Abstract
Introduction
Mindfulness training may reduce smoking rates and lessen the association between craving and smoking. This trial tested the efficacy of mindfulness training via smartphone app to reduce smoking. Experience sampling (ES) was used to measure real-time craving, smoking, and mindfulness.
Methods
A researcher-blind, parallel randomized controlled trial compared the efficacy of mobile mindfulness training with experience sampling (MMT-ES; Craving to Quit) versus experience sampling only (ES) to (1) increase 1-week point-prevalence abstinence rates at 6 months, and (2) lessen the association between craving and smoking. A modified intent-to-treat approach was used for treatment starters (MMT-ES n = 143; ES n = 182; 72% female, 81% white, age 41 ± 12 year).
Results
No group difference was found in smoking abstinence at 6 months (overall, 11.1%; MMT-ES, 9.8%; ES, 12.1%; χ2(1) = 0.43, p = .51). From baseline to 6 months, both groups showed a reduction in cigarettes per day (p < .0001), craving strength (p < .0001) and frequency (p < .0001), and an increase in mindfulness (p < .05). Using ES data, a craving by group interaction was observed (F(1,3785) = 3.71, p = .05) driven by a stronger positive association between craving and cigarettes per day for ES (t = 4.96, p < .0001) versus MMT-ES (t = 2.03, p = .04). Within MMT-ES, the relationship between craving and cigarettes per day decreased as treatment completion increased (F(1,104) = 4.44, p = .04).
Conclusions
Although mindfulness training via smartphone app did not lead to reduced smoking rates compared with control, our findings provide preliminary evidence that mindfulness training via smartphone app may help lessen the association between craving and smoking, an effect that may be meaningful to support quitting in the longer term.
Implications
This is the first reported full-scale randomized controlled trial of any smartphone app for smoking cessation. Findings provide preliminary evidence that smartphone app-based MMT-ES may lessen the association between craving and smoking.
Trial registration
Clinicaltrials.gov NCT02134509.
Introduction
Tobacco use is the leading cause of preventable disease, disability and death in the United States.1 Although 68% of cigarette smokers want to quit, only 7.4% achieve this annually.2 Behavioral treatments teach smokers to avoid triggers, divert attention from cravings, foster positive affective states, reduce negative mood and stress, or substitute other activities for smoking.1 The limited success of these approaches may be because triggers to smoke are prevalent and difficult to avoid; cognitive resources required to divert attention are often depleted in abstinence or with strong affective states; and effective substitutions for smoking are not always readily available.
Mindfulness training may overcome these limitations by targeting the association between craving and smoking instead of using distraction or substitution strategies. Mindfulness means maintaining attention on one’s immediate experience and cultivating an attitude of acceptance toward one’s experience.3 Mindfulness training typically involves the training of attention regulation, body awareness, and emotion regulation.4 For smoking cessation, mindfulness training may help smokers learn to work mindfully with cravings: to pay attention to craving as they arise and accept one’s experience, learning to ride out the cravings rather than to react by smoking.5 Prior work suggests that mindfulness training may be an effective treatment for smoking by targeting the association between craving and smoking.6,7 A randomized controlled trial (RCT) by our group found that mindfulness training led to higher smoking abstinence rates compared with another leading treatment for smoking,6 and reduced the association between craving and smoking across treatment.7
More broadly, a growing body of evidence supports testing mindfulness training for the treatment of substance use disorders. Recent reviews report that mindfulness-based interventions were associated with reduced consumption of substances of abuse as compared with control.8–11 Furthermore, in several studies, mindfulness training led to reduced craving and increased mindfulness, a potential mechanism for clinical outcomes. Limitations included small sample sizes and lack of reported methodological details.11 Nevertheless, overall findings suggest that mindfulness training may be an effective treatment for addictions including smoking.
Despite these promising findings, in-person mindfulness training is challenged by the need for experienced therapists, significant time and cost demands, limited access, and lack of standardization.12 One way to overcome these limitations is to deliver mindfulness training via smartphone app. Smartphone app-based treatments are gaining popularity due to their relatively low cost, ease of use, and availability.12,13 Compared with in-person treatments, smartphone app-based treatments are more accessible; more cost-effective; reduce stigma; improve tracking; improve standardization; are customizable, personalizable and scalable; provide direct access to tools for self-management, real-time interactive support, and social support; and get treatment into the users hand, in-context and potentially just-in-time.12 Furthermore, smartphone ownership rates are high, at 77% of US adults,14 and an estimated 80% among adult smokers motivated to quit.15
Most evidence in support of mobile device-based treatments for smoking is for text messaging, and the efficacy of text messaging to support smoking abstinence is considered to be generally well-established.16–18 Less evidence is available for smartphone apps for smoking cessation; only feasibility or other initial studies and one “pilot trial”19 have been reported. That RCT compared smartphone app-based acceptance and commitment therapy with the National Cancer Institute’s QuitGuide app (N = 196) and found that treatment was feasible to deliver by smartphone, and smoking abstinence rates were promising at 13% for the experimental app and 8% for the comparator.19 Other initial studies support that delivering treatment for smoking via smartphone app is feasible and can reduce smoking.20,21 To our knowledge, this trial is the first full-scale RCT of a smartphone app for smoking cessation. Likewise, although many meditation/mindfulness apps are available, no RCTs of these apps have been reported,22 and none have been tested for use in clinical populations such as smokers.12
This RCT tested Craving to Quit, a smartphone app for mindfulness training for smoking cessation based on our efficacious in-person mindfulness training.6 The app teaches the concept of mindfulness- paying attention to, and accepting momentary experience3; three standard meditation practices for attention regulation, body awareness, and emotion regulation23; and an exercise to work mindfully with cravings. A component of the app is experience sampling (ES) to query smoking, craving, and mindfulness in real time. The control intervention was a smartphone app delivering only ES. ES may bring awareness to craving and smoking, and is similar to other interventions that teach individuals to identify triggers and track their smoking. ES was used as a control in an effort to disentangle the effects of mindfulness training from those of effective self-monitoring.
The primary aim of this RCT was to test the efficacy of smartphone app-based mindfulness training (Craving to Quit) to reduce smoking rates compared with ES-only. The primary hypothesis was that mindfulness training would lead to higher smoking abstinence rates at 6 months versus control. The secondary aim was to test whether mindfulness training reduced the association between craving and smoking. The secondary hypothesis was that mindfulness training would be associated with a change in the prediction of smoking by craving across time points versus control.
Methods
A pragmatic RCT was conducted (NCT02134509). A protocol paper was previously published.24 All study procedures were approved by the Yale Institutional Review Board. All participants provided online informed consent. The study was conducted entirely using smartphone.
Participants
Eligible participants were as follows: age 18–65 years, smoked ≥5 cigarettes/day, had ≤3 months past-year abstinence, owned an iPhone/Android, and were motivated to quit, indicated by ≥8/10 on the Contemplation Ladder25 and ≥4/5 on an Action item of the Readiness to Change Questionnaire26: “I am trying to smoke less than I used to,” 1 = strongly disagree, 5 = strongly agree.
Recruitment and Randomization
Recruitment took place online from November 2014 to August 2015. Recruitment sources were as follows: 46% Google ads, 23% word of mouth/other, 14% Facebook posts, 11% https://smokefree.gov (Accessed June 25, 2018), 2% Twitter, 2% Reddit ads, 1% www. clinicaltrials.gov (Accessed June 25, 2018), 0.4% Huffington Post (blog posts from JB). Ads linked to the study website and screening survey. All surveys were automated (https://yalesurvey.qualtrics.com, Accessed June 25, 2018). As shown in Supplementary Figure S1, N = 5300 completed the screening survey, of whom N = 2200 were eligible, N = 518 consented, and N = 13 were excluded for enrolling more than once, based on contact information and IP address; N = 505 were e-mailed a link to the baseline survey, at which point quit-smoking medications were also recommended.27 At the end of the baseline survey, participants were randomized to receive mobile mindfulness training with experience sampling (MMT-ES; n = 245) or only ES (n = 260).
Sample Size
This study used a modified intent-to-treat (ITT) approach,28 defined as participants who were randomized and completed one day of treatment: either day 1 of MMT-ES or three ES check-ins (the requested minimum number of check-ins/day). Sample size was based on a trial of in-person mindfulness training.6 No smartphone app RCTs were available to estimate attrition, therefore, sample size was increased midway based on high attrition rates at 1 month, to maintain an adequate sample at 6 months to detect medium effects (adjusted sample, N = 505). Some randomized users never opened their app (MMT-ES, n = 95/245, 38.8%; ES n = 42/260, 16.2%; χ2(1) = 32.7, p < .001) or did not complete one day. Final groups: MMT-ES, n = 143; ES, n = 182.
With a sample of n = 150 per group, we have at least 80% power at a two-sided alpha level of .05 to detect a ~10% difference in abstinence due to the intervention, provided a 5% abstinence rate among ES (5% vs. 14.6%), or a 13% difference provided a 15% abstinence rate among ES (15% vs. 28%).
Interventions
Mobile Mindfulness Training With Experience Sampling
The intervention group received MMT-ES (Craving to Quit) for 22 days of training modules (5–15 minutes/day) teaching mindfulness for smoking cessation (see protocol paper24). The app teaches mindfulness and three standard meditation practices: body scan, loving kindness, and breath awareness. Body scan is practiced by bringing awareness to different parts of the body, to foster awareness of body sensations that constitute cravings and affective states. Loving kindness is practiced by directed well-wishing by repeating phrases such as “may X be happy,” to foster acceptance of oneself and others. Breath awareness is practiced by paying attention to the breath wherever one feels it most strongly in the body, to help retrain the mind away from habitual self-related thinking toward a more present-centered awareness. The app also teaches an informal practice to work mindfully with cravings, RAIN: Recognize, Accept, Investigate, and Note what cravings feel like as they arise and pass away. ES is another feature of the app delivered as “check-ins” adapted from the Day Reconstruction Method29 and from prior ES studies30 to measure smoking, craving, and other factors (Supplementary Table S1). For the current analysis, the following two check-in questions were utilized: (1) “When you started this check-in, how much were you craving a cigarette?” (visual analog scale from “Not at all” to “Very much”); and (2) The tracker says you have smoked [#] cigarettes today. Adjust your tracker below if needed. “Today I have smoked: [#].” Users were asked to check-in six times per day for 22 days. They set daily start/end times, their day was divided into six intervals, they were notified to check-in randomly in each interval30 and they were manually sent a text message if their response rate dropped below three check-ins/day, monitored daily across treatment by a blinded researcher.
Automated follow-up surveys including reminders of payment were sent to participants at 1, 3, and 6 months from treatment initiation. At 6 months, participants who reported 1-week abstinence were asked to take part in carbon monoxide (CO) monitoring. They were shipped piCO+ Smokerlyzer breath CO-monitors (Bedfont Scientific Ltd) and set up a video CO-monitoring session with a blinded researcher. Participants were compensated (Amazon.com e-card) after completing the 6-month survey and returning any equipment.
Experience Sampling-Only
The control group received a smartphone app with the same look and feel as MMT-ES, delivering only ES for 22 days, to control for potential effects of ES, expectancy effects and nonspecific effects of using a smartphone for smoking cessation. All study procedures were matched to the intervention group. Upon completion, the ES group received a free download code for Craving to Quit.
Retention
To maximize retention: the importance of follow-up was emphasized throughout; contact information for three referrals was requested at study initiation; surveys and check-ins were compensated; and study payment (up to $116) was provided at 6 months. All randomized participants were followed up by a blinded researcher.
Measurements
The primary outcome measure was 1-week point-prevalence abstinence from smoking at 6 months, verified by video-based CO-monitoring (<10 parts per million [ppm]). Secondary outcome measures included smoking, craving, and mindfulness, from baseline and 6-month surveys. Smoking was measured as cigarettes/day (CPD). Craving strength and craving frequency were measured using the Craving Experience Questionnaire.31 Mindfulness was measured using the Five Facet Mindfulness Questionnaire32 composite score.
An additional analysis was conducted using ES data of smoking (Tracker) and craving (check-in question, “When you started this check-in, how much were you craving a cigarette?”).
Statistical Analysis
All analyses were performed on a modified ITT basis. Demographics were compared using chi-square tests and independent t tests. For the primary outcome, abstinence rates were compared using chi-square tests and a significance level of 0.05, with missing data coded as smoking. Multiple imputation of missing 6 months prevalence data was also considered. A total of five datasets were imputed using SAS PROC MI based on treatment assignment, age, gender, race, education, marital status, employment status, income, age at smoking onset, and baseline CPD. Prevalence rates were then compared between groups using logistic regression for each of the imputed data sets, with results combined and summarized using SAS PROC MIANALYZE. No difference in prevalence rates were detected between the two groups (p = .7).
For the secondary outcomes (eg, CPD, craving, mindfulness), analysis involved five comparisons and used a Bonferroni-adjusted significance level of 0.01. Secondary outcomes were analyzed using linear mixed models with group (ES vs. MMT-ES) as a between-subjects factor, time (baseline vs. 6 months) as a within-subjects factor, and the interaction between group and time. The influences of craving and mindfulness on CPD were tested by entering each in turn as a time-dependent predictor in the above model. Similar models were used when modeling craving as the outcome. In these models, significant interactions were assessed by estimating slopes between the predictor and outcome of interest separately for each group. In all models, the best-fitting variance-covariance structure was determined using information criteria.
An additional analysis was conducted using ES data, where the relationship between average daily craving and total daily cigarettes (log-transformed due to skew) was assessed using a linear mixed model with group as a between-subjects factor and subject as a random effect in order to account for the correlation between multiple (daily) observations within each subject. As only one ES model was tested (ie, craving), this analysis used a significance level of 0.05.
Results
Participant Characteristics
Demographics, baseline smoking, and meditation/mindfulness experience are reported in Table 1. Self-reported use of quit smoking medications is reported in Supplementary Table S2.
Table 1.
Baseline Characteristics for the Modified Intent-to-Treat Sample for Mobile Mindfulness Training With Experience Sampling (MMT-ES) or Experience Sampling-Only (ES)
| MMT-ES (n = 143) | ES (n = 182) | χ2/t | p | |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD) | 43.3 (11.1) | 39.7 (12.6) | 2.7 | .01 |
| Female | 103 | 130 | 0.01 | .91 |
| Caucasian | 115 | 147 | 0.01 | .94 |
| Hispanic | 5 | 9 | 0.41 | .52 |
| Married | 81 | 76 | 7.1 | .01 |
| Working | 28 | 41 | 0.42 | .52 |
| HS or less education | 18 | 34 | 2.2 | .14 |
| Smoking behavior | ||||
| Age of onset, mean (SD) | 17.1 (4.6) | 17.2 (5.4) | −0.22 | .83 |
| Smokers in home, mean (SD) | 0.64 (0.99) | 0.66 (0.79) | −0.16 | .87 |
| Quit attempts, mean (SD) | 7.1 (12.0) | 15.0 (83.1) | −1.1 | .26 |
| Cigarettes per day, mean (SD) | 16.0 (7.1) | 16.2 (8.2) | −0.19 | .85 |
| Meditation/mindfulness | ||||
| Previous meditation experience | 50 | 67 | 0.12 | .73 |
| FFMQ-short | 78.8 (13.1) | 79.6 (10.7) | −0.31 | .76 |
FFMQ = Five Facet Mindfulness Questionnaire, HS = high school.
Retention and Adherence
Retention was defined as answering the primary outcome questions at 6 months. Retention among full ITT was 72.6% (MMT-ES, 78.4%; ES, 74.2%; χ2(1) = 1.2, p = .28) and among modified ITT was 83.7% (MMT-ES, 87.4%; ES, 80.8%; χ2(1) = 2.6, p = .11). Treatment completion, defined as completing 60% of MMT-ES modules or checking in on 60% of treatment days, was 52.9% among modified ITT (MMT-ES, 55.2%; ES, 51.1%; χ2(1) = 0.55, p = .46). No group differences were identified in the number of check-ins completed (MMT-ES = 51 ± 50, ES = 58 ± 51, t(323) = −1.1, p = .26) or number of treatment days checked-in (MMT-ES = 13 ± 10, ES = 14 ± 9, t(323) = −0.69, p = .49). Average check-ins per treatment day checked-in was greater for controls (MMT-ES = 3 ± 2, ES = 4 ± 2, t(309) = −2.2, p = .03). Within MMT-ES, participants completed 11 ± 9 of 22 days; 30 ± 19 of 58 modules; and 53.1% (n = 76/143) completed week 1, 41.3% (n = 59/143) week 2, and 28.7% (n = 41/143) week 3.
Primary Outcome
One-week point-prevalence abstinence from smoking at 6 months verified by CO-monitoring was 11.1% overall and did not differ between groups (MMT-ES, n = 14/143, 9.8%; ES, n = 22/182, 12.1%; χ2(1) = 0.43, p = .51). There was also no difference between groups using a lower CO cutoff (<6 ppm) to indicate abstinence33 (MMT-ES, n = 12/143, 8.4%; ES, n = 17/182, 9.3%; χ2(1) = 0.09, p = .77). Self-reported 1-week point prevalence abstinence at 6 months was 18.2% overall and did not differ between groups (MMT-ES, n = 26/143, 18.2%; ES, n = 33/182, 18.1%; χ2(1) = 0.0001, p = .99). Continuous abstinence (self-reported ≤5 cigarettes since quit date) at 6 months was 18.2% overall and did not differ between groups (MMT-ES, n = 23/143, 16.1%; ES, n = 36/143, 19.8%; χ2(1) = 0.74, p = .39).
Secondary Outcomes
Reduction in Smoking
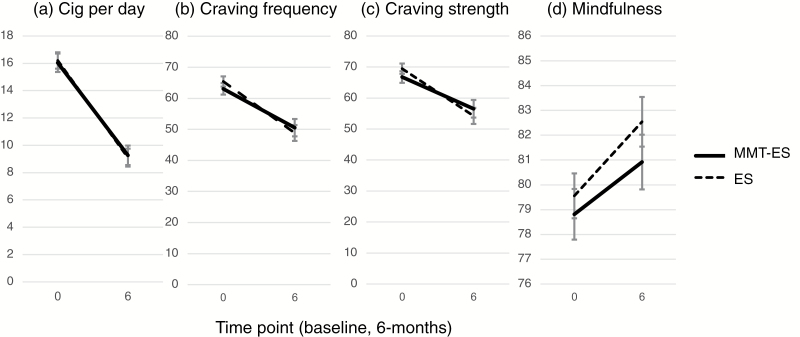
There was an overall reduction in CPD from baseline to 6 months (F(1,261) = 220.4, p < .0001). However, reduction in smoking did not differ between groups (group by time: F(1,261) = 0.13, p = .72). MMT+ES showed a reduction from 16.0 ± 7.1 to 9.0 ± 7.8 CPD (F(1,261) = 97.0, p < .0001), and ES showed a reduction from 16.2 ± 8.2 to 8.8 ± 9.0 CPD (F(1,261) = 125.9, p < .0001; Figure 1a).
Figure 1.
Change in smoking, craving and mindfulness: (a) cigarettes per day; (b) craving frequency; (c) craving strength; and (d) mindfulness, at baseline (0) and 6 months (6) for mobile mindfulness training with experience sampling (MMT-ES) (solid) and experience sampling-only (ES) (dashed).
Reduction in Craving
There was a reduction in craving strength from baseline to 6 months (F(1,323) = 38.95, p < .0001) that did not differ between groups (F(1,323) = 1.5, p = .22), and was significant for MMT-ES (baseline = 66.8 ± 22.8, 6 months = 57.4 ± 27.9; F(1,323) = 11.5, p = .0008) and ES (baseline = 69.5 ± 22.1, 6 months = 53.8 ± 31.9; F(1,323) = 30.8, p < .0001; Figure 1b). Similarly, there was a reduction in craving frequency from baseline to 6 months (F(1,323) = 54.1, p < .0001) that did not differ between groups (F(1,323) = 1.1, p = .30), and was significant for MMT+ES (baseline = 63.1 ± 22.3, 6 months = 51.1 ± 29.1; F(1,323) = 18.3, p < .0001) and ES (baseline = 65.4 ± 22.2, 6 months= 48.3 ± 30.2; F(1,323) = 38.9, p < .0001; Figure 1c).
Association Between Craving and Smoking
Significant overall associations were observed between CPD and craving strength (F(1,231) = 34.8, p < .0001) and frequency (F(1,231) = 51.6, p < .0001). A significant craving by time interaction was observed for craving strength (F(1,231) = 8.1, p = .005) and frequency (F(1,231) = 6.5, p = .01). These latter interactions were driven by stronger positive associations between craving and smoking at 6 months (strength: r = 0.44, p = 0.0001; frequency: r = 0.44, p = 0.0001) versus baseline (strength: r = 0.07, p = 0.22; frequency: r = 0.12, p = 0.03). In both sets of analyses, no main or interactive effects with group were observed.
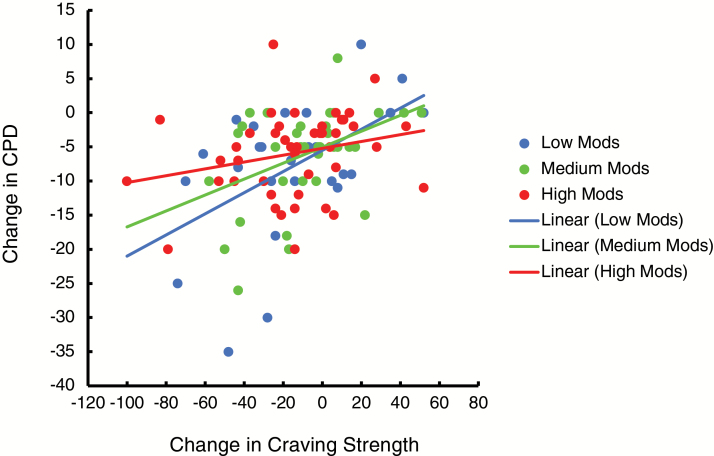
For MMT-ES, a separate analysis showed that the relationship between craving strength and CPD decreased as the number of completed treatment modules increased from low (0–14 modules), to medium (15–41 modules), to high (42+ modules) rates of completion (F(1,104) = 4.44, p = .04; Figure 2). This effect was not significant for craving frequency (F(1,104) = 2.1, p = .15).
Figure 2.
The relationship between change in cigarettes per day (CPD) and change in craving strength grouped by low (0–14 modules), medium (15–41 modules), and high (42+ modules) rates of completion of mobile mindfulness training with experience sampling (MMT-ES) training modules (of 58 modules). The relationship between CPD and craving strength decreased as the number of MMT-ES treatment modules completed increased (p = .04).
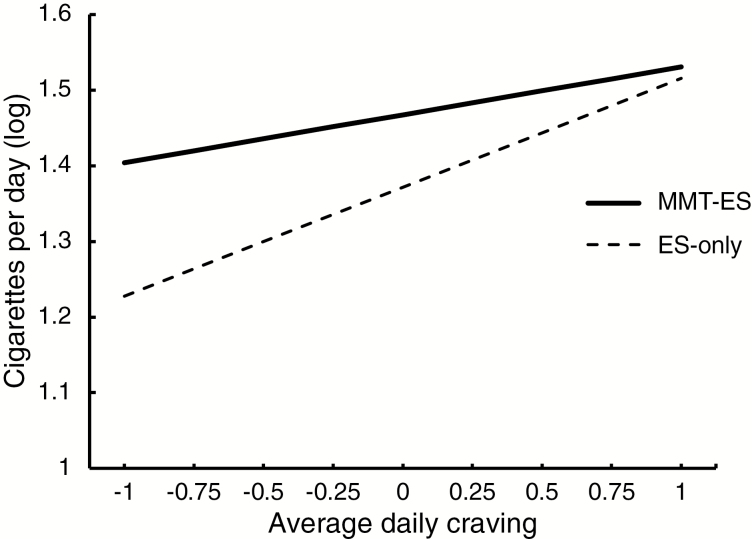
In a separate analysis using the ES data, a significant craving by group interaction (F(1,3785) = 3.7, p = .05) was observed, which was driven by a stronger positive association between average daily craving and total daily smoking among ES (slope = .14 ± .03, p < .0001) compared to MMT-ES (slope = .06 ± .03, p = .04; Figure 3).
Figure 3.
Regression lines for the prediction of total daily smoking (cigarettes per day) by average daily craving across treatment days for each group. This prediction differed between groups (p = .05), with a weaker association between craving and smoking for mobile mindfulness training with experience sampling (MMT-ES) (t = 2.03, p = .04; solid line) as compared with experience sampling-only (ES) (t = 4.96, p < .0001; dashed line).
Increase in Mindfulness
There was an increase in mindfulness from baseline to 6 months (F(1,248) = 13.7, p = .0003) that did not differ between groups (F(1,248) = 0.41, p = .52), and was significant for MMT + ES (baseline = 78.1 ± 13.1, 6 months = 80.7 ± 13.3; F(1,248) = 4.27, p = .04) and ES (baseline = 79.6 ± 10.7, 6 months = 82.4 ± 12.5; F(1,248) = 10.4, p = .002; Figure 1d).
Association Between Mindfulness and Smoking and Craving
No significant relationship was observed between increased mindfulness and reduced smoking (F(1,237) = 2.77, p = .098). Mindfulness did not interact with group (F(1,237) = 0.04, p = .84) or group and time (F(1,237) = 0.05, p = .82). Increased mindfulness was associated with lower craving strength (F(1,323) = 17.3, p < .0001) overall, and interacted with group (F(1,323) = 4.3, p = .039) such that reductions in craving due to increased mindfulness were observed for ES (slope = −.59 ± .13, p < .0001) but not MMT-ES (slope = −.20 ± .13, p = .14). A similar interaction between mindfulness and group was observed for craving frequency (F(1,323) = 4.3, p = .039) owing to a similar inverse association among ES (slope = −.67 ± .13, p < .0001) but not MMT-ES (slope = −.17 ± .13, p = .19).
Discussion
To our knowledge, this is the first reported full-scale RCT of either a smartphone app for smoking cessation or a smartphone app for mindfulness training. MMT-ES (Craving to Quit) was found to reduce smoking and craving and increase mindfulness. MMT-ES was compared with only ES, which was found to comparably reduce smoking and craving and increase mindfulness. Although group differences in smoking abstinence rates at 6 months were not identified, importantly, MMT-ES was found to lessen the association between craving and smoking after treatment versus ES.
These mechanistic findings are consistent with our trial in which in-person mindfulness training was found to lessen the association between craving and smoking.7 Although the centrality of craving to smoking cessation is disputed,34 craving is nevertheless one of the symptoms that smokers seek to alleviate through treatment.35 Quit smoking medications have been found to reduce background craving but not to prevent or alleviate craving induced by cues, thoughts or affective states.35 Nicotine replacement therapies have been found to provide relief from acute craving only once it has been triggered,35 a substitution strategy that may not break the link between craving and smoking.5 Only behavioral treatments that specifically target reduced craving have been found to lessen the association between craving and substance use.36 For example, targeted approaches of monitoring urges and “urge-surfing” were found to reduce the relationship between negative mood and craving, which predicted a reduction in drinking frequency in response to negative mood.37 Similarly, Mindfulness-Based Relapse Prevention was found to reduce the relationship between negative affect and craving, which predicted a later reduction in substance use.38 Finally, our findings are consistent with a study of web-based acceptance and commitment therapy, in which the difference in smoking quit rates versus control were mediated by increases in noticing and not acting on urges to smoke.39
From a mechanistic perspective, mindfulness training targets craving by teaching smokers to recognize cravings as they arise and investigate what they feel like in the body, to learn to tolerate the physical sensations and “ride out” cravings rather than react by smoking.5 In line with this, mindfulness training has been found to reduce reactivity to craving cues40 and stressors41 in individuals with addictions, among other changes4 that may help reduce the relationship between craving and smoking. Cravings may continue to arise, as is evident by a similar level of craving reported by experimental and control groups, yet the positive relationship between craving and smoking was reduced with mindfulness training, more so in individuals who completed a greater number of mindfulness modules. Although this change was not related to greater quit rates, these findings are promising for targeted treatment of craving, and suggest that increased adherence to mobile mindfulness training may improve outcomes.
A control app delivering only ES was found to comparably reduce smoking and craving and increase mindfulness. This finding is surprising given that the control app did not provide an active treatment for smoking cessation, however it is also in line with our hypothesis24 that ES may bring awareness to smoking, craving, and mood, similarly to mindfulness training. This finding is also consistent with a recent study in which notifying smokers of their smoking in real time using smartband data alone led to reduced smoking rates.42 Additionally, the ES check-ins prompted smokers to evaluate their momentary awareness, concentration, feeling tone, and equanimity, all aspects of mindfulness, and the outcome measure (Five Facet Mindfulness Questionnaire) specifically evaluates related aspects of mindfulness, including observing and describing experience, awareness, nonjudgment and nonreactivity.32 These findings indicate some potential reactivity to the ES methods43 that should be considered in future trials, in particular when ES is not being tested as an intervention. Nevertheless, our findings indicate that mindfulness training lessens the association between craving and smoking across treatment, an effect that was not found for ES, suggesting a potential mechanism for the effects of mindfulness training on smoking cessation. Alternative controls could be tested, such as another smartphone app for smoking cessation (as in NCT02037360).
Quit rates were encouraging at 18.2% self-reported and 11.1% CO-verified overall, suggesting a potential high impact of smartphone-based treatments for smoking. These findings are comparable to the only other RCT of a smartphone-based smoking treatment, acceptance and commitment therapy, at self-reported 13% experimental and 8% control.19 Future analyses will test whether individual baseline characteristics relate to differences in smoking abstinence rates.
Although the current trial was not designed to evaluate feasibility, it is an early trial of any smartphone app for smoking cessation, and therefore may be useful to inform other studies. The findings support feasibility, including methods for recruitment, enrollment, and retention. Enrollment rates were high (51 randomized/month), however, the resulting study population was 71.7% female and 80.6% white. More females than males report using health apps44 and downloading quit smoking apps,45 although sex differences may be leveling out with overall increased use of health apps.44 Similarly, the odds of downloading a health app are reduced with race/ethnicity compared to white individuals, although race may be less predictive than other demographics such as education, age, and sex.44 This difference is not attributable to smartphone ownership, as ownership rates are comparable by race/ethnicity.14 Additionally, 84% of participants had greater than high school education, a potentially important factor given that lower educational attainment is associated with higher smoking rates1 and with lower smartphone ownership rates.14 Future studies may consider targeted advertising or other strategies to better balance these factors. Finally, retention rates were also high (73% ITT, 84% modified ITT at 6 months) and were comparable to the only other smartphone app-based smoking cessation trial (84% at 2 months).19
Feasibility was also supported for video-based CO-monitoring. A majority of participants (73%) were CO-verified and confirmed (61%) self-reported abstinence. Abstinence rates were 18.2% self-reported and 11.1% CO-verified. It is possible that those who self-reported abstinence but did not CO-verify were smoking (they were coded as smoking in the analysis). However, there were several barriers to CO-monitoring. Other studies have had success with CO-monitoring via video, for example, in studies of online or mobile phone–based contingency management.46–48 In those studies, participants recorded and uploaded videos of CO-monitoring, which took place regularly across the trial. Here, participants took part in a video chat with a researcher at only one follow-up time-point. It is possible that more regular and indirect sampling may promote adherence. For example, immediate reinforcement for regular video-based CO samples increased adherence in contingency management studies.47,48 Other approaches, such as smartphone-compatible CO-monitors49 or smartband-based smoking detection42 could be considered, as should the necessity of biochemical verification.50
Importantly, our findings support that mindfulness training is feasible to deliver via smartphone app and has a positive impact on mechanisms of smoking (ie, craving). A greater number of mindfulness modules completed was associated with a greater reduction in the association between craving and smoking, an effect that may be meaningful to support quitting and prevent relapse in the longer term. Although there is no comparative data on adherence to smartphone app interventions in RCTs, we consider adherence to MMT-ES moderate (eg, 11/22 days). Given our findings that completing more mindfulness modules leads to potential gains for smoking cessation (ie, reduced relationship between craving and smoking), future studies will work to improve adherence. For both groups, participation in ES was high (55 check-ins across treatment), providing a rich high-resolution dataset to evaluate smoking, craving, mood, and mindfulness in secondary analyses.
Finally, our findings should contribute to the development of clinical practice guidelines for RCTs of smartphone apps for smoking cessation. A limitation of this trial was that guidelines had not yet been established. Available quit smoking apps have low levels of adherence to clinical guidelines for treating tobacco use and dependence,13 including being specific to smoking, advising quitting, assisting with a quit plan, recommending counseling and quit smoking medications, and including text messaging for smoking cessation.51 Craving to Quit contains most of these features, and those not innate to the app were incorporated into the trial, such as recommending quit smoking medications.
Limitations
A number of limitations of this trial should be considered. First, groups were imbalanced at treatment onset, with fewer treatment starters in MMT-ES (n = 143) versus ES (n = 182; p = .007), despite blinding to treatment allocation. Those in ES were directed to the app store to download their app and start the study, whereas those in MMT-ES were first directed to an app website to enter demographic and smoking information (eg, CPD), and were then directed to the app store to download their app and start the study. This additional step may have been a barrier to entry and should be eliminated (or matched) in similar trials. Additionally, the average number of check-ins per day was greater for ES, likely because check-ins were the only feature of the app, whereas MMT-ES was a multi-featured app for mindfulness training with check-ins as one supporting feature. Another limitation was that treatment was delivered only up to the quit date, although subjects could continue to use their apps, and those in MMT-ES were trained on which modules to return to upon relapse.24
Conclusion
These findings provide preliminary evidence that smartphone app-based mindfulness training may help to lessen the association between craving and smoking, an effect that did not lead to reduced abstinence rates compared with control, but may be meaningful to support quitting and prevent relapse in the longer term.
Funding
This study was supported by grants from the American Heart Association [14CRP18200010] and the National Institute on Drug Abuse grant [K12DA00167]. The views presented in the manuscript are not necessarily those of the funding agencies who did not have input into the content of the manuscript outside of funding the proposed research.
Declaration of interests
Judson A. Brewer and Prasanta Pal own stock in Claritas Mindsciences, the company that developed the apps used in this study. All other authors declare that they have no competing interests.
Supplementary Material
References
- 1. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. Printed with corrections, January 2014. [Google Scholar]
- 2. Centers for Disease Control and Prevention. Quitting smoking among adults—United States, 2000–2015. MMWR. 2017;65(52):1457–1464. https://www.cdc.gov/mmwr/volumes/65/wr/mm6552a1.htm. Accessed June, 25, 2018. [DOI] [PubMed] [Google Scholar]
- 3. Bishop SR, Lau M, Shapiro S, et al. . Mindfulness: a proposed operational definition. Clin Psychol. 2004;11(3):230–241. [Google Scholar]
- 4. Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci. 2011;6(6):537–559. [DOI] [PubMed] [Google Scholar]
- 5. Brewer JA, Elwafi HM, Davis JH. Craving to quit: psychological models and neurobiological mechanisms of mindfulness training as treatment for addictions. Psychol Addict Behav. 2013;27(2):366–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brewer JA, Mallik S, Babuscio TA, et al. . Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend. 2011;119(1–2):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elwafi HM, Witkiewitz K, Mallik S, Thornhill TA IV, Brewer JA. Mindfulness training for smoking cessation: moderation of the relationship between craving and cigarette use. Drug Alcohol Depend. 2013;130(1-3):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goyal M, Singh S, Sibinga EM, et al. . Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174(3):357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oikonomou MT, Arvanitis M, Sokolove RL. Mindfulness training for smoking cessation: a meta-analysis of randomized-controlled trials. J Health Psychol. 2017;22(14):1841–1850. [DOI] [PubMed] [Google Scholar]
- 10. Li W, Howard MO, Garland EL, McGovern P, Lazar M. Mindfulness treatment for substance misuse: a systematic review and meta-analysis. J Subst Abuse Treat. 2017;75:62–96. [DOI] [PubMed] [Google Scholar]
- 11. Maglione MA, Maher AR, Ewing B, et al. . Efficacy of mindfulness meditation for smoking cessation: a systematic review and meta-analysis. Addict Behav. 2017;69:27–34. [DOI] [PubMed] [Google Scholar]
- 12. Garrison KA, O’Malley SS, Brewer JA, Potenza MN. Mobile applications for mindfulness training in the treatment of substance use disorders. In: Potenza MN, Faust K, Faust D, eds. The Oxford Handbook of Digital Technologies and Mental Health. Oxford, UK: Oxford University Press; in press. [Google Scholar]
- 13. Abroms LC, Lee Westmaas J, Bontemps-Jones J, Ramani R, Mellerson J. A content analysis of popular smartphone apps for smoking cessation. Am J Prev Med. 2013;45(6):732–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pew Research Center. Mobile Fact Sheet http://www.pewinternet.org/fact-sheet/mobile/. Accessed December 23, 2017.
- 15. Borrelli B, Bartlett YK, Tooley E, Armitage CJ, Wearden A. Prevalence and frequency of mHealth and eHealth use among US and UK smokers and differences by motivation to quit. J Med Internet Res. 2015;17(7):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kong G, Ells DM, Camenga DR, Krishnan-Sarin S. Text messaging-based smoking cessation intervention: a narrative review. Addict Behav. 2014;39(5):907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scott-Sheldon LA, Lantini R, Jennings EG, et al. . Text messaging-based interventions for smoking cessation: a systematic review and meta-analysis. JMIR mHealth uHealth. 2016;4(2):e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spohr SA, Nandy R, Gandhiraj D, Vemulapalli A, Anne S, Walters ST. Efficacy of SMS text message interventions for smoking cessation: a meta-analysis. J Subst Abuse Treat. 2015;56:1–10. [DOI] [PubMed] [Google Scholar]
- 19. Bricker JB, Mull KE, Kientz JA, et al. . Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug Alcohol Depend. 2014;143:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buller DB, Borland R, Bettinghaus EP, Shane JH, Zimmerman DE. Randomized trial of a smartphone mobile application compared to text messaging to support smoking cessation. Telemed J E Health. 2014;20(3):206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ubhi HK, Michie S, Kotz D, Wong WC, West R. A mobile app to aid smoking cessation: preliminary evaluation of SmokeFree28. J Med Internet Res. 2015;17(1):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mani M, Kavanagh DJ, Hides L, Stoyanov SR. Review and evaluation of mindfulness-based iPhone apps. JMIR mHealth uHealth. 2015;3(3):e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gunaratana H. Mindfulness in Plain English. Somerville, MA: Wisdom Publications; 2002. [Google Scholar]
- 24. Garrison KA, Pal P, Rojiani R, Dallery J, O’Malley SS, Brewer JA. A randomized controlled trial of smartphone-based mindfulness training for smoking cessation: a study protocol. BMC Psychiatry. 2015;15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365. [DOI] [PubMed] [Google Scholar]
- 26. Rollnick S, Heather N, Gold R, Hall W. Development of a short ‘readiness to change’ questionnaire for use in brief, opportunistic interventions among excessive drinkers. Br J Addict. 1992;87(5):743–754. [DOI] [PubMed] [Google Scholar]
- 27. Fiore M, JaÈn C, Baker T, et al. . Treating Tobacco Use and Dependence: 2008 Update—Clinical Practice Guidelines 2008; 101–103. Available at: http://bphc.hrsa.gov/buckets/treatingtobacco.pdf. Accessed June 25, 2018.
- 28. Moher D, Hopewell S, Schulz KF, et al. . CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kahneman D, Krueger AB, Schkade DA, Schwarz N, Stone AA. A survey method for characterizing daily life experience: the day reconstruction method. Science. 2004;306(5702):1776–1780. [DOI] [PubMed] [Google Scholar]
- 30. Berkman ET, Dickenson J, Falk EB, Lieberman MD. Using SMS text messaging to assess moderators of smoking reduction: validating a new tool for ecological measurement of health behaviors. Health Psychol. 2011;30(2):186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. May J, Andrade J, Kavanagh DJ, et al. . The craving experience questionnaire: a brief, theory-based measure of consummatory desire and craving. Addiction. 2014;109(5):728–735. [DOI] [PubMed] [Google Scholar]
- 32. Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27–45. [DOI] [PubMed] [Google Scholar]
- 33. Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100(2):159–167. [DOI] [PubMed] [Google Scholar]
- 34. Wray JM, Gass JC, Tiffany ST. A systematic review of the relationships between craving and smoking cessation. Nicotine Tob Res. 2013;15(7):1167–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36(3):235–243. [DOI] [PubMed] [Google Scholar]
- 36. Longabaugh R, Magill M. Recent advances in behavioral addiction treatments: focusing on mechanisms of change. Curr Psychiatry Rep. 2011;13(5):382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Witkiewitz K, Bowen S, Donovan DM. Moderating effects of a craving intervention on the relation between negative mood and heavy drinking following treatment for alcohol dependence. J Consult Clin Psychol. 2011;79(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Witkiewitz K, Bowen S. Depression, craving, and substance use following a randomized trial of mindfulness-based relapse prevention. J Consult Clin Psychol. 2010;78(3):362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bricker J, Wyszynski C, Comstock B, Heffner JL. Pilot randomized controlled trial of web-based acceptance and commitment therapy for smoking cessation. Nicotine Tob Res. 2013;15(10):1756–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, Tindle HA. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Soc Cogn Affect Neurosci. 2011;8(1):73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brewer JA, Sinha R, Chen JA, et al. . Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Subst Abus. 2009;30(4):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dar R. Effect of real-time monitoring and notification of smoking episodes on smoking reduction: a pilot study of a novel smoking cessation app. Nicotine Tob Res. 2018;20(12):1515–1518. [DOI] [PubMed] [Google Scholar]
- 43. Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21(4):486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krebs P, Duncan DT. Health app use among US mobile phone owners: a national survey. JMIR mHealth uHealth. 2015;3(4):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. BinDhim NF, McGeechan K, Trevena L. Who uses smoking cessation apps? A feasibility study across three Countries via smartphones. JMIR mHealth uHealth. 2014;2(1):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kong G, Goldberg AL, Dallery J, Krishnan-Sarin S. An open-label pilot study of an intervention using mobile phones to deliver contingency management of tobacco abstinence to high school students. Exp Clin Psychopharmacol. 2017;25(5):333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dallery J, Raiff BR, Grabinski MJ. Internet-based contingency management to promote smoking cessation: a randomized controlled study. J Appl Behav Anal. 2013;46(4):750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dallery J, Raiff BR, Kim SJ, Marsch LA, Stitzer M, Grabinski MJ. Nationwide access to an internet-based contingency management intervention to promote smoking cessation: a randomized controlled trial. Addiction. 2017;112(5):875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meredith SE, Robinson A, Erb P, et al. . A mobile-phone-based breath carbon monoxide meter to detect cigarette smoking. Nicotine Tob Res. 2014;16(6):766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Society for Research on Nicotine and Tobacco, Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. [DOI] [PubMed] [Google Scholar]
- 51. Whittaker R, McRobbie H, Bullen C, Borland R, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD006611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.