Abstract
Summary
Background
Current efficacy studies of a mosaic HIV-1 prophylactic vaccine require 4 vaccination visits over 1 year, which is a complex regimen that may prove challenging for vaccine delivery at the community level, both for recipients and clinics. In this study, we evaluated the safety, tolerability, and immunogenicity of shorter, simpler regimens of trivalent Ad26.Mos.HIV expressing mosaic HIV-1 Env/Gag/Pol antigens combined with aluminum phosphate-adjuvanted Clade C gp140 protein.
Methods
We conducted a randomised, double-blind, placebo-controlled Phase 1 trial (IPCAVD010/HPX1002) at Beth Israel Deaconess Medical Center in Boston, MA. We included healthy, HIV-uninfected participants (aged 18–50 years) who were considered at low risk for HIV infection. We randomly assigned participants to one of three study groups testing different regimens of trivalent Ad26.Mos.HIV (5 × 1010 viral particles per 0·5 mL) combined with 250 mcg adjuvanted Clade C gp140 protein. Participants and investigators were blinded to the treatment allocation throughout the study. Group 1 received Ad26.Mos.HIV alone at weeks 0 and 12 and Ad26.Mos.HIV plus adjuvanted gp140 at weeks 24 and 48. Group 2 received Ad26.Mos.HIV plus adjuvanted gp140 at weeks 0, 12 and 24. Group 3 received Ad26.Mos.HIV alone at week 0 and Ad26.Mos.HIV plus adjuvanted gp140 at weeks 8 and 24. Participants in the control group received 0·9% saline. All study interventions were administered intramuscularly. Primary endpoints were safety and tolerability of the vaccine regimens and Env-specific binding antibody responses at weeks 28, 52 and 72. All participants who received at least one vaccine dose or placebo were included in the safety analysis; immunogenicity was analyzed using the per-protocol population. The IPCAVD010/HPX1002 trial is registered with ClinicalTrials.gov, number NCT02685020. We also conducted a parallel preclinical study in 30 rhesus monkeys to test the protective efficacy of the shortened Group 3 regimen.
Findings
Between Apr 26, 2016 and Aug 19, 2016, we randomly assigned 36 participants to receive at least one dose of study vaccine or placebo in the IPCAVD010/HPX1002 trial. All vaccine regimens were well tolerated. Mild-to-moderate pain and/or tenderness at the injection site was the most commonly reported solicited local adverse event (28 (93%) and 2 (33%) vaccine and placebo recipients, respectively). Grade 3 solicited systemic AEs were reported by 8 (27%) and 0 vaccine and placebo recipients, respectively; the most commonly reported Grade 3 systemic symptoms were fatigue, myalgia, and chills. There was one unrelated death due to a motor vehicle accident. The shortened regimens (Groups 2 and 3) elicited comparable antibody titers against autologous Clade C Env at peak immunity to the longer, 1-year regimen (41,007 and 49,243 GMT vs. 44,590 GMT, respectively), with this peak occurring earlier in the shortened regimens at week 28. Antibody responses remained elevated (>5,000 GMT) in Groups 2 and 3 at week 52. ADCP, Env-specific IgG3, tier 1A neutralizing activity and broad cellular immune responses were detected in all groups. The shortened Group 3 regimen induced comparable peak immune responses in rhesus monkeys as in humans and resulted in an 83% reduction in per exposure acquisition risk after 6 intrarectal challenges with SHIV-SF162P3 at week 54, more than 6 months after final vaccination.
Interpretation
Shorter 6-month regimens of a mosaic HIV-1 prophylactic vaccine elicited robust HIV-specific immune responses that were similar to responses elicited by a longer 12-month schedule. Preclinical data show partial protective efficacy of one of the short vaccine regimens in rhesus monkeys. Further clinical studies are required to test the suitability of the shortened vaccine regimens in humans.
Funding
Clinical trial site activities were funded by the Ragon Institute of MGH, MIT and Harvard. Janssen Vaccines & Prevention, B.V. (Janssen) was the study sponsor, provided investigational products, data management and clinical site monitoring, and funded the preclinical study.
Introduction
The HIV epidemic continues to take an enormous toll on global health and economic development, with 1·7 million new HIV infections in 2018 and $562·6 billion in global HIV/AIDS spending in the 21st century so far1,2. Despite nearly $10 billion spent on HIV prevention annually, the rate of HIV infections decreased by only five percent between 2016 and 2017. The development of a safe and effective HIV vaccine is therefore part of the UNAIDS 2016–2021 Strategic Plan3.
In 2017, a Phase 2b proof-of-concept study called “Imbokodo” (HVTN 705/HPX2008) was launched among women in sub-Saharan Africa to test the preventive vaccine efficacy of a heterologous vaccination regimen using a tetravalent mosaic adenovirus serotype 26 (Ad26)-based HIV vaccine plus adjuvanted Clade C gp140 protein (NCT03060629)4. The Imbokodo regimen involves 4 vaccinations over 12 months, in keeping with pre-clinical and Phase 1/2a studies of similar mosaic HIV-1 vaccine candidates5. However, a 1-year vaccine regimen may be difficult to implement at the community level, where the delivery of multiple doses of a heterologous vaccine regimen may be challenging. We therefore aimed to evaluate whether shorter 6-month regimens of the mosaic HIV-1 vaccine might be equally immunogenic to the longer, 1-year vaccine regimen. One of the shorter regimens we tested was optimized for maximum simplicity, involving 3 doses of homologous vaccines (Ad26.Mos.HIV plus adjuvanted gp140) given 3 months apart. The other shorter regimen we tested was an accelerated version of the heterologous vaccine regimen used in Imbokodo, involving 1 dose of Ad26.Mos.HIV alone, followed by 2 doses of Ad26.Mos.HIV plus adjuvanted gp140, separated by shorter dosing intervals. We also explored the protective efficacy of a shorter heterologous vaccine regimen in rhesus monkeys.
Methods
Study design and participants
We conducted a single-center, randomised, double-blind, placebo-controlled, parallel-group trial at Beth Israel Deaconess Medical Center (BIDMC) in Boston, Massachusetts. The BIDMC Institutional Review Board approved the study on Jan 21, 2016, and the study was registered on ClinicalTrials.gov on Feb 18, 2016 (NCT02685020). We began recruiting participants on Mar 7, 2016 and completed enrollment Aug 29, 2016. Eligible participants were healthy, 18–50 years old, and at low risk for HIV infection. All participants gave written informed consent and successfully completed a test of understanding before the initiation of study procedures.
The study compared three different vaccine schedules of trivalent Ad26.Mos.HIV and aluminum-phosphate adjuvanted Clade C gp140 (table 1). Group 1 tested a heterologous vaccine regimen with 4 immunizations: Ad26.Mos.HIV was given at weeks 0 and 12, followed by Ad26.Mos.HIV plus adjuvanted gp140 at weeks 24 and 48. Group 2 tested a shorter 3-dose homologous schedule of three identical immunizations: Ad26.Mos.HIV plus adjuvanted gp140 was given at weeks 0, 12 and 24. Group 3 tested a shorter 3-dose heterologous vaccination: Ad26.Mos.HIV was given at week 0, followed by Ad26.Mos.HIV plus adjuvanted gp140 at weeks 8 and 24. Each group was divided into active and placebo sub-groups, with 10 vaccine and 2 placebo recipients per group (N=36 total sample size).
Table 1:
Study design
| Group | Sub-group | N | Week 0 | Week 8 | Week 12 | Week 24 | Week 48 |
|---|---|---|---|---|---|---|---|
| 1 | A | 10 | Ad26 | Ad26 | Ad26 + gp140 |
Ad26 + gp140 |
|
| B | 2 | Placebo | Placebo | Placebo + Placebo |
Placebo + Placebo |
||
| 2 | A | 10 | Ad26 + gp140 |
Ad26 + gp140 |
Ad26 + gp140 |
||
| B | 2 | Placebo + Placebo |
Placebo + Placebo |
Placebo + Placebo |
|||
| 3 | A | 10 | Ad26 | Ad26 + gp140 |
Ad26 + gp140 |
||
| B | 2 | Placebo | Placebo + Placebo |
Placebo + Placebo |
Ad26=5 × 1010 viral particles of Ad26.Mos.HIV. gp140= 250 mcg glycoprotein + 0·425 mg aluminum phosphate. Placebo=normal saline.
Randomization and masking
Participants were randomly assigned to a treatment group and a subgroup based on a computer-generated randomization schedule prepared before the study under the supervision of the sponsor. An interactive web response system assigned a unique treatment code, which dictated the treatment assignment and matching study vaccine for the participant. The clinical staff, investigators, participants, and laboratory personnel were blinded to sub-group assignment until week 72. The sponsor was blinded to sub-group assignment until the week 52 analysis. There was no blinding regarding group assignment. The pharmacist with primary responsibility for study product preparation and dispensing was not blinded to the group or sub-group assignment and an overlay was placed on the syringes prior to dispensing.
Procedures
Participants received one or two doses of vaccine or placebo on three or four timepoints as detailed in table 1. Ad26.Mos.HIV consisted of 5 × 1010 viral particles per 0·5 mL injection. Adjuvanted clade C gp140 consisted of 250 mcg glycoprotein mixed with 0·425 mg aluminum per 0·5 mL injection. Placebo consisted of 0·9% saline per 0·5 mL injection. All study products were administered as intramuscular injections into the deltoid. For visits with one injection of Ad26.Mos.HIV (or placebo), the deltoid from the subdominant arm was used. When two injections were given at one visit, the deltoid from the subdominant arm was used for Ad26.Mos.HIV (or placebo) and the deltoid from the dominant arm was used for adjuvanted clade C gp140 (or placebo). No local or topical anesthetic was used prior to the injections. Ad26.Mos.HIV is composed of adenovirus serotype 26 vector expressing bioinformatically optimized HIV-1 mosaic Env, Gag, and Pol antigens; gp140 is a soluble HIV-1 Env protein based on a clade C HIV-1 strain. Details of vaccine composition and manufacturing are described elsewhere5–8.
Local and systemic reactogenicity safety data were collected for eight days after each vaccine or placebo administration. Data on unsolicited adverse events (AEs) were collected for 28 days after each vaccine or placebo administration. Data on serious adverse events (SAEs) and incident HIV infections were collected during the entire study period (72 weeks). Blood samples for serum chemistry (creatinine, aspartate transaminase and alanine transaminase), hematology and urinalysis were collected at several time-points throughout the study. Electrocardiogram measurements were assessed at screening. Medical monitoring was provided by a Protocol Safety Review Team. Peripheral blood was collected to determine anti-HIV and anti-vector immunity.
Serum binding antibody titers against five HIV Envelope (Env) gp140 antigens were measured by enzyme-linked immunosorbent assays (ELISAs) using vaccine-matched Clade C and Mosaic Env, as well as non-vaccine-matched Clade A, B, and C Env5. Serum binding IgG1–4 subclass responses were measured by vaccine-matched Clade C gp140 Env-specific ELISAs. Serum functional antibody responses were measured by antibody-dependent cellular phagocytosis (ADCP) assays using vaccine-matched Clade C Env9,10, and by TZM-bl assays using non-vaccine-matched Tier 1 Clade B and C viruses11,12. Vector-specific antibody responses were assessed by Ad26 neutralization assays13. The magnitude of IgG binding to HIV epitopes was measured with global HIV-1 peptide microarrays (JPT Peptide Tech)14. HIV-specific T cell responses and epitope mapping were measured by interferon-gamma (IFNγ) enzyme-linked immunospot (ELISPOT) assays using potential T-cell epitopes (PTE) and vaccine-matched mosaic Env, Pol, and Gag peptide libraries15,16. All immunogenicity assays were conducted in a blinded fashion.
Endpoints
The primary safety and tolerability endpoints were AEs for 28 days after each vaccine or placebo administration, local and systemic reactogenicity for eight days after each vaccine or placebo administration, discontinuations from vaccination or study due to AEs, and related AEs, SAEs, and AE of special interest (incident HIV infection) during the course of the study. The primary immunogenicity endpoints were serum Env-specific binding antibody titers in each experimental group at weeks 28, 52 and 72. Secondary immunogenicity endpoints included Env-specific functional antibody responses (ADCP and neutralization), IgG subtypes, and T cell responses. Exploratory endpoints included antibody and T cell epitope mapping and baseline Ad26-specific neutralizing antibody titers. Peak immune responses were estimated to occur at four weeks following the last product administration (week 28 for Groups 2 and 3, and week 52 for Group 1). Endpoints for placebo recipients were pooled into a single “Placebo” group.
Statistical analysis
The sample size was determined to assess the preliminary safety and tolerability of the different vaccine or placebo regimens. With 10 individuals in a group, the observation of 0 significant AEs (e.g., that preclude further dose administration or limit product development) would be associated with a 95% confidence (exact Clopper-Pearson method) that the true rate is less than 26%. For the combined groups (n=30), there would be 95% confidence that the true rate is less than 9.5% when 0 events are observed. Placebo recipients were included to assess safety and to provide control specimens for immunogenicity assays. The statistical analysis of safety data followed the intention-to-treat principle, including all participants that were randomised and received at least one vaccine or placebo dose. For each vaccine or placebo regimen, the number and proportion of participants experiencing AEs, SAEs and laboratory abnormalities were tabulated.
The per protocol immunogenicity (PPI) population included all participants who were randomised and who received all vaccines or placebos according to the protocol-specified vaccination schedule (+/− two weeks) for whom immunogenicity data were available excluding participants with major protocol deviations expecting to impact immunogenicity outcomes. The analysis of the immune responses was performed on the PPI. Immunogenicity data were analyzed descriptively through tabulations of geometric mean with corresponding two-sided 95% confidence intervals (CIs) and/or medians. Response rates and CIs for immunoassays were calculated as the number and proportion of participants meeting the predefined definition of response. CIs were not adjusted for multiplicity. No formal hypothesis on immunogenicity was tested. Differences between groups at specific time-points were tested for exploratory purposes by a 2-sample t-test using GraphPad Prism 8.2.1. All statistical tests were 2-sided and p-values were not adjusted for multiplicity.
Rhesus monkey challenge study
Thirty Indian-origin rhesus monkeys (Macaca mulatta) were immunized with active or sham vaccines using the same regimen as used in Group 3 of the IPCAVD010/HPX1002 clinical study, including a first immunization with Ad26.Mos.HIV alone (week 0) and second and third immunizations (weeks 8 and 24) with Ad26.Mos.HIV combined with adjuvanted Clade C gp140 or saline instead of vaccinations (n=15/Group). At week 54, all monkeys received six weekly intrarectal challenges with 500 TCID50 of the heterologous virus SHIV-SF162P3. Viral loads were determined weekly following challenge in a blinded fashion by a qualified viral load assay. Immunogenicity assays were performed as described above. Ad26 vectors and gp140 protein were produced at Janssen, and the SHIV challenge stock was produced in rhesus peripheral blood mononuclear cells at BIDMC. Institutional Animal Care and Use Committee approvals were obtained. Time-to-infection was analyzed with Cox proportional hazard regression for discrete times, and final infection status was assessed by a two-sided Fisher’s exact test. Prediction of time to infection was based on peak (week 28) Clade C ELISA and PTE Env ELISPOT data of the present study (17–22) that were included in a prediction model based on peak (week 54/56) Clade C ELISA and PTE Env ELISPOT data of historic study 13–195. Additionally, a prediction model using 17–22 ELISA and ELISPOT data alone was generated by using ordinal logistic regression on the two assays to predict the cumulative infection probability after each challenge. ELISA data underlying the statistical analyses were generated in the NHP assay format described previously5.
Role of the funding source
All clinical trial site activities were funded by the Ragon Institute of MGH, MIT and Harvard. Janssen was the study sponsor, and provided Ad26.Mos.HIV, Clade C gp140, and aluminum products, as well as data management and clinical site monitoring. Janssen also participated in data collection, analysis, interpretation and writing of the report. The preclinical study was funded by Janssen. All authors had full access to the data in the study. The BIDMC study leads (KES, DHB) had final responsibility for the decision to submit for publication, which was joint among all coauthors.
Results
The clinical study began on Mar 31, 2016 when the first participant signed the informed consent form, and the study was completed on Jan 23, 2018 when the last participant reached week 72. Seventy (70) participants were screened. Thirty-six (36) participants were randomised and received at least one dose of vaccine or placebo (figure 1). The majority of participants (83%) completed the main study. Six out of 36 participants (17%) discontinued the study prematurely due to one of the following reasons: lost to follow-up (N=3), death due to an unrelated motor vehicle accident (N=1), investigator decision (N=1), and withdrawal of consent (N=1). The majority of participants received all planned product administrations: 87% and 67% of vaccine and placebo recipients, respectively. No unblinding occurred prior to week 72. There were no relevant differences in demographic characteristics observed between the groups (table 2).
Figure 1: Trial profile.
IPCAVD010/HPX1002 clinical study.
Table 2:
Baseline characteristics of the intention-to-treat population
| Total (n=36) | Group 1 (n=10) | Group 2 (n=10) | Group 3 (n=10) | Placebo* (n=6) | |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 27·4 (6·92) | 26·9 (4·43) | 27·4 (8·86) | 28·6 (7·82) | 26·0 (6·51) |
| Range | 20–47 | 20–35 | 20–47 | 21–42 | 21–39 |
| Height (cm) | |||||
| Mean (SD) | 172·5 (8·89) | 169·4 (8·31) | 174·6 (11·35) | 170·2 (7·71) | 178·2 (3·65) |
| Range | 152·4–189·9 | 159·8–185·1 | 152·4–189·9 | 161·0–180·8 | 172·7–184·1 |
| Weight (kg) | |||||
| Mean (SD) | 76·8 (23·33) | 72·2 (9·81) | 81·8 (27·39) | 69·3 (12·01) | 88·8 (40·62) |
| Range | 48·4–170·9 | 57·6–89·1 | 48·4–138·0 | 56·7–88·0 | 65·3–170·9 |
| Body mass index (kg/m2) | |||||
| Mean (SD) | 25·5 (6·07) | 25·1 (1·77) | 26·5 (7·32) | 23·8 (2·89) | 27·6 (11·27) |
| Range | 19·0–50·4 | 22·1–26·9 | 19·0–39·4 | 21·7–30·5 | 20·7–50·4 |
| Sex | |||||
| Female | 16 (44%) | 7 (70%) | 5 (50%) | 4 (40%) | 0 |
| Male | 20 (56%) | 3 (30%) | 5 (50%) | 6 (60%) | 6 (100%) |
| Race | |||||
| White | 20 (56%) | 9 (90%) | 5 (50%) | 4 (40%) | 2 (33%)** |
| Black or African American | 3 (8%) | 0 | 2 (20%) | 1 (10%) | 0 |
| Asian | 8 (22%) | 1 (10%) | 1 (10%) | 4 (40%) | 2 (33%)** |
| Other | 1 (3%) | 0 | 0 | 0 | 1 (17%)** |
| Multiple | 4 (11%) | 0 | 2 (20%) | 1 (10%) | 1 (17%)** |
| Ethnicity | |||||
| Hispanic or Latino | 1 (3%) | 0 | 0 | 0 | 1 (17%) |
| Not Hispanic or Latino | 33 (92%) | 9 (90%) | 10 (100%) | 9 (90%) | 5 (83%) |
| Not Reported | 1 (3%) | 0 | 0 | 1 (10%) | 0 |
| Unknown | 1 (3%) | 1 (10%) | 0 | 0 | 0 |
| Country | |||||
| USA | 36 (100%) | 10 (100%) | 10 (100%) | 10 (100%) | 6 (100%) |
Endpoints for placebo recipients from Groups 1–3 were pooled into a single “Placebo” group.
Percentages may not total 100 due to rounding.
Symptoms of local and systemic reactogenicity were solicited from participants for eight days following each product administration (table 3). Pain and/or tenderness at the injection site was reported by 93% and 33% of vaccine and placebo recipients, respectively. Erythema was reported in one vaccine recipient in Group 2. No cases of swelling/induration were reported. Two out of 30 (7%) vaccine recipients reported a Grade 3 AE of pain and/or tenderness. All other solicited local AEs were Grade 1 or Grade 2 in severity. The most common solicited systemic symptoms were fatigue, myalgia and headache, which were reported by 87%, 73% and 67% of vaccine recipients and 67%, 33%, and 33% of placebo recipients, respectively. The median duration of these symptoms ranged from 1 to 3·5 days, depending on the symptom. In general, the highest incidences of solicited systemic AEs were seen after the first vaccination and decreased with subsequent vaccinations. Grade 3 solicited systemic AEs were reported by 27% and 0% of vaccine and placebo recipients, respectively. The most commonly reported Grade 3 systemic symptoms were fatigue, myalgia, and chills. There were no grade 3 or higher reported fevers. There were no Grade 3 or life-threatening unsolicited AEs considered related to vaccination. There was one unrelated death due to motor vehicle accident. There were no pregnancies or incident HIV infections reported during the study.
Table 3:
Maximum reactogenicity within 8 days of vaccination
| Group 1 (n=10) | Group 2 (n=10) | Group 3 (n=10) | Placebo* (n=6) | |
|---|---|---|---|---|
| Local symptoms | ||||
| Pain/Tenderness | ||||
| Any | 10 (100%) | 8 (80%) | 10 (100%) | 2 (33%) |
| Grade 3 | 0 | 1 (10%) | 1 (10%) | 0 |
| Erythema | ||||
| Any | 0 | 1 (10%) | 0 | 0 |
| Systemic symptoms | ||||
| Fatigue | ||||
| Any | 10 (100%) | 8 (80%) | 8 (80%) | 4 (67%) |
| Grade 3 | 1 (10%) | 2 (20%) | 2 (20%) | 0 |
| Myalgia | ||||
| Any | 8 (80%) | 7 (70%) | 7 (70%) | 2 (33%) |
| Grade 3 | 1 (10%) | 0 | 2 (20%) | 0 |
| Headache | ||||
| Any | 8 (80%) | 7 (70%) | 5 (50%) | 2 (33%) |
| Grade 3 | 0 | 1 (10%) | 1 (10%) | 0 |
| Chills | ||||
| Any | 7 (70%) | 4 (40%) | 5 (50%) | 2 (33%) |
| Grade 3 | 0 | 1 (10%) | 2 (20%) | 0 |
| Nausea | ||||
| Any | 7 (70%) | 3 (30%) | 1 (10%) | 1 (17%) |
| Grade 3 | 0 | 1 (10%) | 1 (10%) | 0 |
| Pyrexia | ||||
| Any | 4 (40%) | 2 (20%) | 1 (10%) | 0 |
Endpoints for placebo recipients from Groups 1–3 were pooled into a single “Placebo” group.
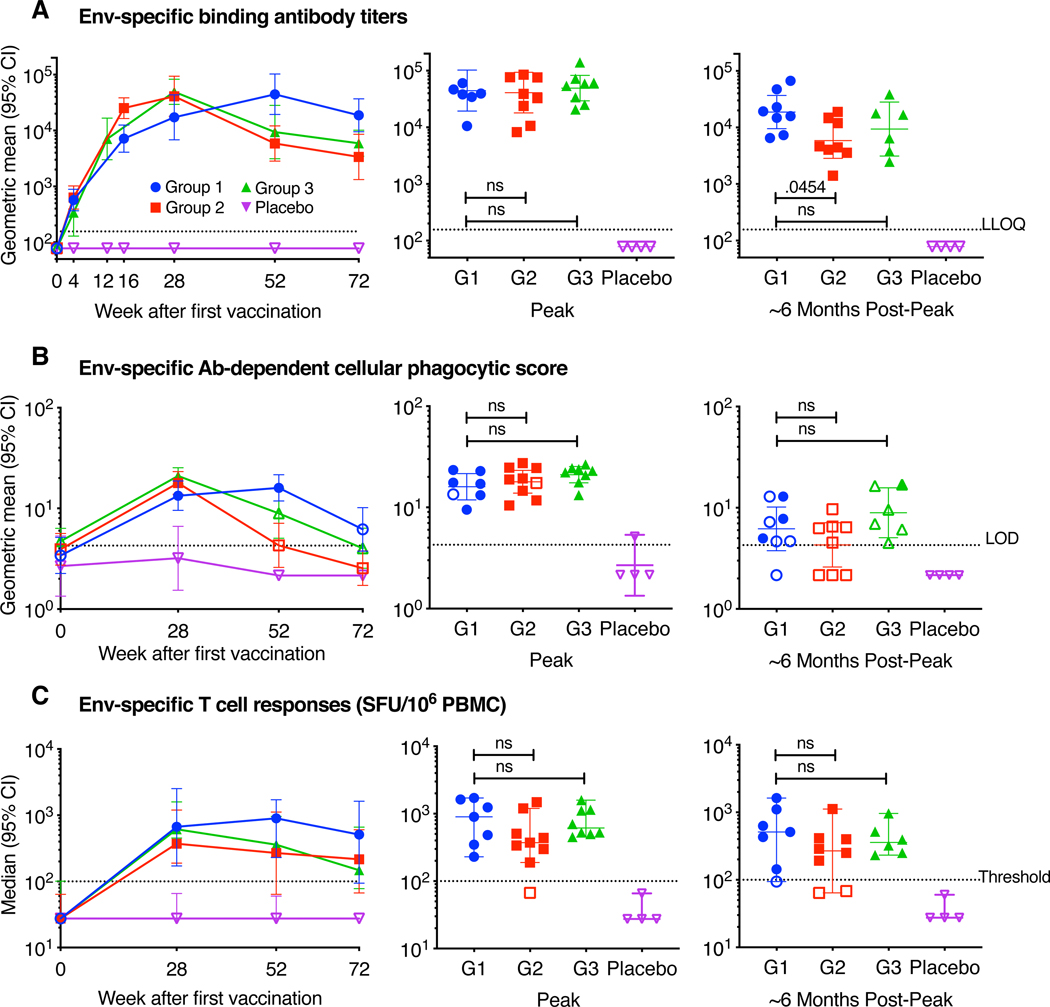
Serum binding antibody responses against vaccine-matched Clade C HIV Env were detected in 100% of vaccine recipients and 0% of placebo recipients at weeks 28, 52, and 72, regardless of which vaccine regimen was received (figure 2A). Peak antibody binding titers at four weeks following last vaccination were comparable across the different vaccine regimens: the geometric mean titer (GMT) in Group 1 was 44,590 (95% CI 19,345—102,781) at Week 52, compared to 41,007 (95% CI 17,959—93,636) at Week 28 in Group 2 and 49,243 (95% CI 29,346—82,630) at Week 28 in Group 3. Approximately six months following peak immunity, antibody titers declined in all groups, as expected: the GMT in Group 1 at week 72 was 18,807 (95% CI 9,555—37,017), compared to 5,872 (95% CI 2,837—12,156) in Group 2 at week 52 and 9,397 (95% CI 3,131—28,200) in Group 3 at week 52. A similar pattern of antibody binding responses was observed against Mosaic Env (matched to the Ad26.Mos.HIV Env sequence), as well as non-vaccine-matched Clades A, B, and C Envs, with responder rates of 100% in all experimental groups at Weeks 28, 52, and 72 (table S1, appendix p. 3–5). Serum binding antibodies were predominantly IgG1 subtype in magnitude and frequency for all experimental groups, although IgG3 subtype was also observed (table S2, appendix p. 6–7). This pattern of IgG1/3 responses was previously observed with Ad26.Mos.HIV and gp1405, and was also elicited in the RV144 HIV-1 vaccine trial showing modest protection against HIV-1 acquisition17,18. No IgG2 or IgG4 were detected, even among Group 2 participants who received repeated vaccination with gp140 Env three times and for whom a class switch from IgG1/3 to IgG4 might have been observed19. IgG1 and IgG3 GMTs in each group followed a similar pattern as total IgG, with Group 2 responses consistently lower in post-peak responses.
Figure 2: Humoral and cellular immune responses against clade C HIV-1 Env following vaccination.
(A) Binding antibody clade C gp140-specific titers by enzyme-linked immunosorbent assays. Dotted line is the LLOQ threshold. Left panel, the symbol reflects the group geometric mean. Middle and right panels, the line reflects the group geometric mean. (B) Clade C gp140-specific antibody-dependent cellular phagocytic score. Dotted line is the LOD. Left panel, the symbol reflects the group geometric mean. Middle and right panels, the line reflects the group geometric mean. (C) T cell responses by interferon-gamma (IFNγ) enzyme-linked immunospot (ELISPOT) assays against Env PTE peptide pools. Dotted line is positivity threshold. Vaccine response was defined as value more than threshold (if baseline is <threshold); otherwise it was defined as value with a three-time increase from baseline (if baseline is ≥ threshold). Left panel, the symbol reflects the group median. Middle and right panels, the line reflects the group median. For all panels, error bars reflect 95% CI. Group 1 responses were compared to Group 2 and 3 responses at peak and ~6 months post-peak using 2-sided t-tests. Closed symbol=vaccine responder. Open symbol=non-responder. CI=confidence interval. NS=not significant. LLOQ=lower limit of quantification. SFU=spot-forming units. PBMC=peripheral blood mononuclear cells. Endpoints for placebo recipients from Groups 1–3 were pooled into a single “Placebo” group.
Serum ADCP activity was detected in the majority of vaccine recipients at week 28 in all experimental groups, e.g., 63%, 89%, and 100% in Groups 1–3, respectively, though responder rates declined more quickly than antibody binding titers in all groups by week 72 (figure 2B, table S3, appendix p. 8). ADCP activity is presented as a phagocytic score, which is a measure of how well monocytes phagocytose Env-coated beads when in the presence of antibodies from a particular clinical sample9. Peak phagocytic scores at four weeks following last vaccination were similar across the different vaccine regimens: the geometric mean score (GMS) in Group 1 was 16·0 (95% CI 11·9—21·6) at Week 52, compared to 17·9 (95% CI 13·8—23·1) at Week 28 in Group 2 and 21·0 (95% CI 17·4—25·3) at Week 28 in Group 3. Approximately six months following peak immunity, phagocytic scores declined in all groups, as expected: the GMS in Group 1 at week 72 was 6·2 (95% CI 3·8—10·2), compared to 4·3 (95% CI 2·6—7·1) in Group 2 at week 52 and 8·9 (95% CI 5·1—15·8) in Group 3 at week 52. Neutralizing antibody titers against Clade C (MW965.26) pseudovirus were detected at peak immunogenicity, and titers were similar across the groups: the GMT in Group 1 was 80·7 (95% CI 25·6—254·7), compared to 46·8 (95% CI 21·8—100·5) in Group 2 and 39·9 (95% CI 13·4—118·6) in Group 3. Neutralizing antibody titers against Clade B (MN-3 and SF162.LS) pseudoviruses were similar to Clade C responses (table S4, appendix p. 9). One (1) participant was seropositive for Ad26 at baseline; this participant was in Group 3 and received vaccine (table S5, appendix p. 10).
Peripheral T cell responses against potential T cell epitope (PTE) Env peptides were detected in >70% of vaccine recipients at weeks 28, 52, and 72, regardless of which vaccine regimen was received (figure 2C). Peak T cell responses at four weeks following last vaccination were as follows across the different vaccine regimens: the median response (SFU/106 PBMC) in Group 1 was 898 (range 230—1711) at Week 52, compared to 372 (range 67–1480) at Week 28 in Group 2 and 614 (range 451—1592) at Week 28 in Group 3. Approximately six months following peak immunity, T cell responses declined slightly: the median response in Group 1 at week 72 was 510 (range 94—1627), compared to 269 (range 64—1118) in Group 2 at week 52 and 359 (range 232—962) in Group 3 at week 52. T cell responses against PTE Gag and Pol peptides showed the same relative response pattern, although Pol-specific responses were higher than and Gag-specific responses were lower than Env-specific responses. T cell responses against vaccine-matched Mosaic1 and partially-matched Mosaic 2 peptides were similar to responses against PTE peptides (table S6, appendix p. 11–16).
In an exploratory immunogenicity analysis of select key comparisons, 2-sided t-tests were performed to compare Env-specific binding antibody titers, ADCP and T cell responses between Group 1 and either Group 2 or 3 at peak and ~6 months post-peak time-points (table S7, appendix p. 17). There were no significant differences between Group 1 and either Group 2 or 3 for any parameter at peak timepoints. At ~6 months post-peak, there was a difference between Group 1 and Group 2 Env-specific binding antibody titers (p=0.0454), but no other differences at ~6 months post-peak were observed. An area under curve analysis of Clade C gp140 binding antibody titers revealed comparable cumulative responses over the course of the study per regimen (data not shown). Additional comparisons of binding antibody titers were performed for week 28, 52, and 72, which showed Group 1 titers were significantly higher than the other groups by week 72 (G1 vs. G2, p=0.0222; G1 vs. G3 p=0.0316), which would be expected given Group 1’s late boost.
The pattern and depth of antibody binding to Env epitopes was measured with global HIV-1 peptide microarrays containing 4031 individual overlapping 15 amino acid linear Env peptides. At week 28, all three groups displayed comparable Env-specific linear antibody binding profiles (figure 3A), with the highest signals observed at two peaks within gp120: position 296–310 in variable region three (V3) and position 456–470 within constant region four (C4). There were few gp41-specific responses. At week 52, following the 4th vaccine visit, Group 1 responses shifted within gp120: peak V3 responses moved to position 304–318, the C4 peak disappeared, and a new peak appeared at position 491–505 of constant region five (C5). In contrast, Group 2 and 3 responses did not change specificity at week 52, but declined in magnitude in parallel with lower antibody titers at this time-point. The depth of peptide responses at four weeks following last vaccination was similar across the different vaccine regimens (figure 3B): the mean number of positive Env peptides in Group 1 at week 52 (peak) was 161.3 (95% CI 11.1—311.5), compared to 118.1 (95% CI 85.72—150.5) in Group 2 at week 28 (peak) and 127.6 (95% CI 82.2—172.9) in Group 3 at week 28 (peak). The depth of responses within the variable loop peptide set (including V1 and V2 peptides) was also similar across the groups, as was the depth of responses within constant region and gp41 peptide sets.
Figure 3: Antibody epitope mapping.
(A) The pattern of antibody binding to linear Env peptides is shown for each group at week 28 (all groups) and week 52 (Group 1 only, after late boost). Each dot represents the average signal intensity per peptide within each group. Baseline responses are plotted on the y axis in the downward direction. Week 28 and 52 responses are plotted on the y axis in the upward direction. The amino acid start position of each peptide (aligned to HXB2 HIV strain) is plotted along the x axis. V3=variable loop 3 region. C4=constant region 4. C5=constant region 5. (B) The depth of antibody binding to linear Env peptides is shown for each participant within each group at week 28 and 52. Each dot represents the total number of positive peptide responses per participant for each peptide set (e.g., all Env (4031 peptides), variable loop (1559 peptides), constant region (1205 peptides) and gp41 (1237 peptides). The threshold for positivity was >5× noise distribution of the sample slide. The line reflects the group median. G1=group 1. G2=group 2. G3=group 3. Wk=Week. Endpoints for placebo recipients from Groups 1–3 were pooled into a single “Placebo” group.
The specificity of T cell responses to Env, Gag, and Pol epitopes was measured by epitope mapping studies with PTE and vaccine-matched peptides aligned by amino acid start position in 10 peptide subpools. At week 28, all three groups displayed T cell responses that were broadly distributed across Env, Gag and Pol, without marked immunodominance at any protein location (figure S1A, appendix p. 18), consistent with previous observations3. T cell breadth (number of positive Env, Gag, or Pol subpools) was similar across the groups at week 28 (figure S1B, appendix p. 18): the median number of total positive subpools in Group 1 was 8 (range 4–20), compared to 8 (range 4–20) in Group 2 and 14 (range 5–30) in Group 3. At week 52, following the 4th vaccine visit, the breadth of Group 1 T cell responses remained similar to week 28 (median 8 positive subpools, range 2–39).
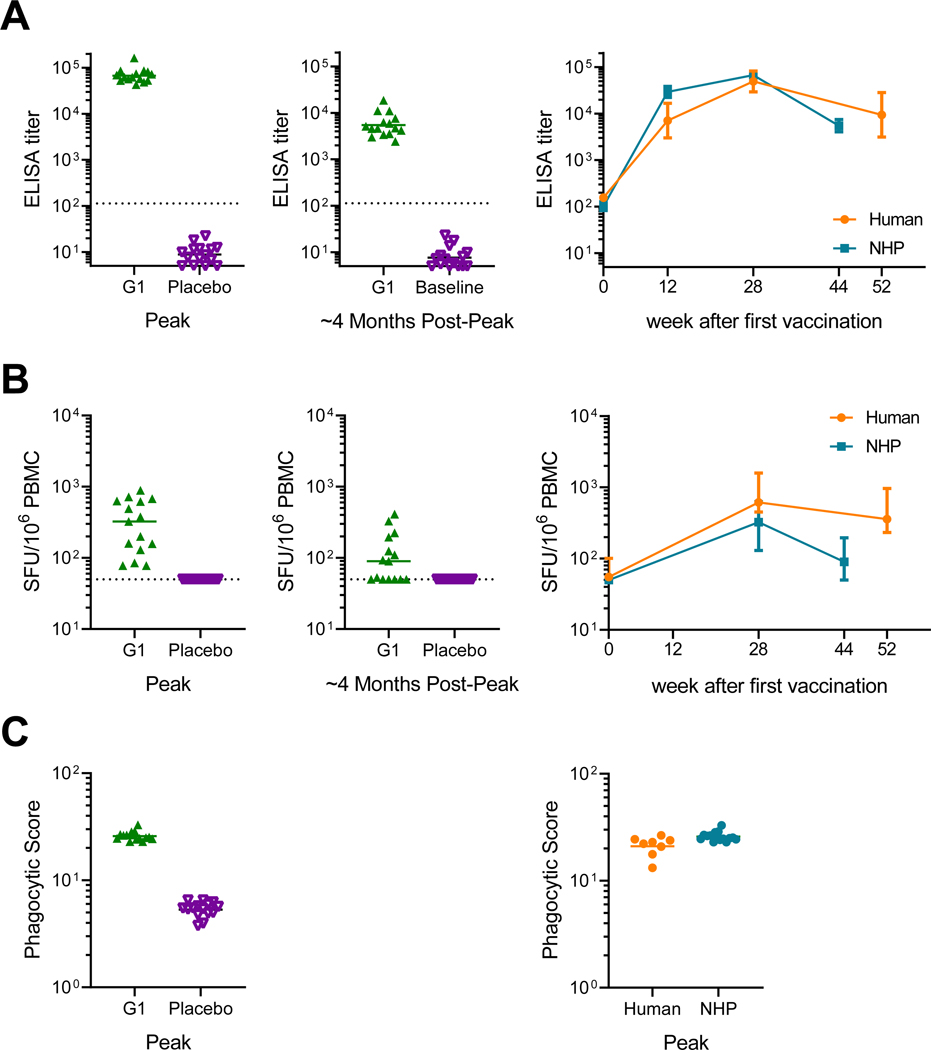
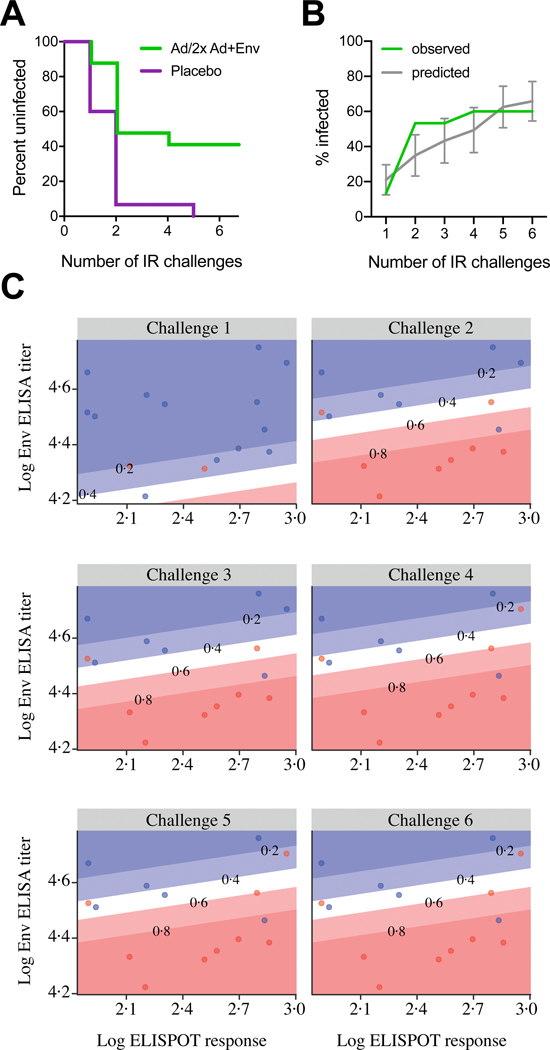
The protective efficacy of the shortened regimen used in Group 3 was then tested in rhesus monkeys. The Group 3 regimen was selected for testing because peak binding antibody responses were highest in this group in the clinical study. Peak binding antibody responses against Clade C gp140 were detected in all vaccinated monkeys by ELISA (Figure 4A) and declined 4 months after the final vaccination (Week 44). Cellular immune responses against HIV-1 Env measured by IFN-γ ELISPOT assays using PTE peptide pools were detected in all monkeys (figure 4B) and contracted between peak immunogenicity (week 28) and pre-challenge time points (week 44). ADCP activity was also detected in all vaccinated animals at peak immunogenicity (figure 4C). Monkeys showed comparable gp140 binding antibody, ELISPOT and ADCP responses at peak time points as in humans, but the durability of cellular immune responses was lower in monkeys than humans. At week 54, which reflects 30 weeks after the final vaccination, all animals were challenged 6 times by the intrarectal route with the heterologous, tier 2 neutralization-resistant virus SHIV-SF162P3. Control monkeys were infected after a median of 2 challenges (range 1 to 5), whereas 6 of 15 vaccinated animals remained uninfected after the complete challenge series (figure 5A). This corresponds to an 83% reduction in per exposure acquisition risk (P=0.004, log-rank test) and 40% complete protection (P=0.017, 2-sided Fishers’ exact test). As expected using a vaccine containing HIV Gag and a SHIV challenge virus containing mismatched SIV Gag, peak viral loads of infected animals did not differ between groups (figure S2A–C, appendix p. 19). In a previous NHP study (13–19)5, a 4-immunization regimen was assessed in the same challenge model. The present study showed comparable peak viral loads, time to infection kinetics and per exposure risk reduction (figure S2C–E, appendix p. 19). We predicted the time to infection of the present (17–22) study by including 17–22 immunogenicity data into the prediction model based on 13–19 study data. Despite the small size of the present study, the predicted time to infection data were consistent with the observed protection data, thus confirming the overall value of the prediction model (figure 5B). A prediction model generated on the basis of ELISA and ELISPOT data of the present study alone led to a distinct model (model fit of both assays p=0.011; figure 5C). The linear predictor defined with these two assays strongly correlated with observed data (Spearman correlation ρ=71%).
Figure 4: Humoral and cellular immune responses in rhesus monkeys and comparison to humans.
(A) Binding antibody clade C gp140-specific titers determined by enzyme-linked immunosorbent assays (ELISA). Dotted line is the LLOQ threshold. (B) T cell responses by interferon-gamma (IFN-γ) enzyme-linked immunospot (ELISPOT) assays against Env PTE peptide pools. Dotted line reflects the positivity threshold. (C) Clade C gp140-specific antibody-dependent cellular phagocytic (ADCP) score. Horizontal lines indicate geometric mean titers for ELISA and ADCP and medians for ELISPOT responses. LLOQ=lower limit of quantification. SFU=spot-forming units. PBMC=peripheral blood mononuclear cells. Error bars depict the 95% confidence interval.
Figure 5: Protective efficacy in rhesus monkeys and correlates of risk reduction.
Six weekly intrarectal challenges were administered to rhesus monkeys at weeks 1, 2, 3, 4, 5 and 6. (A) Kaplan-Meier plot of the level of protection the short vaccine regimen offered to the 15 rhesus monkeys at risk per group, assessed one week after each challenge. No animals were censored. (B) Mean predicted infection frequency compared to observed infection frequency per time point (vaccine group only). Predictions are based on a model that was generated with historic data using peak immunogenicity Clade C ELISA and PTE Env ELISPOT data5. Error bars depict the 95% confidence interval. (C) Humoral and cellular immune responses measured by clade C ELISA and PTE Env ELISPOT at week 28, the week following each of six challenges (at weeks 54–59) of monkeys from the vaccine group. Blue dots represent uninfected monkeys per timepoint, and red dots indicate monkeys that were infected following each challenge. The diagonal lines display model-derived probabilities of infection, modeled on ELISA and ELISPOT responses.
Discussion
TheAd26.Mos.HIV/Ad26.Mos.HIV plus gp140 HIV vaccine was recently shown to be safe and immunogenic in humans, and it provided 67% protection against simian-human immunodeficiency virus (SHIV) repeated challenge in rhesus monkeys5. This vaccine is administered as a 4-vaccine series over 12 months, which may be difficult to implement in a real-world setting. To explore shortened and simplified regimens, we performed a randomised controlled trial to compare three different vaccine schedules of mosaic Ad26 plus gp140 (table 1). Group 1 tested the current 12-month, 4-vaccine schedule, described above, and Group 2 and 3 tested 6-month, 3-vaccine schedules. Group 2 was also a simpler vaccine regimen as the products given at each visit were always the same. We found that the vaccines were safe and well-tolerated across regimens, in line with previously reported data on Ad26.Mos.HIV and Clade C gp140 in humans5. We found that peak immune responses were not decreased in the alternative vaccine schedules. Binding antibody titers, ADCP activity, and T cell responses were comparable at peak levels, regardless of whether participants received a 3 or 4 vaccine series, and whether the regimen included mosaic Ad26 alone or mosaic Ad26 plus gp140 as the first dose. We initially hypothesized that the epitope breadth and specificity of the antibody and T cell responses might be different among the groups, even if the magnitude of the response was equivalent. However, we found that antibody and T cell breadth were very similar at peak levels across the groups. Together, these data suggest that the shorter and simpler vaccine schedules did not lead to a marked change in peak immune responses. In fact, it is possible that this accelerated 3-vaccine schedule may induce peak immune responses sooner than the 4-vaccine schedule.
Consistent with our earlier observations5, humans and monkeys showed comparable levels and kinetics of peak immune responses. The main difference between species was reduced durability of cellular immune responses in monkeys compared to humans, which suggests a high stringency of the monkey model, since immune responses contracted at the time of viral challenge compared to peak levels. Significant per exposure infection risk reduction was nevertheless achieved by the 3-dose regimen in monkeys (point estimate 83%; 95% confidence interval 38.7 – 95%), which is comparable to the efficacy observed in a previous preclinical efficacy study testing several 4-dose regimens of the mosaic vaccine in the same model (point estimate 94.4%; 95% confidence interval 71.5 – 98.9%). Moreover, similar to previous studies, binding antibody titers strongly correlated with time to infection5. Nevertheless, the relevance of vaccine protection in rhesus monkeys to clinical efficacy in humans will remain unclear until validated by clinical efficacy trials.
While shorter and simpler 3-vaccine schedules did not diminish peak immune responses in the clinical study, there were trends that the durability of the immune responses were negatively impacted by the inclusion of gp140 with the Ad26 prime in clinical study Group 2. Group 2 decreased more than in the other groups at 6 months following peak immunity. We also observed that median Group 2 responses were ranked the lowest of the three groups across most immune parameters and timepoints tested, with the exception of Gag- and Pol-specific T cell breadth. These data suggest that co-administering mosaic Ad26 with soluble Env protein (gp140) at the first vaccine visit – as done in Group 2 – may inhibit optimal immune priming, though the mechanism of this inhibition is unclear.
The interpretation of these data is limited by the small sample size of the clinical study (36 participants), the relatively short follow up (12 months total), and the demographic characteristics of the study population (predominantly white and non-Hispanic participants from Boston, MA). For example, it is possible that the late boost at week 48 in Group 1 may prove important for long-term durability, as evidenced by the trend towards a slower decline in antibody titers in Group 1 compared to the other groups between peak levels and samples obtained 6 months after final vaccination. In addition, there may be subtle differences in the phenotype of the immune response that could prove to have clinical significance in efficacy studies. For instance, the late boost was associated with a shift in the specificity of Env-specific antibodies as measured by linear peptide microarray, perhaps reflecting antibody maturation in the setting of repeated antigen exposure which may signal increased durability. Follow-up of study participants may help provide additional insight regarding these differences. Future clinical studies should also be performed to test these observations in a larger sample size.
In summary, peak immune responses elicited by the mosaic Ad26.Mos.HIV/Ad26.Mos.HIV plus gp140 vaccine were not affected by whether participants received the 3-vaccine or 4-vaccine series. These data suggest that there is flexibility in the dosing of the mosaic Ad26/Ad26 plus gp140 vaccine. In fact, only one of the first two Ad26.Mos.HIV alone vaccinations may be necessary to elicit high magnitude responses and may delay peak immunity. However, there may still be a benefit to keeping a first immunization with Ad26.Mos.HIV without co-administration with gp140, and a longer interval before the final vaccination, in order to elicit durable immune responses. Further studies are planned to simplify the delivery of this HIV vaccine candidate pending efficacy data from ongoing clinical trials.
Supplementary Material
Acknowledgements
We thank the clinical trial participants and staff at the Center for Virology and Vaccine Research Clinical Trials Unit and the Harvard Catalyst Clinical Research Center. This work was supported by the Ragon Institute of MGH, MIT, and Harvard; Janssen Vaccines & Prevention BV; National Institutes of Health grants AI060354, AI124377, AI126603, AI128751, A129797, OD024917, and TR001102; and Harvard Catalyst. The content is solely the responsibility and opinions of the authors. We acknowledge generous advice and assistance from the BIDMC research and clinical teams and the Ragon Institute, as well as the following members of the Janssen clinical and pre-clinical teams: Colleen Spicer, Olive Yuan, Karin Feddes-de Boer and Sietske Rosendahl Huber.
Declaration of Interests
DHB has received grant funding from the National Institutes of Health, the Bill & Melinda Gates Foundation, and Janssen Vaccines & Prevention BV. KES has received grant funding from the National Institutes of Health. DHB is a coinventor on HIV-1 vaccine antigen patents that have been licensed to Janssen Vaccines & Prevention BV. FW, FT, JH, SN, CT, JT, RZ, DJS, MGP and HS are employees of Janssen, pharmaceutical companies of Johnson & Johnson. LL is a consultant to Janssen, pharmaceutical companies of Johnson & Johnson. All other authors declare no competing interests.
References
- 1.Progr Joint U. N.. HIV/AIDS. 2018. UNAIDS Data 2018. Geneva: Joint U. N. Progr. HIV/AIDS. https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf [Google Scholar]
- 2.Global Burden of Disease Health Financing Collaborator N. Spending on health and HIV/AIDS: domestic health spending and development assistance in 188 countries, 1995–2015. Lancet. 2018;391(10132):1799–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Progr Joint U. N.. HIV/AIDS. 2015. UNAIDS 2016–2021 Strategy: On the Fast-Track to End AIDS. Geneva: Joint U. N. Progr. HIV/AIDS. [Google Scholar]
- 4.National Institutes of Health. Press Release: NIH and partners launch HIV vaccine efficacy study: Public-private partnership begins clinical trial in sub-Saharan Africa (Thursday, November 30, 2017). Thursday, November 30, 2017. URL: https://www.nih.gov/news-events/news-releases/nih-partners-launch-hiv-vaccine-efficacy-study [Google Scholar]
- 5.Barouch DH, Tomaka FL, Wegmann F, Stieh DJ, Alter G, Robb ML, et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13–19). Lancet. 2018;392(10143):232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155(3):531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer W, Perkins S, Theiler J, Bhattacharya T, Yusim K, Funkhouser R, et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med. 2007;13(1):100–6. [DOI] [PubMed] [Google Scholar]
- 8.Barouch DH, O’Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med. 2010;16(3):319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackerman ME, Moldt B, Wyatt RT, Dugast A-S, McAndrew E, Tsoukas S, et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366(1–2):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dugast A-S, Chan Y, Hoffner M, Licht A, Nkolola J, Li H, et al. Lack of protection following passive transfer of polyclonal highly functional low-dose non-neutralizing antibodies. PloS one. 2014;9(5):e97229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarzotti-Kelsoe M, Bailer RT, Turk E, Lin CL, Bilska M, Greene KM, et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods. 2014;409:131–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Current protocols in immunology / edited by John E Coligan [et al. ]. 2005;Chapter 12:Unit 12.1. [DOI] [PubMed] [Google Scholar]
- 13.Sprangers MC, Lakhai W, Koudstaal W, Verhoeven M, Koel BF, Vogels R, et al. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. Journal of clinical microbiology. 2003;41(11):5046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephenson KE, Neubauer GH, Reimer U, Pawlowski N, Knaute T, Zerweck J, et al. Quantification of the epitope diversity of HIV-1-specific binding antibodies by peptide microarrays for global HIV-1 vaccine development. J Immunol Methods. 2015;416:105–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. 2012;482(7383):89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li F, Malhotra U, Gilbert PB, Hawkins NR, Duerr AC, McElrath JM, et al. Peptide selection for human immunodeficiency virus type 1 CTL-based vaccine evaluation. Vaccine. 2006;24(47–48):6893–904. [DOI] [PubMed] [Google Scholar]
- 17.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6(228):228ra38. [DOI] [PubMed] [Google Scholar]
- 18.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–20. [DOI] [PubMed] [Google Scholar]
- 19.Methot SP, Di Noia JM. Molecular Mechanisms of Somatic Hypermutation and Class Switch Recombination. Adv Immunol. 2017;133:37–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.