Abstract
Introduction
Existing treatments can aid tobacco smoking cessation, but they have low efficacy. Because there is a network of neural systems involved in tobacco addiction, combination treatments may provide greater efficacy. Chronic nicotine and amitifadine have each been shown to significantly reduce nicotine self-administration in rats. This study was conducted to determine if the combination of chronic nicotine with amitifadine, a triple monoamine reuptake inhibitor with CYP2B inhibitory effects, would reduce nicotine self-administration to a greater extent than either alone or placebo.
Methods
This study tested the combination of nicotine plus amitifadine in young adult female Sprague-Dawley rats self-administering nicotine (0.03 mg/kg/infusion). This combination was compared with each treatment alone and the vehicle during continuing nicotine self-administration as well as during resumption of self-administration after a week of enforced abstinence, modeling a quit attempt. Finally, we studied the residual effects of these therapies after discontinuation of treatment.
Results
Treatment with either chronic nicotine or amitifadine alone significantly reduced nicotine self-administration relative to controls. The combination of the treatments significantly enhanced this effect. After treatment withdrawal, all of the groups showed increases in nicotine self-administration, but only the combined treatment group remained significantly below control rates of nicotine self-administration.
Conclusions
This study showed the promise of amitifadine as a possible new treatment for smoking cessation and suggested that amitifadine is more effective when given with chronic nicotine. The improved efficacy of the amitifadine and nicotine combination may be potentiated by amitifadine’s inhibitory effects on CYP2B, which slows nicotine metabolism.
Implications
This study replicated the effects that chronic nicotine or chronic amitifadine, a triple reuptake inhibitor, significantly reduces nicotine self-administration in rats. It extends those findings by showing that the combination of chronic nicotine plus amitifadine causes significantly greater reduction in nicotine self-administration than either drug treatment alone. The combination of chronic amitifadine and chronic nicotine also causes a persistent significant reduction in nicotine self-administration after the end of treatment. The amitifadine and nicotine treatment should be assessed in humans to determine whether this combination provides greater efficacy in smoking cessation than transdermal nicotine treatment alone.
Introduction
Pharmacological treatments for smoking cessation started with nicotine replacement and later expanded to bupropion and varenicline1–3 Each aids smoking cessation, but abstinence rates remain low. At present, other treatments that affect nonnicotinic neural systems have shown promise in preclinical studies including drugs that target serotonergic,4 dopaminergic,5,6 histaminergic,7,8 and glutamatergic systems.9,10 Because the neural circuits key for the reinforcing effects of nicotine include a variety of different receptors, combination treatments acting on more than one system may be more effective. Previously, it was shown that a combination therapy is more effective than individual drugs in reducing alcohol intake.11 Because more than one neurotransmitter system is involved in tobacco addiction, then a therapeutic approach that targets more than one system may be more effective in reducing nicotine intake than one drug addressing a single system.
Treatments such as the D1 antagonist SCH-23390, the serotonin 5HT2C agonist lorcaserin, and the H1 antagonist pyrilamine all had their efficacy boosted by coadministration with chronic nicotine infusion.5,8 Single drugs affecting multiple transmitter systems have also been shown to effectively reduce nicotine self-administration. For example, dextromethorphan, which is an antagonist at N-methyl-D-aspartate glutamate receptors and α3β4 nicotinic acetylcholine receptors, an agonist at sigma-1 receptors and a serotonin reuptake inhibitor, effectively reduces nicotine self-administration.9
Amitifadine has actions on multiple neurotransmitter systems, which has been shown to effectively reduce nicotine self-administration. Amitifadine inhibits presynaptic reuptake of dopamine, serotonin, and norepinephrine,12 without detected effects on nicotinic receptors.13 Amitifadine has antidepressant-like effects in a rat model14 and has been shown in a clinical study to have antidepressant effects.15 Amitifadine may be effective in treating nicotine addiction as well, as blocking reuptake of monoamines could reduce the acute reinforcing effects of nicotine and could promote sustained abstinence by reducing negative effect, which is modulated by monoamines and which have been linked to smoking relapse.16 We showed that amitifadine given either acutely or chronically significantly reduces nicotine self-administration in rats.13 Amitifadine also significantly reduces ethanol consumption in rats14 and reduces binge drinking and impulsivity in mice17
On the basis of our previous findings,13 we hypothesized that the combination of nicotine infusion plus amitifadine would provide greater and more persistent reductions in nicotine self-administration than amitifadine alone. Chronic nicotine infusions would serve to keep nicotinic receptors desensitized blunting the effects of nicotine self-administration, whereas chronic amitifadine as a triple reuptake inhibitor would provide a higher tone of dopamine, serotonin, and norepinephrine blunting the consequence nicotine-induced catecholamine release. To test this hypothesis, we compared the efficacy of amitifadine alone to the efficacy of amitifadine with chronic nicotine to reduce nicotine self-administration in rats. Effectiveness was measured during continuing intravenous nicotine self-administration, resumption after a week of enforced abstinence, as well as during the week after withdrawal of the drug treatments.
Materials and Methods
Subjects
Young adult female Sprague-Dawley rats were used. Animals were individually housed in accordance with standard laboratory conditions. Housing was located in a temperature- and humidity-controlled room adjacent to the testing room to minimize the stress induced by transportation. Animals were singly housed to prevent damage to catheters by cage-mates. All animals were kept on a reverse 12:12 day:night cycle (lights on from 6 pm to 6 am), with behavioral testing occurring during the dark cycle, that is, the “active” phase of their diurnal cycle. All animals were given water ad libitum at all times outside of their 1-hour experimental sessions. Initially ad libitum, all animals were kept on a scheduled diet of standard rat chow, given approximately 30 minutes after the completion of experimental session, once behavioral training commenced. With this scheduled diet, the animals were maintained at approximately 85% of the age-adjusted free-feeding weight for female Sprague-Dawley rats, increasing feeding from 8 to 16 g/day as the rats grew. Animals healthy gained weight throughout the study. These studies were conducted under protocols approved by the institutional animal care and use committee of Duke University and met the requirements of state, federal, and international regulatory bodies.
Drug Preparation
Nicotine bitartrate solutions for intravenous self-administration were prepared bi-weekly in sterilized isotonic solutions. The pH of nicotine solutions was adjusted using NaOH to 7.0. Solutions were then passed through a 0.22-µm Nalgene filter (Nalgene Nunc International, Rochester, NY) to ensure sterilization. Doses of nicotine for self-administration sessions were calculated as a function of the nicotine base weight. All nicotine solutions were kept covered to prevent photodegradation and all solutions were refrigerated between experiments. Amitifadine was dissolved in saline solution and was injected in dose of 10 mg/kg in a volume of 1 ml/kg.
Study Design
The study tested the interactions of chronic nicotine infusions with repeated injections of amitifadine Table 1. Osmotic pumps, filled with nicotine ditartrate or the saline vehicle placed in pockets underneath the animal’s skin, were used in this study to model nicotine patches; both forms of administration exhibit zero-order kinetics. The drug treatments continued for 4 weeks with an additional week of behavioral testing after the end of drug treatment.
Table 1.
The Sequence of Experimental Procedures
| Week | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Pretraining | SA Training |------------------SA testing----------------| | |-----------SA testing-------------| | |||||
| Catheter placement | Abstinence resumption posttreatment | ||||||
| |------------------------------------Nicotine treatment----------------------------------| | |||||||
| |-------------------Amitifadine treatment---------------| | |||||||
A period of forced abstinence from self-administration trials was implemented to model the self-enforced abstinence of people trying to quit smoking. During the hiatus from nicotine reinforcement, rats continued to receive cessation treatments, similar to how an individual would continue to use treatments for a period following cessation of smoking. After 2 weeks of treatments combined with access to nicotine self-administration, there was a week of enforced abstinence during which treatments were discontinued and the rats were not given access to nicotine for self-administration. Following the hiatus from nicotine reinforcement, rats were reintroduced to nicotine self-administration. The last part of the experiment evaluated the nicotine-seeking behavior of rats after discontinuation of treatment.
Behavioral Training
Before surgery and nicotine self-administration trials began, all rats were trained to lever-press in a standard dual-lever experimental chamber. Each chamber was equipped with two levers (one active and one inactive), two cue lights, one located above each lever, a house light above the food container and a tone generator. A computer programmed with MED-PC software managed experimental events and data collection. Initially, rats were trained to respond to a lever to receive a 45 mg food pellet reward under a fixed ratio 1 schedule of reinforcement for operant conditioning. Approximately half the animals were trained to respond to the right lever, and the other half was trained to respond to the left lever. The cue light over the correct lever was illuminated, whereas the cue light over the incorrect lever remained dark.
Once rats met a criterion of at least 50 correct lever responses in three consecutive 1-hour training sessions, they went under catheter surgeries to allow them to receive intravenous nicotine infusions and began nicotine self-administration trials. Nicotine self-administration sessions were conducted in the same dual-lever experimental chambers used for pellet training. Following catheterization, animals began self-administration sessions with nicotine as a reinforcer. During each session, an illuminated light over the active lever indicated the correct lever to press. Responding to the active lever resulted in a 0.5-second tone generation, and a 50-µL infusion of nicotine (0.03 mg/kg/infusion) for less than 1 second. Each infusion was followed by a 1-minute time-out phase, where the cue light was turned off and responses were recorded, but no reinforcement was given for responses. Inactive lever presses were also recorded, but resulted in no infusion deliveries, serving only as a control. Each self-administration session lasted for 1 hour. Five baseline sessions were administered to each rat before test sessions were conducted with repeated injection with amitifadine or saline in combination with chronic nicotine infusion or saline treatment.
Surgery
Each animal went through two surgeries, one for implanting an intravenous catheter for voluntary nicotine self-administration and one for implanting an osmotic pump for passive infusion of either nicotine or saline subcutaneously for 28 consecutive days.
Following completion of training with food reinforcements, animals were anesthetized using 60 mg/kg of ketamine (Fort Dodge Animal Health, Fort Dodge, IA) and 0.15 mg/kg of dexdomitor (Pfizer, New York, NY) given via i.p. injections and a catheter (Strategic Application Inc., Libertyville, IL) was implanted into the jugular vein using aseptic techniques to facilitate nicotine infusions. After anesthetization, a small incision slightly lateral to the frontal midline was made, and the jugular vein was exposed via blunt dissection. The area of jugular vein distal to the desired region was tied off to prevent bleeding, and an incision was made in the vein for catheter insertion. The catheter was inserted until the tip was close to the heart. Once in place, the catheter was sutured to both the vein and deep muscle using silk thread. The remaining portion of catheter was routed subcutaneously around the back, through a skin pocket created by blunt instrument, and threaded through a small incision made between the scapulae. The catheter was then attached to an infusion harness (SAI Infusion Technologies, Libertyville, IL), which could be tethered to the infusion pump during experimental sessions. Polypropylene thread was used to suture surgical wounds and to tether harnesses to each animal. Each rat was administered ketoprofen (5 mg/kg, s.c.) for postoperative pain, a topical anesthetic, bupivacaine, and a topical antibiotic was also applied. All catheters were flushed daily before self-administration sessions with a 0.3-mL solution containing 100 U/mL heparinized saline (Baxter Health Corporation, Deerfield, IL). Postsessions, the nicotine solution remaining in each animal’s harness was removed and replaced with a 0.3-mL sterile lock containing heparinized saline 500 U/mL with 8 mg/mL gentamicin (American Pharmaceutical Partners, Schaumberg, IL). On completion of self-administration sessions, animals were tested for jugular-catheter patency using phenobarbital before sacrifice. Data were only included from animals with verified patent catheters.
After five sessions of nicotine self-administration training, rats underwent another surgery to implant osmotic pumps (Alzet Model 2ML4; Durect Corporation, Palo Alto, CA) to infuse either saline or nicotine (2.5 mg/kg/day) for 28 consecutive days. Nicotine dose was calculated as a function of nicotine base weight. Pumps were implanted subcutaneously into a pocket on the back, created by a blunt instrument through an incision. Rats were anesthetized using ketamine (60 mg/kg) and dexdormitor (0.15 mg/kg) given via intraperitoneal injections. The incision was closed once the pump was implanted using a combination of polypropylene thread and surgical clips, and a topical antibiotic was applied. Each animal was administered ketoprofen (5 mg/kg, s.c.) for potential postoperative pain, and a topical anesthetic, bupivacaine.
Chronic Nicotine Infusion
After five baseline sessions of nicotine self-administration, rats began receiving passively either saline or 2.5 mg/kg/day of nicotine for 28 consecutive days via subcutaneous osmotic pump. The nicotine dose was calculated as a function of the nicotine base weight at the time of the surgery. The dose was chosen to simulate plasma levels of nicotine observed in chronic heavy smokers18 and has been shown to alter cholinergic receptor expression in adult rats.19 Previously, we have found that this dose of chronic nicotine causes significant, but not maximal, reduction of nicotine self-administration in rats.8 After their osmotic pump surgeries, all animals continued with nicotine self-administration sessions.
Amitifadine Injections
Starting after five sessions of baseline training for nicotine self-administration, drug tests began. Nicotine or placebo osmotic pump implantation surgery was performed. Approximately 20 minutes before the initiation of a nicotine self-administration session, rats were injected with the saline vehicle or amitifadine (10 mg/kg, s.c.) during weeks 2–4 of chronic nicotine (or saline) infusion. This dose of amitifadine previously shown to significantly reduce nicotine self-administration in rats.13 Each solution was injected subcutaneously in a volume of 1 ml/kg body weight. Thus, each animal received injections of saline or amitifadine while chronically receiving subcutaneous infusion of nicotine or saline, during self-administration trials, as well as the period during nicotine self-administration abstinence.
After 2 weeks of receiving chronic pump treatment with nicotine or saline and 1 week of receiving injections of amitifadine or saline with nicotine self-administration trials, rats were put on 1-week hiatus from those trials. Although on hiatus, rats received amitifadine or saline according to their group (five injections over the course of the week) as well as having continued nicotine or saline infusions via osmotic pump.
After the enforced abstinence, rats were reintroduced to nicotine self-administration trials for 5 consecutive days while receiving saline or amitifadine subcutaneous. injections 20 minutes before beginning each session.
Once the reinstatement week was completed, the osmotic pumps were removed under general anesthesia using ketamine and dexdormitor as described earlier. Once the pump was removed, the incision was sutured shut using polypropylene thread, topical antibiotics were applied, and ketoprofen was administered for postoperative pain. Rats were given at least 24 hours to recover from this surgery. Rats then returned to complete another 5 consecutive days of nicotine self-administration without receiving any treatment. After completion of the last session, each rat’s catheter was tested for patency using phenobarbital before sacrifice. Data were only included from animals with verified patent catheters.
Data Analysis
Analysis of variance was used to determine statistical significance of the collected data (nicotine infusions per session). Drug treatment was a between-subjects factor for chronic nicotine and amitifadine injections treatments. Planned comparisons were made between-subject groups. As recommended by Snedecor and Cochran, interactions of p values of less than .10 were followed up tests of the simple main effects.20 An alpha level of p value of less than .05 (two-tailed) was used as a cutoff for statistical significance.
Results
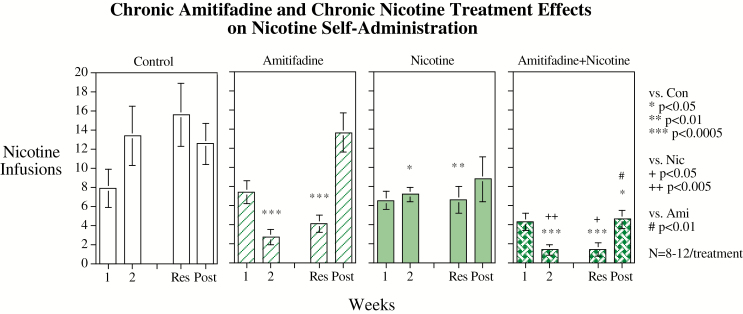
Chronic subcutaneous infusions of nicotine via osmotic pump (2.5 mg/kg/day) significantly decreased nicotine self-administration relative to controls (F (1,37) = 8.34, p < .01). There was also a significant main effect of amitifadine reducing nicotine self-administration relative to controls (F (1,37) = 9.62, p < .005). The main effect of week of testing was significant (F (3,111) = 13.27, p < .0005). The two-way interactions of nicotine × week (F (3,111) = 3.89, p < 0.025) and amitifadine × week (F(3,111) = 11.66, p < .0005) were also significant. Finally, there was a significant three-way interaction of nicotine × amitifadine × week (F (3,111) = 4.92, p < .005). As the highest order significant interaction, this was followed up by tests of the simple main effects of each treatment at each week of testing (Figure 1).
Figure 1.
Repeated amitifadine subcutaneous injections interacting with chronic subcutaneous nicotine infusion, weekly means. Data represent mean ± SEM, N = 8–12 per treatment. “1” and “2” refer to the first and second weeks of treatment. “Res” refers to the week of resumed access to nicotine for self-administration after the week of enforced abstinence. “Post” refers to the week of continued nicotine self-administration after the end of therapeutic treatment.
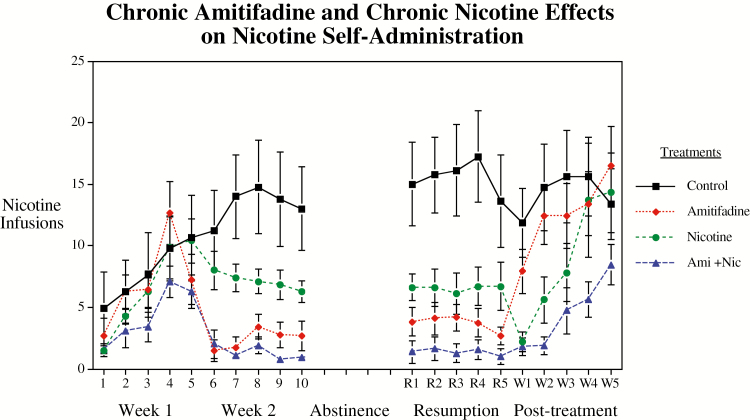
During the first week of treatment with nicotine infusion before amitifadine was added, there were no significant effects on nicotine self-administration. During the second week of treatment with chronic nicotine infusion alone a significant (p < .05) decrease in nicotine self-administration relative to controls was observed. Amitifadine alone caused a significant (p < .0005) decrease in nicotine self-administration relative to control. These treatments attenuated the increase in nicotine self-administration seen with continued access in the vehicle-treated control group. The combination of nicotine and amitifadine caused a significant decrease in nicotine self-administration relative to control (p < .0005) and nicotine alone (p < .01), but not amitifadine alone. A similar array of effects was seen during the week after enforced abstinence with nicotine alone (p < .01), amitifadine alone (p < 0.0005), and nicotine and amitifadine (p < .0005) all significantly lowering nicotine self-administration relative to controls. As seen, before the week of enforced abstinence, the combination of nicotine and amitifadine significantly lowered nicotine self-administration relative to nicotine alone (p < .05) but not relative to amitifadine alone. During the week after the end of nicotine infusion and amitifadine treatment, all groups increased their nicotine self-administration but only the nicotine and amitifadine treatment group maintained a nicotine self-administration rate significantly (p < .025) lower than controls. The combined treatment group had significantly lower nicotine self-administration than the group that had formerly received amitifadine alone (p < 0.01) but not the group that had previously received nicotine alone. Daily session data that composed the weekly scores are shown in Figure 2.
Figure 2.
Repeated amitifadine subcutaneous injections interacting with chronic subcutaneous nicotine infusion daily sessions. Data represent mean ± SEM, N = 8–12 per treatment.
Discussion
The results of this study provide promising insight into the combination pharmacotherapy for the treatment of nicotine addiction. This study replicated prior findings8,13 that the individual treatments of chronic amitifadine or chronic nicotine therapy significantly reduced nicotine self-administration when compared to vehicle treated controls. Importantly, this study showed that the combination treatment of amitifadine plus nicotine caused more long-lasting significant decreases in nicotine self-administration compared with amitifadine alone.
Previously, we have seen that chronic nicotine co-treatment enhances and prolongs the efficacy of another drug therapy to combat nicotine self-administration. We found that chronic nicotine infusion that mimics the continuous zero-order kinetics of the nicotine skin patch significantly potentiated and prolonged the efficacy of the serotonin 5HT2c agonist lorcaserin for reducing self-administration of nicotine.5
In addition to the reduction in withdrawal effects mediated by triple reuptake inhibition, a plausible explanation for the potentiation of the effects observed with nicotine and amitifadine relates to the metabolism of nicotine in the rat and amitifadine properties on cytochrome CYP2B. It is known that amitifadine is a reversible human CYP2B6 inhibitor with a Ki of 1.8 µM.12 In humans, CYP2B6 plays a minor role in the metabolism of nicotine21; however, in the rat, CYP2B1, which corresponds to human CYP2B6, plays the major metabolic role in the production of cotinine from nicotine.22 A recent study demonstrated the correlation between rat CYP2B inhibition and increases in brain nicotine levels23 mimicking the effects of nicotine slow metabolism. Nicotine slow metabolizers have fewer withdrawal effects than normal nicotine metabolizers and are highly responsive to nicotine replacement therapy.24
Future studies could provide insight into the extent prolongation of nicotine’s kinetic profile plays in the added efficacy seen with the combination of nicotine and amitifadine. In addition, it would be useful to know the efficacy of this combination for reducing nicotine self-administration, which has been established for longer periods of time.
Very important, with regard to possible therapeutic efficacy of the nicotine and amitifadine treatment was the persistent effect of the combined treatments even after treatment stopped. Drug treatment for smoking cessation has a limited time therapy. It has largely been the case with currently used treatments that efficacy is not maintained after withdrawal of the treatment.25 The combination of chronic amitifadine and chronic nicotine continued to produce significantly reduced nicotine self-administration after withdrawal of the treatment, whereas either drug alone did not. Amitifadine alone was quite effective in reducing nicotine self-administration during the period of its delivery. However, after withdrawal, the animals that had previously received amitifadine quickly recovered to control-level nicotine self-administration. The addition of nicotine to amitifadine also caused substantial reductions in nicotine self-administration during therapy. There was a modest rise in nicotine self-administration, but it remained significantly below control levels of nicotine self-administration. These observations may relate to the reversibility of the effects on CYP2B.
Combination therapies show promise in attenuating nicotine intake even after the end of therapy and should be studied further to explore new and more effective treatment options for smoking cessation. Key to long-term success with drugs aiding smoking cessation is demonstration of the efficacy of the drug therapy and prolonged maintenance of the effect after the end of the period of drug treatment to minimize the risk of relapse.
Funding
This research was supported by the P50 grant DA027840 from National Institute on Drug Abuse.
Declaration of Interests
Anthony McKinney is founder, CEO and shareholder of Ethismos Research, Inc., the IND holder and developer of amitifadine. The other authors have no conflicts of interest.
References
- 1. Krumpe P, Malani N, Adler J, et al. Efficacy of transdermal nicotine as an adjunct for smoking cessation in heavily nicotine addicted smokers. Am. Rev. Resp. Dis. 1989;139:A337. [Google Scholar]
- 2. Jorenby DE, Hays JT, Rigotti NA, et al. ; Varenicline Phase 3 Study Group Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. [DOI] [PubMed] [Google Scholar]
- 3. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985–994. [DOI] [PubMed] [Google Scholar]
- 4. Levin ED, Johnson J, Slade S, et al. Lorcaserin decreases nicotine self-administration in female rats. J. Pharmacol. Exp. Therap. 2011;338:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DiPalma D, Rezvani AH, Willette B, et al. Persistent attenuation of nicotine self-administration in rats by co-administration of chronic nicotine infusion with the dopamine D1 receptor antagonist SCH-23390 or the serotonin 5-HT2C agonist lorcaserin. Pharmacol Biochem Behav. 2019;176:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall BJ, Slade S, Allenby C, Levin ED. Neuro-anatomic mapping of dopamine D1 receptor involvement in nicotine self-administration in rats. Neuropharmacology. 2015;99:689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levin ED, Slade S, Wells C, et al. Histamine H1 antagonist treatment with pyrilamine reduces nicotine self-administration in rats. Eur. J. Pharmacol. 2011;650:256–260. [DOI] [PubMed] [Google Scholar]
- 8. Levin ED, Hall BJ, Chattopadhyay A, et al. Reduction of nicotine self-administration by chronic nicotine infusion with H1 histamine blockade. Psychopharmacology. 2016;233:3009–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Briggs SA, Wells C, Slade S, et al. Dextromethorphan interactions with serotonergic and histaminergic treatments to reduce nicotine self-administration. Pharmacol. Biochem. Behav. 2016;142:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rezvani AH, Tizabi Y, Slade S, Getachew B, Levin ED. Sub-anesthetic doses of ketamine attenuate nicotine self-administration in rats. Neurosci. Lett. 2018;668:98–102. [DOI] [PubMed] [Google Scholar]
- 11. Rezvani AH, Overstreet DH, Mason GA, et al. Combination pharmacotherapy: a mixture of small doses of naltrexone, fluoxetine, and a thyrotropin-releasing hormone analogue reduces alcohol intake in three strains of alcohol-preferring rats. Alcohol Alcohol. 2000;35(1):76–83. [DOI] [PubMed] [Google Scholar]
- 12. Golembiowska K, Kowalska M, Bymaster FP. Effects of the triple reuptake inhibitor amitifadine on extracellular levels of monoamines in rat brain regions and on locomotor activity. Synapse. 2012;66(5):435–444. [DOI] [PubMed] [Google Scholar]
- 13. Levin ED, Wells C, Johnson JE, Rezvani AH, Bymaster FP, Rose JE. Amitifadine, a triple monoamine reuptake inhibitor, reduces nicotine self-administration in female rats. Eur. J. Pharmacol. 2015;764:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Warnock KT, Yang AR, Yi HS, et al. Amitifadine, a triple monoamine uptake inhibitor, reduces binge drinking and negative affect in an animal model of co-occurring alcoholism and depression symptomatology. Pharmacol Biochem Behav. 2012;103(1):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tran P, Skolnick P, Czobor P, et al. Efficacy and tolerability of the novel triple reuptake inhibitor amitifadine in the treatment of patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Psychiatr Res. 2012;46(1):64–71. [DOI] [PubMed] [Google Scholar]
- 16. Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72(2):192–201. [DOI] [PubMed] [Google Scholar]
- 17. O’Tousa DS, Warnock KT, Matson LM, et al. Triple monoamine uptake inhibitors demonstrate a pharmacologic association between excessive drinking and impulsivity in high-alcohol-preferring (HAP) mice. Addict Biol. 2015;20(2):236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murrin LC, Ferrer JR, Zeng WY, Haley NJ. Nicotine administration to rats: methodological considerations. Life Sci. 1987;40(17):1699–1708. [DOI] [PubMed] [Google Scholar]
- 19. Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851(1-2):9–19. [DOI] [PubMed] [Google Scholar]
- 20. Snedecor GW, Cochran WG.. Statistical Methods. Ames, Iowa: Iowa State University Press; 1967. [Google Scholar]
- 21. Al Koudsi N, Tyndale RF. Hepatic CYP2B6 is altered by genetic, physiologic, and environmental factors but plays little role in nicotine metabolism. Xenobiotica. 2010;40(6):381–392. [DOI] [PubMed] [Google Scholar]
- 22. Hammond DK, Bjercke RJ, Langone JJ, Strobel HW. Metabolism of nicotine by rat liver cytochromes P-450. Assessment utilizing monoclonal antibodies to nicotine and cotinine. Drug Metab Dispos. 1991;19(4):804–808. [PubMed] [Google Scholar]
- 23. Garcia KL, Coen K, Miksys S, Lê AD, Tyndale RF. Effect of brain CYP2B inhibition on brain nicotine levels and nicotine self-administration. Neuropsychopharmacology. 2015;40(8):1910–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kubota T, Nakajima-Taniguchi C, Fukuda T, et al. CYP2A6 polymorphisms are associated with nicotine dependence and influence withdrawal symptoms in smoking cessation. Pharmacogenomics J. 2006;6(2):115–119. [DOI] [PubMed] [Google Scholar]
- 25. Malmlöf K, Golozoubova V, Peschke B, et al. Increase of neuronal histamine in obese rats is associated with decreases in body weight and plasma triglycerides. Obesity (Silver Spring). 2006;14(12):2154–2162. [DOI] [PubMed] [Google Scholar]