Abstract
Background
Recent research findings have improved the understanding of the diagnosis, pathophysiology, genetics, etiology, and treatment of peripheral, central, and functional vestibular vertigo syndromes.
Method
A literature search, with special attention to the current classification, treatment trials, Cochrane analyses, and other meta-analyses.
Results
There are internationally accepted diagnostic criteria for benign positional paroxysmal vertigo, Menière’s disease, bilateral vestibulopathy, vestibular paroxysmia, and functional dizziness. Whether an acute vestibular syndrome is central or peripheral can usually be determined rapidly on the basis of the history and the clinical examination. “Cerebellar vertigo” is a clinically important entity. For bilateral vestibulopathy, balance training is an effective treatment. For Menière’s disease, preventive treatment with betahistine (48 mg and 144 mg per day) is not superior to placebo. For vestibular paroxysmia, oxcarbazepine has been shown to be effective. Treatments that are probably effective for functional dizziness include vestibular rehabilitation, cognitive behavioral therapy, and serotonin reuptake inhibitors.
Conclusion
The diagnostic assessment of vestibular syndromes is much easier for clinicians now that it has been internationally standardized. There is still a lack of randomized, controlled trials on the treatment of, for example, Menière’s disease, vestibular migraine, and “cerebellar vertigo.”
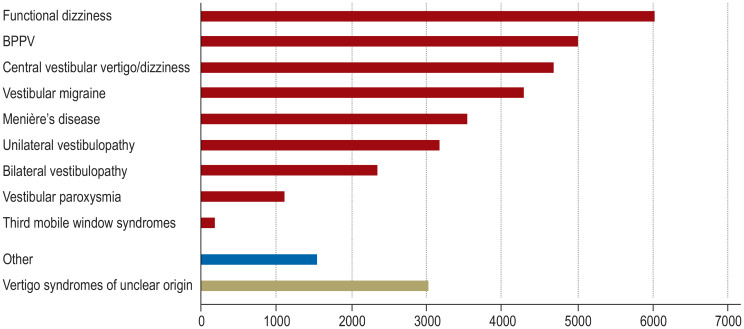
Vertigo or dizziness is not a disease in itself, but rather a leading symptom of various diseases of differing etiologies. These include dysfunction of the vestibular system, both peripheral (inner ear, vestibular nerve) and central (brainstem, cerebellum); functional dizziness; and diseases of other causes, including blood pressure regulation disorders, such as orthostatic dizziness, and adverse drug effects. Vertigo is often wrongly attributed to an isolated polyneuropathy or to poor vision, as these conditions can also cause gait instability. Its incidence is approximately 11% per year; individuals with have a markedly higher mortality (adjusted for age, sex, and comorbidities) than those without vertigo or dizziness (adjusted odds ratio 1.7) (1). The lifetime prevalence of moderate to severe vertigo and dizziness is approximately 30% (e1, e2). The relative frequency of the individual diseases is shown in Figure 1.
Figure 1.
The frequency of various vestibular syndromes among 34 860 patients in a specialized outpatient clinic
Absolute frequencies of various vertigo/dizziness syndromes in the supraregional specialized outpatient clinic of the German Center for Vertigo and Balance Disorders and the Department of Neurology, LMU, Munich, Germany (1998–2019). BPPV, Benign paroxysmal positional vertigo.
Learning objectives
The diagnostic evaluation of vestibular disorders.
This is based on a combination of the clinical history with four key questions, physical examination of the vestibular, ocular motor, and cerebellar systems, a video head-impulse test, and, if the latter is normal, caloric testing.
Incidence.
The incidence of vertigo and dizziness is approximately 11% per year.
After reading this article, the reader should:
Have a new attitude to vertigo and dizziness: on the basis of the history and physical examination, even the non-specialist physician can now, in many cases, diagnose vertigo and dizziness rapidly and easily and apply effective treatment.
Have better knowledge/skills in the following areas: internationally accepted diagnostic criteria, physical examination of the vestibular system, and treatment options, including physical therapy, drugs, psychotherapy, and, rarely, surgery.
Diagnosis and current classification
The diagnostic assessment of the various vestibular syndromes is based on the patient’s history and the clinical examination of the vestibular, ocular motor, and cerebellar systems. When the history is taken, the patient should be asked about the nature and temporal course of the symptoms. Did they begin suddenly? Are they continuous, or do they occur episodically? It should be determined which particular disorder is present: spinning vertigo, dizziness, postural imbalance, or light-headedness. The modulatory factors are also important, e.g., changes of body position or the activities the patient was engaged in when the symptoms arose. One should also ask about any accompanying symptoms and their localization. Is the inner ear, the brainstem, or the cerebellum affected? Or are the attacks typical of migraine? (box).
BOX. The differential diagnosis of frequent vestibular syndromes.
Temporal course
-
Attacks
seconds to minutes: BPPV (< 1 min*), vestibular paroxysmia (< 1 min*), third mobile window syndromes
minutes to hours: vestibular migraine (5 min to 72 h*), Menière’s disease (20 min to 12 h*), rarely episodic ataxia type 2
-
Acute onset, duration of days to a few weeks
acute unilateral vestibulopathy, brainstem or cerebellar stroke
-
Persistent symptoms: > 3 months
bilateral vestibulopathy, functional dizziness, neurodegenerative diseases (cerebellar vertigo, extrapyramidal disorders)
Accompanying symptoms
-
Hypacusis, tinnitus, pressure sensation in the ear
Menière’s disease
-
Double vision, ataxia, dysarthria, hemiparesis, hemihypesthesia
central vertigo, e.g., acute central vestibular syndrome or cerebellar vertigo
-
Headache attacks, migraine, photophobia, phonophobia, aura
vestibular migraine
-
Oscillopsia even at rest
acute unilateral vestibulopathy, Menière’s disease (during attacks), vestibular migraine, patients with spontaneous nystagmus, e.g., downbeat nystagmus
-
Oscillopsia on movement
bilateral vestibulopathy
Precipitating and modulating factors
-
Symptoms are present even at rest
attack of Menière’s disease or vestibular migraine, acute unilateral vestibulopathy, vestibular paroxysmia, acute central vestibular syndrome
-
Evoked by changes in body position
BPPV, central positional vertigo, orthostatic dizziness
-
Evoked by changes in pressure or loud noises
third mobile window syndromes (semicircular canal dehiscence, perilymph fistula)
-
On standing, walking, and running
bilateral vestibulopathy (with exacerbation in the dark and no symptoms when lying or sitting)
-
Certain situations (crowds, wide open spaces)
functional dizziness
-
Amelioration by distraction, sporting activities, or mild alcohol consumption, and generally less severe in the morning
functional dizziness
*Current diagnostic criteria: www.jvr-web.org/ICVD.html BPPV, benign paroxysmal positional vertigo
Diagnosis.
The diagnostic assessment has been simplified by the current classification of vestibular disorders of the Bárány Society (www.jvr-web.org/ICVD.html).
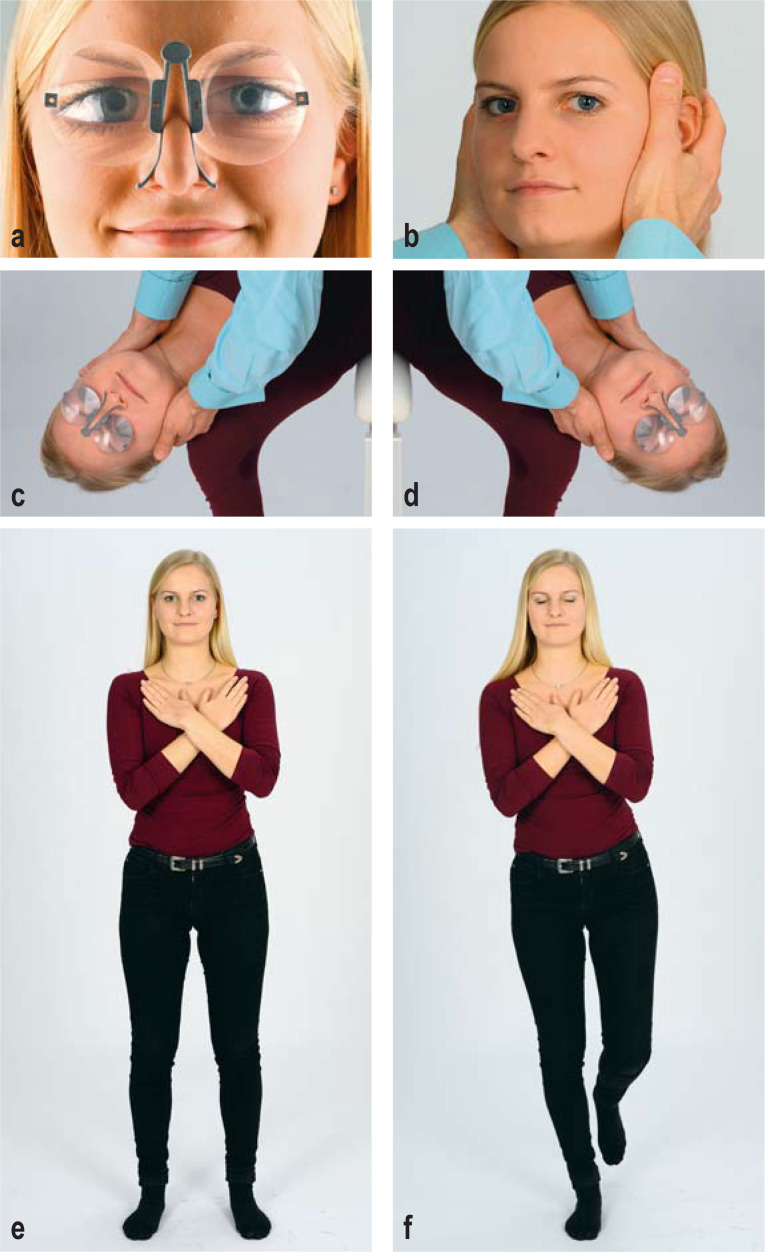
In the physical examination, special attention should be paid to whether spontaneous or positionally induced nystagmus is present. Frenzel’s goggles and M glasses have proved to be simple, practical aids to nystagmus testing (Figure 2a) (2). The vestibulo-ocular reflex (VOR) should be evaluated with the head-impulse test (Figure 2b) and the diagnostic positional maneuvers (Figure 2c, d). Finally, stance and gait should be examined (Figure 2e, f). Basic, initial screening for hearing impairment can be performed with a tuning fork.
Figure 2.
The clinical examination of a patient complaining of vertigo, dizziness, or postural imbalance.
a) Inspection for spontaneous nystagmus. This should be performed with and without fixation, with the aid of Frenzel goggles (M glasses, illustrated).
b) Clinical head-impulse test. The examiner should look for possible refixation saccades, which indicate dysfunction of the vestibulo-ocular reflex (VOR).
c, d) All patients, regardless of their history, should be investigated for positional vertigo with rotation of the head by 45° and simultaneous positioning in the opposite direction. Diagnostic positioning maneuvers (SémontPlus) are shown for c) the right posterior semicircular canal and d) the left posterior semicircular canal.
e, f) Romberg test: e) basic test condition with the legs apart and the eyes open; f) advanced test condition with the patient standing on one leg and keeping both eyes closed.
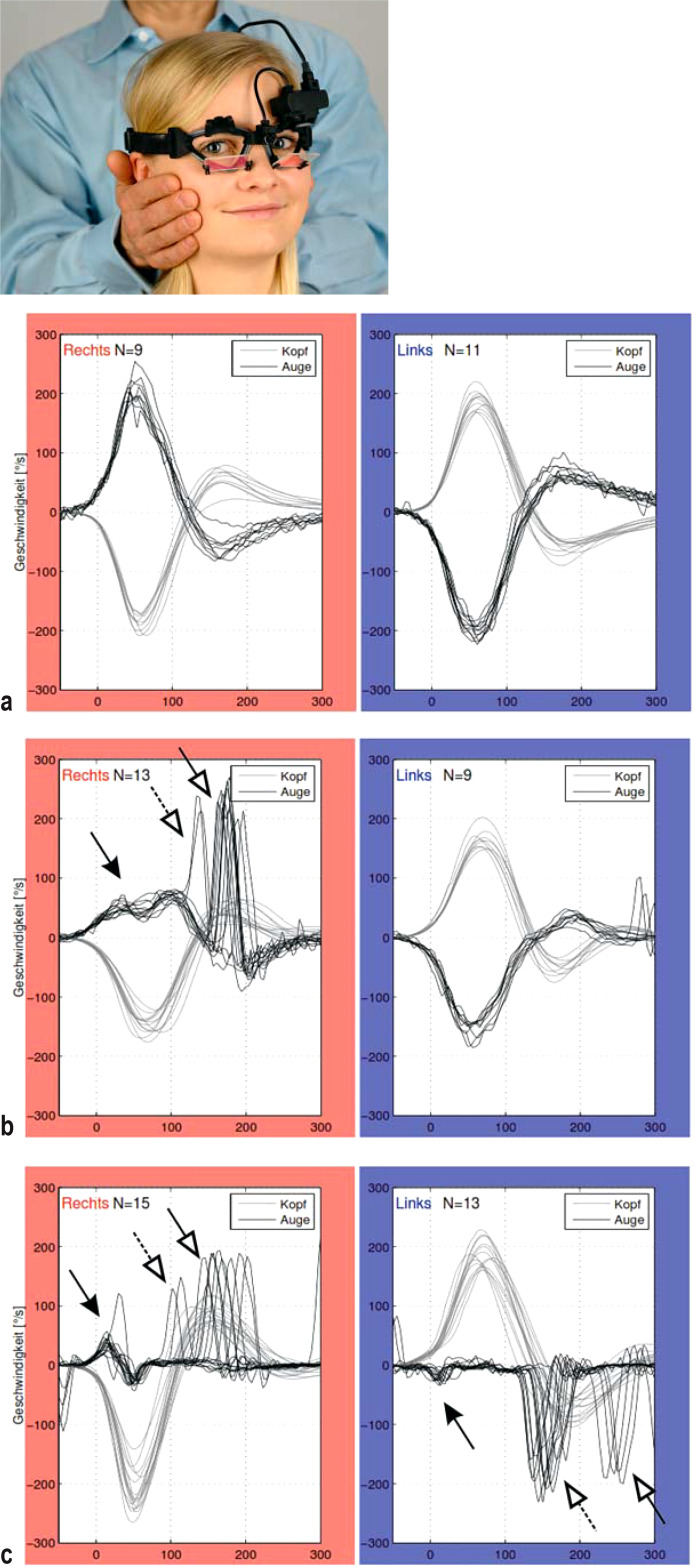
The two main ancillary laboratory tests for the function of the VOR are the video head-impulse test (e3) (efigure), which is superior to clinical examination (3), and caloric testing of the horizontal semicircular canals. Finally, diagnostic assessment has been standardized and further simplified in the entirely clinically oriented, internationally accepted diagnostic criteria of the Bárány Society (free download: www.jvr-web.org/ICVD.html). Cochrane analyses and German clinical practice guidelines are listed in eTables 1 and 2.
eFigure.
Video head-impulse test (vHIT):
Left: Quantitative investigation of the function of the horizontal vestibulo-ocular reflex (hVOR). The angular velocities of eye and head movements are measured simultaneously, and their quotient — called “gain” — is calculated.
Below: Original recordings, with values on head turning to the right (marked in red) and to the left (marked in blue).
Normal findings: The angular velocities of eye movement (black curve) and head movement (gray curve) are nearly identical: gain approximately 1.
A patient with right unilateral vestibulopathy The right-sided h-VOR gain is reduced to 0.3 (black arrow), and there are both covert and overt correcting saccades (white arrows).
A patient with bilateral vestibulopathy (BVP) Reduced VOR gain bilaterally: 0.05 (black arrow), with both covert and overt correcting saccades (white arrows)
eTabLe 1. AWMF guidelines on vertigo (www.awmf.org, as of 30 March 2020).
| Title | Registration number | Validity | Issuing society |
| Acute vertigo in general medical practice (S3) | 053–018 | Until 29 November 2020 | German Society for General Practice and Family Medicine (Deutsche Gesellschaft für Allgemeinmedizin) und Familienmedizin, DEGAM) |
| Vestibular disorders (S2k, forthcoming) | 017–078 | Forthcoming;as of 29 April 2019: guideline documents submitted, review not yet completed, completion expected by 31 December 2020 | German Society for Neurology (Deutsche Gesellschaft für Neurologie) and German Society for Otorhinolaryngology and Head and Neck Surgery (Deutsche Gesellschaft für Hals-Nasen-Ohren-Heilkunde, Kopf- und Halschirurgie e.V.) |
eTable 2. Cochrane reviews on vertigo (www.cochranelibrary.com, as of 30 March 2020).
| Diagnosis | Title | Date of publication |
| Acute unilateral vestibulopathy | Corticosteroids for the treatment of idiopathic acute vestibular dysfunction (vestibular neuritis) | 11 May 2011 |
| Unilateral peripheral vestibular dysfunction | Vestibular rehabilitation for unilateral peripheral vestibular dysfunction | 13 January 2015 |
| Benign paroxysmal positional vertigo | The Epley (canalith repositioning) manoeuvre for treatment of benign paroxysmal positional vertigo | 8 December 2014 |
| Modifications of the Epley (canalith repositioning) manoeuvre for posterior canal benign paroxysmal positional vertigo (BPPV) | 18 April 2012 | |
| Menière’s disease | Betahistine for Menière’s disease or syndrome | 22 January 2001; update 2008, 2011 |
| Diuretics for Menière’s disease or syndrome | 19 July 2006; update 2010 | |
| Intratympanic gentamicin for Menière’s disease or syndrome | 16 March 2011 | |
| Intratympanic steroids for Menière’s disease or syndrome | 6 July 2011 | |
| Surgery for Menière’s disease | 28 February 2013 | |
| Positive pressure therapy for Menière’s disease or syndrome | 10 March 2015 | |
| Restriction of salt, caffeine and alcohol intake for the treatment of Menière’s disease or syndrome | 31 December 2018 | |
| Vestibular migraine | Pharmacological agents for the prevention of vestibular migraine | 21 June 2015; update 2018 |
| General | Betahistine for symptoms of vertigo | 21 June 2016 |
We would like to emphasize that, contrary to widespread belief, the diagnosis of the more common vertigo syndromes is actually a simple (though by no means trivial) matter. A physician who is not a specialist in this area can usually make the right diagnosis. The new diagnostic criteria are particularly useful as a practical aid to the “closely listening and carefully examining clinician”. Consultation of a neurologist, otolaryngologist, or specialized center becomes necessary only if specialized ancillary testing is required, if the diagnosis is in doubt, or if the patient has not responded to a course of appropriate treatment.
Physical examination.
In the physical examination, special attention should be paid to spontaneous or positionally induced nystagmus. M glasses are a simple, practical aid to nystagmus testing.
Common vestibular syndromes of peripheral origin
Three types of peripheral vestibular disorders with typical signs and clinical symptoms can be differentiated on pathophysiological and functional grounds:
Bilateral loss of function (partial or total) of the vestibular nerve and/or vestibular end organs: bilateral vestibulopathy;
“Acute unilateral vestibulopathy” manifesting itself as an “acute peripheral vestibular syndrome”;
Unilateral paroxysmal pathological excitation or, more rarely, inhibition of the vestibular nerve and/or vestibular end organs (BPPV, Menière’s disease, vestibular paroxysmia, third mobile window syndromes) (4).
In the following sections, the current methods of diagnostic assessment, pathophysiology, etiology, and treatment of the more common peripheral vestibular syndromes will be presented in the light of the above classification.
Bilateral vestibulopathy
The diagnostic criteria for bilateral vestibulopathy are as follows (5):
A chronic vestibular syndrome with at least two of the following symptoms: instability of stance or gait and/or motion-induced blurred vision or oscillopsia while walking or on rapid head movement and/or worsening of instability in the dark and/or on uneven ground.
No symptoms while sitting or lying under static conditions.
Bilaterally impaired or absent function of the horizontal vestibulo-ocular reflex (VOR), as documented by a bilaterally pathological video head-impulse test (vHIT) for the horizontal semicircular canal (VOR gain < 0.6) and/or bilaterally reduced caloric excitability (the sum of the peak slow phase velocity of the calorically induced nystagmus amounts to < 6°/s for each ear).
Symptoms not better explained by another disease.
The diagnosis of bilateral vestibulopathy requires quantitative testing of vestibular function (5) (efigure), as the bedside head-impulse test is of low sensitivity and specificity (3). In the absence of quantitative testing, only “probable bilateral vestibulopathy” can be diagnosed.
The six most common peripheral vestibular syndromes, in order of decreasing incidence:
Benign paroxysmal positional vertigo, Menière’s disease, acute unilateral vestibulopathy/vestibular neuritis, bilateral vestibulopathy, vestibular paroyxsmia, and the third mobile window syndromes.
The differential diagnosis of common vestibular disorders.
Important clues to the diagnosis are to be sought in the temporal course of the symptoms, any accompanying symptoms, and precipitating or modulating factors.
The various causes of bilateral vestibulopathy were analyzed in a retrospective series of 154 patients (6). Twenty different causes were found, identified with certainty in 47% of cases and considered only possible causes in another 22%; in the remaining 31%, the etiology remained unclear. Ototoxic drugs (in particular gentamicin), were most common, followed by genetic causes and bilateral Menière’s disease. Bilateral vestibulopathy can arise at any age. The average age at diagnosis lies between 50 and 60 years (for an overview, see [5]).
The four principles that guide treatment are as follows:
Explaining the cause of the symptoms to the patient.
Primary prevention, i.e., avoidance of ototoxic substances.
Optimal treatment of the underlying disease, e.g., Menière’s disease.
Physiotherapy (level I evidence) with daily balance training, particularly including head turning in all three planes, in order to train the vestibulo-ocular reflex (VOR training). Older patients, in particular, should additionally undergo training to prevent falls under the guidance of a physiotherapist (e4, 7, 8).
Acute unilateral vestibulopathy/vestibular neuritis
Acute unilateral vestibulopathy is characterized by spinning vertigo of acute onset that lasts for at least 24 h (9), accompanied by oscillopsia, a tendency to fall, and nausea. Furthermore, horizontal-torsional spontaneous nystagmus is seen, with the fast phase beating toward the presumably unaffected side; this can be suppressed by fixation. The head-impulse test demonstrates unilateral peripheral vestibular dysfunction of the vestibulo-ocular reflex (vHIT gain < 0.7) (efigure) and/or a > 25% difference between the two sides on caloric testing. Moreover, the subjective visual vertical is pathologically tilted toward the side of the affected labyrinth.
No signs of a central ocular motor disturbance or acute hearing impairment are found.
This disease is also referred to as vestibular neuritis, neuronitis, or neuropathy.
In patients with clinical manifestations of an acute vestibular syndrome, the main differential diagnosis after acute unilateral vestibulopathy is a lesion of the brainstem or cerebellum (usually ischemic).
The probable, though not yet directly proven, etiology of acute unilateral vestibulopathy is reactivation of a latent herpes simplex virus 1 (HSV-1) infection. This hypothesis is supported, among other evidence, by a link to genetic variants of a host factor for HSV-1 replication that was revealed by a genome-wide association study (10).
Acute unilateral vestibulopathy/vestibular neuritis.
Acute unilateral vestibulopathy is characterized by spinning vertigo that arises acutely and lasts for days to weeks, peripheral vestibular horizontal-torsional spontaneous nystagmus, a pathological head-impulse test, and the exclusion of an acute central vestibular syndrome.
The efficacy of steroids as an aid to the recovery of peripheral vestibular function in acute unilateral vestibulopathy has been shown in a randomized, controlled trial (11) but nonetheless remains controversial; at least one more randomized, controlled trial is needed. A prospective observational study indicated that the success of steroid treatment depends on the latency between symptom onset and the initiation of treatment (12). The efficacy of balance training in the treatment of acute unilateral vestibulopathy has been documented in multiple randomized trials. A particularly important component of balance training is rotation of the head with fixation of a visual target to enhance the central vestibular tonus imbalance, which is the adequate stimulus for initiating central vestibular compensation (e4, 8).
Benign paroxysmal positional vertigo
Recurrent attacks of spinning vertigo induced by changes in head position relative to the gravitational axis, lasting for several seconds each, are the main manifestation of benign paroxysmal positional vertigo. Nowadays videos of eye movements taken by the patient himself or herself, with the aid of screening techniques (smart devices [e5]) or with a smartphone (e6) can also be helpful to make the diagnosis.
Approximately 95% of all cases of benign paroxysmal positional vertigo are idiopathic. A multivariate analysis has shown that this condition is significantly associated with age, prior falls, and reduced physical activity (e7). Individuals with migraine are 2.5 times more likely to develop benign peripheral paroxysmal positional vertigo than others (e8).
The diagnostic criteria for benign paroxysmal positional vertigo of the posterior semicircular canal are as follows (13): The patient suffers from recurrent attacks of rotational vertigo induced by lying down supine or turning into supine position while lying down. Each attack lasts less than 1 min. Positional nystagmus is present, arising with a short latency after the lateral positioning maneuver (the diagnostic Sémont maneuver) (figure 2) or the Dix–Hallpike maneuver. This nystagmus consists of a torsional component (the upper pole of the eyes beats toward the lower ear) combined with vertical upbeating nystagmus, and it typically lasts less than 1 min. Finally, the symptoms cannot be attributed to any other disorder.
Treatment recommendation for acute unilateral vestibulopathy.
The treatment of choice is steroid administration as soon as possible after the onset of symptoms, combined with balance training to reinforce central compensation.
The treatment of choice for benign paroxysmal positional vertigo of the posterior canal is the Sémont or Epley maneuver (level 1 evidence) (14). When these are correctly performed, the success rate is above 95%. Despite the efficacy of these repositioning maneuvers, older patients in particular tend to suffer much longer from the consequences of benign paroxysmal positional vertigo, with marked resulting impairment (e7, e9); follow-up appointments are therefore needed. Treating benign paroxysmal positional vertigo of the posterior canal with sedating antivertiginous drugs is not recommended for any more than a short time, and only if the patient suffers from marked nausea. There is no need to restrict the patient’s movements or positions during sleep (14).
Benign paroxysmal positional vertigo of the horizontal semicircular canal is much rarer. It causes linear, horizontal nystagmus (13).
Menière’s disease
The diagnostic criteria for Menière’s disease are as follows (15):
Two or more spontaneous attacks of vertigo lasting 20 min to 12 h
Audiometrically documented hearing loss for frequencies below 2000 Hz bone conduction (> 30 dB worse than the other ear in at least two neighboring frequencies), associated with vertigo attack
Fluctuating tinnitus or fullness in the affected ear
No evidence of another cause.
If low-frequency hearing loss has not been demonstrated audiometrically, then only the diagnosis of probable Menière’s disease can be made according to these criteria. It is very helpful that useful portable, iPad-based audiometric devices are now available with which patients can test their own hearing (e10).
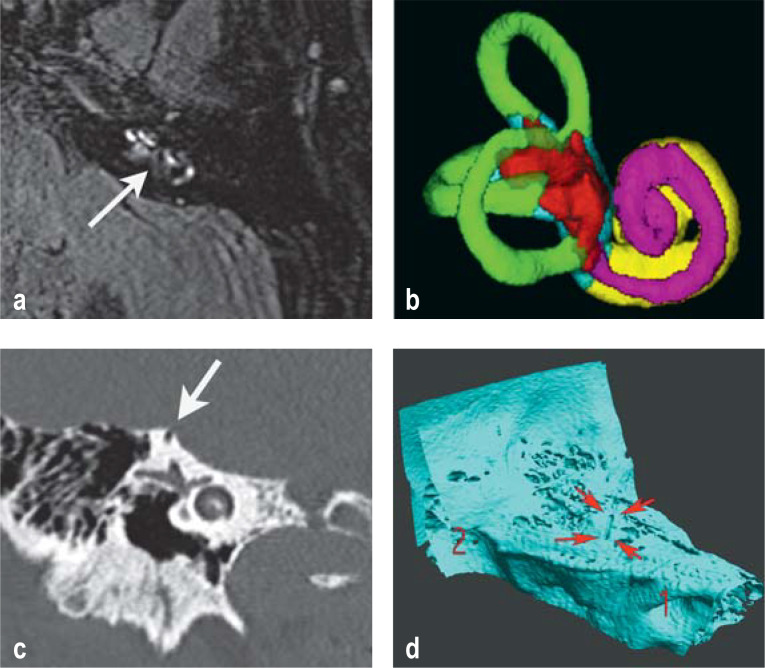
The pathogenesis of Menière’s disease is thought to involve a multifactorial disequilibrium of inner-ear homeostasis (e11), with endolymphatic hydrops as the final common pathway. The latter can be seen on delayed contrast-enhanced magnetic resonance imaging (MRI) of the inner ear (figure 3). The specificity of such findings for the diagnosis of Menière’s disease is a matter of controversy, because imaging studies reveal endolymphatic hydrops in the region of the saccule in 10% of normal persons and in 40% of patients with a sensorineural hearing loss of > 45 dB without vestibular symptoms (16).
Figure 3.
Imaging of the inner ear
a) and b) Contrast-enhanced MRI of a patient with Menière’s disease: a) evidence of vestibular (arrow) and cochlear endolymphatic hydrops in a high-resolution FLAIR sequence 4 h after the administration of intravenous contrast medium; b) 3D reconstruction of a segmented, contrast-enhanced MRI-based visualization of the endo- and perilymphatic spaces (endolymphatic hydrops shown in red and purple).
c) and d) High-resolution computer-tomographic imaging of the petrous bone, revealing dehiscence of the left superior semicircular canal; c) the petrous bone CT depicts the bony defect (arrow); d) the 3D reconstruction shows that the defect occupies a considerable length of the superior semicircular canal.
Benign paroxysmal positional vertigo of the horizontal semicircular canal.
Benign paroxysmal positional vertigo of the horizontal canal is much rarer. It causes linear, horizontal nystagmus.
Benign paroxysmal positional vertigo.
Benign paroxysmal positional vertigo can be well treated by properly performed positioning maneuvers. Patients should, however, always be asked to give feedback concerning the outcome of treatment. If symptoms persist, further follow-up is indicated.
Moreover, although nearly all patients with definite Menière’s disease have endolymphatic hydrops, the latter can also be seen in patients with vertigo syndromes of other etiologies, e.g., vestibular migraine (e12).
There is no consensus yet on the preventive treatment of Menière’s disease. The efficacy of individual treatments is hard to assess, because of the clinical heterogeneity of the disease, its fluctuating course, and its high rate of response to placebo (up to 70% [17]). At present, a stepwise concept is recommended, beginning with conservative treatment (e.g., betahistine), then non-destructive techniques (e.g., transtympanic cortisone application), and, finally, invasive destructive techniques (e.g., labyrinthectomy). There is no evidence to date for the efficacy of a low-salt diet, abstinence from coffee or alcohol, diuretic use, or endolymphatic sac surgery (8– 20, e13, e14).
Treatment with betahistine
Betahistine in a dose of 48 or 144 mg/d is not superior to placebo, according to a randomized, controlled trial on 221 patients in which a strong placebo effect was found (17).
Uncontrolled observational studies have shown that higher doses are associated with higher efficacy. Individual treatment trials suggest that combining orally administered betahistine with the MAO-B inhibitor selegiline may enhance its efficacy: this can be understood in light of the fact that 99% of orally ingested betahistine is metabolized in the gastrointestinal tract by MAO-B/A. This approach has received support from experimental studies in animals in a model of acute unilateral vestibulopathy (21). On the whole, more randomized and controlled trials are needed to study the therapeutic effects of betahistine.
Administration of gentamicin and steroids
The transtympanic administration of gentamicin reduces the frequency of the vertigo attacks (22). This is an ablative treatment that works by vestibulotoxic impairment of vestibular function. In approximately 20% of patients, there is ototoxic hearing loss. The utility of gentamicin is limited for this reason, and also because 45% of patients with Menière’s disease develop the condition bilaterally.
Transtympanic corticosteroid administration is an alternative treatment with demonstrated efficacy in double-blind, randomized, controlled trials (22). It is neither cochleotoxic nor vestibulotoxic.
Surgery
Pathogenesis of Menière’s disease.
The pathogenesis of Menière’s disease is thought to involve a multifactorial disequilibrium of inner-ear homeostasis, of which endolymphatic hydrops is the final common pathway.
If conservative treatment, including orally administered betahistine and transtympanic corticosteroids, is ineffective, surgery may be indicated (e13, e14). Endolymphatic sac surgery is recommended by many surgeons, but its efficacy has not yet been definitively demonstrated (20).
In advanced Menière’s disease, labyrinthectomy and simultaneous cochlear implantation leads to the cessation of the vertigo attacks and to an improvement of hearing.
Vestibular paroxysmia
This term, along with its clinical characteristics and pathophysiology, was introduced by Brandt and Dieterich in 1994 (23). The current diagnostic criteria for vestibular paroxysmia are as follows: At least ten attacks of spontaneous spinning or non-spinning vertigo; duration less than 1 min; stereotyped phenomenology in a particular patient; response to treatment with a sodium-channel blocker; and not better accounted for by another diagnosis (24). The presumed cause is contact between a blood vessel and the vestibular nerve in the cerebellopontine angle, although imaging studies reveal such a contact in as many as 45% of normal individuals (e15, e16). For the latter reason, MRI in this condition is indicated mainly to rule out other etiologies. A randomized, controlled trial demonstrated the significant therapeutic efficacy of oxcarbazepine at a dosage of 900 mg/day (attack frequency halved), although poor tolerability of the drug led to a 60% dropout rate (25). Lacosamide (100–200 mg/day) seems to be a well-tolerated alternative, as individual cases of off-label use have shown (26).
Third mobile window syndromes (semicircular canal dehiscence and, rarely, perilymph fistula)
The main manifestations of third mobile window syndromes are recurrent attacks of vertigo induced by changes in intracranial/middle-ear pressure or loud noises, autophony, increased bone conduction, and/or pulsatile tinnitus. Dehiscence of the superior semicircular canal is the most common subtype and is caused by a bony defect between the superior semicircular canal and the middle cranial fossa (figure 3), creating a “third window” in the inner ear (in addition to the round and oval windows) (27). Alongside the clinical history, the diagnosis is based on a combination of at least one test finding typical of this syndrome, e.g., elevated amplitudes and/or reduced thresholds of cervical/ocular vestibular evoked myogenic potentials (VEMP) (e17), and high-resolution computer tomographic imaging of the petrous bone (slice thickness ≤ 0.6 mm). It is important to note that on CT about 2% of healthy subjects also show a thin bone on top of the superior canal.
Betahistine.
Betahistine in a dose of 48 or 144 mg/d is not superior to placebo, according to a randomized, controlled trial on 221 patients in which a strong placebo effect was found.
For patients with mild symptoms, it usually suffices to explain the mechanism of the disease to them and for them to avoid marked pressure changes. For patients who are more severely impaired, surgical treatment is available (27).
Central vestibular syndromes
Central vestibular syndromes (e18) can manifest themselves as follows:
Spinning vertigo and/or instability of stance and/or gait, due to ischemic or inflammatory lesions of the brainstem or cerebellum
Recurrent attacks, as in vestibular migraine or episodic ataxia type 2
A persistent syndrome, as in cerebellar dizziness.
Acute vestibular syndrome.
In acute vestibular syndrome, the most important differential diagnosis of an acute peripheral vestibulopathy is a central lesion in the brainstem or cerebellum, usually due to a stroke.
Acute central vestibular syndrome: ischemia of the brainstem or cerebellum
In the acute vestibular syndrome, the most important differential diagnosis of an acute peripheral vestibulopathy is a central lesion in the brainstem or cerebellum, usually due to a stroke. The following features point to a stroke as the more likely cause: age over 60 years, a history of arterial hypertension or diabetes mellitus, accompanying symptoms referable to the central nervous system, and spontaneous and acute onset of the symptoms for the first time ever. In the clinical examination, the clinician should look for skew deviation/vertical divergence of the eyes, in which the vertical position of one eye is higher than that of the other. Furthermore, the nature of the spontaneous nystagmus is very important: if it cannot be suppressed by fixation, it is not a spontaneous nystagmus due to a peripheral vestibular disorder. Important further clues indicating a central lesion are a normal head-impulse test and a vertical nystagmus as well as a head-shaking nystagmus beating in the opposite direction to the spontaneous nystagmus.
Acute central vestibular syndrome is an emergency calling for immediate referral to a hospital that has the relevant diagnostic imaging and, ideally, a stroke unit. It should be emphasized here that, in patients with acute central vestibular syndrome due to a small stroke, MRI, including diffusion-weighted sequences, can often be normal in 50% of cases in the first 24 h (28). This underlines the importance of the patient’s history and clinical examination in determining the correct diagnosis.
Vestibular migraine
Vestibular paroxysmia.
Vestibular paroxysmia consists of recurrent (as many as 100 times per day), spontaneously arising, brief attacks of vertigo. The demonstration of neurovascular conflict by MRI is not specific to this entity. A convincing response to a sodium-channel blocker supports the diagnosis.
Vestibular migraine is the most common cause of recurrent, spontaneous attacks of vertigo. Its 1-year prevalence is 2.7% among adults in the USA; the average age of individuals suffering from vestibular migraine is 40.9 years, and 64.1% of them are women (29). Vestibular migraine is defined as follows, according to the current diagnostic criteria (30):
The patient has had at least five episodes of vestibular symptoms of moderate or severe intensity lasting from 5 min to 72 h.
The patient has, or has had, migraine with or without aura as defined by the criteria of the International Classification of Headache Disorders (ICHD).
At least 50% of the vestibular episodes are preceded by one or more migraine symptoms: headache with at least two of the following features (unilaterality, pulsatile character, moderate or severe intensity, aggravation by routine physical activities), photophobia, phonophobia, visual aura.
The symptoms are not attributable to another vestibular or ICHD diagnosis.
A single one of the following two criteria suffices for the diagnosis of probable vestibular migraine: a history of migraine, or migraine symptoms during the attack.
The main differential diagnosis of vestibular migraine is Menière’s disease. Some patients meet the diagnostic criteria for both of these disorders; this suggests a pathophysiological link between them. Vestibular migraine might, for example, lead to a disturbance in the inner ear by way of the trigeminovascular system, increasing the probability of endolymphatic hydrops (e19). As there is no evidence yet to support any specific treatment for vestibular migraine (Cochrane Review [31]), this disorder should currently be treated like any other type of migraine.
Cerebellar dizziness and vertigo
Dizziness, vertigo, and imbalance due to cerebellar disease present a particular differential-diagnostic challenge. These patients typically do not present with the full spectrum of cerebellar symptoms. The entity of “cerebellar dizziness and vertigo” was, therefore, investigated more precisely in a study encompassing 369 patients (32), 81% of whom suffered from persistent dizziness, 31% from attacks of vertigo or dizziness, and 21% from both. Ninety-five percent had signs of a central ocular motor disorder (e.g., saccadic smooth pursuit, omnidirectional gaze-evoked nystagmus, central fixation nystagmus, and, in particular, downbeat nystagmus). The diagnosis cerebellar dizziness/vertigo is clinically relevant because many types can be treated with drugs, e.g., episodic ataxia type 2 (e20) and downbeat nystagmus (e21) with 4-aminopyridine, or certain other types of ataxia with acetyl-DL-leucine (33, 34).
Functional dizziness
Cerebellar dizziness and vertigo.
The key to this diagnosis is the examination of eye movements, as practically all patients have at least one oculomotor abnormality of cerebellar origin, such as saccadic ocular pursuit, gaze-evoked nystagmus, or downbeat nystagmus.
Somatoform/psychogenic vertigo has been internationally designated as functional dizziness since 2017 and is probably the single most common type of vertigo (Figure); the term covers several subtypes, including phobic postural dizziness or persistent postural-perceptual dizziness. The Bárány Society, on the basis of many years of experience and in view of the many common features of phobic postural vertigo (phobischer Schwankschwindel) (e22) in Germany, chronic subjective dizziness in the USA, and visual vertigo in the UK (35), has established another entity called “persistent postural-perceptual dizziness” (PPPD) (36). This disorder is characterized by persistent dizziness and/or unsteadiness on most days over a period of 3 months or longer, with the symptoms lasting for hours per day, but not necessarily the whole day. The symptoms arise spontaneously but may be worsened by upright posture, active or passive body movements, or exposure to moving visual stimuli. Acute or chronic organic vestibular disorders, neurological or medical illness, or psychological distress may precede these symptoms, appear simultaneously with them, and/or outlast them. The symptoms cause marked functional impairment. They cannot be better explained by any other diagnosis.
Patients often complain of more or less permanent imbalance or light-headedness, which is usually less severe during sporting activities, on distraction, after the consumption of a small amount of alcohol, or in the morning, but is exacerbated by certain situations (e.g., in a crowd or in a department store); this can lead to avoidance behavior (“phobic postural dizziness”). Functional dizziness can be pathophysiologically explained as a result of heightened self-observation of balance. It typically affects individuals with perfectionist personality traits. As many as 45% of patients have an organic disease before the onset of symptoms: this condition is called secondary functional dizziness.
A detailed diagnostic evaluation is part of the effective treatment of this disorder. The work-up frees the patient from the fear that the symptoms are due to an underlying organic disease. Moreover, the treating physician should provide psychoeducation by explaining the mechanism of the disorder to the patient. The physician should encourage the patient to participate in sporting activities regularly, and a course of vestibular rehabilitation may be advisable (37). Both of these measures help the patient regain confidence in his or her own ability to maintain balance. If necessary, cognitive behavioral therapy or desensitization by auto-exposure can be performed. In the event of accompanying anxiety or simultaneous panic attacks, treatment with a selective serotonin reuptake inhibitor (38) or other antidepressants may be beneficial.
Approximately 70% of the affected patients can be treated successfully in this manner. There have not yet been any prospective, controlled trials of the treatment of this disorder (39).
Functional dizziness.
Functional dizziness is probably the single most common vestibular syndrome. It can usually be diagnosed easily on the basis of the current “positive” diagnostic criteria, and its treatment after correct diagnosis is often successful.
Supplementary Material
CASE ILLUSTRATION
History
A 46-year-old engineer reports that he has been suffering from severe, persistent spinning vertigo for the past 24 h. The vertigo arose suddenly; it is accompanied by instability of stance and gait, with a tendency to fall to the right, and nausea. When he tries to fixate on a target, everything seems to move in front of his eyes. On questioning, he states that his hearing is normal, and he has neither tinnitus nor fullness in the ears. Photophobia and phonophobia are likewise absent, and he does not suffer from migraine. He has no vascular risk factors.
Physical examination
On central gaze, there is a low-amplitude spontaneous nystagmus with the fast phase beating to the left and a clockwise torsional component (from the examiner’s point of view). When the patient wears M glasses, the intensity of the spontaneous nystagmus increases markedly. The head-impulse test is pathological toward the right. There are no signs of central ocular motor disorders: in particular, the alternating cover test reveals no skew deviation, nor is there any gaze-evoked nystagmus on rightward gaze, i.e., in the direction opposite to the rapid phase of the spontaneous nystagmus. The Rinne and Weber tuning fork tests are normal. The Romberg test reveals instability, with a tendency to fall to the right, that is much worse with the eyes closed.
Ancillary diagnostic studies
Video head-impulse test
The gain of the rightward horizontal vestibulo-ocular reflex (VOR) is reduced to 0.3 eFigure, (below, b); values less than 0.7 indicate a clinically relevant unilateral deficit and obviate the need for caloric testing. As neither the history nor the physical examination give any reason to suspect a central disturbance—in particular, a stroke in the brainstem or cerebellum—no neuroimaging is necessary (and, in any case, the MRI findings are often normal in the first 24 h in patients who present with spinning vertigo or dizziness as their main symptom of a brainstem stroke).
Diagnosis and differential diagnosis
On the basis of the history and physical findings, an acute unilateral vestibulopathy on the right is diagnosed.
The three major differential diagnoses are:
Treatment
Course
Four weeks later, the patient reports that his symptoms have markedly improved. He has no spinning vertigo any more, and physical examination no longer reveals spontaneous nystagmus. The rightward VOR gain is now 0.7, and there is still a mild tendency to fall to the right on the Romberg test with the eyes closed.
Acute central vestibular syndrome
Menière’s disease (the lack of hearing loss makes this diagnosis less likely, although Menière’s disease can begin with only mild symtoms)
Vestibular migraine (not supported by the history).
To treat nausea and vertigo: dimenhydrinate, 50 mg po tid for 1 day.
To promote the recovery of peripheral vestibular function: methylprednisolone, initially 100 mg po qd, tapering by -20 mg every 4th day to off.
Balance training for at least 15 min three times a day for 4 weeks.
Further information on CME.
Participation in the CME certification program is possible only over the Internet: cme.aerzteblatt.de. This unit can be accessed until 24 April 2021. Submissions by letter, e-mail or fax cannot be considered.
The following CME units can still be accessed for credit:
“The Diagnosis and Treatment of Glaucoma” (issue 13/2020) until 21 June 2020
“The Treatment of Gallstone Disease” (issue 9/2020) until 24 May 2020
“Hip Pain in Children” (issue 5/2020) until 26 April 2020
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN), which is found on the CME card (8027XXXXXXXXXXX). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or else entered in “Meine Daten,” and the participant must agree to communication of the results.
CME credit for this unit can be obtained via cme.aerzteblatt.de until 24 April 2021.
Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
A 38-year-old school teacher reports that she has had approximately 10 attacks of spinning vertigo in the past 12 months, usually lasting several hours each. She has also noted hearing loss, tinnitus, and fullness in the left ear. Audiological testing reveals a 40-dB low-frequency
hearing deficit in the left ear.
What is the probable diagnosis?
Recurrent transient ischemic attacks
Acute unilateral vestibulopathy
Menière’s disease
Functional dizziness
Superior canal dehiscence syndrome
Question 2
A 44-year-old colleague of yours sustains mild head trauma in a bicycle accident. The next morning, he experiences spinning vertigo while turning over in bed; the symptoms subside at rest. He vomits repeatedly. On questioning, he states that the attacks of vertigo are triggered by turning over to the left from a supine position and last approximately 30 s each. The clinical examination is normal. A few days later, the patient sends you a video showing a nystagmus beating upward and torsionally toward the left ear when he is lying in the left lateral decubitus position. The nystagmus lasts approximately 20 s.
What is the diagnosis?
Post-traumatic loss of labyrinthine function
Panic attacks
Vertebral artery dissection
Orthostatic dizziness
Benign paroxysmal positional vertigo
Question 3
Frequent, spontaneously arising vertigo attacks of a few seconds’ duration characterize what disorder?
Vestibular paroxysmia
Functional dizziness
Vestibular migraine
Episodic ataxia type 2
Bilateral vestibulopathy
Question 4
A 28-year-old nurse reports that she has suffered from severe, persistent spinning vertigo for 2 days, accompanied by apparent movement of her surroundings and a tendency to fall to the right. Your examination reveals spontaneous nystagmus with the fast phase beating toward the left. The head-impulse test is pathological when the head is turned to the right. The patient’s hearing and eye movements are normal.
What is the diagnosis?
Downbeat nystagmus syndrome
Acute unilateral vestibulopathy
Visual dizziness
Semicircular canal dehiscence syndrome
Cervicogenic vertigo
Question 5
A 76-year-old man reports that he has been suffering from increasing instability of stance and gait for the past 5 years. When asked, he further states that he perceives apparent motion of the environment while walking. The instability is worse in the dark and when he walks on uneven ground. He has already fallen three times. On examination, you find a bilaterally pathological head-impulse test and a pathological Romberg test with the eyes closed. There is no spontaneous nystagmus, and the eye movements are normal.
What is the diagnosis?
Subcortical vascular encephalopathy
Recurrent syncope
Downbeat nystagmus syndrome
Bilateral vestibulopathy
Spinal canal stenosis
Question 6
An 18-year-old high-school student states that, for the past 5 years, she has had attacks of dizziness lasting many minutes to many hours, approximately twice per month. The attacks are usually accompanied by a pulsating right-sided headache that is exacerbated by physical exertion. She also reports that she suffers from migraine with aura. Your examination reveals a mild central ocular motor disorder, with mildly saccadic smooth pursuit movements in all gaze directions.
What is the diagnosis?
Paroxysmal brainstem attacks
Vestibular migraine
Upbeat nystagmus syndrome
Menière’s disease
Third mobile window syndrome
Question 7
What type of vertigo is most frequently diagnosed?
Bilateral vestibulopathy
Functional dizziness
Menière’s disease
Unilateral peripheral vestibulopathy
Vestibular migraine
Question 8
What is the presumed pathogenesis of Menière’s disease?
Abony defect in a semicircular canal
Labyrinthitis
Endolymphatic hydrops
Labyrinthine ischemia
A lesion in the vestibular cortex
Question 9
A 24-year-old IT specialist reports having suffered from practically continuous light-headedness of fluctuating intensity over the past 2 years. On questioning, he states that the problem is less severe in the morning, worsens when he goes to a department store, but improves during sporting activities or after the consumption of a small amount of alcohol. The physical examination is unremarkable.
What is the probable diagnosis?
Persistent unilateral vestibulopathy
Cerebellar dizziness
Third mobile window syndrome
Incipient Parkinson’s disease
Functional dizziness
Question 10
Which of the following is a treatment for downbeat nystagmus?
4-Aminopyridine
Diuretics
Betahistine
The Sémont maneuver
Gentamicin
►Participation is possible only over the Internet: cme.aerzteblatt.de
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Strupp owns stock in Intra Bio, as well as related patents. He serves as a paid consultant for Abbott, Actelion, AurisMedical, Heel, IntraBio, and Sensorion. He has received lecture honoraria from Abbott, Actelion, Auris Medical, Biogen, Eisai, Grünenthal, GSK, Hennig Pharma, Interacoustics, MSD, Mylan, Otometrics, Pierre-Fabre, TEVA, and UCB. He has received financial support from Abbott, Decibel Interacoustics, and Natus for a research project that he initiated, as well as third-party research support from Auris Medical, Biogen, Decibel, and Heel. He is the distributor of the M glasses.
Prof. Dlugaiczyk has received reimbursement of meeting participation fees and of travel and accommodation expenses from Hennig Arzneimittel and Otometrics, and lecture honoraria from Otometrics. She has received financial support for the performance of clinical studies from Otonomy.
Prof. Ertl-Wagner has a personal relationship with Siemens Healthineers.
Prof. Westhofen has received reimbursement of travel and accommodation expenses from Hennig Arzneimittel and lecture honoraria from Heel. His institution received financial support for a research project from Otonomy, and materials support for another study from Diatec/Interacoustics.
Prof. Dieterich has received financial support for the performance of clinical studies from Schwabe and Heel.
Prof. Rujescu states that he has no conflict of interest.
References
- 1.Corrales CE, Bhattacharyya N. Dizziness and death: an imbalance in mortality. Laryngoscope. 2016;126:2134–2136. doi: 10.1002/lary.25902. [DOI] [PubMed] [Google Scholar]
- 2.Strupp M, Fischer C, Hanss L, Bayer O. The takeaway frenzel goggles: a fresnel-based device. Neurology. 2014;83:1241–1245. doi: 10.1212/WNL.0000000000000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yip CW, Glaser M, Frenzel C, Bayer O, Strupp M. Comparison of the bedside head-impulse test with the video head-impulse test in a clinical practice setting: a prospective study of 500 outpatients. Front Neurol. 2016;7 doi: 10.3389/fneur.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strupp M, Mandala M, Lopez-Escamez JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165–173. doi: 10.1097/WCO.0000000000000649. [DOI] [PubMed] [Google Scholar]
- 5.Strupp M, Kim JS, Murofushi T, et al. Bilateral vestibulopathy: diagnostic criteria consensus document of the Classification Committee o f the Barany Society. J Vestib Res. 2017;27:177–189. doi: 10.3233/VES-170619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucieer F, Vonk P, Guinand N, Stokroos R, Kingma H, van de Berg R. Bilateral vestibular hypofunction: insights in etiologies, clinical subtypes, and diagnostics. Front Neurol. 2016;7 doi: 10.3389/fneur.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall CD, Herdman SJ, Whitney SL, et al. Vestibular rehabilitation for peripheral vestibular hypofunction: an evidence-based clinical practice guideline: from the American Physical Therapy Association Neurology Section. J Neurol Phys Ther. 2016;40:124–155. doi: 10.1097/NPT.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlap PM, Holmberg JM, Whitney SL. Vestibular rehabilitation: advances in peripheral and central vestibular disorders. Curr Opin Neurol. 2019;32:137–144. doi: 10.1097/WCO.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 9.Strupp M, Magnusson M. Acute unilateral vestibulopathy. Neurol Clin. 2015;33:669–685. doi: 10.1016/j.ncl.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Rujescu D, Hartmann AM, Giegling I, et al. Genome-wide association study in vestibular neuritis: involvement of the host factor for HSV-1 replication. Front Neurol. 2018;9 doi: 10.3389/fneur.2018.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strupp M, Zingler VC, Arbusow V, et al. Methylprednisolone, valacyclovir, or the combination for vestibular neuritis. N Engl J Med. 2004;351:354–361. doi: 10.1056/NEJMoa033280. [DOI] [PubMed] [Google Scholar]
- 12.Sjogren J, Magnusson M, Tjernstrom F, Karlberg M. Steroids for acute vestibular neuronitis-the earlier the treatment, the better the outcome? Otol Neurotol. 2019;40:372–374. doi: 10.1097/MAO.0000000000002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Brevern M, Bertholon P, Brandt T, et al. Benign paroxysmal positional vertigo: diagnostic criteria. J Vestib Res. 2015;25:105–117. doi: 10.3233/VES-150553. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (Update) Otolaryngol Head Neck Surg. 2017;156(3):S1–S47. doi: 10.1177/0194599816689667. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere‘s disease. J Vestib Res. 2015;25:1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
- 16.Attye A, Eliezer M, Medici M, et al. In vivo imaging of saccular hydrops in humans reflects sensorineural hearing loss rather than Meniere‘s disease symptoms. Eur Radiol. 2018;28:2916–2922. doi: 10.1007/s00330-017-5260-7. [DOI] [PubMed] [Google Scholar]
- 17.Adrion C, Fischer CS, Wagner J, Gurkov R, Mansmann U, Strupp M. Efficacy and safety of betahistine treatment in patients with Meniere’s disease: primary results of a long term, multicentre, double blind, randomised, placebo controlled, dose defining trial (BEMED trial) BMJ. 2016;352 doi: 10.1136/bmj.h6816. h6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain K, Murdin L, Schilder AG. Restriction of salt, caffeine and alcohol intake for the treatment of Meniere‘s disease or syndrome. Cochrane Database Syst Rev. 2018;12 doi: 10.1002/14651858.CD012173.pub2. CD012173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thirlwall AS, Kundu S. Diuretics for Meniere‘s disease or syndrome. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD003599.pub2. CD003599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pullens B, Verschuur HP, van Benthem PP. Surgery for Meniere’s disease. Cochrane Database Syst Rev. 2013;2 doi: 10.1002/14651858.CD005395.pub3. CD005395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tighilet B, Leonard J, Watabe I, Bernard-Demanze L, Lacour M. Betahistine treatment in a cat model of vestibular pathology: pharmacokinetic and pharmacodynamic approaches. Front Neurol. 2018;9 doi: 10.3389/fneur.2018.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoo DP, Tan GX, Ehrenburg MR, Pross SE, Ward BK, Carey JP. Intratympanic (IT) Therapies for Meniere‘s Disease: some consensus among the confusion. Curr Otorhinolaryngol Rep. 2017;5:132–141. doi: 10.1007/s40136-017-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandt T, Dieterich M. Vestibular paroxysmia: vascular compression of the eighth nerve? Lancet. 1994;343:798–799. doi: 10.1016/s0140-6736(94)91879-1. [DOI] [PubMed] [Google Scholar]
- 24.Strupp M, Lopez-Escamez JA, Kim JS, et al. Vestibular paroxysmia: diagnostic criteria. J Vestib Res. 2016;26:409–415. doi: 10.3233/VES-160589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayer O, Bremova T, Strupp M, Hufner K. A randomized double-blind, placebo-controlled, cross-over trial (Vestparoxy) of the treatment of vestibular paroxysmia with oxcarbazepine. J Neurol. 2018;265:291–298. doi: 10.1007/s00415-017-8682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strupp M, Elger C, Goldschagg N. Treatment of vestibular paroxysmia with lacosamide. Neurol Clin Pract. 2019;9:539–541. doi: 10.1212/CPJ.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward BK, Carey JP, Minor LB. Superior canal dehiscence syndrome: lessons from the first 20 years. Front Neurol. 2017;8 doi: 10.3389/fneur.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saber Tehrani AS, Kattah JC, Kerber KA, et al. Diagnosing stroke in acute dizziness and vertigo: pitfalls and pearls. Stroke. 2018;49:788–795. doi: 10.1161/STROKEAHA.117.016979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Formeister EJ, Rizk HG, Kohn MA, Sharon JD. The epidemiology of vestibular migraine: a population-based survey study. Otol Neurotol. 2018;39:1037–1044. doi: 10.1097/MAO.0000000000001900. [DOI] [PubMed] [Google Scholar]
- 30.Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22:167–172. doi: 10.3233/VES-2012-0453. [DOI] [PubMed] [Google Scholar]
- 31.Maldonado FM, Birdi JS, Irving GJ, Murdin L, Kivekas I, Strupp M. Pharmacological agents for the prevention of vestibular migraine. Cochrane Database Syst Rev. 2015;6 doi: 10.1002/14651858.CD010600.pub2. CD010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feil K, Strobl R, Schindler A, et al. What is behind cerebellar vertigo and dizziness? Cerebellum. 2019;18:320–332. doi: 10.1007/s12311-018-0992-8. [DOI] [PubMed] [Google Scholar]
- 33.Strupp M, Teufel J, Zwergal A, Schniepp R, Khodakhah K, Feil K. Aminopyridines for the treatment of neurologic disorders. Neurology: Clinical Practice. 2017;7:65–76. doi: 10.1212/CPJ.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalla R, Strupp M. Aminopyridines and acetyl-DL-leucine: new therapies in cerebellar disorders. Curr Neuropharmacol. 2019;17:7–13. doi: 10.2174/1570159X16666180905093535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dieterich M, Staab JP. Functional dizziness: from phobic postural vertigo and chronic subjective dizziness to persistent postural-perceptual dizziness. Curr Opin Neurol. 2017;30:107–113. doi: 10.1097/WCO.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 36.Staab JP, Eckhardt-Henn A, Horii A, et al. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the Committee for the Classification of Vestibular Disorders of the Barany Society. J Vestib Res. 2017;27:191–208. doi: 10.3233/VES-170622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nada EH, Ibraheem OA, Hassaan MR. Vestibular rehabilitation therapy outcomes in patients with persistent postural-perceptual dizziness. Ann Otol Rhinol Laryngol. 2019;128:323–329. doi: 10.1177/0003489418823017. [DOI] [PubMed] [Google Scholar]
- 38.Yu YC, Xue H, Zhang YX, Zhou J. Cognitive behavior therapy as augmentation for sertraline in treating patients with persistent postural-perceptual dizziness. Biomed Res Int. 2018;2018 doi: 10.1155/2018/8518631. 8518631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popkirov S, Stone J, Holle-Lee D. Treatment of persistent postural-perceptual dizziness (PPPD) and related disorders. Curr Treat Options Neurol. 2018;20 doi: 10.1007/s11940-018-0535-0. [DOI] [PubMed] [Google Scholar]
- E1.Neuhauser HK. The epidemiology of dizziness and vertigo. Handb Clin Neurol. 2016;137:67–82. doi: 10.1016/B978-0-444-63437-5.00005-4. [DOI] [PubMed] [Google Scholar]
- E2.Hulse R, Biesdorf A, Hormann K, et al. Peripheral vestibular disorders: an epidemiologic survey in 70 million individuals. Otol Neurotol. 2019;40:88–95. doi: 10.1097/MAO.0000000000002013. [DOI] [PubMed] [Google Scholar]
- E3.Halmagyi GM, Chen L, MacDougall HG, Weber KP, McGarvie LA, Curthoys IS. The video head impulse test. Front Neurol. 2017;8 doi: 10.3389/fneur.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Lehnen N, Kellerer S, Knorr AG, et al. Head-movement-emphasized rehabilitation in bilateral vestibulopathy. Front Neurol. 2018;9 doi: 10.3389/fneur.2018.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Feil K, Feuerecker R, Goldschagg N, et al. Predictive capability of an iPad-Based Medical Device (medx) for the diagnosis of vertigo and dizziness. Front Neurol. 2018,;9 doi: 10.3389/fneur.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Shah MU, Lotterman S, Roberts D, Eisen M. Smartphone telemedical emergency department consults for screening of nonacute dizziness. Laryngoscope. 2019;129:466–469. doi: 10.1002/lary.27424. [DOI] [PubMed] [Google Scholar]
- E7.Park MK, Lee DY, Kim YH. Risk factors for positional vertigo and the impact of vertigo on daily life: The Korean National Health and Nutrition Examination Survey. J Audiol Otol. 2019;23:8–14. doi: 10.7874/jao.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E8.Kim SK, Hong SM, Park IS, Choi HG. Association between migraine and benign paroxysmal positional vertigo among adults in South Korea. JAMA Otolaryngol Head Neck Surg. 2019;145:307–312. doi: 10.1001/jamaoto.2018.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Sim E, Tan D, Hill K. Poor treatment outcomes following repositioning maneuvers in younger and older adults with benign paroxysmal positional vertigo: a systematic review and meta-analysis. J Am Med Dir Assoc. 2019;20 doi: 10.1016/j.jamda.2018.11.019. [DOI] [PubMed] [Google Scholar]
- E10.Tse D, Ramsay T, Lelli DA. Novel use of portable audiometry to track hearing fluctuations in Meniere‘s disease: a pilot study. Otol Neurotol. 2019;40:e130–e134. doi: 10.1097/MAO.0000000000002080. [DOI] [PubMed] [Google Scholar]
- E11.Frejo L, Martin-Sanz E, Teggi R, et al. Extended phenotype and clinical subgroups in unilateral Meniere disease: A cross-sectional study with cluster analysis. Clin Otolaryngol. 2017;42:1172–1180. doi: 10.1111/coa.12844. [DOI] [PubMed] [Google Scholar]
- E12.Gurkov R, Kantner C, Strupp M, et al. Endolymphatic hydrops in patients with vestibular migraine and auditory symptoms. Eur Arch Otorhinolaryngol. 2104;271:2661–2667. doi: 10.1007/s00405-013-2751-2. [DOI] [PubMed] [Google Scholar]
- E13.Lim MY, Zhang M, Yuen HW, Leong JL. Current evidence for endolymphatic sac surgery in the treatment of Meniere‘s disease: a systematic review. Singapore Med J. 2015;56:593–598. doi: 10.11622/smedj.2015166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.Sood AJ, Lambert PR, Nguyen SA, Meyer TA. Endolymphatic sac surgery for Meniere‘s disease: a systematic review and meta-analysis. Otol Neurotol. 2014;35:1033–1045. doi: 10.1097/MAO.0000000000000324. [DOI] [PubMed] [Google Scholar]
- E15.Best C, Gawehn J, Kramer HH, et al. MRI and neurophysiology in vestibular paroxysmia: contradiction and correlation. J Neurol Neurosurg Psychiatry. 2013;84:1349–1356. doi: 10.1136/jnnp-2013-305513. [DOI] [PubMed] [Google Scholar]
- E16.Sivarasan N, Touska P, Murdin L, Connor S. MRI findings in vestibular paroxysmia—an observational study. J Vestib Res. 2019;29:137–145. doi: 10.3233/VES-190661. [DOI] [PubMed] [Google Scholar]
- E17.Fife TD, Colebatch JG, Kerber KA, et al. Practice guideline: cervical and ocular vestibular evoked myogenic potential testing: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017;89:2288–2296. doi: 10.1212/WNL.0000000000004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E18.Brandt T, Dieterich M. The dizzy patient: don‘t forget disorders of the central vestibular system. Nat Rev Neurol. 2017;13:352–362. doi: 10.1038/nrneurol.2017.58. [DOI] [PubMed] [Google Scholar]
- E19.Nakashima T, Pyykko I, Arroll MA, et al. Meniere‘s disease. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.28. 16028. [DOI] [PubMed] [Google Scholar]
- E20.Strupp M, Kalla R, Claassen J, et al. A randomized trial of 4-aminopyridine in EA2 and related familial episodic ataxias. Neurology. 2011;77:269–275. doi: 10.1212/WNL.0b013e318225ab07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E21.Claassen J, Spiegel R, Kalla R, et al. A randomised double-blind, cross-over trial of 4-aminopyridine for downbeat nystagmus—effects on slowphase eye velocity, postural stability, locomotion and symptoms. J Neurol Neurosurg Psychiatry. 2013;84:1392–1399. doi: 10.1136/jnnp-2012-304736. [DOI] [PubMed] [Google Scholar]
- E22.Brandt T, Dieterich M. Phobischer Attacken-Schwankschwindel, ein neues Syndrom. Münch Med Wochenschr. 1986;128:247–250. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CASE ILLUSTRATION
History
A 46-year-old engineer reports that he has been suffering from severe, persistent spinning vertigo for the past 24 h. The vertigo arose suddenly; it is accompanied by instability of stance and gait, with a tendency to fall to the right, and nausea. When he tries to fixate on a target, everything seems to move in front of his eyes. On questioning, he states that his hearing is normal, and he has neither tinnitus nor fullness in the ears. Photophobia and phonophobia are likewise absent, and he does not suffer from migraine. He has no vascular risk factors.
Physical examination
On central gaze, there is a low-amplitude spontaneous nystagmus with the fast phase beating to the left and a clockwise torsional component (from the examiner’s point of view). When the patient wears M glasses, the intensity of the spontaneous nystagmus increases markedly. The head-impulse test is pathological toward the right. There are no signs of central ocular motor disorders: in particular, the alternating cover test reveals no skew deviation, nor is there any gaze-evoked nystagmus on rightward gaze, i.e., in the direction opposite to the rapid phase of the spontaneous nystagmus. The Rinne and Weber tuning fork tests are normal. The Romberg test reveals instability, with a tendency to fall to the right, that is much worse with the eyes closed.
Ancillary diagnostic studies
Video head-impulse test
The gain of the rightward horizontal vestibulo-ocular reflex (VOR) is reduced to 0.3 eFigure, (below, b); values less than 0.7 indicate a clinically relevant unilateral deficit and obviate the need for caloric testing. As neither the history nor the physical examination give any reason to suspect a central disturbance—in particular, a stroke in the brainstem or cerebellum—no neuroimaging is necessary (and, in any case, the MRI findings are often normal in the first 24 h in patients who present with spinning vertigo or dizziness as their main symptom of a brainstem stroke).
Diagnosis and differential diagnosis
On the basis of the history and physical findings, an acute unilateral vestibulopathy on the right is diagnosed.
The three major differential diagnoses are:
Treatment
Course
Four weeks later, the patient reports that his symptoms have markedly improved. He has no spinning vertigo any more, and physical examination no longer reveals spontaneous nystagmus. The rightward VOR gain is now 0.7, and there is still a mild tendency to fall to the right on the Romberg test with the eyes closed.
Acute central vestibular syndrome
Menière’s disease (the lack of hearing loss makes this diagnosis less likely, although Menière’s disease can begin with only mild symtoms)
Vestibular migraine (not supported by the history).
To treat nausea and vertigo: dimenhydrinate, 50 mg po tid for 1 day.
To promote the recovery of peripheral vestibular function: methylprednisolone, initially 100 mg po qd, tapering by -20 mg every 4th day to off.
Balance training for at least 15 min three times a day for 4 weeks.