Abstract
Introduction
Non-daily intermittent smokers (ITS) comprise 30% of US adult smokers. ITS smoke for nicotine and have trouble quitting, but tend to smoke in particular situations. This study tested the effect of nicotine gum, used to prevent or react to situational temptations, for helping ITS quit.
Methods
ITS (smoking 4–27 days/month) seeking help quitting were randomized to 2 mg nicotine gum (n = 181) or placebo (n = 188), to be used to anticipate or react to temptations to smoke, for 8 weeks. Participants received up to six sessions of behavioral counseling. The primary outcome was 6-month biochemically verified continuous abstinence; analyses also examined 14-day point-prevalence abstinence at multiple time points, and used event-history analyses to assess progression to abstinence, lapsing, and relapsing. Analyses adjusted for group differences in age and baseline smoking, and considered several potential moderators of treatment effects.
Results
Nicotine gum did not significantly improve outcomes on any measure. Biochemically verified 6-month continuous abstinence rates were 7.2% for active gum and 5.3% for placebo (AOR = 1.39, 0.58–3.29, p > .25). ITS with any degree of dependence (Fagerstrom Test of Nicotine Dependence scores >0) showed poorer outcomes on multiple endpoints, and did more poorly on active gum on some outcomes. Gum use was low, starting at 1 gum per day on average and declining over time.
Conclusions
Nicotine gum (2 mg), used intermittently, did not improve cessation rates among ITS, including those demonstrating some degree of dependence.
Implications
Nicotine replacement has been extensively tested with daily smokers, especially those who smoke relatively heavily. Nondaily smoking is now common, creating a need for treatment for ITS. Despite evidence that ITS’ smoking is motivated by nicotine-seeking, a theoretically and empirically derived situational approach to using acute nicotine replacement was not successful at helping ITS quit. Gum use was low; whether higher or more frequent dosing is needed, or whether an entirely different approach is needed, is not clear. Effective treatment options are needed for ITS, especially those with some degree of dependence.
Introduction
Cigarette smoking is typically considered to be maintained by nicotine dependence1,2 wherein smokers need nicotine to maintain homeostasis, and smoke regularly to maintain nicotine levels and avoid nicotine withdrawal.3 Consistent with this model, quitting smoking is very difficult, and, in general population samples, only about 7% of daily smokers (DS) succeed when trying to quit.4 Temporarily providing nicotine via nicotine replacement therapy (NRT) improves quit rates,5 especially in the most dependent smokers,6 confirming the important role of nicotine dependence and withdrawal in hindering smoking cessation.
This account of smoking and cessation is complicated by emerging understanding regarding nondaily or intermittent smokers (ITS), who now comprise approximately one-third of adult smokers in the United States.7 Despite modest cigarette consumption, ITS suffer significant smoking-related mortality.8,9 ITS do not maintain steady-state nicotine levels, by virtue of going days without smoking.10 They do not suffer nicotine withdrawal or increased craving when voluntarily abstaining for periods averaging 5 days,11 and show scant evidence of nicotine dependence by traditional dependence measures.12 Nevertheless, ITS, who attempt quitting more often than DS, have only slightly better success.13 ITS’ difficulty quitting might be related to the strong stimulus control that environmental and internal stimuli exercise over their smoking14; i.e., associations with such cues, rather than nicotine withdrawal, might prompt resumption of smoking.14
A central role for stimulus control in ITS’ smoking and relapse does not necessarily exclude a role for nicotine. ITS absorb nicotine from cigarettes in amounts comparable to DS and show normal nicotine metabolism rates.15 Furthermore, a recent study established that ITS’ smoking is motivated by nicotine-seeking. Compared with ITS provided with cigarettes delivering normal nicotine levels, ITS randomized to very low nicotine cigarettes reduced their cigarette consumption, and sought nicotine from other sources.16 ITS clearly do not seek nicotine to maintain steady-state nicotine levels and ward of nicotine withdrawal, but likely seek nicotine for its acute reinforcing effects, particularly in the presence of relevant cues.15
This account of ITS’ smoking suggests an approach to help them quit smoking: providing nicotine, via NRT, on an acute basis, in situations where cues stimulate craving and might trigger relapse. NRT is usually conceptualized as delivering steady-state levels of nicotine to ward off withdrawal.17,18 The directions even for acute dosing forms of NRT, such as nicotine gum, dictate dosing at regular intervals to maintain nicotine levels.19 However, acute NRT forms can also provide immediate relief in high-craving situations: when strong craving is provoked in abstinent DS by exposure to smoking cues, post-cue use of nicotine gum reduces the associated craving.20,21 This acute craving-relief would be expected to reduce the likelihood of smoking in situations where cues provoke intense craving.22 Thus, we hypothesized that ITS’ quit rates could be improved by strategic use of nicotine gum in situations where cues might trigger smoking. We report a randomized, placebo-controlled, double-blind trial of this strategy among ITS trying to quit smoking.
We considered several potential moderators of a nicotine gum effect. We considered how outcome might be affected by the amount of gum actually used. Randomized studies of DS typically show that using more gum is associated with improved outcomes.5 Additionally, we hypothesized that ITS who had a history of daily smoking,10 and those who were more dependent, as indicated by a nonzero score on the Fagerstrom Test for Nicotine Dependence (FTND),23 might be more responsive to a pharmacological intervention with nicotine. Finally, because African-American smokers have poorer outcomes in smoking cessation24,25 and are also more likely to be ITS,26 we explored whether outcomes might differ for African-American participants.
Methods
Data for this 6-month randomized, controlled, double-blind intervention trial were collected between June 2015 and January 2019, at which time treatment condition was unblinded and analyses begun. The study was approved by the University of Pittsburgh Institutional Review Board, and protocol and analysis plan were pre-registered at ClinicalTrials.gov (NCT02168855).
Upon completion of a 2-week, prequit baseline period of normal smoking, participants were randomized 1:1 to receive either active 2 mg nicotine gum or an inactive, placebo gum. For the following 6 weeks, participants’ gum use and smoking were assessed, and smoking status was further ascertained after 3 and 6 months.
Subjects
Participants were adult ITS (≥18 years old) interested in help quitting smoking, willing to use nicotine gum and to abstain from use of any form of tobacco, recruited from the Pittsburgh area via various methods, who reported smoking non-daily (4–27 days per month, at any quantity) for ≥1 year, and smoking for ≥3 years. Those who had received smoking cessation counseling or used any form of NRT in the previous 2 months were ineligible, as were those with contraindications for NRT (Buerger’s disease; recent heart attack or new heart condition diagnosed); unstable psychiatric status (past-month hospitalization, severe, or unstable mental illness); and women pregnant, breastfeeding, or planning to become pregnant.
Procedures
The active phase of the study lasted 8 weeks: the quit date fell after 2 weeks of baseline smoking, at which time participants were provided with gum. After enrollment, participants attended six sessions (weeks −1, 0, 1, 2, 4, and 6, relative to the quit date) at which smoking was assessed and carbon monoxide (CO) readings were taken. Longer-term follow-up visits occurred at 12 and 24 weeks. Participants were compensated up to $345, based on session attendance, and independent of abstinence status.
Treatment
Gums
Participants were to use their assigned gum for 8 weeks following the designated quit day. The active gum was FDA-approved Zonnic brand 2 mg nicotine mint gum (Niconovum, Inc., Helsingborg, Sweden). No matched placebo was available; the control group received Dentyne Ice Arctic Chill mint gum, which was selected because it provided a persistent “tingle,” as described in the directions for nicotine gum. The gums were not exactly identical in appearance; to maintain blinding, they were dispensed in opaque envelopes, so that neither participants nor counselors could see or compare the gums. At the first dispensing visit participants were given enough gum to account for at least twice their cigarette consumption, plus a margin for a late or missed dispensing visit (median = 30 pieces); at subsequent visits, additional gum was dispensed as needed to maintain frequent use with a margin to avoid any shortfall (total dispensed averaged 8.5 gums/day).
Participants were directed to use the gum as needed when they encountered situations or cues that tempted them to smoke, or in anticipation of such situations, and to use as much gum as they needed. To encourage use, participants first tried the gum in a counseling session, and were provided with a small container to make it convenient to carry gum with them.
Behavioral treatment.
At each of the six visits after enrollment, through week 6 after the quit date, participants were provided with 15–30 min of standardized behavioral counseling, delivered by trained counselors blind to treatment assignment. Counseling emphasized the importance of cues in triggering smoking, and the use of the provided gum to avoid, mitigate, or respond to threats to abstinence.
Assessments
Smoking history and FTND were assessed at enrollment. Self-reported smoking status was assessed at all study visits. At visits during active treatment, participants also completed a time-line follow-back report of cigarettes and gum use on a tablet computer displaying a calendar-formatted entry form for dates going back to the prior assessment.27 Additionally, participants were provided with a mobile phone (BLU Dash-4.0-D270A) programmed to implement an ecological momentary assessment (EMA)28,29 closely following the protocol in Shiffman et al.30 Briefly, participants were to record each episode of smoking (lapse), each episode of temptation to smoke, and each occasion of gum use after the quit date, in real time, and were also provided a daily opportunity to report any entries otherwise missed. At end of their participation, participants were asked to guess whether they had received active or placebo gum.
Expired carbon monoxide (CO) readings (Vitalograph Inc., Lenexa, KS) were taken at all visits, and 3- and 6-month urine samples were sent for cotinine analysis via liquid chromatography-tandem mass spectrometry31 (Clinical Pharmacology Lab, University of California San Francisco) for participants claiming abstinence and meeting CO criteria.
Outcomes and Analyses
Outcomes and analyses were defined prior to the end of data collection and programmed on blinded data. All outcome analyses adjusted for observed baseline differences (age and cigarettes per day) between treatment groups.
Outcomes were assessed and analyzed in three ways.
The primary outcome, analyzed by logistic regression, was 6-month continuous abstinence, with a 2-week “grace period” following the start of treatment.32,33 Participants were abstinent if they self-reported abstinence throughout the period, demonstrated CO levels ≤3 ppm each time they were tested, and demonstrated urinary cotinine levels ≤25 ng/mL when tested at 3 and/or 6 months. For the primary analysis, participants lost to follow-up were considered to be smoking, consistent with the common standard for assessing smoking cessation outcomes. However, sensitivity analyses considered a range of possible odds ratios (1, 2, and 5) linking loss to follow-up and smoking.34
A secondary outcome was 14-day point-prevalence abstinence, verified by CO ≤ 3 ppm, over multiple assessments 2, 4, 6, 12, and 24 weeks post-treatment. (Analysis of ad libitum smoking patterns among ITS11 indicated that 7-day periods of abstinence are relatively common, whereas 14-day periods are not.) These data were analyzed using multi-level generalized linear mixed models (SAS PROC GLIMMIX), allowing for multiple observations per subject of the correlated dependent variable (essentially an “area under the curve” analysis). Parameters included assessment time (linear and quadratic trends), treatment assignment, and their interaction. Missing assessments were not imputed; GLIMMIX allows for incomplete data across time and uses full maximum likelihood estimation. This allows for missing at random (MAR) in which the missingness can be related to model covariates as well as observed values of the dependent variable.35
Finally, during the first 6 weeks of treatment (during EMA data collection), we assessed key cessation milestones proposed by Shiffman et al.36 to separately examine key behavioral milestones in trajectories of smoking cessation success or failure, namely achievement of initial abstinence (7 consecutive days), lapsing (any smoking) after achieving initial abstinence, and relapsing (smoking at ≥50% of baseline levels) following a lapse. (Previous definitions of relapse,37 based on consecutive days smoking, are unsuitable for ITS, who might not meet such criteria even when smoking ad libitum.) Achievement of cessation milestones (abstinence, lapsing, and relapsing) during the primary treatment period of 6 weeks was analyzed via event-history (“survival”) analyses using Cox proportional hazards models. Time to initial abstinence was counted from the first treatment day; time to lapse from the first day of initial abstinence, among those who achieved that milestone; time to relapse from the day of first lapse, among those who lapsed. For subjects who were not observed to reach a milestone (e.g., subjects who did not achieve abstinence by end of study or time of drop out), time was censored as of the last day of observation.
Potential Moderators
Three individual difference variables were hypothesized to potentially moderate the treatment effects: Degree of dependence was expressed as a dichotomy, distinguishing subjects with an FTND score of 0 from those with higher scores. Previous research has indicated that this distinction is behaviorally relevant among ITS.12 Self-reported history of previous daily smoking (for at least 6 months) has been shown to predict ITS’ behavior10 and smoking cessation outcome.13
The amount of gum used influences outcomes in smoking cessation.38,39 Because gum use can change over time, sometimes in response to relapse (creating a reverse-causality relationship with outcome),40 we focused on gum use in the first week of treatment. Whereas DS are directed to use at least 9 pieces per day initially,19 ITS in this study were not given a target number of pieces. Thus, there is no absolute standard against which to assess the amount of gum used; we analyzed gum use by strata based on the observed range of gum use (0 pieces per day, <0.5/day, 0.5–1.0/day, >1/day).
In addition to these hypothesized moderators, differences between African-American and Caucasian participants were explored, both as main effect and as potential moderators of treatment effects.
Results
Supplementary Figure 1 shows subject disposition. A total of 26.9% of enrolled participants were lost prior to randomization; 90.5% of those randomized attended the 6-month follow-up. Table 1 shows the subject characteristics of randomized subjects, who were a mean age of 43.8 years, 43.1% male, and 49.3% from racial and ethnic minorities. On average, randomized participants averaged 1.9 (1.5) cigarettes per day (3.6 cigarettes on 3.6 days per week); 62.7% had an FTND score of 0, and 54.7% had previously been DS. Subject characteristics generally did not differ by treatment group, with the exception that placebo subjects were slightly older and slightly heavier smokers at entry to the study; all outcome analyses adjusted for those differences, and we report the adjusted statistics.
Table 1.
Subject characteristics
| All randomized subjects n = 369 |
Placebo group n = 188 |
Active group n = 181 |
p a | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, mean (SD) | 43.8 (14.5) | 45.4 (15.0) | 42.2 (13.8) | .04 |
| Gender, N (%) male | 159 (43.1) | 86 (45.7) | 73 (40.3) | .29 |
| Race, N (%) | ||||
| White | 187 (50.7) | 93 (49.5) | 94 (51.9) | .32 |
| African-American | 148 (40.1) | 81 (43.1) | 67 (37.0) | |
| Other | 34 (9.2) | 14 (7.5) | 20 (11.1) | |
| Hispanic, N (%) | 20 (5.4) | 9 (4.8) | 11 (6.1) | .58 |
| Education, N (%) | .86 | |||
| High school or less | 88 (23.8) | 45 (24.0) | 43 (23.8) | |
| Some college | 139 (37.7) | 73 (38.8) | 66 (36.4) | |
| Bachelor’s or above | 142 (38.5) | 70 (37.2) | 72 (39.8) | |
| Annual incomeb, $1,000s, mean (SD) | 30.2 (26.8) | 28.4 (26.2) | 32.0 (27.4) | .20 |
| Smoking at enrollment | ||||
| Days smoking/week, mean (SD)c | 3.6 (1.2) | 3.6 (1.2) | 3.6 (1.1) | .95 |
| Number of cigarettes on smoking days, mean (SD)c | 3.6 (2.5) | 3.9 (2.9) | 3.3 (2.0) | .02 |
| Average cigarettes per day across all days (including days at 0), mean (SD)c | 1.9 (1.5) | 1.9 (1.5) | 1.8 (1.5) | .24 |
| Smoke mentholdN (%) | 239 (65.5) | 124 (66.7) | 115 (64.3) | .63 |
| Years smoked, mean (SD) | 21.2 (14.2) | 22.1 (15.2) | 20.3 (13.1) | .23 |
| Past daily smoker, N (%) | 202 (54.7) | 104 (55.3) | 98 (54.1) | .82 |
| Fagerstrom Test for Nicotine Dependence= 0, N (%) | 230 (62.7) | 119 (63.6) | 111 (61.7) | .70 |
Abbreviations: SD = standard deviation; N = number.
aComparison between active and placebo groups. Chi-square test for categorical variables, t-test for continuous variables.
bComputed from categorical reports, using midpoints from 12 categories, from <$5,000 to >$80,000.
cBased on time-line follow-back (TLFB) reporting in the 28 days prior to study enrollment.
dMenthol smoking was reported by 94% of African-American participants; among other ethnicities, this was 43%.
Supplemental Table 1 shows the amount of gum used over the first 6 weeks of treatment. Gum use started at an average of one piece per day, and declined over time, primarily due to decline in number of days gum was used. There were no differences in gum use between active and placebo groups.
Primary Outcome
Continuous abstinence to 6 months
There was no significant effect of active treatment on continuous 6-month abstinence, imputing smoking to those with missing smoking status: 7.2% of active gum participants and 5.3% of inactive gum participants were abstinent (AOR = 1.39, 0.58–3.29, p > .40). Among the moderators, only dependence was itself associated with poorer outcomes (FTND > 0; AOR = 0.24, 0.07–0.84, p < .03). None of the hypothesized moderators significantly moderated the effect of nicotine treatment. Only 4.3% of participants’ 6-month status was missing; sensitivity testing for imputation of these participants’ smoking status did not change the results.
Secondary Outcomes
Point-prevalence abstinence over time
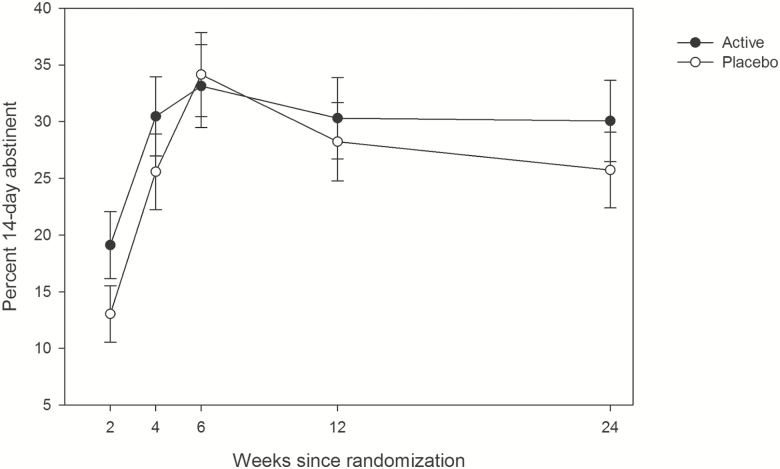
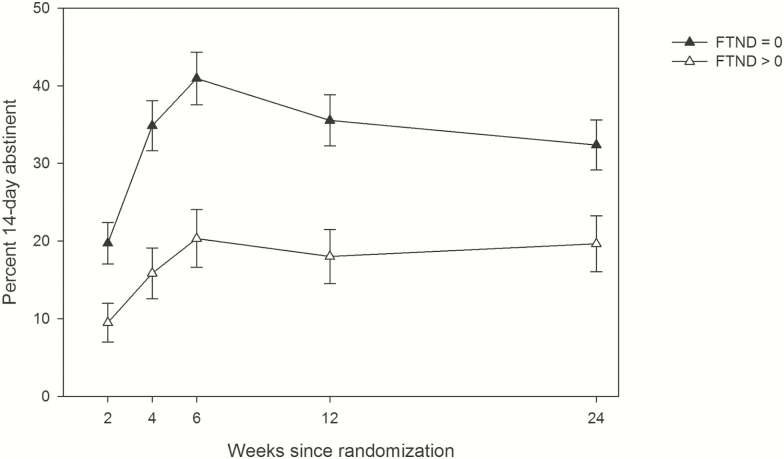
As seen in Figure 1, observed CO-verified 14-day abstinence rates varied over time in a nonlinear way, initially rising over the course of active treatment, and declining thereafter (quadratic curvature, p < .0001). Tests showed no differences by treatment in this multi-timepoint analysis (AOR = 1.12, 0.60–2.08), and dependence again was associated with lower abstinence rates (AOR = 0.23, 0.12–0.45, p < .0001; Figure 2). Furthermore, FTND interacted with treatment, such that those with FTND > 0 had slightly lower abstinence rates on active treatment versus placebo (Supplementary Figure 2).
Figure 1.
Percent of participants achieving CO-verified 14-day abstinence at each time-point by treatment group. Analyses considering all time-points showed no significant treatment effect (P > .70, active vs placebo AOR: 1.12, 0.60–2.08). Error bars represent standard errors.
Figure 2.
Percent of participants achieving 14-day CO-verified abstinence, by FTND score. FTND = Fagerstrom Test of Nicotine Dependence. Analyses considering all time points showed a significant effect (p < .0001, FTND > 0 vs FTND = 0 AOR: 0.23, 0.12–0.45). Error bars represent standard errors.
In this analysis, African-American participants demonstrated consistently poorer outcomes across time than Caucasian participants (AOR = 0.38, 0.19–0.78, p < .01); Supplementary Figure 3.
The amount of gum used in the first week was related to abstinence in a seemingly paradoxical direction: those using more gum had poorer outcomes, regardless of whether it was active gum or placebo (i.e., no interaction with treatment); Supplementary Figure 4. A follow-up analysis provided an explanation: the individuals using more gum were the ones experiencing more frequent temptations (excluding occasions where they smoked; p < .001; Supplementary Figure 5), consistent with their also being more likely have FTND > 0 (53% of those using more than one piece per day, vs. 30% of those using less).
History of daily smoking was unrelated to abstinence and, other than FTND, none of the examined individual differences moderated the treatment effect.
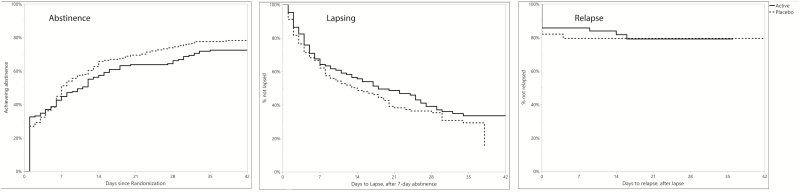
Smoking cessation milestones
Figure 3 shows the Kaplan–Meier survival curves for achievement of 7-day abstinence, progression to a lapse once abstinent, and progression to relapse once lapsed. None of the milestones were significantly affected by treatment. However, dependence (FTND > 0) was associated with a 34% lower chance of achieving initial abstinence (AHR = 0.66, 0.51–0.87) (Supplementary Figure 6). Moreover, there was a significant interaction between dependence and treatment (p < .01), illustrated in Supplementary Figure 7. Among participants with FTND = 0, the active and placebo gum groups were similar (AOR = 1.06, 0.79–1.42), whereas among those with FTND > 0, active treatment was associated with a lower odds of achieving initial abstinence (AOR = 0.48, 0.31–0.75). As seen for overall point-prevalence abstinence, the amount of gum used in the first week was inversely associated with achievement of abstinence, whether it was active gum or placebo: the more gum used, the lower the likelihood of achieving abstinence (Supplementary Figure 8).
Figure 3.
Kaplan–Meier survival curves for achievement of 7-day abstinence, progression to a lapse once abstinent, and progression to relapse once lapsed, by treatment group. Abstinence was defined as achieving 7 days of abstinence, and time is counted from the first day of the 7-day series; the hazard ratio for treatment was AHR = 0.79, 0.62–1.01. Lapsing reflects any smoking after achieving 7-day abstinence, and is time counted from the end of that 7-day abstinence series (AHR = 0.83, 0.61–1.13). Relapse is counted as smoking at 50% or more of participants’ baseline smoking rate, and time is counted from the first lapse (AHR = 1.03, 0.50–2.11).
Neither treatment nor any of the moderators affected the risk of lapsing or relapsing.
Blinding
About half of participants correctly guessed their treatment assignment (Supplementary Table 2); this was more likely among those on active treatment. Correct guessing had no relationship with the primary outcome in either condition, and there was no interaction with condition. (In open-ended statements, participants predominantly attributed their guess about condition to perceived efficacy or perceived subjective effects of their gum.)
Adverse Events
Three well-known side effects of nicotine gum (gastric upset, throat irritation, and nausea)19 were reported more often in the active gum group. Otherwise, event rates were similar by group, and unremarkable (Supplementary Table 3).
Discussion
This randomized controlled trial of nicotine gum for smoking cessation among non-DS did not show any statistically significant effects of treatment, whether assessed by a strict criterion of long-term biochemically verified continuous absolute abstinence or by 14-day abstinence at various time points over a 6-month period. Nor did treatment appear to increase or accelerate achievement of initial abstinence or decrease or slow progression to lapsing and relapse among those who were abstinent.
Dozens of studies have demonstrated the efficacy of nicotine replacement, including nicotine gum, in improving smoking cessation outcomes.5 This has also been demonstrated in relatively light (<10 cigarettes per day) DS.41 One possible explanation for the lack of effect in the current study with an ITS population could be that ITS’ smoking is not primarily motivated by nicotine-seeking, which would undermine the rationale for nicotine replacement. However, evidence demonstrating that switching ITS to very-low-nicotine cigarettes results in reduced smoking (much as it does for DS),42 and caused ITS to seek nicotine in other products,16 suggests that ITS do indeed smoke to get nicotine.
It is possible that the as-needed dosing regimen recommended in this study provided inadequate dosing. However, even when instructed to dose regularly, DS seem to dose according to perceived need,17 and yet are helped by nicotine gum. Furthermore, regular dosing around the clock is inconsistent with how ITS smoke, and would have substantially increased their nicotine exposure, both in amount and in frequency.
Participants in the study used very little gum. They averaged about one piece per day in the first week, and the frequency of use declined thereafter. Although participants were not directed to use a certain minimum number of pieces of gum, they were advised to use gum whenever they might smoke or be tempted to smoke. Participants’ average use of 1 piece of gum or less per day seems likely to be under-dosing, given that participants averaged about 2 cigarettes per day at baseline. With ITS, as with DS, use of inadequate amounts of medication may be a significant barrier to treatment efficacy.
It is possible that a more aggressive, proactive, or regular dosing regimen would have improved outcomes. An ITS cessation trial with higher-dose NRT, including nicotine patch, is being conducted.43 However, evidence suggests that ITS’ smoking is strongly driven by environmental cues,14 making situational use appropriate. Participants’ use of gum may have been too little, too late, to fend off smoking once such cues were experienced, particularly as it takes the currently approved nicotine gum up to 30 min to reach maximum blood nicotine concentrations,44 and 10–15 min to achieve craving relief.21 Thus, using gum reactively may have been less than optimal for these highly cue-driven smokers.
Aside from the absence of significant effects of NRT in this study, the very low long-term continuous abstinence rate seen in both treatment groups is striking. Population data suggest that ITS generally have quit success rates slightly higher than DS, yet even studies with heavy DS have observed higher abstinence rates than observed here.5 It could be that ITS who have enough difficulty quitting that they seek intensive behavioral and pharmacological treatment, which is rare even in DS,45 may be unusually resistant to quitting, perhaps because their smoking is under very strong stimulus control by cues.14
A consistent finding was that ITS with even very slight signs of dependence (FTND > 0) were less successful at quitting. This confirms clinically the conclusions from prior studies, suggesting that such small degrees of dependence are meaningful.12 It may be that the instruction to use gum as needed may have led to under-dosing for these individuals who have a greater need for nicotine. Some analyses suggested that using active gum may even have been associated with poorer outcomes among those with any degree of dependence. Speculatively, it is possible that using nicotine gum could have primed craving in this group, particularly as they were accustomed to intermittent nicotine intake, such that, in combination with inadequate dosing, it actually undermined cessation. One analysis suggested that less-severely-dependent smokers with high cue exposure may not benefit from adding acute NRT to patch.46 An appropriate balance may need to be found between adequate nicotine dosing in abstinence and avoiding dosing that increases ITS’ nicotine intake too far beyond their typical low and intermittent pattern of their smoking. Individualized precision-medicine treatment approaches that take account of the heterogeneity among ITS may yield better outcomes.
It was also notable that participants who used more gum—whether active or placebo—during the first week of treatment were less likely to achieve abstinence. This does not indicate that using gum undermined abstinence: Since participants were advised to use gum when they were tempted to smoke, the ones who used gum frequently were those who were experiencing many temptations. This was confirmed by analysis of real-time reports of temptation episodes (discounting occasions that may have actually led to smoking, which would have introduced some circularity). Thus, the association between gum use and outcome likely represents reverse causation, partly due to confounding by indication.40,47 Furthermore, when participants recorded a temptation, they were asked whether they had used gum, and if not, why not, which may have prompted gum use.
African-American and Caucasian ITS were not differentially affected by nicotine treatment, but African-Americans demonstrated lower point-prevalence abstinence rates. We previous reported48 that African-American ITS were more dependent than Caucasian ITS. It has also been observed that African Americans are more dependent at lower levels of cigarette consumption than Caucasians,49 and may have lower cessation rates.24,25 These factors, as well as others (e.g., socioeconomic status, stress, menthol smoking), warrant exploration as causes of the observed ethnic disparity.
All participants in the study were provided with behavioral counseling focused on avoiding and coping with situational temptations that might lead to lapsing. This was based on the observation14 that ITS’ smoking is highly situation bound. The effect of that behavioral approach was not evaluated in this study. Further research may be needed to evaluate this and other behavioral approaches to helping ITS quit.
The study had several limitations. Foremost was limited sample size. While the study found no significant effect on continuous abstinence (i.e., could not exclude an odds ratio of 1.0), we cannot exclude the possibility of a smaller but clinically meaningful effect: the confidence interval around the estimated AOR reached into the range of effects typically seen for nicotine gum.50 Thus, the study’s limited sample size and power may preclude firm conclusions about the inefficacy of NRT for ITS. The study sample was comprised of volunteers from a single geographic area and may not be nationally representative. Finally, the placebo gum was not perfectly matched to the active gum, though steps were taken to maintain blinding, and correct guessing of treatment assignment had no relation to outcome. Participants also underwent repeated CO testing and were tracking smoking and temptations through EMA, which might have affected their quit rates. The study also had considerable strengths, including well-balanced treatment groups, and the use of multiple measures and analytic strategies to assess efficacy.
In summary, this randomized trial did not find a statistically reliable benefit from treating non-DS with nicotine gum used to react to or anticipate temptations to smoke. Identifying pharmacological and behavioral interventions that can help non-DS quit continues to be important.
Funding
This work was supported by a grant (R01 DA034629) to H.T. and B.P. from the National Institute on Drug Abuse at the National Institutes of Health (NIH). NIH had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Declaration of Interests
SS, through Pinney Associates, consults on tobacco cessation and harm reduction (including NRT and digital vapor products; by contract, combusted cigarettes are excluded) to Niconovum USA, RJ Reynolds Vapor Company, and RAI Services Company, all subsidiaries of Reynolds American, Inc. and British American Tobacco. Previously, S.S. consulted to NJOY on e-cigarettes, and to GlaxoSmithKline Consumer Healthcare on smoking cessation medications and treatments, including nicotine gum. S.S. holds patents for a novel nicotine gum that is not under commercial development. H.A.T. provided input to Achieve Life Sciences regarding study design for a future possible cessation trial of cytisine, and has led or co-led studies using smoking cessation medication donated by the manufacturer (e.g., Pfizer). S.G.F. has worked as a consultant to GlaxoSmithKline Consumer Healthcare and Chrono Therapeutics on matters relating to smoking cessation, has received researcher-initiated project grant funding, and travel funds, from Pfizer, and has served on an advisory board for Johnson & Johnson. Other authors report no competing interests. Niconovum provided medication for this study at no cost, but had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Supplementary Material
Acknowledgments
The authors are grateful to Allison Brown for assistance overseeing the study, to Jessica Manriquez Richard, Jessica Cheng, Christina Warner, and Jacob Burns, research assistants who conducted research sessions; to James Moorehead for data management and preparation; to Alexsys Hoesch for administrative assistance; and to support provided by the National Institute on Drug Abuse (P30 DA012393) and the National Center for Research Resources (S10 RR026437) for laboratory resources at the University of California, San Francisco. We also appreciate the contributions of members of the study Data and Safety Monitoring Board, Peter Callas (University of Vermont), Jonathan Foulds (Pennsylvania State University), and John Hughes (University of Vermont, Chair). Niconovum, makers of Zonnic nicotine gum, kindly provided nicotine gum for the study at no cost.
References
- 1. Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl). 1995;117(1):2–10; discussion 14. [DOI] [PubMed] [Google Scholar]
- 3. Benowitz NL. Clinical pharmacology of nicotine: Implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83(4):531–541. [DOI] [PubMed] [Google Scholar]
- 4. Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults – United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–1464. [DOI] [PubMed] [Google Scholar]
- 5. Stead L, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11(CD000146). [DOI] [PubMed] [Google Scholar]
- 6. Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. BMJ. 1995;311(7001):363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reyes-Guzman CM, Pfeiffer RM, Lubin J, et al. Determinants of light and intermittent smoking in the United States: Results from three pooled national health surveys. Cancer Epidemiol Biomarkers Prev. 2017;26(2):228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inoue-Choi M, Liao LM, Reyes-Guzman C, Hartge P, Caporaso N, Freedman ND. Association of long-term, low-intensity smoking with all-cause and cause-specific mortality in the national institutes of health-AARP diet and health study. JAMA Intern Med. 2017;177(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schane RE, Ling PM, Glantz SA. Health effects of light and intermittent smoking: A review. Circulation. 2010;121(13):1518–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C. Characteristics and smoking patterns of intermittent smokers. Exp Clin Psychopharmacol. 2012;20(4):264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiffman S, Dunbar MS, Tindle HA, Ferguson SG. Nondaily smokers’ experience of craving on days they do not smoke. J Abnorm Psychol. 2015;124(3):648–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiffman S, Ferguson SG, Dunbar MS, Scholl SM. Tobacco dependence among intermittent smokers. Nicotine Tob Res. 2012;14(11):1372–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tindle HA, Shiffman S. Smoking cessation behavior among intermittent smokers versus daily smokers. Am J Public Health. 2011;101(7):e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shiffman S, Dunbar MS, Li X, et al. Smoking patterns and stimulus control in intermittent and daily smokers. PLoS One. 2014;9(3):e89911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiffman S, Dunbar MS, Benowitz NL. A comparison of nicotine biomarkers and smoking patterns in daily and nondaily smokers. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shiffman S, Kurland BF, Scholl SM, Mao JM. Nondaily smokers’ changes in cigarette consumption with very low-nicotine-content cigarettes: A randomized double-blind clinical trial. JAMA Psychiatry. 2018;75(10):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer J Clin. 2005;55(5):281–99; quiz 322. [DOI] [PubMed] [Google Scholar]
- 18. Molyneux A. Nicotine replacement therapy. BMJ. 2004;328(7437): 454–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Food and Drug Administration. Nicorette gum approved labeling. 1998; Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018612s061_020066s042lbl.pdf
- 20. Niaura R, Sayette M, Shiffman S, et al. Comparative efficacy of rapid-release nicotine gum versus nicotine polacrilex gum in relieving smoking cue-provoked craving. Addiction. 2005;100(11):1720–1730. [DOI] [PubMed] [Google Scholar]
- 21. Shiffman S, Shadel WG, Niaura R, et al. Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology (Berl). 2003;166(4):343–350. [DOI] [PubMed] [Google Scholar]
- 22. Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36(3):235–243. [DOI] [PubMed] [Google Scholar]
- 23. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The fagerström test for nicotine dependence: A revision of the fagerström tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 24. Fu SS, Kodl MM, Joseph AM, et al. Racial/ethnic disparities in the use of nicotine replacement therapy and quit ratios in lifetime smokers ages 25 to 44 years. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1640–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kulak JA, Cornelius ME, Fong GT, Giovino GA. Differences in quit attempts and cigarette smoking abstinence between whites and African Americans in the United States: Literature review and results from the international tobacco control US survey. Nicotine Tob Res. 2016;18(Suppl 1):S79–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trinidad DR, Pérez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101(4):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sobell LC, Sobell MB, Maisto SA, eds. Time-line follow-back assessment methods. Washington, D.C.: National Institute on Alcoholism and Drug Abuse DHHS Publications; 1985. [Google Scholar]
- 28. Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21(4):486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Ann of Behav Med. 1994;16(3):199–202. [Google Scholar]
- 30. Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64(2):366–379. [DOI] [PubMed] [Google Scholar]
- 31. Jacob P 3rd, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3’-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: Biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(3-4):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 33. West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: Proposal for a common standard. Addiction. 2005;100(3):299–303. [DOI] [PubMed] [Google Scholar]
- 34. Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: Missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102(10):1564–1573. [DOI] [PubMed] [Google Scholar]
- 35. Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shiffman S, Scharf DM, Shadel WG, et al. Analyzing milestones in smoking cessation: Illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol. 2006;74(2):276–285. [DOI] [PubMed] [Google Scholar]
- 37. Ossip-Klein DJ, Bigelow G, Parker SR, Curry S, Hall S, Kirkland S. Classification and assessment of smoking behavior. Health Psychol. 1986;5(Suppl):3–11. [PubMed] [Google Scholar]
- 38. Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Arch Intern Med. 2002;162(11):1267–1276. [DOI] [PubMed] [Google Scholar]
- 39. Shiffman S, Paty JA, Rohay JM, Di Marino ME, Gitchell J. The efficacy of computer-tailored smoking cessation material as a supplement to nicotine polacrilex gum therapy. Arch Intern Med. 2000;160(11):1675–1681. [DOI] [PubMed] [Google Scholar]
- 40. Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34(2):102–111. [DOI] [PubMed] [Google Scholar]
- 41. Krupski L, Cummings KM, Hyland A, Carlin-Menter S, Toll BA, Mahoney MC. Nicotine replacement therapy distribution to light daily smokers calling a quitline. Nicotine Tob Res. 2013;15(9):1572–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Donny EC, Denlinger RL, Tidey JW, et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nollen NL, Cox LS, Mayo MS, Ellerbeck EF, Madhusudhana S, Ahluwalia JS. A randomized clinical trial of counseling and nicotine replacement therapy for treatment of African American non-daily smokers: Design, accrual, and baseline characteristics. Contemp Clin Trials. 2018;70:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Benowitz NL, Porchet H, Sheiner L, Jacob P 3rd. Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44(1):23–28. [DOI] [PubMed] [Google Scholar]
- 45. Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Individual differences in adoption of treatment for smoking cessation: Demographic and smoking history characteristics. Drug Alcohol Depend. 2008;93(1-2):121–131. [DOI] [PubMed] [Google Scholar]
- 46. Loh WY, Piper ME, Schlam TR, et al. Should all smokers use combination smoking cessation pharmacotherapy? Using novel analytic methods to detect differential treatment effects over 8 weeks of pharmacotherapy. Nicotine Tob Res. 2012;14(2):131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Signorello LB, McLaughlin JK, Lipworth L, Friis S, Sørensen HT, Blot WJ. Confounding by indication in epidemiologic studies of commonly used analgesics. Am J Ther. 2002;9(3):199–205. [DOI] [PubMed] [Google Scholar]
- 48. Cheng J, Shiffman S, King W, Scholl S. Interaction between ethnicity and smoker type with dependence: A comparison of daily and intermittent African American and Caucasian smokers. Psychol Addict Behav. 2018;32(4):410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luo Z, Alvarado GF, Hatsukami DK, Johnson EO, Bierut LJ, Breslau N. Race differences in nicotine dependence in the Collaborative Genetic study of Nicotine Dependence (COGEND). Nicotine Tob Res. 2008;10(7):1223–1230. [DOI] [PubMed] [Google Scholar]
- 50. Silagy C, Stead L, Mant D, Fowler G, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004;3:CD000146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.