Abstract
Introduction
Preliminary trial data suggest group-delivered acceptance and commitment therapy (ACT) might be effective for smoking cessation. If so, this could offer a viable alternative to mainstream behavioral therapies, such as those grounded in cognitive behavioral therapy (CBT). The goal of the current study was to compare the effectiveness of group-delivered ACT versus group-delivered CBT in a rigorous randomized trial design with long-term follow-up.
Methods
Participants (n = 450) were recruited from the Kaiser Permanente Washington health care system and randomized to either ACT-based group counseling or an attention-matched CBT-based group program. All were prescribed an 8-week course of nicotine patches. The primary outcome was self-reported 30-day point prevalence abstinence at 12 months post-randomization assessed with missing values imputed as smoking. Sensitivity analyses using multiple imputation and complete cases were examined, as were biochemically confirmed and 6-month outcomes.
Results
Thirty-day point prevalence abstinence rates at the 12-month follow-up did not differ between study arms in the primary analysis (13.8% ACT vs. 18.1% CBT, adjusted odds ratio = 0.68 [95% CI = 0.35 to 1.27], p = .23) or the sensitivity analyses.
Conclusions
Group-based ACT and CBT had similar long-term quit rates in this methodologically rigorous randomized trial. Group-based ACT is a reasonable alternative to group-based CBT for smoking cessation.
Implications
This study compared the effectiveness of group-based ACT with group-based CBT for smoking cessation using a rigorous, large-scale, attention-matched, randomized trial with 1-year follow-up. One-year cessation rates did not differ between group-based ACT and CBT, suggesting ACT-based intervention is a reasonable alternative to CBT-based counseling for smoking cessation. The results add to the nascent but growing literature assessing ACT and other mindfulness-based treatments for smoking cessation.
Introduction
Group therapy remains a common format for delivering smoking cessation interventions. It offers a context for learning skills to cope with cravings, avoid relapse, and maintain motivation for quitting as well as offering and receiving support from fellow group members.1 To date, the predominant behavioral approach for group-delivered smoking cessation treatment is rooted in the principles of cognitive behavioral therapy (CBT). CBT-based interventions focus on identifying and altering the attitudes, beliefs, and behaviors that keep people smoking or cause them to lapse or relapse.2 Intervention typically includes helping smokers understand their reasons for smoking and for quitting, problem-solving and coping skills training, and the provision of social support. Smokers are also taught to avoid situations that elicit cravings to smoke; to try to reduce or control the sensations, thoughts, and emotions that trigger their smoking; and to engage in distracting activities when they cannot avoid or control stimuli that put them at risk for smoking.
CBT-based counseling is effective for smoking cessation,2 particularly when paired with pharmacotherapy, and group-based interventions are better than self-help and less intensive interventions.1 But in a recent Cochrane Review, average quit rates associated with group-based treatments ranged from 9% to 20%, with higher rates associated with concomitant use of pharmacotherapy.1 Clearly, more effective group-based behavioral treatment approaches are needed. This includes treatments with greater efficacy than currently available CBT-based interventions, as well as alternative treatment options, even if only equally effective.
Acceptance and commitment therapy (ACT) may hold promise as an alternative treatment for smoking cessation. ACT uses a mindfulness-based approach to teach people to be more accepting of their unwanted thoughts and behavior.3,4 People are also encouraged to commit to actions that are consistent with their personal values and to remain committed to these actions even in the presence of difficult cravings, thoughts, or emotions.4,5 In the context of smoking cessation, smokers are taught to observe, acknowledge, and accept their cravings to smoke, emotions, or thoughts while allowing them to come and go without smoking. People are encouraged to identify what they value about not smoking and use this to motivate their value-based decision to quit. Thus, although both CBT and ACT focus on smokers’ thoughts and behaviors, ACT is conceptually distinct from CBT.
To date, only three trials of group-based ACT for smoking cessation have been published. The first was a promising pilot trial that compared group-delivered ACT without pharmacotherapy to nicotine-replacement therapy (NRT) without counseling among 76 adult smokers.6 When missing values were imputed as smoking, the self-report 24-hour quit rate was 21% for ACT versus 9% for the NRT arm at 1 year (odds ratio [OR] = 2.62 [95% CI = 0.70 to 9.88]). In the second trial, 303 smokers were assigned to bupropion or bupropion plus ACT and functional analytic psychotherapy.7 The 1-year outcomes (biochemically confirmed, 7-day point prevalence abstinence [PPA]) with missing data imputed as smoking) also favored ACT (31.6% vs. 17.5% for bupropion, p < .05), as did continuous abstinence in a complete case analysis (36.6% vs. 17.5%, p = .03). In the third trial, ACT was compared against CBT among 81 smokers; no pharmacotherapy was provided.8 The 1-year quit rate (biochemically confirmed, 30-day PPA) was 30.2% for ACT vs. 13.2% for CBT (p = .02) when missing values were imputed as smokers and 48.1% for ACT and 17.2% for CBT in a complete case analysis (p = .01). However, each of these studies had important limitations including small sample sizes,6,8 use of a quasi-experimental design,8 and lack of a usual-care counseling condition,6,7 and the largest of these studies assessed ACT as a treatment component and not the sole counseling intervention.7 Moreover, none of the studies followed best-practice recommendations to offer all smokers (intervention and control) combined counseling and pharmacotherapy.9
In sum, more rigorous, large-scale, randomized trials of group-delivered ACT are needed, particularly those that compare this intervention to effective usual-care treatments such as CBT. Prior research has compared other forms of mindfulness-based addiction treatments to CBT10 and one recent study compared an ACT-based online cessation program to a CBT-based online intervention,11 but the current study is the first to compare the effectiveness of ACT to CBT when both were delivered as group counseling or paired with NRT and evaluated in a large, methodologically rigorous trial. On the basis of the promising preliminary evidence, we hypothesized that ACT would result in higher smoking abstinence rates than CBT.
Methods
Setting and Participants
All participants were recruited from Kaiser Permanente Washington (KPWA). KPWA is a large, non-profit health care system in Washington State. As a comparative-effectiveness trial, our goal was to compare the ACT intervention against a typical “usual care” CBT program. Therefore, consistent with usual-care group cessation counseling in this health care system, all in-person intervention activities were scheduled at KPWA clinics and NRT was provided through the mail-order pharmacy. Screening, enrollment, and CBT intervention were delivered by staff at the Kaiser Permanente Washington Health Research Institute. Staff from the Fred Hutchinson Cancer Research Center delivered the ACT intervention, collected follow-up surveys and saliva samples for cotinine analysis, and analyzed outcome data. All research activities were approved by the institutional boards of KPWA and Fred Hutchinson Cancer Research Center. The trial is registered at Clinicaltrials.gov (NCT01533974).
Recruitment and Enrollment
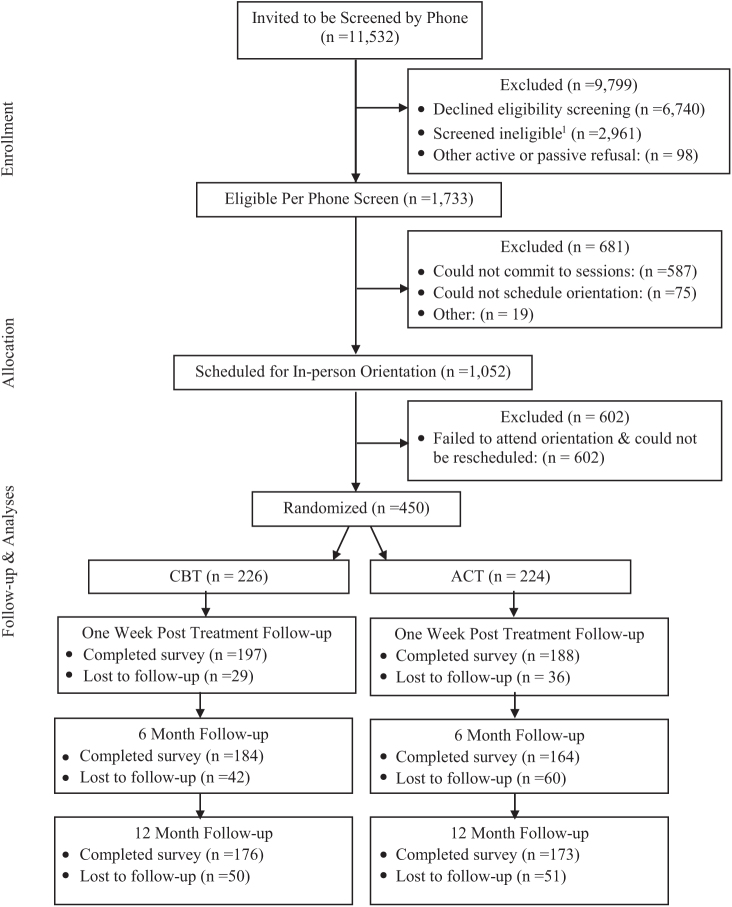
Potential participants were identified via automated medical records and sampled if they were noted as a smoker in the past year, at least 18 years old, and lived or sought care at a KPWA clinic in the Seattle area. Only one person per household was sampled to prevent treatment contamination that could occur from recruiting housemates into different intervention arms. Individuals who met the preliminary screening criteria were mailed an invitation letter and study brochure (n = 11 532; Figure 1). Those not opting out of further contact were called and screened for eligibility. Persons were potentially eligible if they smoked at least 10 cigarettes a day, wanted to quit smoking in the next month, could read and speak in English, were not currently using treatment to quit smoking, had no contraindications for NRT, and had no cognitive or physical impairment precluding participation. Final eligibility required attendance at an in-person orientation session and subsequent agreement to participate in group counseling sessions.
Figure 1.
Study consolidated standards of reporting trials diagram. 1Primary reasons were not smoking enough (n = 1135), unwilling to attend sessions (n = 954), not ready to quit (n = 818), declined NRT (n = 611), allergy to NRT patch (n = 335), all other (n = 1796). Exclusions were not mutually exclusive. NRT = nicotine-replacement therapy.
Four hundred and fifty people met the inclusion criteria and were randomized to receive ACT counseling and nicotine patch (experimental arm) or CBT counseling and nicotine patch (control arm). Primary reasons for exclusion are noted in Figure 1. Randomization was stratified by gender, quit attempts in the past 12 months (yes/no), binge alcohol use (yes/no), positive screen for anxiety or depression symptoms (yes/no), and number of cigarettes per day (less than a pack vs. a pack or more). Participants were informed of their group assignment following randomization and prior to their first in-person session.
Interventions
Nicotine-Replacement Therapy
Each participant was prescribed nicotine patch following the health care system’s standard dosing protocol. Persons smoking more than 10 cigarettes a day were prescribed 21-mg patch × 4 weeks, 14-mg patch × 2 weeks, and 7-mg patch × 2 weeks. Persons smoking less than 10 cigarettes a day were prescribed 14-mg patch × 6 weeks and 7-mg patch × 2 weeks.
Group Counseling Implementation
Participants in each arm were randomly assigned to a five-session, weekly group counseling program. The number and timing of sessions was chosen to match that of the usual-care CBT program offered to Kaiser Permanente patients. Five counseling sessions is also equivalent to standard care offered through most tobacco quitlines and was, therefore, deemed a reasonable program length for real world dissemination. Each session lasted approximately 90 minutes and was led by a master’s level counselor (ie, each had a master’s degree) and training in CBT or ACT, as appropriate to the treatment arm. Separate counselors were used in each arm and were supervised by different licensed clinical psychologists with expertise in either CBT or ACT to prevent contamination. The study counselors were chosen to ensure their training and skill level were comparable to one another and comparable to that of the usual-care counselors conducting smoking cessation groups in the health care system, to increase generalizability of the study results. Treatment in both arms followed a standardized session outline.
CBT Group Counseling
Consistent with relapse prevention theory12 and the Public Health Service Treatment Guideline,9 the CBT intervention followed a standard problem-solving and coping skills training approach. Discussion topics included motivational content (the risks of smoking and benefits of quitting, understanding one’s personal reasons for quitting), behavioral exercises (tracking one’s smoking), and psychoeducational content (how to use the nicotine patch, how to set a quit date). Participants were taught how to manage cravings and nicotine withdrawal symptoms by using the ACE strategies (avoid, cope or escape). For example, smokers were told to avoid environmental stimuli that were likely to trigger cravings, such as hanging out with other smokers, drinking alcohol, or visual cues to smoke (eg, ashtrays, lighters). When avoidance was not possible, participants were taught to cope using strategies such as distraction and deep breathing. Finally, if avoidance and coping were not effective, smokers were advised to escape, or leave, situations that tempted them to smoke. These strategies reflect the basic principles of CBT-based intervention.
Sessions 1 and 2 focused on preparing for one’s quit date and sessions 3 through 5 focused on addressing medication side effects, provision of social support, and relapse prevention. Each participant was encouraged to set their quit date following the second session. Individuals who slipped were encouraged to recommit to quitting smoking and start again, rather than giving up.
ACT Group Counseling
Each ACT session focused on two broad themes: acceptance (acceptance, awareness, and being present) and commitment (values, committed action). Participants learned these concepts through a series of metaphors and experiential exercises led by the counselor. Sample exercises and metaphors are summarized in Table 1. The overarching metaphor of a car journey was used to help participants understand the quit process. It was explained that each participant was the driver of their own car, but the counselor was the front seat passenger, there to help them navigate the journey. Along the way, participants would choose their own destination and path (based on their values) and would learn how to not be distracted by the baggage and passengers in the back seat (ie, unwanted thoughts, emotions, and sensations) that could detour their journey. Each session covered several new ACT exercises targeting processes of acceptance or commitment, and these exercises were practiced as a group with discussion and feedback afterwards. Each session ended with an action plan review during which participants were encouraged to cut back on their smoking, set a quit date, use NRT, and take other committed actions toward quitting and remaining abstinent. Unlike the CBT intervention, and in line with ACT’s emphasis on flexibility, the ACT group did not employ a shared group window for the quit date.
Table 1.
Sample Metaphors and Exercises from ACT Intervention
| Session No. | Topic/exercise | Goal |
| 1 | Why am I Quitting | Understand what really matters and how smoking fits into your life (Values) |
| Action Plan | Set a quit date, cut-back on smoking, and use NRT but be open to “detours” (Commit to Values-based Action) | |
| 2 | Trigger Tracker | Learn to identify and track triggers to smoke (Awareness) |
| Are You Willing? | Learn to notice triggers without responding, so can focus on more important things in life (Acceptance) | |
| Take BREAKS | Learn to stop, notice physical sensations (cravings), and experience them without smoking (Awareness and Acceptance) | |
| Chinese Finger Traps | Using Chinese finger traps as an example, learn the value of willingness and not fighting back against urges to smoke (Acceptance) | |
| Just Noticing | Learn to deep breathe and notice senses, thoughts, and emotions (Awareness and Acceptance) | |
| Holding an Unlit Cigarette | Practice noticing the feelings, thoughts, and emotions associated with holding a cigarette. Learn to let these pass by without action (Awareness and Acceptance) | |
| 3 | Getting Unstuck from Thoughts | Learn to identify maladaptive thoughts (eg, “I need to smoke”) and let them pass by without action (Awareness and Acceptance) |
| Word Exercise | Identify words that trigger desire to smoke (eg, “incompetent”) and repeat them out loud to desensitize participants to their power (Awareness and Acceptance) | |
| I’m Having the Thought That … | Learn to observe triggering thoughts by telling yourself, “I’m having the thought that …” (Awareness and Acceptance) | |
| Thoughts on a Stream | Learn to observe thoughts and let them pass like leaves floating down a stream (Awareness and Acceptance) | |
| 4 | Sky and Weather Exercise | Learn to observe thoughts, emotions, and sensations and let them go without emotion (Awareness and Acceptance) |
| Ocean Exercise | Learn to observe thoughts, emotions, and sensations as waves on the ocean—unrelated to the calm underneath the surface. See self as the ocean and let the waves pass (Awareness and Acceptance) | |
| Awareness | Learn to be an observer of own thoughts (Awareness) | |
| 5 | Letter Exercise | Think about what words of wisdom your future self would share with your current self (Values) |
| Committed Action | Review plans for staying committed to values and action plans for quitting (Commitment to Values-based Action) |
NRT = nicotine-replacement therapy.
Fidelity Monitoring
All counseling sessions were audiotaped and 20% were randomly chosen to be coded for fidelity. Each session was coded using two treatment checklists—each reflecting the key themes and planned content for either the ACT or CBT intervention. Each session was coded by two independent reviewers. Ratings reflected adherence to the planned discussion topics and overall adherence to the principles of each therapeutic approach. By coding each session against the checklist for both interventions, we were able to assess both adherence and contamination.
Assessment
Baseline assessment included participant demographics, current depression,13 generalized anxiety,14 nicotine dependence,15 cigarettes per day, number of quit attempts in the prior year, alcohol use,16 and commitment to quitting. The latter was assessed with the Commitment to Quitting Scale.17 Acceptance of physical, emotional, and cognitive triggers to smoke were assessed with the 27-item adaptation of the Avoidance and Inflexibility Scale.6,18
The primary outcome measure was self-reported 30-day point prevalence abstinence (PPA) at 12 months. Secondary outcomes included biochemically confirmed PPA at 1-year and 30-day PPA (self-reported and biochemically confirmed) at 6 months. Participants who self-reported not smoking at long-term follow-up were mailed a saliva collection kit for cotinine analysis. Saliva cotinine concentrations less than 15 ng/mL were considered indicative of abstinence. Self-reported 7-day PPA was also assessed at end of treatment.
Treatment utilization was assessed as the number of counseling sessions attended, the proportion of participants who attended all five sessions, and whether participants self-reported using the provided nicotine patches. At end of treatment, participants rated their satisfaction with their provided treatment, how useful their assigned group was for quitting smoking, and whether they would recommend their assigned treatment to a friend. The former two items were rated on a 5-point Likert scale from not at all (0) to very much (5).
Three hundred and eighty-five participants completed the 1-week follow-up, 348 completed the 6-month follow-up, and 349 completed the 1-year follow-up (see Figure 1).
Analyses
Descriptive statistics were used to characterize the sample, treatment utilization, and treatment fidelity. Baseline variables were compared by arm using two-sample t-tests for continuous variables and Fisher’s exact test for categorical variables. To quantify treatment differences for the two main outcomes, logistic mixed effects regression models were used to estimate odds ratios. Models adjusted for the five prespecified baseline covariates that were used in stratified randomization, and random effects were included to account for potential therapist and group effects. All analyses used an intent-to-treat approach with participants classified based on their assigned arm, regardless of actual treatment exposure. The primary analyses relied on self-report with missing values imputed as smokers. Although this is common practice in tobacco cessation trials, this use of “penalized imputation” has been criticized because it is sensitive to disproportionate missing data across arms.19 As such, two sensitivity analyses were also conducted examining 1-year cessation outcomes, each using a different approach to handle missing data. The first analysis was limited to participants with observed outcome data (complete case analysis with no imputation) and the second used multiple imputation methods to impute missing outcomes. Ten multiply imputed datasets were created and pooled for analysis using the R package “mi.”20 Secondary analyses also included biochemically confirmed abstinence outcomes. Finally, we conducted post hoc analyses to investigate the role of NRT use, adherence to all counseling sessions, and gender on smoking abstinence (30-day PPA) at 1 year (primary outcome) and our secondary abstinence outcomes (30-day PPA at 6 months and 7-day PPA at 1-week posttreatment follow-up). All outcomes were self-reported cessation with missing values imputed as smoking. To explore these relationships, we compared treatment effects by arm among the subgroup of participants who reported use of NRT (n = 253 at 1 year, n = 257 at 6 months, and n = 296 1 week). Mediation analyses were used to investigate whether attendance at all five group counseling sessions mediated the treatment effects at each follow-up.21 Finally, we assessed whether gender moderated the observed treatment effects at each follow-up. Post hoc analyses were conducted with SAS version 9.4.
Results
Participant Baseline Characteristics
Baseline characteristics are listed in Table 2. Participants (n = 450) were middle aged (mean age = 51.3), 47.3% were male, most were white (82.6%), were employed (72.4%), and had less education than a bachelor’s degree (73.5%). The average nicotine-dependence score (4.9) indicated a moderate level of dependence. Nearly one-third (29.6%) screened positive for current depression, 11.8% screened positive for current anxiety, and 8.2% endorsed binge drinking based on gender-adjusted levels using the AUDIT-C.16
Table 2.
Baseline Characteristics
| CBT N = 226 |
ACT N = 224 |
All N = 450 |
||
| M (SD) | M (SD) | M (SD) | p | |
| Age | 50.8 (12.2) | 51.8 (12.0) | 51.3 (12.1) | .38 |
| Nicotine-dependence scorea | 4.8 (2.0) | 4.9 (2.0) | 4.9 (2.0) | .53 |
| Quit attempts in past year | 2.1 (7.9) | 1.8 (4.3) | 1.9 (6.4) | .72 |
| Commitment to quitting b | ||||
| ACT measure | ||||
| Acceptance of physical triggers | 2.2 (0.5) | 2.1 (0.5) | 2.1 (0.5) | .19 |
| Acceptance of emotional triggers | 2.1 (0.5) | 2.1 (0.5) | 2.1 (0.5) | .31 |
| Acceptance of cognitive triggers | 2.1 (0.5) | 2.1 (0.5) | 2.1 (0.5) | .86 |
| Acceptance mean score | 2.2 (0.4) | 2.1 (0.4) | 2.1 (0.4) | .29 |
| n (%) | n (%) | n (%) | ||
| Female | 119 (52.7) | 118 (52.7) | 237 (52.7) | >.99 |
| Hispanic/Latino | 9 (4.0) | 8 (3.6) | 17 (3.8) | >.99 |
| White | 189 (84.0) n = 225 | 180 (81.1), n = 222 | 369 (82.6), n = 447 | .44 |
| Education | ||||
| HS education or less | 56 (24.8) | 45 (20.1) | 101 (22.4) | .28 |
| Some college or associate’s degree | 109 (48.2) | 120 (53.6) | 229 (50.9) | .30 |
| Bachelor’s degree | 34 (15.0) | 36 (16.1) | 70 (15.6) | .87 |
| Advanced degree | 27 (11.9) | 22 (9.8) | 49 (10.9) | .57 |
| Married | 127 (57.0), n = 223 | 119 (54.6), n = 218 | 246 (55.8), n = 441 | .69 |
| Currently employed | 161 (71.2) | 165 (73.7) | 326 (72.4) | .64 |
| Current depressionc | 73 (32.3) | 60 (26.8) | 133 (29.6) | .24 |
| Current anxietyd | 26 (11.5) | 27 (12.1) | 52 (11.8) | .97 |
| Ever used medication to quit smoking | 75 (33.2) | 76 (34.1), n = 223 | 151 (33.6), n = 449 | .92 |
| Ever used counseling to quit smoking | 168 (74.3) | 155 (69.2) | 323 (71.8) | .27 |
| Binge drinkinge | 17 (7.5) | 20 (8.9) | 37 (8.2) | .71 |
CBT = cognitive behavioral therapy; ACT = acceptance and commitment therapy; M = mean; SD = standard deviation.
aAssessed via Fagerström Test for Nicotine Dependence.
bAssessed via Commitment to Quitting Smoking Score.
cAssessed via Center for Epidemiologic Studies Depression Scale (CESD-20). Participants were characterized as having current depression if their CESD-20 scores were at least 16.
dAssessed via the Generalized Anxiety Disorder 7-item scale (GAD-7). Participants were characterized as having current anxiety if their GAD-7 scores were at least 10.
eAssessed as five or more drinks per day in the past 30 days for males and four or more drinks per day for females.
Indicators of Trial Integrity
Intervention Fidelity and Quality
Both treatment arms scored highly in terms of adherence to their respective treatment protocol (4.9 out of 5 for ACT vs. 4.9 for CBT; p = .97), implementation fidelity (4.7 out of 5 for ACT vs. 4.8 for CBT; p = .42), and group leader facilitation skill (4.7 out of 5 for ACT vs. 4.6 for CBT; p = .48). There was no evidence of treatment contamination between arms. As expected, ACT sessions were rated higher than the CBT sessions for adherence to core ACT processes (3.9 out of 5 for ACT vs. 1.5 for CBT; p = .0001) and rated lower for adherence to core CBT processes (1.2 out of 5 for ACT vs. 4.3 for CBT; p = .0001). Facilitators in both groups had similar ratings of competence for addressing NRT-related issues and medication side effects (3.5 out of 5 for ACT vs. 4.0 CBT, p =.09). The proportion of sessions during which target quit dates were discussed did not differ (12.0% for ACT vs. 34.6% CBT, p =.12), nor did ratings of general discussions about planning to quit smoking (3.3 out of 5 for ACT vs. 3.1 for CBT; p = .64). Overall interrater overall agreement was high (range: .83–1.0).
Intervention Utilization
The average number of sessions attended did not differ (3.5 [SD = 1.4] ACT vs. 3.8 [SD = 1.4] CBT, p = .16), but a higher proportion of CBT participants attended all five sessions (43% vs. 32%, p = .04). ACT participants were less likely to report use of NRT at the end of treatment (67.5% for ACT vs. 84.3% for CBT, p < .001).
Participant Retention
Participant retention rates are included in Figure 1. Eighty-six percent (83.9% ACT vs. 87.2% CBT, p = .04) provided self-report follow-up data at the 1-week (end of treatment) follow-up; 77.3% at 6 months (73.2% ACT vs. 81.4%, p = .03), and 77.6% at 12 months (77.2% vs. 77.8%, p = .68). Saliva collection for biochemical confirmation at 1 year trended lower among ACT participants than CBT participants (61.9% vs. 80.2%, p = .09). Return of cotinine collection kits did not significantly differ at 6 months (73% CBT vs. 82% ACT, p = .35).
Acceptance of Cravings
Both arms had a similar level of increase, from baseline to end of treatment, in acceptance of cravings to smoke (0.30 for ACT vs. 0.30 for CBT; p = .787). Each one-unit increase in the acceptance of cravings, from baseline to end of treatment, was strongly associated with self-reported 30-day PPA rates at the 12-month follow-up (OR = 2.17 [1.41 to 3.34]; p = .0004).
Satisfaction
Most participants endorsed being at least somewhat satisfied with their assigned counseling (92.4% for ACT vs. 95.3% for CBT; p = .33), but fewer people in the ACT arm found their intervention useful for quitting smoking (88.0% ACT vs. 94.3% CBT; p = .04).
Abstinence
Self-reported 30-day PPA rates did not differ between study arms at 1 year (13.8% ACT vs. 18.1% CBT, adjusted OR = 0.68 [0.35 to 1.27], p = .23; Table 3).
Table 3.
Smoking Cessation Outcomes at 12-Month Follow-up
| Outcome variable | Overall N = 450 n (%) |
ACT N = 224 n (%) |
CBT N = 226 n (%) |
Adjusted OR (95% CI)a |
p |
| 30-day PPA, missing = smokingb | 72 (16.0) | 31 (13.8) | 41 (18.1) | 0.67 (0.32 to 1.39) | .28 |
| 30-day PPA, complete casec | 72 (20.6) | 31 (17.9) | 41 (23.7) | 0.64 (0.28 to 1.49) | .31 |
| 30-day PPA, multiple imputation | 99 (22.0) | 42 (18.75) | 57 (25.2) | 0.63 (0.28 to 1.38) | .24 |
| 7-day PPA, missing = smoking | 89 (19.8) | 40 (17.9) | 49 (21.7) | 0.77 (0.44 to 1.37) | .38 |
| 7-day PPA, complete casec | 89 (25.5) | 40 (23.1) | 49 (27.8) | 0.78 (0.42 to 1.46) | .44 |
ACT = acceptance and commitment therapy; CBT = cognitive behavioral therapy; OR= odds ratio; PPA = point prevalence abstinence
aOdds ratios are adjusted for the five factors used in stratified randomization: stratified by gender, quit attempts in the past 12 months (yes/no), binge alcohol use (yes/no), positive screen for anxiety or depression symptoms (yes/no), and number of cigarettes per day (less than a pack vs. a pack or more).
bMissing equals smoking was specified a priori as the primary outcome as recommended by the Russell Standard.
cComplete case analyses included n = 173 ACT and n = 176 CBT participants.
Abstinence results were similar at 12 months in the secondary complete case and multiple imputation sensitivity analyses. Biochemically confirmed 30-day PPA rates with missing values imputed as smokers were lower in the ACT arm (6.3% for ACT vs. 12.4% for CBT, adjusted OR = 0.46 [0.21 to 0.99], p = .05), reflecting a differential rate of return of saliva collection kits at 1 year.
At 6-month follow-up, self-reported 30-day PPA rates were lower in the ACT arm (14.7% for ACT vs. 23.0% for CBT, adjusted OR = 0.56 [0.34 to 0.91], p = .02), but biochemically confirmed PPA rates were not significantly different (9.4% for ACT vs. 13.7% for CBT, adjusted OR = 0.61 [0.34 to 1.11], p = .10).
At 1-week posttreatment follow-up, self-reported 7-day PPA rates were lower in the ACT arm (27.7% for ACT vs. 44.2% for CBT, adjusted OR = 0.44 [0.30 to 0.67], p = .0001).
Post Hoc Analyses
Given the differences in NRT use between treatment arms, we compared self-reported quit rates between ACT (n = 129) and CBT (n = 167) participants who used NRT during treatment, to see whether group differences still emerged when everyone used pharmacotherapy. CBT participants who used NRT self-reported higher 7-day PPA at 1 week (50% vs. 39%, adjusted OR = 0.55 [0.34 to 0.90], p = .02), but no significant differences were observed at 6 or 12 months (data not shown). Similarly, we did not find that attending all five counseling sessions mediated treatment effects on abstinence (data not shown). Finally, there was no evidence that gender moderated the abstinence effects at any follow-up time point.
Discussion
The current trial is the first to compare the effectiveness of ACT group counseling to CBT group counseling for smoking cessation when both were paired with NRT and tested in a large, methodologically rigorous, randomized controlled trial. Despite early outcomes favoring CBT, the results did not show a statistically significant difference in cessation between ACT and CBT at 1-year follow-up. This finding was unchanged in sensitivity analyses that used alternative methods to account for missing data.
The results are consistent with two recent large trials in which mindfulness-based smoking cessation programs were compared to CBT. The first of these compared online ACT versus CBT intervention (Smokefree.gov) and found them to be not significantly different in their effectiveness at 1 year (self-reported 30-day PPA: 24% ACT vs. 26% CBT, p = .33).11 The second compared group-delivered mindfulness counseling (mindfulness-based addiction treatment [MBAT]) to group CBT for smoking cessation and found no difference in biochemically confirmed 7-day PPA at 26 week follow-up when analyzed with missing values imputed as smokers and in a complete-case analysis (13.0% MBAT vs. 15% CBT, p = .43).10 MBAT and ACT are different in many ways, but they share the philosophy that by noticing emotions, cognitions, perceptions, and sensations in a nonjudgmental manner, individuals can learn that these phenomena are transient and will pass without smoking.22 Thus, the emerging evidence suggests that mindfulness interventions, including ACT, may be comparable to CBT for smoking cessation. This is an important finding and could offer smokers who have tried and failed traditional CBT interventions an alternative treatment option.
The significance of offering smokers more empirically validated treatment options should not be underestimated. Knowing that new treatments exist could encourage smokers to make quit attempt, particularly if they have not been successful with CBT-based programs. This is important because one’s chances of successfully quitting increases with each quit attempt.
Because treatment intensity is associated with cessation23 and NRT use enhances quit rates, particularly when paired with counseling,23,24 both of these factors could have contributed to the higher initial quit rates observed in the CBT group. However, we note that attendance of all five counseling sessions (yes/no) did not mediate group differences and any treatment advantage provided by NRT use was dissipated after the 1-week follow-up. Thus, we cannot attribute the more favorable early outcomes in the CBT group to these factors alone. Nevertheless, adherence is an important factor in treatment outcome and it is, therefore, important to understand why there were differences in adherence to the counseling and pharmacotherapy between study arms. We believe this may have been due to a couple of factors. First, the CBT intervention was more directive and explicitly instructed people to set a quit date and use their NRT. In contrast, our ACT protocol8 allowed participants more flexibility to decide how to quit smoking. Participants had the option to gradually reduce their smoking by one-third each week or set a specific quit date. They also had the option of quitting “cold turkey.” This approach is consistent with the foundational ACT principle that people should direct their own committed action. Moreover, the core philosophy of ACT is to teach people how to be willing to experience uncomfortable physical sensations, such as nicotine-withdrawal symptoms. Thus, some ACT participants may have considered use of NRT counter to this philosophy, because nicotine replacement is intended to lessen the uncomfortable effects of withdrawal. These factors may help explain why more CBT participants attended all of the counseling sessions and used NRT, but does not explain why ACT and CBT outcomes were not significantly different at 1 year.
Future Research
Future research should seek to replicate the findings from this trial. In addition, it will be important to explore ways to enhance the effectiveness of ACT-based treatments. One question to be answered is whether there are specific groups for whom ACT may be more effective than CBT, so these individuals can be targeted for treatment. In a prior pilot study, we found smokers who had lower levels of acceptance (ie, greater avoidance) of internal smoking cues had significantly greater quit rates after receiving ACT-based treatment compared to CBT-based intervention.25 Although we did not replicate this finding in the current study (data not shown), we did find a strong association between 1-year abstinence rates and each one-unit increase in participants’ acceptance of cravings from baseline to end of treatment. This suggests that learning to accept cravings is critical to long-term abstinence. Future research should explore how to strengthen smokers’ acceptance of cravings early in the treatment process. In addition, future research may need to explore ways to maximize treatment adherence among ACT participants, particularly regarding use of pharmacotherapy.
Limitations and Strengths
The trial had important methodological limitations. First, only 25.9% of those eligible based on an initial phone screening were randomized into the trial. The main reason for not being included was a lack of interest in or inability to attend in-person sessions (72.9% of those excluded). Thus, the results may not generalize to smokers who are not interested in group-based treatment programs. Similarly, the sample was not racially diverse and was limited to smokers with medical insurance. So, we cannot comment on the extent to which our findings might generalize to minorities or those without public or private health insurance. Next, the differential response rate between study arms at 6 months may have biased the results in favor of the CBT arm because missing values were imputed as smokers, but attrition rates were equivalent at end of treatment and 1 year, so there does not appear to have been a systematic attrition bias, and 1-year equivalence provides greater confidence in the long-term results. This confidence is further supported by the fact that similar results were observed in the primary and sensitivity analyses, each of which used different methods to account for missing data. Another limitation is the reliance on self-reported abstinence outcomes as the primary outcome at 1 year instead of biochemically confirmed rates.26 Although biochemical confirmation was collected, the lower participation rate among ACT nonsmokers (61.9% vs. 80.2%) calls into question the validity of the biochemically confirmed 1-year abstinence rates.
The current study has several notable methodological strengths. These include the large sample, randomized design, attention-matched counseling protocol, and inclusion of pharmacotherapy. In addition, the counseling was delivered by separate counselors in each arm to prevent contamination. Adherence to both counseling protocols was high. The study was also conducted under “real world” conditions within a health care system. That is, all participants had medical insurance, treatment groups met within their local clinics, NRT was dispensed through the health plan pharmacy, and the counselors were chosen to match the skill/training level of counselors, leading the usual-care group therapy sessions within the health plan. All of this increases the generalizability of the results to other health care settings where group counseling might be offered.
Conclusion
In sum, this study is the first to compare the effectiveness of ACT-based group counseling to CBT-based group counseling for smoking cessation using a rigorous, large-scale, attention-matched, randomized trial. Similar to other recent trials comparing mindfulness-based smoking cessation interventions to CBT, we found no significant difference in long-term cessation rates between those receiving ACT group counseling compared to CBT group counseling. The results add to the nascent evidence base suggesting ACT may be a viable alternative to CBT for smoking cessation. Future research should explore how the effects of this therapeutic approach can be maximized to better help smokers achieve abstinence.
Funding
This study was funded by the National Cancer Institute (R01CA151251 to JB, PI). At the time of this study, Kaiser Permanente Washington was known as Group Health Cooperative.
Acknowledgments
We thank the people who worked on this study at Kaiser Permanente Washington Health Research Institute (KPWHRI) and Fred Hutchinson Cancer Research Center (FHCRC), including Jackie St. John, Karen Riggs, Joe Webster, Mary Shea, Amy Mohelnitzky, June BlueSpruce, Janice Bayley Renderos, Katrina Akioka, Madelon Bolling, Wade Copeland, Jessica Harris, Emily Whitish, and the KPWHRI Survey Research Program. We are also grateful to KPWA and the KPWA pharmacy for providing NRT for this trial and to Bryan Comstock, for his assistance with the initial analytic plan and randomization scheme. Additional thanks to the members of the Data and Safety Monitoring Board (Sean David, Gary Swan, and Brian LeRoux) and our study consultants (Steven Hayes and Janice Blalock). Finally, we thank all of the volunteers who participated in this trial.
Declaration of Interests
In July 2016, JB was a consultant to GlaxoSmithKline, the makers of a nicotine-replacement therapy. He now serves on the Scientific Advisory Board of Chrono Therapeutics, the makers of a nicotine-replacement therapy device. JLH has also received support from Pfizer. No other authors have conflicts to disclose.
References
- 1. Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev. 2017;3:CD001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perkins KA, Conklin CA, Levine MD.. Cognitive-Behavioral Therapy for Smoking Cessation: A Practical Guidebook to the Most Effective Treatments. New York, NY: Routledge; 2008. [Google Scholar]
- 3. Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1–25. [DOI] [PubMed] [Google Scholar]
- 4. Hayes SC, Strosahl KD, Wilson KG.. Acceptance and Commitment Therapy: The Process and Practice of Mindful Change. New York, NY: The Guilford Press; 2012. [Google Scholar]
- 5. Dindo L, Van Liew JR, Arch JJ. Acceptance and commitment therapy: a transdiagnostic behavioral intervention for mental health and medical conditions. Neurotherapeutics. 2017;14(3):546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gifford E, Kohlenberg B, Hayes S, et al. Acceptance-based treatment for smoking cessation. Behav Ther. 2004;35(4):689–705. [Google Scholar]
- 7. Gifford EV, Kohlenberg BS, Hayes SC, et al. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behav Ther. 2011;42(4):700–715. [DOI] [PubMed] [Google Scholar]
- 8. Hernandez-Lopez M, Luciano MC, Bricker JB, Roales-Nieto JG, Montesinos F. Acceptance and commitment therapy for smoking cessation: a preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychol of Addict Behav. 2009;23(4):723–730. [DOI] [PubMed] [Google Scholar]
- 9. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 10. Vidrine JI, Spears CA, Heppner WL, et al. Efficacy of mindfulness-based addiction treatment (MBAT) for smoking cessation and lapse recovery: a randomized clinical trial. J Consult Clin Psychol. 2016;84(9):824–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bricker JB, Mull KE, McClure JB, Watson NL, Heffner JL. Improving quit rates of web-delivered interventions for smoking cessation: full-scale randomized trial of WebQuit.org versus Smokefree.gov. Addiction. 2018;113(5):914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marlatt GA, Gordon JR.. Relapse Prevention: Maintenance and Strategies in the Treatment of Addictive Behaviors. New York, NY: The Guilford Press; 1985. [Google Scholar]
- 13. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Apply Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 14. Spitzer RL, Kroenke K, Williams JBW, Lowe B. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166(10) 1092–1097. [DOI] [PubMed] [Google Scholar]
- 15. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 16. Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–1217. [DOI] [PubMed] [Google Scholar]
- 17. Kahler CW, Lachance HR, Strong DR, Ramsey SE, Monti PM, Brown RA. The commitment to quitting smoking scale: initial validation in a smoking cessation trial for heavy social drinkers. Addict Behav. 2007;32(10):2420–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bricker J, Wyszynski C, Comstock B, Heffner JL. Pilot randomized controlled trial of web-based acceptance and commitment therapy for smoking cessation. Nicotine Tob Res. 2013;15(10):1756–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson DB, Partin MR, Fu SS, Joseph AM, An LC. Why assigning ongoing tobacco use is not necessarily a conservative approach to handling missing tobacco cessation outcomes. Nicotine Tob Res. 2009;11(1):77–83. [DOI] [PubMed] [Google Scholar]
- 20.Su YS, Gelman A, Hill J, Yajima M. Multiple imputation with diagnostics (mi) in R: Opening windows into the black box. J Stat Softw. 2011;45(2). https://www.jstatsoft.org/article/view/v045i02 . Accessed January 2, 2019. [Google Scholar]
- 21. VanderWeele TJ. A unification of mediation and interaction: a four-way decomposition. Epidemiology. 2014;25(5):749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heppner W, Spears CA, Vidrine J, Wetter DW. Mindfulness and emotion regulation. In: Ostafin B, Robinson MB, Meier B, eds. Handbook of Mindfulness and Self-regulation. New York, NY: Springer; 2015:107–120. [Google Scholar]
- 23. Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3:CD008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. [DOI] [PubMed] [Google Scholar]
- 25. Bricker JB, Bush T, Zbikowski SM, Mercer LD, Heffner JL. Randomized trial of telephone-delivered acceptance and commitment therapy versus cognitive behavioral therapy for smoking cessation: a pilot study. Nicotine Tob Res. 2014;16(11):1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. [DOI] [PubMed] [Google Scholar]