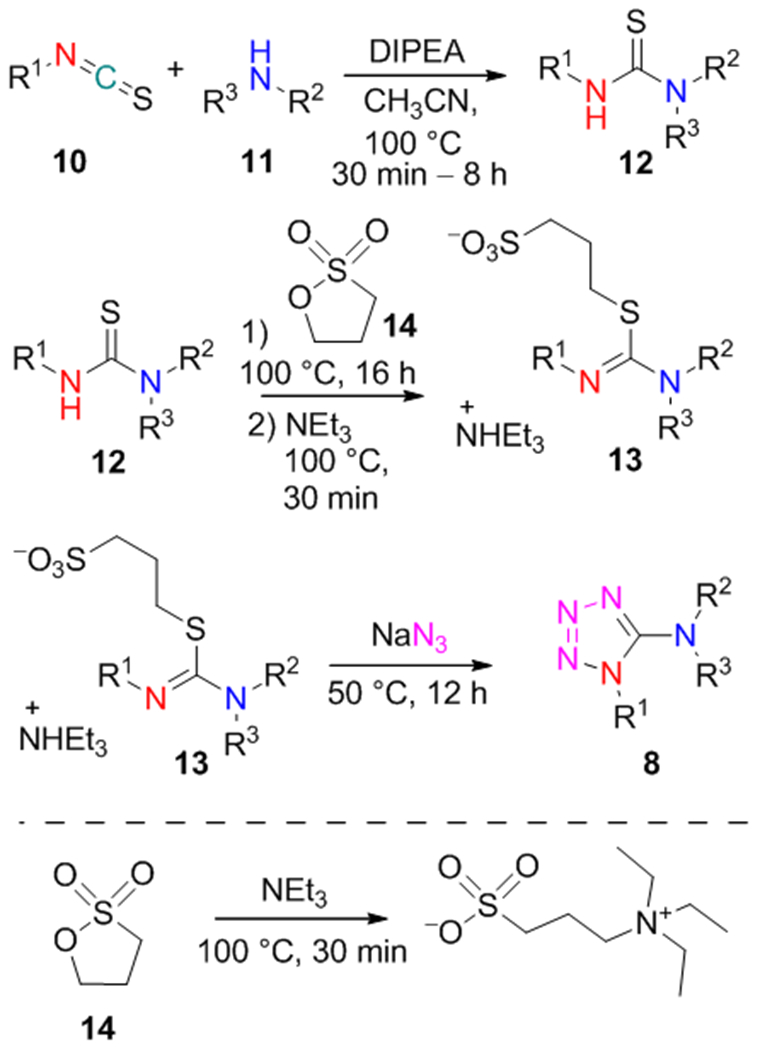
Synthesis of the compounds 8 by the isothiocyanate-based method.
To validate the efficiency of the proposed method, various aliphatic isothiocyanates 10 (58 examples) and secondary aliphatic amines 11 (280 examples) were used. The representative substrates 10 and 11 are listed in Figure 2. As a result, 161 out of 430 experiments allowed obtaining the target tetrazole-containing products (37% success rate, up to 81% yield). For some derivatives, moderate reaction yields could be caused by volatile nature of isothiocyanates with low molecular weight. Besides, in several cases the presence of intermediates 12, 13 and the corresponding carbamates (formed by hydrolysis of 13) was indicated by LC–MS along with the target tetrazoles 8. It was also noticed that branching at the α-carbon atom of isothiocyanates 10 was not crucial for determining the reaction outcome. However, because of the modest success rate, it was difficult to evaluate the precise relationship between structure and reactivity of the reagents 10/11. Therefore, additional modifications of the protocol were further considered.
Figure 2.
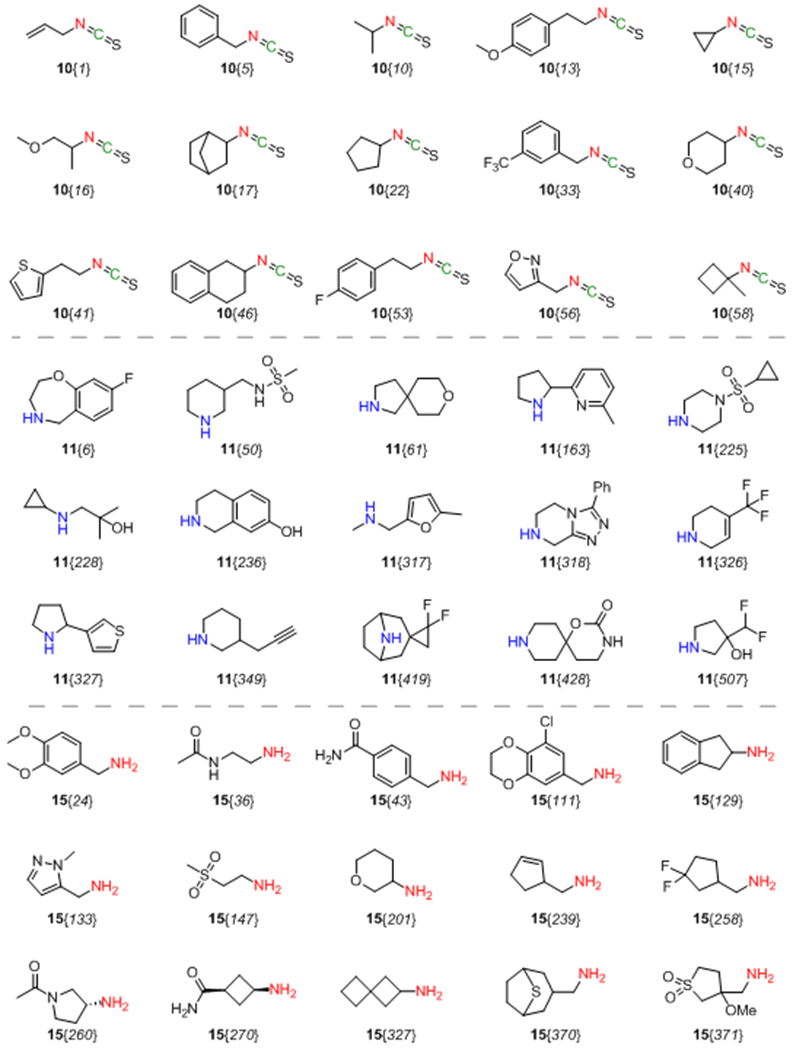
Representative reagents 10, 11, and 15
We have suggested that low efficiency of the first step (i.e. thiourea 12 preparation) in the isothiocyanate-based method might be a possible reason for its modest success rate. Recently, our group had reported the efficient application of bis(2,2,2-trifluoroethyl)carbonate (BTC) and -thiocarbonate (BTTC) as reagents for preparation of unsymmetrical ureas40,41 and thioureas,39 respectively. Therefore, a stepwise reaction of amines 15 and 11 with BTTC was envisaged for the preparation of 12 (BTTC-based method, Scheme 4, A). The reagent is commercially available and can be also synthesized in one step from thiophosgene and trifluoroethanol (Scheme 4, B).
Scheme 4.
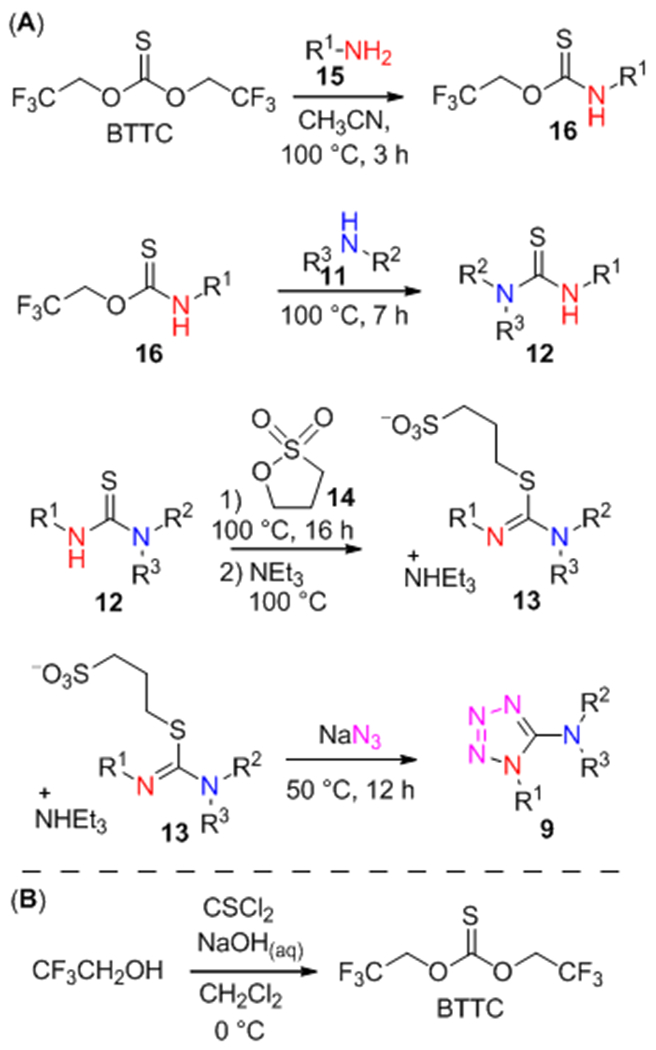
Synthesis of the compounds 9 by BTTC-based method.
To ensure regioselectivity of 5-aminotetrazole formation, primary and then – secondary amines were used sequentially in the reaction with BTTC. Both primary (15, 378 compounds) and secondary (11, 262 compounds) amines were randomly selected from our building block collection; their representative examples are given in Figure 2. It should be noted that only aliphatic amines were used, whereas less nucleophilic anilines and heteroaromatic amines were avoided according to their previously noted inefficiency.39 Further transformations of generated in situ thioureas 12 followed those for the isothiocyanate-based method. As a result, a total of 559 target products out of 830 experiments were successfully synthesized (67% success rate, up to 92% yield).
It was found that amines 15 with either primary or secondary aliphatic substituents showed an almost equal success rate for the BTTC-based method (Table 1); amines with tertiary aliphatic groups were not tested for this procedure. In the case of secondary amines, representatives 11a–d had similar reactivity. More α-branched amines 11e demonstrated lower efficiency, although this result should be considered with precaution due to paucity of the corresponding reagents in our set. The presence of functional groups like alcohol, carboxylic acid ester, primary, secondary or tertiary amide, carbamate, sulfone and sulfonamide in amines 11 and 15 was found to be compatible with the reaction conditions. Considering electronic properties, it was found that electron-withdrawing substituents (e.g. di- and trifluoroethyl) in amine 15 were unfavorable for the discussed transformations. Additionally, an attempt to carry out the reaction sequence with O-alkylhydroxylamines was unsuccessful in all cases.
Table 1.
Influence of amines 11 and 15 substitution pattern on the reaction success rate.
| Substitution pattern | Success rate | Total number of experiments | |
|---|---|---|---|
| 15a |  |
68% | 685 |
| 15b | 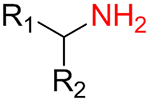 |
64% | 138 |
| 11a | 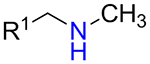 |
70% | 131 |
| 11b | 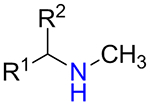 |
66% | 38 |
| 11c | 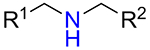 |
68% | 598 |
| 11d | 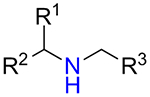 |
78% | 40 |
| 11e | 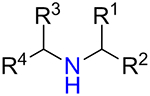 |
40% | 15 |
A subset of selected products obtained by both discussed synthetic approaches is depicted in Figure 3. When comparing the two aforementioned methods, application of BTTC-based sequence not only improved the success rate of the approach but also extended the accessible pool of the starting reagents. The wide availability of primary amines from commercial sources compared to the corresponding isothiocyanates makes BTTC-based protocol a powerful tool for achieving a great variety of final products. Additionally, because the developed protocol is tolerant to a wide range of polar functional groups and produces a wide range of lead-like compounds (95% of the synthesized products had MW 200–350 Da and cLogP 1–3, Figure 4),42 it provides a new potent method for lead-oriented synthesis. The calculated cLogP drift value (i.e. the difference of the mean cLogP of a synthesized array and the mean cLogP of the designed array)42 of 0.017 units was also beneficial for BTTC-based approach.
Figure 3.
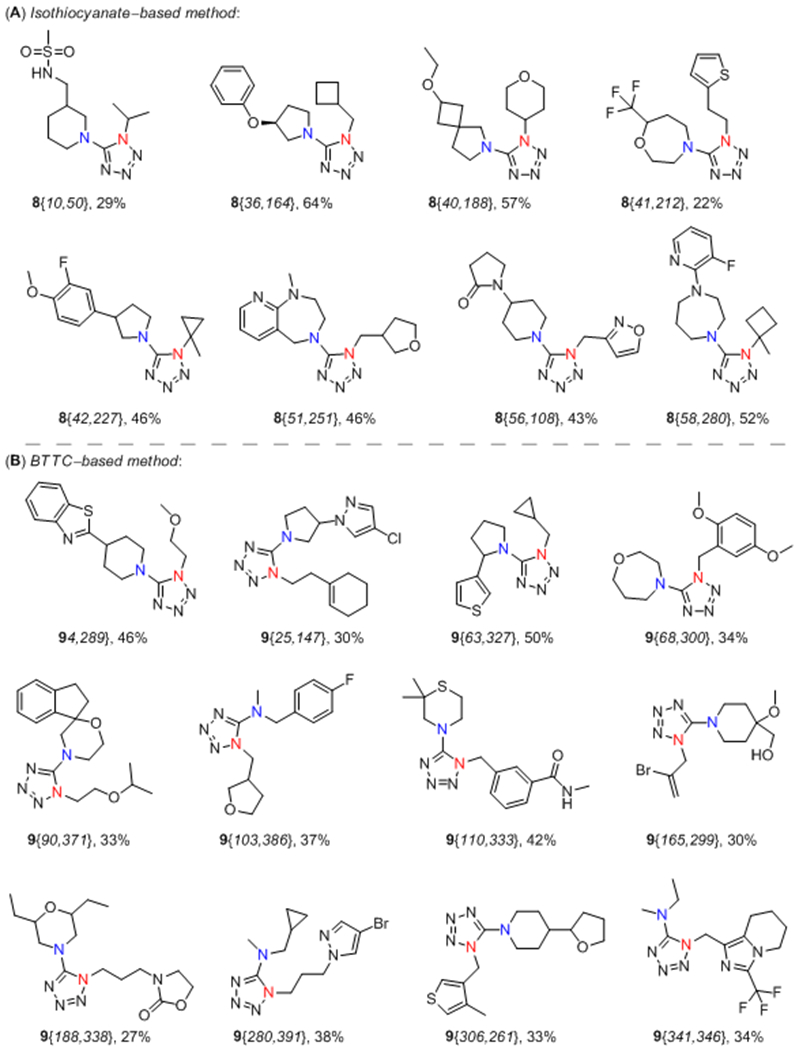
Representative 1-substituted 5-aminotetrazoles 8 and 9 derived products prepared by isothiocyanate-based (A) and BTTC-based (B) methods.
Figure 4.
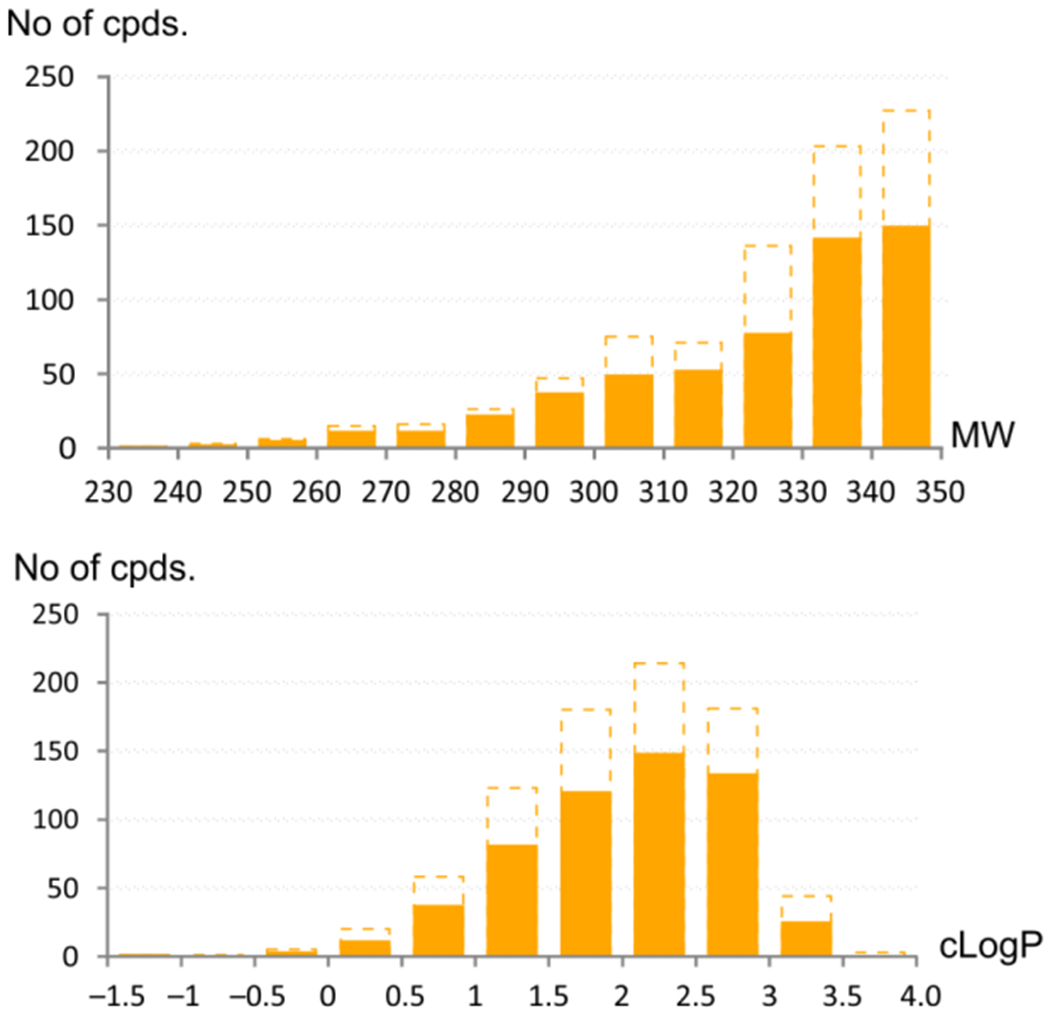
MW and cLogP characteristics of the synthesized (filled bars) and designed arrays (hollowed dashed bars).
Expanding chemical space of synthetically feasible 5-aminotetrazoles.
The major role of parallel synthesis has been providing a great number of new structurally related compounds for facile application in biological, physicochemical and other investigations. Nowadays, well-elaborated combinatorial protocols can also provide a base for prediction of all theoretical structures from already known starting materials. In several cases, it is more practical to model potentially accessible virtual chemical space,43 analyze its properties by in silico methods,44 and pick only the most promising representatives before carrying out the synthetic part. This approach not only avoids the waste of resources on the preparation of redundant compounds, but also facilitates the discovery of better hits due to an increased pool of processed ligands by virtual high-throughput screening (vHTS).45,46 For this reason, REAL database47 (REAL = readily accessible) was developed by us to accumulate highly feasible chemical compounds which can be obtained from available reagents by well-validated combinatorial chemistry protocols. Recently, the structure-based docking of 170 million feasible compounds from this database against AmpC and the D4 dopamine receptor allowed to find the most potent AmpC reversible inhibitor, as well as 158 thousand potential D4 ligands with sub-1-μM predicted affinity values.48 Thus, a further objective of the current work was to demonstrate the extension of accessible chemical space by the developed method.
The current commercial availability and literature coverage of 5-aminotetrazoles was estimated by substructural searches in eMolecules49 and PubChem50 databases, which revealed 17,219 and 55,163 compounds, respectively (Figure 5). To estimate the number of 5-aminotetrazoles feasible by isothiocyanate-based and BTTC-based methods, the combinations of commercially available secondary amines 11 with isothiocyanates 10 or primary amines 15, respectively, were selected by in-house developed algorithms based on our previous data on the reactivity of the reagents.39–41 Because the abundance of primary amines is significantly greater than amount of isothiocyanates, it was not surprising that the number of BTTC-derived products (7,097,307) was 20 times larger than that of isothiocyanate-derived (326,702). Additionally, because the probability of successful preparation of the target products by the BTTC-based method was 67%, these 7 million virtual structures can be considered as REAL substituted 5-aminotetrazoles.
Figure 5.
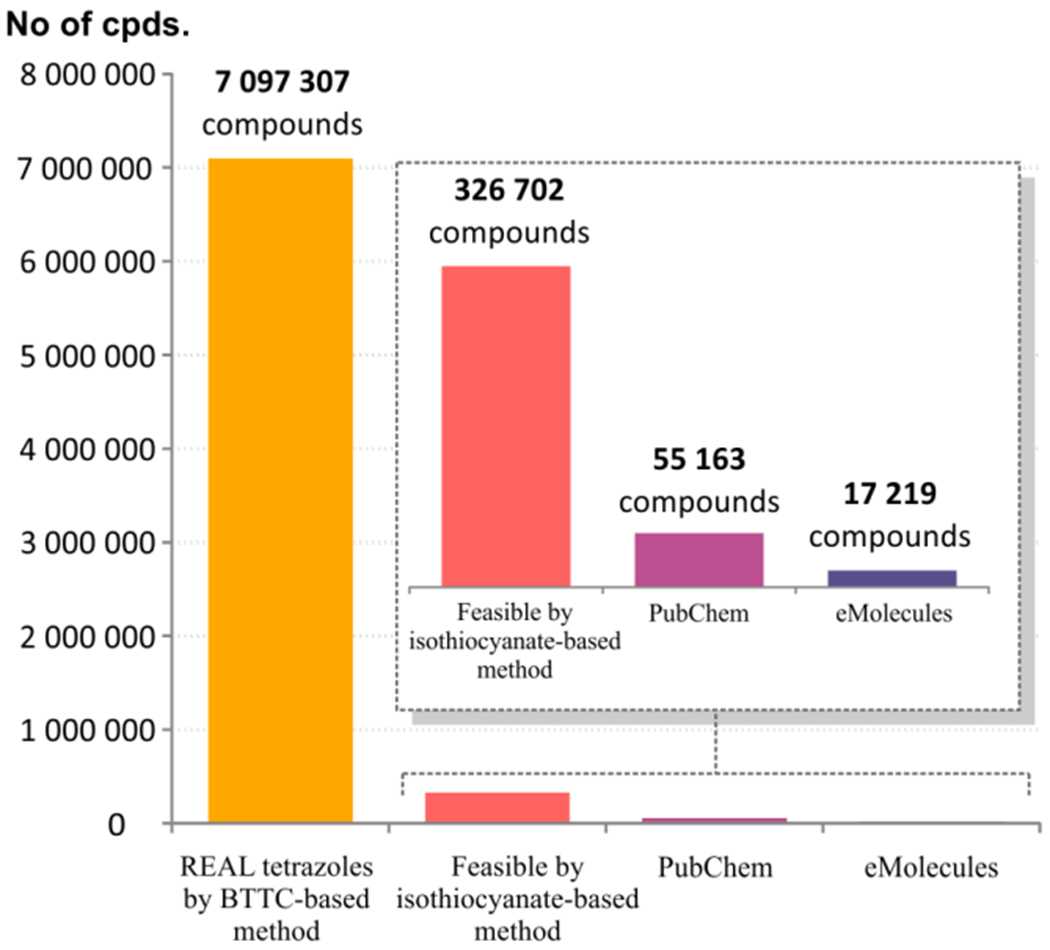
Substituted 5-aminotetrazoles in commercial, public, and the REAL databases.
The utility of the generated REAL database subset for vHTS is ensured by the high percentage of structures passing PAINS51 (99%) and Lilly52 (95%) filters. The database scaffold diversity was represented by 809,308 Bemis – Murcko frameworks and included 56% of singletons. These characteristics make the REAL 5-aminotetrazole database a useful tool for lead discovery and optimization programs.
CONCLUSIONS
Two approaches for the combinatorial synthesis of 5-(dialkylamino)tetrazoles commencing from aliphatic isothiocyanates and 2,2,2-trifluoroethylthiocarbamate (BTTC) were developed. Of these protocols, the BTTC-based method was found to be superior. This approach involved the sequential reaction of primary and then – secondary amines with 2,2,2-trifluoroethylthiocarbamate, subsequent alkylation of the resulting unsymmetrical thiourea with 1,3-propane sultone, and cyclization with azide anion. As a result, a 559-member 5-aminotetrazole library were obtained with up to 92% yield (67% success rate). Wide accessibility of the starting reagents from commercial sources, together with tolerance to a broad range of polar functional groups make the BTTC-based protocol a new potent method for lead-oriented library synthesis. Immense expansion of chemical space of synthetically feasible aminotetrazoles was demonstrated by generation of the REAL (readily accessible) database containing over 7 million structures, 95% of which satisfied common medicinal chemistry filters.
EXPERIMENTAL SECTION
General Methods.
The solvents were purified according to the standard procedures.53 All starting materials were taken at Enamine Ltd. The success rate was calculated as the number of successful experiments divided by the number of failed experiments. Reverse phase column chromatography was performed using C18-modified silica gel as a stationary phase. 1H, 19F, and 13C NMR spectra were recorded at 500 MHz, 470, and 126 MHz, respectively. Chemical shifts are reported in ppm downfield from TMS as internal standards. Mass spectra were recorded on an LC–MS instrument with chemical ionization (CI). LC–MS data were acquired on an Agilent 1200 HPLC system equipped with DAD/ELSD/LCMS-6120 diode matrix and mass-selective detector. Melting points were measured on a MPA100 OptiMelt automated melting point system. Elemental analyses were performed at the Laboratory of Organic Analysis, Department of Chemistry, Taras Shevchenko National University of Kyiv.
General protocol for 5-aminotetrazole synthesis (isothiocyanate-based method).
Isothiocyanate 10 (1.00 mmol) and amine 11 (1.00 mmol) were added to a sealable vial containing CH3CN (0.7 mL). If the amine 11 was used as a salt, i-Pr2NEt (1.20 mmol) was also added. After sealing, the vial was heated at 100 °C for 10 min and shaken vigorously. The reaction mixture was heated at 100 °C until full dissolution, and then – for additional 30 min. 1,3-Propane sultone (2.00 mmol) was added to the solution, the reaction mixture was shaken at 50 °C overnight and then was heated at 100 °C for 16 h. Et3N (3.00 mmol) was added to the resulting mixture and the reaction mixture was heated at 100 °C for 30 min. NaN3 (1.10 mmol) was added, the mixture was shaken at rt overnight and then stirred at 50 °C for 12 h. The resulting reaction mixture was evaporated and further dissolved in CHCl3 (3 mL). The organic layer was washed with H2O (2×4 mL). The organic phase was evaporated and subjected to HPLC purification on a C18-column using MeOH − H2O as eluent to give 8 with >90% purity.
General protocol for 5-aminotetrazole synthesis (BTTC-based method).
Amine 15 (1.00 mmol) and 2,2,2-trifluoroethylthiocarbamate (1.00 mmol) were added to a sealable vial containing Et3N (1.00 mmol) in CH3CN (0.5 mL). If the amine 15 was used as a salt, Et3N (1.20 mmol for mono- and 2.20 mmol for dihydrochlorides) was also added. After sealing, the vial was shaken at rt overnight and then heated at 100 °C for 3 h. Next, amine 11 (1.00 mmol) was added to the reaction mixture. If the amine 11 was used as a hydrochloride – additional Et3N (1.10 mmol) was added. After heating at 100 °C for 7 h, the reaction mixture was evaporated under vacuum and the residue was dissolved in CH3CN (0.7 mL). 1,3-Propane sultone (2.00 mmol) was added to the solution, the reaction mixture was shaken at 50 °C overnight and then heated at 100 °C for 16 h. Et3N (3.00 mmol) was added, and the reaction mixture was heated at 100 °C for 30 min. NaN3 (1.10 mmol) was added, the mixture was shaken at rt overnight and then stirred at 50 °C for 12 h. The resulting reaction mixture was evaporated and further dissolved in CHCl3 (3 mL). The organic layer was washed with H2O (2×4 mL). The organic phase was evaporated and subjected to HPLC purification on a C18-column using MeOH − H2O as eluent to give 9 with >90% purity.
Supplementary Material
ACKNOWLEDGMENT
The work was funded by Enamine Ltd and NIH grant GM133836 (to Prof. John J. Irwin and Y.S.M.). O.O.G. was also funded by Ministry of Education and Science of Ukraine (Grant No. 19BF037-03). The authors thank Prof. Andrey A. Tolmachev for his encouragement and support.
ABBREVIATIONS
- BTC
bis(2,2,2-trifluoroethyl)carbonate
- BTTC
2,2,2-trifluoroethylthiocarbamate
- REAL
readily accessible
Footnotes
Supporting Information. The following files are available free of charge: Tables of reagents, products, and copies of NMR spectra (PDF)
REFERENCES
- (1).Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57 (24), 10257–10274. [DOI] [PubMed] [Google Scholar]
- (2).Taylor RD; MacCoss M; Lawson ADG Rings in Drugs. J. Med. Chem. 2014, 57 (14), 5845–5859. [DOI] [PubMed] [Google Scholar]
- (3).Ostrovskii VA; Koldobskii GI; Trifonov RE Tetrazoles In Comprehensive Heterocyclic Chemistry III; Elsevier, 2008; pp 257–423. [Google Scholar]
- (4).Ostrovskii VA; Trifonov RE; Popova EA Medicinal Chemistry of Tetrazoles. Russ. Chem. Bull. 2012, 61 (4), 768–780. [Google Scholar]
- (5).Wei CX; Bian M; Gong GH Tetrazolium Compounds: Synthesis and Applications in Medicine. Molecules 2015, 20 (4), 5528–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Mohite PB; Bhaskar VH Potential Pharmacological Activities of Tetrazoles in the New Millennium. Int. J. PharmTech Res. 2011, 3 (3), 1557–1566. [Google Scholar]
- (7).Ostrovskii VA; Popova EA; Trifonov RE Developments in Tetrazole Chemistry (2009–16) In Advances in Heterocyclic Chemistry; Elsevier Ltd, 2017; Vol. 123, pp 1–62. [Google Scholar]
- (8).Neochoritis CG; Zhao T; Dömling A Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119 (3), 1970–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Patil P; Mishra B; Sheombarsing G; Kurpiewska K; Kalinowska-Tłuścik J; Dömling A Library-to-Library Synthesis of Highly Substituted α-Aminomethyl Tetrazoles via Ugi Reaction. ACS Comb. Sci. 2018, 20 (2), 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Rainoldi G; Begnini F; de Munnik M; Lo Presti L; Vande Velde CML; Orru R; Lesma G; Ruijter E; Silvani A Sequential Multicomponent Strategy for the Diastereoselective Synthesis of Densely Functionalized Spirooxindole-Fused Thiazolidines. ACS Comb. Sci. 2018, 20 (2), 98–105. [DOI] [PubMed] [Google Scholar]
- (11).Wang Y; Patil P; Kurpiewska K; Kalinowska-Tluscik J; Dömling A Two Cycles with One Catch: Hydrazine in Ugi 4-CR and Its Postcyclizations. ACS Comb. Sci. 2017, 19 (3), 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Patil P; Kurpiewska K; Kalinowska-Tłuścik J; Dömling A Ammonia-Promoted One-Pot Tetrazolopiperidinone Synthesis by Ugi Reaction. ACS Comb. Sci. 2017, 19 (5), 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shaaban S; Negm A; Ashmawy AM; Ahmed DM; Wessjohann LA Combinatorial Synthesis, in Silico , Molecular and Biochemical Studies of Tetrazole-Derived Organic Selenides with Increased Selectivity against Hepatocellular Carcinoma. Eur. J. Med. Chem. 2016, 122, 55–71. [DOI] [PubMed] [Google Scholar]
- (14).Patil P; de Haan M; Kurpiewska K; Kalinowska-Tłuścik J; Dömling A Versatile Protecting-Group Free Tetrazolomethane Amine Synthesis by Ugi Reaction. ACS Comb. Sci. 2016, 18 (3), 170–175. [DOI] [PubMed] [Google Scholar]
- (15).Pérez-Labrada K; Méndez Y; Brouard I; Rivera DG Multicomponent Ligation of Steroids: Creating Diversity at the Linkage Moiety of Bis-Spirostanic Conjugates by Ugi Reactions. ACS Comb. Sci. 2013, 15 (6), 320–330. [DOI] [PubMed] [Google Scholar]
- (16).Gunawan S; Petit J; Hulme C Concise One-Pot Preparation of Unique Bis-Pyrrolidinone Tetrazoles. ACS Comb. Sci. 2012, 14 (3), 160–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Molteni G; Del Buttero P 1,3-Dipolar Cycloadditions of MeOPEG-Bounded Azides. Tetrahedron 2005, 61 (21), 4983–4987. [Google Scholar]
- (18).Gavrilyuk JI; Evindar G; Chen JY; Batey RA Peptide-Heterocycle Hybrid Molecules: Solid-Phase-Supported Synthesis of Substituted N -Terminal 5-Aminotetrazole Peptides via Electrocyclization of Peptidic Imidoylazides. J. Comb. Chem. 2007, 9 (4), 644–651. [DOI] [PubMed] [Google Scholar]
- (19).Herr RJ 5-Substituted-1H-Tetrazoles as Carboxylic Acid Isosteres: Medicinal Chemistry and Synthetic Methods. Bioorg. Med. Chem. 2002, 10 (11), 3379–3393. [DOI] [PubMed] [Google Scholar]
- (20).Tymtsunik AV; Bilenko VA; Kokhan SO; Grygorenko OO; Volochnyuk DM; Komarov IV 1-Alkyl-5-((Di)Alkylamino) Tetrazoles: Building Blocks for Peptide Surrogates. J. Org. Chem. 2012, 77 (2), 1174–1180. [DOI] [PubMed] [Google Scholar]
- (21).Gallou I Unsymmetrical Ureas. Synthetic Methodologies And Application in Drug Design. Org. Prep. Proced. Int. 2007, 39 (4), 355–383. [Google Scholar]
- (22).Wang X-M; Mao S; Cao L; Xie X-X; Xin M-H; Lian J-F; Cao Y-X; Zhang S-Q Modification of N -(6-(2-Methoxy-3-(4-Fluorophenylsulfonamido)Pyridin-5-Yl)-[1,2,4]Triazolo[1,5- a ]Pyridin-2-Yl)Acetamide as PI3Ks Inhibitor by Replacement of the Acetamide Group with Alkylurea. Bioorg. Med. Chem. 2015, 23 (17), 5662–5671. [DOI] [PubMed] [Google Scholar]
- (23).Utsui Y; Ohya S; Magaribuchi T; Tajima M; Yokota T Antibacterial Activity of Cefmetazole Alone and in Combination with Fosfomycin against Methicillin- and Cephem-Resistant Staphylococcus Aureus. Antimicrob. Agents Chemother. 1986, 30 (6), 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Li L-H; Yen M-Y; Ho C-C; Wu P; Wang C-C; Maurya PK; Chen P-S; Chen W; Hsieh W-Y; Chen H-W Non-Cytotoxic Nanomaterials Enhance Antimicrobial Activities of Cefmetazole against Multidrug-Resistant Neisseria Gonorrhoeae. PLoS One 2013, 8 (5), e64794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Campoli-Richards DM; Lackner TE; Monk JP Ceforanide. Drugs 1987, 34 (4), 411–437. [DOI] [PubMed] [Google Scholar]
- (26).Muytjens HL; van der Ros-van de Repe J Comparative Activities of 13 Beta-Lactam Antibiotics. Antimicrob. Agents Chemother. 1982, 21 (6), 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Shinohara Y; Katayama Y; Uchiyama S; Yamaguchi T; Handa S; Matsuoka K; Ohashi Y; Tanahashi N; Yamamoto H; Genka C; Kitagawa Y; Kusuoka H; Nishimaru K; Tsushima M; Koretsune Y; Sawada T; Hamada C Cilostazol for Prevention of Secondary Stroke (CSPS 2): An Aspirin-Controlled, Double-Blind, Randomised Non-Inferiority Trial. Lancet Neurol. 2010, 9 (10), 959–968. [DOI] [PubMed] [Google Scholar]
- (28).Park SY; Lee HR; Lee WS; Shin HK; Kim HY; Hong KW; Kim CD Cilostazol Modulates Autophagic Degradation of β-Amyloid Peptide via SIRT1-Coupled LKB1/AMPKα Signaling in Neuronal Cells. PLoS One 2016, 11 (8), e0160620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Miller AE; Feeney DJ; Ma Y; Zarcone L; Aziz MA; Magnuson E Synthetic Communications : An International Journal for Rapid Communication of Synthetic Organic Chemistry The Synthesis of Aminoiminoethanenitriles , Hydroxyguanidines from Aminoiminomethanesulfonic Acids. 2006, No. October 2013, 217–226. [Google Scholar]
- (30).Erle H-E UmsetzungenN-Acyl-Substituierter α-Chlorformamidine Mit Nucleophilen Reagenzien. Liebigs Ann. der Chemie 1982, 1982 (2), 201–210. [Google Scholar]
- (31).Katritzky AR; Rogovoy BV; Kovalenko KV. A General Route to 5-Aminotetrazoles. J. Org. Chem. 2003, 68 (12), 4941–4943. [DOI] [PubMed] [Google Scholar]
- (32).Chaudhari PS; Pathare SP; Akamanchi KGO -Iodoxybenzoic Acid Mediated Oxidative Desulfurization Initiated Domino Reactions for Synthesis of Azoles. 2012. [DOI] [PubMed] [Google Scholar]
- (33).Nasrollahzadeh M; Habibi D; Shahkarami Z; Bayat Y A General Synthetic Method for the Formation of Arylaminotetrazoles Using Natural Natrolite Zeolite as a New and Reusable Heterogeneous Catalyst. Tetrahedron 2009, 65 (51), 10715–10719. [Google Scholar]
- (34).Congreve MS Synthesis of 5-N-Substituted Tetrazole Derivatives of the Potent NK1 Receptor Antagonist GR203040. Synlett 1996, 1996 (04), 359–360. [Google Scholar]
- (35).Joo Y; Shreeve JM 1-Substituted 5-Aminotetrazoles: Syntheses from CNN 3 with Primary Amines. Org. Lett. 2008, 10 (20), 4665–4667. [DOI] [PubMed] [Google Scholar]
- (36).Karaghiosoff K; Klapo TM; Mayer P; Piotrowski H; Polborn K; Willer RL; Weigand JJ; July RVN -Nitroso- and N -Nitraminotetrazoles. 2006, 1295–1305. [DOI] [PubMed] [Google Scholar]
- (37).Pathare RS; Ansari AJ; Verma S; Maurya A; Maurya AK; Agnihotri VK; Sharon A; Pardasani RT; Sawant DM Sequential Pd(0)/Fe(III) Catalyzed Azide–Isocyanide Coupling/Cyclization Reaction: One-Pot Synthesis of Aminotetrazoles. J. Org. Chem. 2018, 83 (16), 9530–9537. [DOI] [PubMed] [Google Scholar]
- (38).Sathishkumar M; Shanmugavelan P; Nagarajan S; Dinesh M; Ponnuswamy A Water Promoted One Pot Three-Component Synthesis of Tetrazoles. New J. Chem. 2013, 37 (2), 488–493. [Google Scholar]
- (39).Bogolyubsky AV; Savych O; Zhemera AV; Pipko SE; Grishchenko AV; Konovets AI; Doroshchuk RO; Khomenko DN; Brovarets VS; Moroz YS; Vybornyi M Facile One-Pot Parallel Synthesis of 3-Amino-1,2,4-Triazoles. ACS Comb. Sci. 2018, 20 (7), 461–466. [DOI] [PubMed] [Google Scholar]
- (40).Bogolubsky AV; Moroz YS; Mykhailiuk PK; Granat DS; Pipko SE; Konovets AI; Doroschuk R; Tolmachev A Bis(2,2,2-Trifluoroethyl) Carbonate as a Condensing Agent in One-Pot Parallel Synthesis of Unsymmetrical Aliphatic Ureas. ACS Comb. Sci. 2014, 16 (6), 303–308. [DOI] [PubMed] [Google Scholar]
- (41).Bogolubsky AV; Moroz YS; Savych O; Pipko S; Konovets A; Platonov MO; Vasylchenko OV; Hurmach VV; Grygorenko OO An Old Story in the Parallel Synthesis World: An Approach to Hydantoin Libraries. ACS Comb. Sci. 2018, 20 (1), 35–43. [DOI] [PubMed] [Google Scholar]
- (42).Nadin A; Hattotuwagama C; Churcher I Lead-Oriented Synthesis: A New Opportunity for Synthetic Chemistry. Angew. Chem., Int. Ed. 2012, 51 (5), 1114–1122. [DOI] [PubMed] [Google Scholar]
- (43).Bleicher KH; Böhm H-J; Müller K; Alanine AI Hit and Lead Generation: Beyond High-Throughput Screening. Nat. Rev. Drug Discov. 2003, 2 (5), 369–378. [DOI] [PubMed] [Google Scholar]
- (44).Gromski PS; Henson AB; Granda JM; Cronin L How to Explore Chemical Space Using Algorithms and Automation. Nat. Rev. Chem. 2019, 3 (2), 119–128. [Google Scholar]
- (45).Damm-Ganamet KL; Arora N; Becart S; Edwards JP; Lebsack AD; McAllister HM; Nelen MI; Rao NL; Westover L; Wiener JJM; Mirzadegan T Accelerating Lead Identification by High Throughput Virtual Screening: Prospective Case Studies from the Pharmaceutical Industry. J. Chem. Inf. Model. 2019, 59 (5), 2046–2062. [DOI] [PubMed] [Google Scholar]
- (46).Berto R; Barbiero G The Biophilic Quality Index: A Tool to Improve a Building from “Green” to Restorative. Visions Sustain. 2017, 8, 38–45. [Google Scholar]
- (47).Hoffmann T; Gastreich M The next Level in Chemical Space Navigation: Going Far beyond Enumerable Compound Libraries. Drug Discov. Today 2019, 24 (5), 1148–1156. [DOI] [PubMed] [Google Scholar]
- (48).Lyu J; Wang S; Balius TE; Singh I; Levit A; Moroz YS; O’Meara MJ; Che T; Algaa E; Tolmachova K; Tolmachev AA; Shoichet BK; Roth BL; Irwin JJ Ultra-Large Library Docking for Discovering New Chemotypes. Nature 2019, 566 (7743), 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).eMolecules database (data retrieved on 20 May 2019): https://www.emolecules.com/.
- (50).PubChem database (data retrieved on 20 May 2019): https://pubchem.ncbi.nlm.nih.gov/.
- (51).Baell JB; Holloway GA New Substructure Filters for Removal of Pan Assay Interference Compounds (PAINS) from Screening Libraries and for Their Exclusion in Bioassays. J. Med. Chem. 2010, 53 (7), 2719–2740. [DOI] [PubMed] [Google Scholar]
- (52).Bruns RF; Watson IA Rules for Identifying Potentially Reactive or Promiscuous Compounds. J. Med. Chem. 2012, 55 (22), 9763–9772. [DOI] [PubMed] [Google Scholar]
- (53).Armarego WLF. Purification of Laboratory Chemicals Eighth Edition; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


