Abstract
Introduction
Nicotine can robustly increase responding for conditioned reinforcers (CRs), stimuli that acquire reinforcing properties based on association with primary reinforcers. Menthol and licorice are tobacco flavoring agents also found in sweet foods (eg, candy and ice cream), making them putative CRs before they are consumed in tobacco. We sought to determine if intravenous self-administration (IVSA) of nicotine was enhanced by the inclusion of oral tobacco flavor CRs.
Methods
Menthol (160 or 320 µM) or licorice root extract (0.1% or 1%) were established as CRs (paired with 20% sucrose) or “neutral” stimuli (paired with water) in separate groups. During subsequent IVSA tests, nicotine was delivered in conjunction with oral presentations of the CR.
Results
In experiment 1, a menthol CR significantly shifted the peak nicotine dose from 15 µg/kg/infusion (Neutral group) to 3.25 µg/kg/infusion (CR group). In experiment 2, a menthol CR significantly increased operant licks for nicotine (3 µg/kg/infusion) relative to control groups. In experiment 3, both licorice and menthol CRs significantly increased operant licks for nicotine (7.5 µg/kg/infusion) relative to an “inactive” sipper. The licorice CR increased nicotine IVSA in proportion to the strength of the flavor, but both menthol concentrations increased nicotine IVSA to a similar extent.
Conclusion
Tobacco flavor additives with conditioned reinforcing properties promote acquisition of nicotine self-administration at low unit doses and may have robust impact on tobacco consumption when nicotine yield is low.
Implications
Tobacco flavor additives are found in rewarding foods (eg, ice cream) and gain palatability based on associations with primary rewards (eg, sugar) making them “conditioned reinforcers.” Nicotine increases the motivation for flavor conditioned reinforcers and the present studies show that tobacco flavor additives can interact with nicotine to promote more nicotine self-administration. The interaction between flavors additives and nicotine may promote nicotine exposure and subsequently dependence.
Introduction
Menthol cigarettes account for almost 25% of all cigarettes sold in the United States with a disproportionate share consumed by African American, female, and youth smokers.1 Many menthol smokers admit that they would not smoke if menthol cigarettes were not commercially available2 and menthol additives may promote smoking because of their familiar incentive status (eg, “tastes like a peppermint patty”3). In smoked tobacco, menthol is perceived by the smoker and menthol products are used by individuals who do not like the taste of traditional combusted tobacco products.4 In non-menthol cigarettes, flavor additives account for approximately 10% of filler weight and increase the total particulate matter by approximately 15%,5 suggesting that they remain intact and make a substantial contribution to the flavor of the inhaled smoke. Adding these flavors to both smoked and smokeless tobacco preparations (snus, snuff, chewing tobacco) ensures that consuming the tobacco product will engage the incentive motivational systems of the brain. These systems guide approach and choice behaviors, meaning that tobacco products that are flavored with incentive stimuli are more likely to be approached, chosen, and consumed in the future.
In humans, nicotine is traditionally self-administered orally (transmucosal or inhalation), making chemosensory palatability vital to nicotine delivery. Research relating the psychophysics of taste perception to tobacco use suggests that individuals who are more sensitive to the unpalatable flavors in tobacco smoke fewer cigarettes, have lower scores on nicotine dependence scales, and report reduced sensitivity to the reinforcing effects of nicotine6,7). Denicotinized tobaccos have been commercially unsuccessful; one reason may be that the tobacco produces a taste that is subjectively “disliked” by most smokers.8 On the other hand, smokers show tremendous loyalty to specific brands and tobacco products meaning that repeated use may make the specific chemosensory features of some products preferred to others, especially those that are used during initiation and experimentation with nicotine.9
Nicotine is the principal reinforcing ingredient in tobacco products, but it has complex behavioral effects in humans10,11 and nonhuman animals.12,13 As a primary reinforcer, nicotine increases behaviors that result in delivery of nicotine to the central nervous system.14 This effect of nicotine also can be conferred to neutral stimuli, making them “conditioned reinforcers”—stimuli that acquire reinforcing properties based on their history of association with primary reinforcers.15 An important secondary effect of nicotine is that it promotes the incentive properties of nondrug conditioned reinforcers. For example, nicotine injections increase the acquisition of a new operant response for conditioned reinforcers that have previously been paired with food16 or water.17 In addition, nicotine can increase approach responses toward stimuli that are associated with primary rewards such as sucrose.18,19 This increase in “sign-tracking” observed after nicotine treatment suggests that the effect of the drug increases the incentive motivational properties of reward-associated cues, increasing their ability to elicit approach behaviors.
Despite extensive research demonstrating that nicotine increases responding for nondrug rewards16,17,20–23 and approach toward cues associated with nondrug rewards,24,25 few studies have addressed orosensory cues, which are an important aspect of tobacco self-administration in humans. Ksir26 found that sucrose was a critical ingredient for consumption of commercial chewing tobacco in Syrian hamsters. Chen and colleagues27 found that intravenous (IV) nicotine infusions dose-dependently reduced self-administration of a saccharin and cocoa mixture in an operant lick paradigm. However, of the four nicotine doses (0, 15, 30, and 60 µg/kg/infusion) investigated, no ascending limb was observed—as nicotine dose increased, licks systematically decreased, but no nicotine dose increased behavior relative to placebo (0 mg/kg/infusion). One possible explanation for these findings is that the gustatory reward was more reinforcing than nicotine, in which case, one would only expect the satiating or nonspecific effects of nicotine—those that result in the descending limb of the dose–response relationship—to reduce operant behavior. In that study, there were no manipulations used to further probe the effects of nicotine on operant responding (eg, lower doses and more effortful schedules).
An ecologically valid alternative would be to present gustatory conditioned reinforcers (CRs) during nicotine self-administration. For clarity, we use the term gustatory to refer to the sensation of taste rather than eating. Smokers who try menthol cigarettes have undoubtedly been exposed to comparable tastes in the past—menthol is an extract of peppermint and corn mint and mint is a common ingredient in ice cream, candy, and other sweet/high-calorie foods. In addition, the orosensory effects of menthol are commonly considered to be “cleansing” and palatable, making menthol a common ingredient in oral hygiene products such as toothpaste and breath mints. All of these properties of menthol imbue the flavor with conditioned reinforcing properties in humans. Licorice is also a putative CR in humans and is one of the most common tobacco “casing” ingredients—base flavors, which are added to all products before they are individuated with the addition of product-specific ingredients (so-called “top-dressing”). When menthol, licorice, and other flavors are included in tobacco products they are “unsweetened”; meaning that their influence on palatability depends both on physical properties (coolness or smoothness) but also on their associative learning history. Because nicotine can enhance the incentive properties of gustatory CRs,18 a flavoring agent with incentive properties would be expected to promote nicotine self-administration. The goal of the present studies was to establish evidence for this prediction using gustatory stimuli that are commonly used in tobacco products. The stimuli were established as CRs before being included as an oral stimulus during nicotine self-administration. We predicted that the flavors would increase self-administration of nicotine, but only if they were previously established as CRs.
Methods
Subjects
A total of 140 male CD rats were purchased from Charles River (Mattawan, MI) at 250–275 g on arrival and were individually housed in a temperature and humidity controlled colony room on a reverse 12:12 light:dark cycle. All testing was performed in the dark part of the cycle. After a week of habituation to the colony, the rats were feed restricted (~17 g/day), but water was continuously available throughout the experiment. Protocols were approved by the East Tennessee State University Animal Care and Use Committee and followed the guidelines set forth by the Guide for the Care and Use of Animals (National Institutes of Health).
Drugs and Flavor Solutions
dl(–)Menthol (160 or 320 µM; Sigma, St. Louis, MO; CAS: 89-78-1) was mixed in tap water and ethanol (0.5% v/v), approximating the menthol content in the mainstream smoke of tobacco products.28 Licorice root extract (0.1% or 1% v/v; Gaia Herbs, Brevard, NC; herb equivalency 500 mg/ml, 30% ethanol, v/v). Licorice extract has been reported as 1%–4%,29 it “enhances and harmonizes the smoke flavor,”29 and at least 35 pyrolysates are in the mainstream smoke of combustible tobacco.30 Grape (Kool-Aid; 0.05%–0.5% w/v) flavor was purchased from a local market and dissolved in tap water. Sucrose (20% w/v) was purchased from a local market and included in the solutions depending on group assignment (see later). (–) Nicotine hydrogen tartrate (Sigma) was dissolved in sterile isotonic saline and adjusted to a pH of 7 (±0.2) with dilute sodium hydroxide. Nicotine infusions were administered at a volume of 0.2 ml/kg, dose was calculated as the base form. Heparin was diluted to 30 international units per milliliter and mixed with timentin (ticarcillin + clavulanate, 36 mg/ml). The cocktail was infused into catheters at a volume of 0.1 ml/infusion daily to maintain patency.
IV Catheters and Surgical Procedures
IV catheters were constructed from blunted 25-g needles, a 3.2-cm (diameter) polyester patch, and 17 cm of silastic tubing (3.05-mm ID, Dow Plastics). All surgical procedures were similar to those previously described for IV catheter implantation.31 Rats were allowed to recover for 7–10 days before self-administration testing began.
Apparatus
Intravenous self-administration (IVSA) was conducted in 10 standard operant conditioning chambers (Med Associates, St. Albans, VT) equipped with IV drug and oral fluid delivery equipment and lickometer controllers. On the front wall (experiment 1) two nose pokes and a receptacle with a liquid dipper (0.1-ml cup) were located 2 cm above the chamber floor. The rear wall (experiments 2–3) included two polyvinylchloride panels with standard curved sipper tubes attached, a stopper magnetically fixed the sipper to the outside wall of the chamber. The lickometer controller was alligator clipped to the back side of the sipper. A solenoid (grainger.com) opened for 0.5 in. allowed 0.12 ml of solution to flow through chemical resistant tubing into the sipper tube.
Procedure
Strategy
To investigate the effects of flavor CRs on nicotine self-administration, we paired the tobacco flavor additives (menthol or licorice) with sucrose. To tightly control the associative status of the tobacco additives, we used a flavor discrimination procedure (CR flavor vs. Grape) and “Neutral” control groups that received reversed discrimination training. Thus, group name refers to the status of the tobacco additive flavor (menthol or licorice) presented during nicotine self-administration. For CR groups, the additive was paired with sucrose during flavor conditioning; grape was presented in tap water. For Neutral groups, Grape Kool-Aid was paired with sucrose and the additive flavor (menthol or licorice) was presented in water. There are three important reasons for using this strategy. First, rats will often avoid novel flavor stimuli (neophobia),32 a phenomenon that could confound nicotine self-administration if drug infusions are paired with a flavor the rats have never encountered. Second, novel flavors paired with nicotine injections can very easily acquire aversive motivational effects,33 familiarity with the flavor can reduce this effect.34 Third, this strategy equates access to sucrose, caloric availability, and sucrose–flavor pairings, in the control groups without changing the associative status of the target flavor (menthol or licorice).
Flavor Conditioning
All rats were randomly assigned to one of two groups (CR or Neutral). For the CR groups, the target flavors (menthol or licorice) were paired with 20% sucrose, a separate solution contained unsweetened grape. For the Neutral groups, grape was paired with 20% sucrose, target flavors were presented in tap water. Flavor-testing sessions lasted 40 minutes per day in the home cage, for 24 days. IV catheters were implanted after 20 days of conditioning, four additional tests were conducted during the surgical recovery period. Supplementary Figure 1 shows a schematic of experiments 1–3.
IVSA Experiment 1
For all rats, responding on the active nose poke resulted in presentation of IV nicotine and oral menthol (320 µM, unsweetened) or water in a liquid dipper. Rats were assigned to one of three groups based on the flavor conditioning phase; CR (IV nicotine + menthol CR, n = 9), Neutral (IV nicotine + Neutral menthol, n = 8), and Water (IV nicotine + oral water, n = 8). Rats in the Water groups were taken from the CR (n = 4) and Neutral (n = 4) groups from flavor conditioning. All rats acquired IVSA with the 30 µg/kg/infusion nicotine delivered under a fixed ratio (FR) 1 schedule of reinforcement for the first 3 days, followed by 3 days under FR2 and 4 days under FR5. A 30-second time-out was signaled by extinction of the nose key light. Infusion dose was manipulated following FR5 tests; each dose was tested for 3 or more days in randomized order. The lowest unit doses (1.5–3.25 µg/kg) were tested twice and averaged to confirm observed response rates.
IVSA Experiment 2
Rats in the CR group from flavor conditioning were randomly assigned to one of two infusion conditions (CR + NIC; 3.5 µg/kg/infusion, n = 8) or CR + SAL (0 µg/kg/infusion, n = 8). Rats in the Neutral group self-administered nicotine (Neutral + NIC; 3.5 µg/kg/infusion, n = 8). The IV infusion and oral solution (320-µM menthol) were delivered for licks at the sipper tube (FR5). Stimulus lights were illuminated for a 15-second time-out period after each reinforcer. Testing was discontinued after the sixth day after an experimenter error.
IVSA Experiment 3
All rats acquired IVSA with 7.5 µg/kg/infusion nicotine. A second sipper tube and lickometer controller were added to the chamber as an “inactive” response option. The IV infusion and oral solutions were delivered on an incrementally FR schedule (FR2, FR5, and FR10)—increases in the ratio were enforced after a minimum of three sessions and stable responding was observed (no group-wise linear trends). In this experiment, 28 rats self-administered IV nicotine with oral menthol (160 µM, n = 14; 320 µM, n = 14) the remaining 34 rats self-administered IV nicotine with oral licorice (0.1%, n = 17; 1%, n = 17).
Experiment 4
Each group (CR or Neutral, n = 10/group) self-administered oral licorice (1%) with IV saline infusions (0 µg/kg/infusion) on an FR2 to confirm that the flavor served as a conditioned reinforcer.
Refinement
Over the course of three experiments, we altered and refined the protocol for presenting flavor CRs during self-administration in a manner that placed the CR within the same contingency as nicotine (Table 1).
Table 1.
Refinement of Testing Procedures Across Experiments
| Experiment | Active | Inactive | Intravenous reinforcer during acquisition | Target oral stimulus |
|---|---|---|---|---|
| 1 | Nose poke | Nose poke | 30 µg/kg/infusion | 320 µM menthol |
| 2 | Lick | — | 3.5 µg/kg/infuion | 320 µM menthol |
| 3 | Lick | Lick | 7.5 µg/kg/infusion | 160 or 320 µM menthol |
| 0.1 or 1% licorice root extract | ||||
| 4 | Lick | Lick | 0 µg/kg/infusion | 1% licorice root extract |
Data Analyses
All IVSA data were analyzed with mixed analysis of variance (ANOVA) using a diagonal repeated covariance type. Session was a within-subject factor; group (CR vs. Neutral) was the primary between-subjects factor of interest; however, intensity and drug were also included as between-subjects factors where appropriate. For experiment 4, a preference ratio was calculated for licks (flavor–water/flavor + water) and one-sample t tests were used to compare the preference ratio in each group (CR or Neutral) to the hypothetical mean ratio of 0 (equal licks on each sipper). Nose pokes or licks (responses) were the principal dependent measures.
Results
Experiment 1: Acquisition
All three groups (CR, Neutral, and Water) acquired nicotine IVSA during the acquisition tests (sessions 1–10, Supplementary Figure 4). This was confirmed by significant main effects of session and nose poke (ps ≤ .005) during FR1 and FR2 tests. No other main effects or interactions were significant (ps ≥ .14). For FR5 tests, the significant group × nose poke × session interaction [F(6,66) = 2.73, p = 0.02] indicated that the CR group increased active nose pokes across sessions, relative to the Water controls. Second-order contrasts confirmed that the CR group had more active nose pokes than the Water group on session 10 (p = .014); no other comparisons were significant (ps ≥ .18).
Experiment 1: Dose–Response
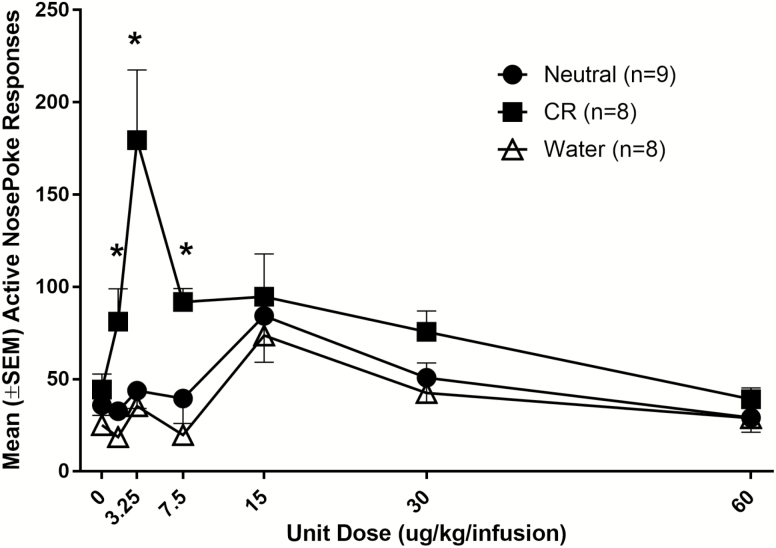
The CR group increased responding at low nicotine doses, relative to the Neutral and Water groups (Figure 1). This was confirmed by two-way ANOVA with significant main effects of group [F(2,22) = 11.26, p < .001] and dose [F(6,132) = 7.8, p < .001] as well as a significant group × dose interaction [F(12,132) = 3.83, p < .001]. Second-order contrasts showed that the CR group made significantly more active nose pokes than Water controls for the 1.5, 3.25, and 7.5 µg/kg/infusion nicotine doses (ps < 0.001) but did not differ from the Water group on any other dose tested (ps ≥ .09). The Neutral group did not differ from the Water controls on any dose tested (ps ≥ 0.16). All groups also responded more on the active, relative to the inactive nose key at the acquisition dose (30 µg/kg/infusion) and the peak dose (15 µg/kg/infusion), but only the CR group discriminated between the active and inactive keys at lower doses. This was confirmed by a three-way group × dose × nose key interaction (p < .001). Follow-up contrasts (data not shown) confirmed that increased responding at the active, relative to the inactive nose key was only observed for all groups from 15 to 30 µg/kg/infusion (corrected ps < .05) but only the CR responded significantly more at the active nose key at lower doses (0–7.5 µg/kg/infusion, corrected ps < .05).
Figure 1.
Average (±SEM) responses for intravenous nicotine and menthol (Neutral and conditioned reinforcer [CR] groups) or tap water (Water group) presented in the liquid dipper over unit nicotine test doses (µg/kg/infusion). * CR group made significantly more active nose pokes than Neutral and Water groups (p < .05).
Experiment 2: Lick Response
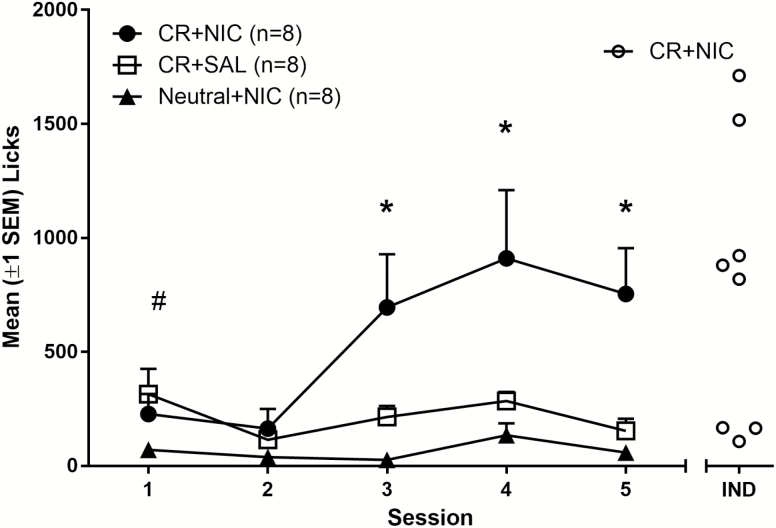
Acquisition of IVSA was only observed in the CR + NIC group (Figure 2). The CR + NIC group made more lick responses across sessions than the CR + SAL group, confirming that nicotine increased responding for a CR. This was confirmed by two-way ANOVA with significant main effects of group [F(2,21) = 9.9, p < .01] and session [F(4,84) = 4.5, p < 0.01] as well as a significant group × session interaction [F(8,84) = 3.2, p < 0.01]. The planned contrasts revealed that CR + NIC rats made more lick responses than the CR + SAL group on sessions 3–5 (ps ≤ .02). The Neutral + NIC rats made fewer responses than the CR + SAL group on the first session (p = .03), but these groups made similar numbers of lick responses on all other sessions (ps ≥ .33). We noted that rats in the CR + NIC group tended to separate themselves into three cohorts: low responders (n = 3), moderate responders (n = 3), and high responders (n = 2). Therefore, plotted individual licks (Figure 2, right abscissa) that showed that only five of the eight rats showed an increase in self-administration relative to controls.
Figure 2.
Average (±SEM) lick responses for rats self-administering intravenous (IV) nicotine (7.5 µg/kg/infusion) and oral menthol (320 µM, Neutral + NIC and CR + NIC groups) or IV saline and oral menthol (320 µM, CR + SAL group). IND (right side) represents data from individuals in the CR + NIC group averaged over the final two test days. #CR + SAL rats made more licks than Neutral + NIC controls (p < .05). *CR + NIC group made significantly more licks than both control groups (p < .05). CR, conditioned reinforce.
Experiment 3: Menthol Intensity
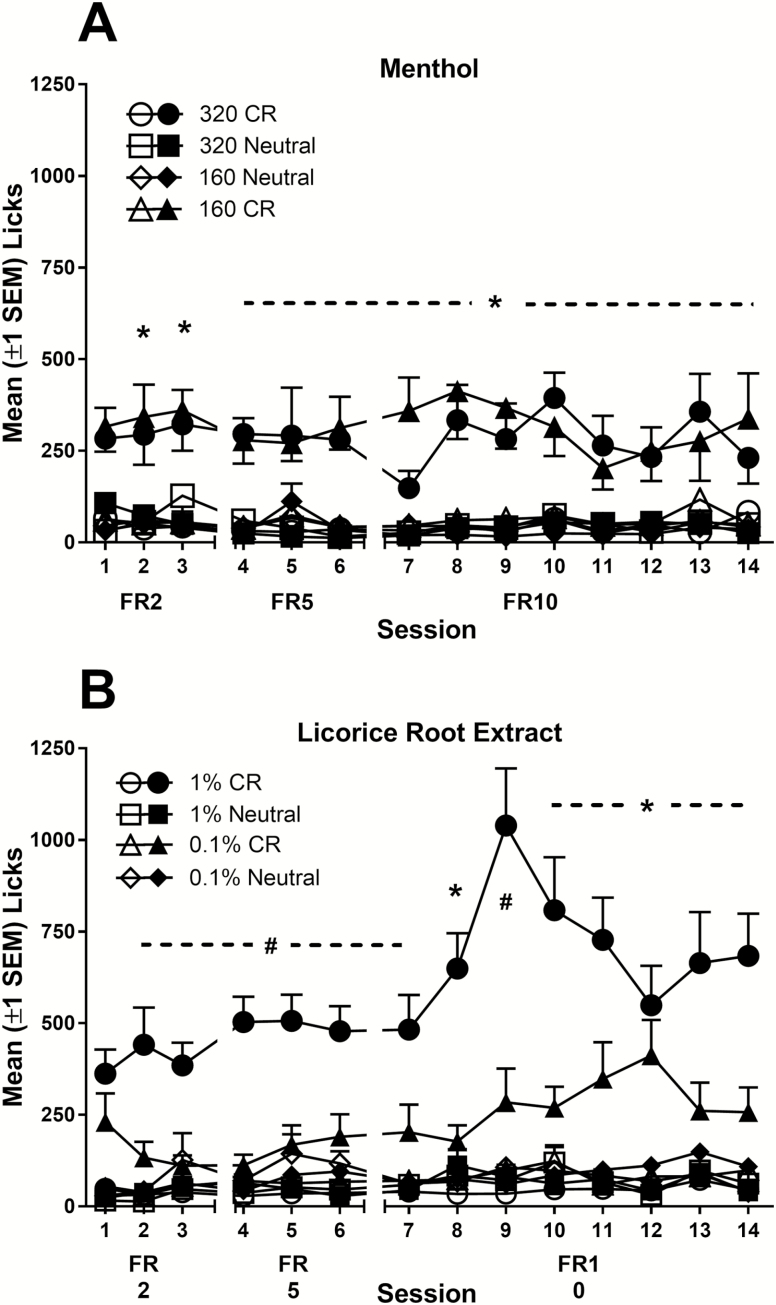
CR groups responded more at the active sipper than the inactive sipper regardless of flavor concentration. In contrast, the Neutral groups did not acquire nicotine IVSA (Figure 3A). This was confirmed by ANOVA with significant main effects of group [F(1,23) = 12.5, p < .01] and sipper [F(1,23) = 20.3, p < .001] and a significant group × sipper × session [F(2,46) = 3.34, p = .04] interaction. Follow-up contrasts confirmed that only rats in the CR groups (160 and 320) made more active sipper responses, relative to inactive sipper responses on sessions 2–3 (ps ≤ 0.01). A similar pattern emerged during FR5 tests (sessions 4–6), but the three-way interaction including session was not significant (p = 0.34). For FR10 tests, the main effects of group and sipper and the sipper × group interaction were significant (ps < .001).
Figure 3.
Average (±SEM) lick responses that self-administered nicotine (7.5 µg/kg/infusion) with menthol (A) or licorice root extract (B). Filled symbols represent licks at the active sipper tube and open symbols represent licks at the inactive sipper tube. *Responses at active sipper significantly differ from responses at inactive sipper for both stimulus intensities (high and low concentrations) in conditioned reinforcer (CR) groups (ps < .05). #Active responses at active sipper differ from inactive sipper for CR group, but only at the higher stimulus intensity (1% licorice root extract, Panel B, ps < .05).
Experiment 3: Licorice Root Extract Intensity
The 1% licorice root extract increased nicotine self-administration in CR rats, relative to the 0.1% concentration (Figure 3B). Neutral groups did not acquire nicotine self-administration. The ANOVA revealed that all main effects and interactions involving sipper (active vs. inactive) without session were significant (sipper × group × flavor intensity, ps ≤ .02). There was also a significant main effect of group [F(1,31) = 18.6, p < .001] and a significant group × flavor intensity interaction [F(1,31) = 8.5, p < .001]. Second-order contrasts compared licks at the active versus inactive sipper tubes at each level of group (CR or Neutral) by each level of flavor intensity (0.1% or 1%). Only CR rats with access to 1% licorice root extract responded more at the active sipper than the inactive sipper on sessions 2–3 (ps ≤ .001). During FR5 tests, the identical pattern of main effects and interactions was observed—the three-way group × flavor intensity × sipper interaction was significant [F(1,31) = 6.4, p = .02]. Simple contrasts again revealed that only CR rats with access to 1% licorice root extract responded more at the active sipper than the inactive sipper (ps ≤ .01). During FR10 tests (sessions 7–14), there was a significant group × flavor intensity × sipper interaction [F(1,31) = 9.13, p < .01]. The simple contrasts revealed that CR rats with access to 1% licorice root extract responded more at the active relative to the inactive sipper on all eight tests (ps < .001). CR rats with access to 0.1% licorice root extract made more responses at the active sipper on sessions 8 and 10–14 (ps ≤ .04). Neutral rats never discriminated between the two sippers during these tests (ps ≥ .22).
Experiment 4: Tests for Conditioned Reinforcement
We confirmed that flavor conditioning established a reliable preference for the 1% licorice root extract flavor for the CR group [t(4) = 24.5, p < .001] (CR group), but there was no preference or aversion for the Neutral group [t(4) = 0.2, p = .8, (Supplementary Figure 5)].
Discussion
The present studies are the first to demonstrate that flavor CRs can promote IV nicotine self-administration. This observation was specific to low unit nicotine doses with no observable primary reinforcing effects (3–7.5 µg/kg/infusion) and was based on the incentive motivational effects of the CRs, as neutral flavors with identical physical properties did not result in self-administration. The three experiments reported here refined novel experimental techniques for presenting oral gustatory CRs during IV nicotine self-administration. In experiment 1, presentation of a menthol CR adjacent to the nose poke (liquid dipper) did not alter acquisition of operant responding for IV nicotine (30 µg/kg/infusion). However, robust increases in responding for nicotine were observed when the unit dose was reduced (1.5–7.5 µg/kg/infusion). In experiment 2, presentation of the gustatory CR in a sipper tube that also served as the operant response robustly increased self-administration of a low unit dose (3 µg/kg/infusion), but this dose appeared to be at a “threshold” for acquisition (Figure 2). Experiment 3 extended these findings to a more traditional discriminative drug self-administration task, other tobacco additive flavors (licorice), and explored the intensity of the gustatory CRs. Finally, experiment 4 confirmed that these procedures18,35–37 established the flavors as CRs. Several of the present findings are worthy of further discussion, but the most critical issues are the range of doses self-administered and their relevance to human use of tobacco and nicotine vapor products.
The dose range that supported operant responding with flavor CRs (experiments 2–3) had no primary reinforcing effects when presented alone. Our current working hypothesis for this effect is that the CRs support enough behavior for a cumulative effect of nicotine to eventually increase the salience of the CR and its ability to elicit approach behavior and operant responding.38 This effect suggests that nicotine may serve in a contextual or modulatory role in human smoking, which is in agreement with recent findings that show brain levels of nicotine remain relatively constant after successive tobacco puffs.39 Moreover, there is acute tolerance to the subjective effects of nicotine,40 which may coincide with rapid desensitization of brain nicotinic cholinergic receptors41,42—meaning that each puff is not producing the same subjective effect as the preceding one. Therefore, it is reasonable to consider the effects of nicotine as contextual and modulatory, rather than as a discrete reward arising from the “bolus” dose.43 In human smokers, the primary reinforcing effects of nicotine only account for part of the satisfaction derived from smoking,44 sensations associated with smoking (eg, taste, smells, and airway stimulation) play an important role in reinforcement,45 and smoked nicotine can enhance the reinforcing effects of nondrug stimuli.10,11 All of these findings point to a complex interaction in which nicotine plays contextual/modulatory role influencing the impact of nondrug incentives.
An important pitfall of the present studies is that we did not establish a relationship between operant responding and nicotine dose in a between-subjects design.46 However, the goal of these studies was to offer proof of concept that gustatory CRs can enhance nicotine self-administration. The gustatory CRs used in these studies are the same as those used in tobacco products, and the interaction was replicated multiple times. Another potential pitfall of experiments 2–3 is that we have not yet demonstrated that nicotine alone, or with a neutral flavor stimulus, supports acquisition of a lick response across the dose–response curve. Previous investigators have already established licking for nicotine using an FR1 schedule47 and response rates were comparable to the present studies. However, it is unclear if rats-associated individual licks or some other aspect of their behavior (eg, bursts or rate of responding48) with the effects of nicotine. In the present studies, the use of partial reinforcement schedules (FR2-FR10) was based on the topography of lick responses, which tend to occur in rapid bursts with inter-response times that are shorter than 250 ms.48 Microstructural analysis of lick topography in experiment 3 (data not shown) did not reveal any difference in burst size between CR and Neutral groups, suggesting there was no change in the hedonic properties of the flavor CR.
One finding of note is that the interaction between nicotine and licorice was a systematic function of CR intensity, but this was not true for menthol. The complex orosensory effects of menthol (astringent, oral cooling) may be more potent, especially in rodents, which may explain the lack of differences in menthol flavor intensities in these studies. On the other hand, licorice contains chemicals called glycyrrhizins that are perceived as sweet in humans but may not be perceived as such by rodents.49 A full parametric analysis of taste reactions to menthol, licorice, and other tobacco and vapor additives would be helpful for investigators who are interested in modeling the effects of CRs on nicotine intake. However, the species differences in flavor perception highlight an important feature of the present studies—it may be more important to understand how flavor CRs contribute to self-administration in animal models, relative to the physical properties of the flavors themselves. Given the divergence of chemosensory systems between humans and rodents,50 the specific physical properties of the flavors are probably less critical than their acquired meaning in tobacco and electronic nicotine delivery system models. For example, preliminary findings in our laboratory have shown that unsweetened blueberry extract is meaningless, or even slightly aversive, in rats. However, pairings between blueberry and sugar convert blueberry into a robust CR that maintains operant behavior over long periods of time, even without additional sweetening. It is worthwhile to determine whether the physical properties of these flavors interact with their status and strength as CRs, but until they have some appetitive or aversive status, their physical properties are most likely irrelevant to nicotine self-administration.
These findings raise important questions for the role of flavor ingredients in tobacco and vapor products. Our paradigm may have more ecological validity for electronic nicotine delivery system, in which flavor stimuli are experienced only with nicotine and the selected “e-juice,” compared to smoking, in which flavor stimuli are part of a complex aerosol containing more than 4000 chemical components.46,51 Notably, adults using electronic cigarettes to quit smoking tend to increase their preference for fruit flavors, relative to “tobacco”-based flavors, shortly after switching from combusted tobacco products to electronic nicotine delivery system.52 This effect could be magnified if nicotine is paired with sweet-associated flavors at critical developmental periods. If the flavor CRs increase nicotine exposure, they may promote use, exposure, and dependence, and therefore be considered unsafe additives for electronic nicotine delivery system.
Funding
This work was supported by the National Institutes of Health and Food and Drug Administration (R15 DA038843).
Declaration of Interests
There are no competing interests to report.
Supplementary Material
Acknowledgments
All procedures were approved by the East Tennessee State University Care and Use of Animals Committee. The authors would like to thank Amy Patterson, Moss Sanders, and Dakota Myers for their assistance conducting the studies reported in this manuscript.
References
- 1. Lawrence D, Rose A, Fagan P, Moolchan ET, Gibson JT, Backinger CL. National patterns and correlates of mentholated cigarette use in the United States. Addiction. 2010;105 (suppl 1):13–31. [DOI] [PubMed] [Google Scholar]
- 2. O’Connor RJ, Bansal-Travers M, Carter LP, Cummings KM. What would menthol smokers do if menthol in cigarettes were banned? Behavioral intentions and simulated demand. Addiction. 2012;107(7):1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richter P, Beistle D, Pederson L, O’Hegarty M. Small-group discussions on menthol cigarettes: listening to adult African American smokers in Atlanta, Georgia. Ethn Health. 2008;13(2):171–182. [DOI] [PubMed] [Google Scholar]
- 4. Kreslake JM, Wayne GF, Alpert HR, Koh HK, Connolly GN. Tobacco industry control of menthol in cigarettes and targeting of adolescents and young adults. Am J Public Health. 2008;98(9):1685–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rustemeier K, Stabbert R, Haussmann HJ, Roemer E, Carmines EL. Evaluation of the potential effects of ingredients added to cigarettes. Part 2: chemical composition of mainstream smoke. Food Chem Toxicol. 2002;40(1):93–104. [DOI] [PubMed] [Google Scholar]
- 6. Mangold JE, Payne TJ, Ma JZ, Chen G, Li MD. Bitter taste receptor gene polymorphisms are an important factor in the development of nicotine dependence in African Americans. J Med Genet. 2008;45(9):578–582. [DOI] [PubMed] [Google Scholar]
- 7. Snedecor SM, Pomerleau CS, Mehringer AM, Ninowski R, Pomerleau OF. Differences in smoking-related variables based on phenylthiocarbamide “taster” status. Addict Behav. 2006;31(12):2309–2312. [DOI] [PubMed] [Google Scholar]
- 8. Donny EC, Jones M. Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug Alcohol Depend. 2009;104(1–2):23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DiFranza JR, Eddy JJ, Brown LF, Ryan JL, Bogojavlensky A. Tobacco acquisition and cigarette brand selection among youth. Tob Control. 1994;3(4):334–338. [Google Scholar]
- 10. Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology (Berl). 2013;228(3):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perkins KA, Karelitz JL. Sensory reinforcement-enhancing effects of nicotine via smoking. Exp Clin Psychopharmacol. 2014;22(6):511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donny EC, Chaudhri N, Caggiula AR, et al. . Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl). 2003;169(1):68–76. [DOI] [PubMed] [Google Scholar]
- 13. Palmatier MI, Evans-Martin FF, Hoffman A, et al. . Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl). 2006;184(3–4):391–400. [DOI] [PubMed] [Google Scholar]
- 14. Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palmatier MI, Coddington SB, Liu X, Donny EC, Caggiula AR, Sved AF. The motivation to obtain nicotine-conditioned reinforcers depends on nicotine dose. Neuropharmacology. 2008;(8). doi:S0028-3908(08)00409-7 [pii] 10.1016/j.neuropharm.2008.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaudhri N, Caggiula AR, Donny EC, et al. . Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl). 2006;189(1):27–36. [DOI] [PubMed] [Google Scholar]
- 17. Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology (Berl). 2004;173(1-2):98–104. [DOI] [PubMed] [Google Scholar]
- 18. Palmatier MI, Lantz JE, O’Brien LC, Metz SP. Effects of nicotine on olfactogustatory incentives: preference, palatability, and operant choice tests. Nicotine Tob Res. 2013;15(9):1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palmatier MI, Kellicut MR, Brianna Sheppard A, Brown RW, Robinson DL. The incentive amplifying effects of nicotine are reduced by selective and non-selective dopamine antagonists in rats. Pharmacol Biochem Behav. 2014;126:50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barret ST, Bevins RA. Nicotine enhances operant responding for qualitatively distinct reinforcers under maintenance and extinction conditions. Pharmacol Biochem Behav. 2013;114-115:9–15. [PMC free article] [PubMed] [Google Scholar]
- 21. Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR. beta2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology (Berl). 2006;184(3–4):328–338. [DOI] [PubMed] [Google Scholar]
- 22. Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl). 2004;171(2):173–178. [DOI] [PubMed] [Google Scholar]
- 23. Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF. Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology. 2008;33(9):2139–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmatier MI, Marks KR, Jones SA, Freeman KS, Wissman KM, Sheppard AB. The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology (Berl). 2013;226(2):247–259. [DOI] [PubMed] [Google Scholar]
- 25. Guy EG, Fletcher PJ. The effects of nicotine exposure during Pavlovian conditioning in rats on several measures of incentive motivation for a conditioned stimulus paired with water. Psychopharmacology (Berl). 2014;231(11):2261–2271. [DOI] [PubMed] [Google Scholar]
- 26. Ksir C. Taste and nicotine as determinants of voluntary tobacco use by hamsters. Pharmacol Biochem Behav. 1983;19(4):605–608. [DOI] [PubMed] [Google Scholar]
- 27. Chen H, Sharp BM, Matta SG, Wu Q. Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology. 2011;36(13):2629–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jenkins RW Jr, Newman RH, Chavis MK. Smoke distribution and mainstream pyrolytic composition of added 14C-Menthol. Beitr Tab Int. 1970;5(6):299–301. [Google Scholar]
- 29. Carmines EL, Lemus R, Gaworski CL. Toxicologic evaluation of licorice extract as a cigarette ingredient. Food Chem Toxicol. 2005;43(9):1303–1322. [DOI] [PubMed] [Google Scholar]
- 30. Green C, Best F. Pyrolysis products of licorice. RDM. 1974;1974 (20):508476955–508476960. [Google Scholar]
- 31. Weeks JR. Chapter 6. Long-term intravenous infusion. In: Meyers RD, ed. Methods in Psychobiology: Specialized Laboratory Techniques in Neuropsychology and Neurobiology. 1st ed. Elsevier; 1972. [Google Scholar]
- 32. Capaldi ED, Owens J, Palmer KA. Effects of food deprivation on learning and expression of flavor preferences conditioned by saccharin or sucrose. Anim Learn Behav. 1994;22(2):173–180. [Google Scholar]
- 33. Messier C, White NM. Contingent and non-contingent actions of sucrose and saccharin reinforcers: effects on taste preference and memory. Physiol Behav. 1984;32(2):195–203. [DOI] [PubMed] [Google Scholar]
- 34. Smith BK, Volaufova J, West DB. Increased flavor preference and lick activity for sucrose and corn oil in SWR/J vs. AKR/J mice. Am J Physiol Regul Integr Comp Physiol. 2001;281(2):R596–R606. [DOI] [PubMed] [Google Scholar]
- 35. Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl). 2006;184(3–4):353–366. [DOI] [PubMed] [Google Scholar]
- 36. Rose JE, Mukhin AG, Lokitz SJ, et al. . Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarettes containing 11C-nicotine. Proc Natl Acad Sci USA. 2010;107(11):5190–5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perkins KA, Epstein LH, Grobe J, Fonte C. Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacol Biochem Behav. 1994;47(1):107–112. [DOI] [PubMed] [Google Scholar]
- 38. Marks MJ, Grady SR, Collins AC. Downregulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther. 1993;266(3):1268–1276. [PubMed] [Google Scholar]
- 39. Collins AC, Marks MJ. Are nicotinic receptors activated or inhibited following chronic nicotine treatment? Drug Dev Res. 1996;38(3–4):231–242. [Google Scholar]
- 40. Russell MA, Feyerabend C. Cigarette smoking: a dependence on high-nicotine boli. Drug Metab Rev. 1978;8(1):29–57. [DOI] [PubMed] [Google Scholar]
- 41. Rose JE, Behm FM, Westman EC, Bates JE, Salley A. Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacol Biochem Behav. 2003;76(2):243–250. [DOI] [PubMed] [Google Scholar]
- 42. Przulj D, McRobbie H, Hajek P. The effect of sensorimotor replacement on smoking cessation and craving. Open Addict J. 2012;5(1):41–50. [Google Scholar]
- 43. Matta SG, Balfour DJ, Benowitz NL, et al. . Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl). 2007;190(3):269–319. [DOI] [PubMed] [Google Scholar]
- 44. Levin ED, Hampton D, Rose JE. IV nicotine self-administration in rats using the consummatory operant licking response. Physiol Behav. 2010;101(5):755–758. [DOI] [PubMed] [Google Scholar]
- 45. Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106(1):217–228. [PubMed] [Google Scholar]
- 46. Hoffmann D, Wynder EL. Chemical constituents and bioactivity of tobacco smoke. IARC Sci Publ. 1986;( 74):145–165. PMID: 3623665. [PubMed] [Google Scholar]
- 47. Stedman RL. The chemical composition of tobacco and tobacco smoke. Chem Rev. 1968;68(2):153–207. [DOI] [PubMed] [Google Scholar]
- 48. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: An internet survey. Int J Environ Res Public Health. 2013;10(12):7272–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener Preference of C57BL/6ByJ and 129P3/J Mice. Chem Senses. 2001;26(7):905–913. doi:10.1093/chemse/26.7.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Young JM, Friedman C, Williams EM, Ross JA, Tonnes-Priddy L, Trask BJ. Different evolutionary processes shaped the mouse and human olfactory receptor gene families. Hum Mol Genet. 2002;11(5):535–546. doi:10.1093/hmg/11.5.535 [DOI] [PubMed] [Google Scholar]
- 51. Stedman RL. Chemical composition of tobacco and tobacco smoke. Chem Rev. 1968;68(2):153–207. [DOI] [PubMed] [Google Scholar]
- 52. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int J Environ Res Public Health. 2013;10(12):7272–7282. doi:10.3390/ijerph10127272 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.