Abstract
Introduction
Tobacco use improves mood states and smoking cessation leads to anhedonia, which contributes to relapse. Animal studies have shown that noncontingent nicotine administration enhances brain reward function and leads to dependence. However, little is known about the effects of nicotine self-administration on the state of the reward system.
Methods
To investigate the relationship between nicotine self-administration and reward function, rats were prepared with intracranial self-stimulation electrodes and intravenous catheters. The rats were trained on the intracranial self-stimulation procedure and allowed to self-administer 0.03 mg/kg/infusion of nicotine. All rats self-administered nicotine daily for 10 days (1 hour/day) and were then switched to an intermittent short access (ShA, 1 hour/day) or long access (LgA, 23 hour/day) schedule (2 days/week, 5 weeks).
Results
During the first 10 daily, 1-hour sessions, nicotine self-administration decreased the reward thresholds, which indicates that nicotine potentiates reward function. After switching to the intermittent LgA or ShA schedule, nicotine intake was lower in the ShA rats than the LgA rats. The LgA rats increased their nicotine intake over time and they gradually consumed a higher percentage of their nicotine during the light phase. The nicotinic acetylcholine receptor (nAChR) antagonist mecamylamine induced a larger increase in reward thresholds (ie, anhedonia) in the LgA rats than the ShA rats. In the LgA rats, nAChR blockade with mecamylamine decreased nicotine intake for 2 hours and this was followed by a rebound increase in nicotine intake.
Conclusions
A brief period of nicotine self-administration enhances reward function and a high level of nicotine intake leads to dependence.
Implications
These animal studies indicate that there is a strong relationship between the level of nicotine intake and brain reward function. A high level of nicotine intake was more rewarding than a low level of nicotine intake and nicotine dependence was observed after long, but not short, access to nicotine. This powerful combination of nicotine reward and withdrawal makes it difficult to quit smoking. Blockade of nAChRs temporarily decreased nicotine intake, but this was followed by a large rebound increase in nicotine intake. Therefore, nAChR blockade might not decrease the use of combustible cigarettes or electronic cigarettes.
Introduction
Tobacco addiction is a chronic disorder that is characterized by compulsive smoking and affective withdrawal signs on smoking cessation. Clinical studies show that anhedonia associated with smoking cessation is a risk factor for relapse.1–3 Therefore, it is important to determine the relationship between nicotine intake and negative mood states during abstinence from chronic nicotine intake.
In most animal studies, the effect of nicotine on mood states was investigated by administering nicotine noncontingently via injections or minipumps.4 Studies that used the intracranial self-stimulation (ICSS) procedure showed that acute nicotine administration lowers the ICSS thresholds, which indicates that nicotine potentiates reward function.5,6 Chronic administration of nicotine leads to the development of dependence and cessation of nicotine administration leads to affective and somatic withdrawal signs.7,8 In the ICSS procedure, nicotine withdrawal is reflected in elevated brain reward thresholds, which is indicative of an anhedonic state.7 Animal studies with noncontingently administered drugs have contributed to a better understanding of the role of the brain reward system in the development and maintenance of tobacco addiction.9 However, there is evidence that the effects of noncontingently administered drugs differ from those of self-administered drugs.10–12 Very few nicotine self-administration studies have been conducted to explore the relationship between nicotine intake and brain reward function. The reinforcing effects of nicotine have been investigated using limited or short access (ShA, 1 hour) and long access (LgA, 21–23 hours) self-administration procedures.13–15 The ShA model has been widely used to study the neurobiological mechanisms that mediate the acute reinforcing effects of nicotine in nondependent animals. The LgA procedure models human smoking more closely as humans titrate their nicotine levels throughout the day. Nicotine self-administration under the LgA model leads to high levels of nicotine intake and the development of dependence as indicated by mecamylamine precipitated somatic withdrawal signs.14 Rodents are nocturnal and nicotine intake is higher during the dark phase than the light phase.14 However, in animals with LgA to nicotine, nicotine intake gradually increases during the light phase.14 This increase in nicotine intake during the light phase is most pronounced in animals with high levels of nicotine intake.14 This is in line with the observation that highly dependent smokers smoke more during the night than less dependent smokers.16
Animal studies have shown that daily LgA to either cocaine, heroin, or methamphetamine leads to the escalation of drug intake and an enhanced motivation for drug intake.17–19 Although rats with daily LgA to nicotine self-administer more nicotine than animals with ShA, they do not escalate their intake.14,20 However, they escalate their nicotine intake when they have intermittent LgA to nicotine.21 In the intermittent LgA procedure, the animals have continuous access to nicotine for 21 hours and then several days off between self-administration sessions.21 Little is known about the relationship between intermittent ShA and LgA to nicotine and the state of the reward system. The main goal of the present studies was to investigate the relationship between nicotine self-administration and brain reward function in rats. The rats had initially daily ShA (1 hour, 5 days/week) to nicotine and this was followed by intermittent LgA (23 hours, 2 days/week) in the first group and intermittent ShA (1 hour, 2 days/week) in the second group. In addition to this, we determined whether circadian stability of nicotine intake changed over time in the LgA group. Humans typically smoke during the day but a subgroup of highly dependent smokers also smoke during the night.16 Therefore, it was expected that the LgA rats would gradually increase their nicotine intake during the light phase (ie, inactive period) and thereby decrease circadian stability of nicotine intake. The noncompetitive nicotinic acetylcholine receptor (nAChR) antagonist mecamylamine decreases nicotine intake in nondependent rats with ShA to nicotine and nAChR blockade has shown promise as an add-on treatment for smoking cessation in humans.13,22 Therefore, we also investigated whether mecamylamine decreases nicotine intake in dependent rats with intermittent LgA to nicotine.
Methods
Animals
Male Wistar rats (200–225 g, Charles River, Raleigh, NC) were housed in a climate-controlled vivarium on a reversed 12-hour light–dark cycle (light off at 8 am). The animals were housed individually to protect the catheters. Food and water were available ad libitum in the home cage and the rats had access to water during the nicotine self-administration sessions. The experimental protocols were approved by the University of Florida Institutional Animal Care and Use Committee.
Drugs
Nicotine and mecamylamine were purchased from Sigma (Sigma-Aldrich, St. Louis, MO). Nicotine and mecamylamine were dissolved in sterile saline (0.9% sodium chloride). Mecamylamine was administered subcutaneously (sc) in a volume of 1 ml/kg body weight. Nicotine doses are expressed as base and mecamylamine doses are expressed as salt.
Intermittent LgA to Nicotine and Reward Function
Adult male rats were prepared with ICSS electrodes in the medial forebrain bundle and trained on the ICSS procedure (Supplementary Figure 1).23 After completing the ICSS training, the rats were prepared with intravenous (iv) catheters and then trained to respond for food pellets (45 mg, F0021; Bio-Serv, Frenchtown, NJ) under an FR5-TO20s schedule in 30–60-minute sessions.24 After completing the food training sessions, the food operant sessions were reduced to 20 minutes. Baseline brain reward thresholds and response latencies were determined on 3 days during which the rats did not respond for food or nicotine. Then the thresholds and latencies were assessed immediately after 20 minutes of operant responding for food (3 days) and before and after operant responding for food (1 day). The rats self-administered nicotine daily for 10 days (0.03 mg/kg/infusion, 1 h per session, 5 days/week) starting at 9 am. During the first two sessions, that maximum number of infusions was set to 12 to prevent the rats from taking an excessive amount of nicotine. The thresholds and latencies were assessed immediately before and after nicotine self-administration. After completing the daily self-administration sessions, the rats were switched to an intermittent LgA schedule. The rats had continuous access to nicotine for 23 hours and had 2–3 days off between the self-administration sessions.21 The rats had access to water during the LgA sessions. The cue light (65 lux) above the right lever (RL, active lever) was turned on during the 20 seconds time-out period. Thresholds and latencies were assessed immediately before, immediately after, and 1 day after the self-administration sessions. The thresholds were assessed 1 day after the self-administration sessions because negative affective withdrawal signs are mostly likely to be detected at this timepoint.25 After 5 weeks of intermittent LgA to nicotine, it was investigated if nAChR blockade with mecamylamine (0, 1.5, 3 mg/kg, sc) precipitates withdrawal. Mecamylamine was administered according to a Latin-square design, 2 hours after the self-administration sessions. There were at least 2 days between the mecamylamine injections. After the withdrawal study, the effect of nAChR blockade with mecamylamine on nicotine self-administration was examined. Mecamylamine (0, 1.5, 3 mg/kg, sc) was administered according to a Latin-square design, 10 minutes before the LgA self-administration session.
Intermittent ShA to Nicotine and Reward Function
This second group of rats received the same treatments as the first group of rats, with the exception that after the daily self-administration sessions, the animals had intermittent ShA (1 hour), instead of LgA (23 hours), to nicotine. Briefly, adult male (N = 10) rats were trained on the ICSS procedure, received iv catheters, and were trained to respond for food pellets. The rats self-administered nicotine daily for 10 days (0.03 mg/kg/infusion, 1 hour per session). The brain reward thresholds and response latencies were assessed at the same timepoints as in the first group of rats. The rats were then switched to an intermittent ShA nicotine self-administration schedule. The rats self-administered nicotine for 1 hour/day and had 2–3 days off between self-administration sessions. The thresholds and latencies were assessed immediately before, immediately after, and 1 day after the self-administration sessions. After 5 weeks of intermittent ShA to nicotine, it was investigated whether blockade of nAChRs with mecamylamine (0, 1.5, 3 mg/kg, sc) precipitates withdrawal. Mecamylamine was administered according to a Latin-square design, 2 hours after the self-administration sessions, and there were at least 2 days between mecamylamine injections.
Electrode Implantations and ICSS Procedure
Rats were anesthetized with an isoflurane and oxygen vapor mixture and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Electrodes were implanted in the medial forebrain bundle with the incisor bar set 5 mm above the interaural line (anterior–posterior –0.5 mm, medial–lateral ±1.7 mm, dorsal–ventral –8.3 mm from dura).26 After the recovery period, the rats were trained on a modified discrete-trial ICSS procedure in operant conditioning chambers (Med Associates, Georgia, VT). Brain stimulation was delivered by constant current stimulators (Model 1200C, Stimtek, Acton, MA). Each test session provided a brain reward threshold and response latency. The reward threshold (µA) was defined as the midpoint between stimulation intensities that supported responding and stimulation intensities that failed to support responding. The response latency (s) was defined as the time interval between the beginning of the noncontingent stimulus and a positive response. Elevations in brain reward thresholds reflect anhedonia.27 Drugs that have sedative effects or induce motor impairments increase the response latency and stimulants decrease the response latency.5,28
Intravenous Catheter Implantation
Rats were anesthetized with an isoflurane–oxygen vapor mixture and prepared with a catheter in the right jugular vein.29 The catheters consisted of silastic tubing (length 13.5 cm, 0.51 mm inside diameter, 0.94 mm outside diameter, Dow Corning, Midland, MI) that was connected to a 22-gauge stainless steel guide cannula, which was molded onto a durable polyester fiber mesh (Plastics One, Roanoke, VA). The tubing was passed from the mid-scapular region to the ventral thorax and inserted into the right jugular vein (4.0 cm). The rats received daily iv infusions with the antibiotic Gentamycin (2 mg/kg, 0.2 ml, Sigma-Aldrich) during the 1-week recovery period. To ensure long-term patency of the catheters, 0.04-ml sterile glycerol (Amresco Inc., Solon, OH) with 100 units of heparin per milliliter (Sagent Pharmaceuticals, Schaumburg, IL) was infused into the catheter after the administration of the antibiotic. The catheters were capped with a short piece of tubing (Saint-Gobain Performance Plastics, Valley Forge, PA) that was plugged with a piece of monofilament.
Operant Responding for Food and Nicotine Self-administration
Food training and nicotine self-administration sessions were conducted as described before.29 First, the rats were trained to respond for food pellets. For the duration of the food training, the rats were fed 17–20 g (75%–85% of baseline ad libitum calories) of laboratory chow per day.30 The rats were trained to respond on the RL (active lever) to receive food pellets. Responding on the left lever (LL, inactive lever) did not have scheduled consequences. Training started under a fixed-ratio 1, time-out 1 second (FR1 TO1-s) reinforcement schedule and the schedule was increased until the rats responded under an FR5 TO20-s schedule. After completion of the food training sessions, the rats were fed 20 g of laboratory chow per day. The rats then self-administered nicotine at the 0.03 mg/kg/infusion dose for 10 days. Responding on the RL resulted in the delivery of a nicotine infusion (0.1 ml infused over a 5.6-second time period). All nicotine self-administration sessions were conducted under an FR5-TO20s schedule. The delivery of an infusion was paired with a cue light, which remained illuminated throughout the 20-second time-out period. During the time-out period, both levers were retracted.
Statistics
The ICSS parameters (thresholds and latencies) were expressed as a percentage of the 3-day baselines. The ICSS parameters were analyzed with repeated measures two-way analyses of variance (ANOVA) with time (before vs. after nicotine intake or days 1–10) or mecamylamine dose as within-subject factors. The effect of time (days 1–10) on nicotine intake, responding on the RL (active lever), and responding on the LL (inactive lever) was analyzed with one-way repeated measures ANOVAs. The effect of Access schedule (ShA vs. LgA) and time (before vs. after nicotine intake) on nicotine intake and responding on the RL and LL was analyzed with a two-way repeated measures ANOVA with Access schedule as between-subject factor and time as within-subject factor. The effect of mecamylamine on responding on the levers was analyzed with a two-way repeated measures ANOVA with mecamylamine dose and time as within-subject factors. The effect of mecamylamine dose on total nicotine intake and total responses on the RL and LL was analyzed with one-way repeated measures ANOVAs with the mecamylamine dose as within-subject factor. Statistically significant results in the ANOVA’s were followed by the Bonferroni post hoc tests (p < .05). The ANOVAs and post hoc tests were conducted with GraphPad Prism 7 software for Windows.
A nonparametric statistical algorithm (JTK_CYCLE) was used to determine whether the responses on the RL and LL cycled (ie, circadian rhythm) during the LgA period.31 JTK_CYCLE was developed to identify cycling variables in large datasets.31 In our previous studies, we used this statistical method to determine circadian temperature stability in humans.32,33 A decrease in circadian stability means that the difference in nicotine intake between the dark and light phase becomes smaller. A decrease in circadian stability is indicative of the development of dependence as night smoking is most common in people with a high level of dependence.16 JTK_CYCLE provides Bonferroni-corrected p values (p < .05), which indicates whether responses on the levers cycled. The negative natural log (ln) of the adjusted p values was used to determine if circadian stability (measure of circadian rhythmicity) of nicotine intake changed over time (day 1–3 vs. day 8–10 of LgA). The stability of each animal’s circadian rhythm is reflected by the variation of the oscillatory patterns of circadian data over several days.31,33
Results
Intermittent LgA to Nicotine and Reward Function
The effects of nicotine self-administration (daily, 1 hour) on the reward thresholds and response latencies were investigated. Nicotine intake (F9,63 = 4.637, p < .0001;, Supplementary Figure 2a) and responding on the RL (F9,63 = 4.762, p < 0001; Supplementary Figure S2b) initially decreased and then increased. Responding on the LL was very low and did not change over time (Supplementary Figure 2b). The self-administration of nicotine did not affect the brain reward thresholds or the response latencies (Supplementary Figure 3a and b). A correlational analysis was conducted to determine if there was a relationship between nicotine intake (mg/kg) and changes in reward thresholds and response latencies. During days 1–10, there was no relationship between nicotine intake and changes in reward thresholds or response latencies. During the first 2 days of nicotine self-administration, a high percentage of rats (60% on day 1 and 50% on day 2) reached the maximum number of infusions allowed. This plateau level of nicotine intake may have affected the outcome of the correlational analysis. After the exclusion of days 1 and 2, there was a negative correlation between nicotine intake and changes in reward thresholds (r = –.38, p < .01) and response latencies (r = –.38, p < .01). This indicates that sessions with the highest level of nicotine intake led to the largest decrease in reward thresholds and response latencies.
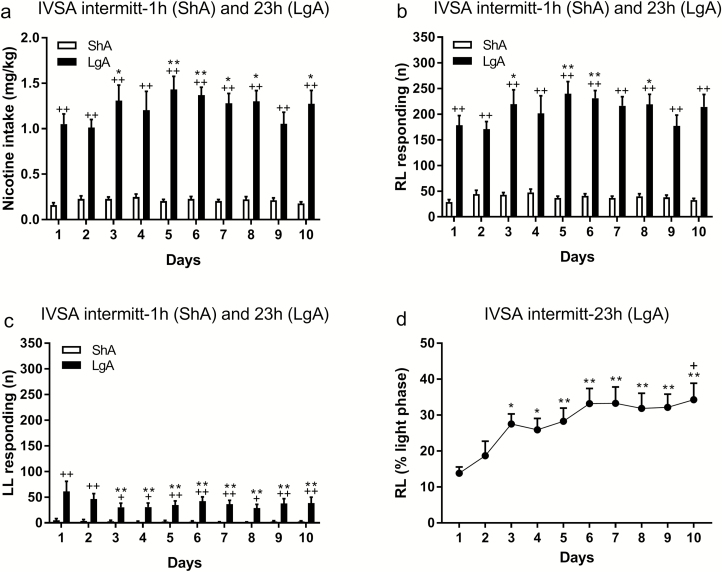
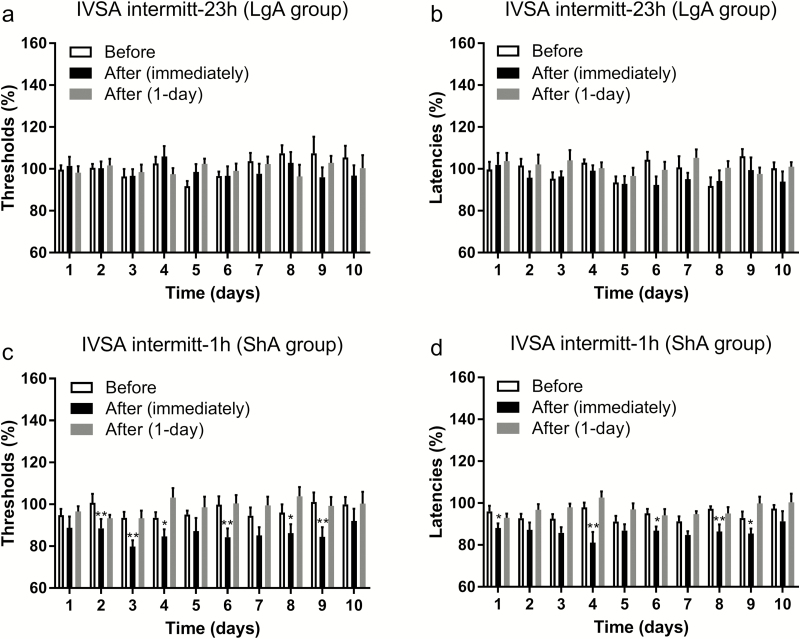
After the rats had had access to nicotine for 1 hour/day for 10 days, they were switched to an intermittent LgA schedule. Switching the rats from the daily ShA schedule to the intermittent LgA schedule led to a dramatic increase in nicotine intake. During the last ShA session, the average number responses on the RL was 35 and it was 179 during the first LgA session. During the LgA period, nicotine intake (F9,63 = 3.09, p < .01; Figure 1a) and responding on the RL increased over time (F9,63 = 3.016, p < .01; Figure 1b), and responding on the LL decreased over time (F9,63 = 2.605, p < .05; Figure 1c). During the first hour of the LgA session, responding on the RL initially decreased but then stabilized (F9,63 = 2.84, p < .01; Supplementary Figure 4). Responding on the LL did not change over time. We also determined whether nicotine intake during the light period, as a percentage of total nicotine intake, changed over time. Responding on the RL during the light period increased over time (F9,63 = 5.36, p < .0001; Figure 1d) and responding on the LL did not change. Figure 1d shows that during the first LgA session, the rats consumed 14% of the nicotine during the light phase and during the 10th session this was 34%. This indicates that the animals gradually consumed a higher percentage of their nicotine during the light phase. We also conducted an analysis with JTK_CYCLE to determine whether circadian stability of nicotine self-administration changed over time. When the data from days 1–10 were combined, all eight rats had a significant circadian rhythm in RL responding and three rats had a circadian rhythm in LL responding (Supplementary Table 1). To investigate if the circadian rhythm changed over time, we compared the negative ln of the p value for days 1–3 with days 8–10 (Supplementary Table 2). The animals displayed higher circadian stability for RL responding than LL responding (F1,14 = 12.76, p < .01) and circadian stability decreased over time (F1,14 = 10.11, p < .01). The post hoc analysis indicated that the decrease in circadian stability was because of a significant decrease in circadian stability for RL responding (Supplementary Figure 5). The brain reward thresholds and response latencies were assessed immediately before, after, and 1 day after the intermittent LgA nicotine self-administration sessions. The reward thresholds were the same immediately before, immediately after, and 1 day after nicotine self-administration (Figure 2a). The response latencies were decreased after nicotine self-administration (F2,14 = 11.93, p < .001; Figure 2b), but the post hoc tests did not reveal effects at any timepoints. An additional ANOVA showed that the latencies were decreased immediately after nicotine self-administration (F1,7 = 19.39, p < .01; Figure 2b) but not 1 day later.
Figure 1.
Higher levels of nicotine intake in the intermittent long access (LgA) rats than in the intermittent short access (ShA) rats. Rats had ShA or LgA to nicotine for 2 days per week and total nicotine intake (a), responding on the RL (active lever, b) and LL (inactive lever, c) lever was recorded. Plus signs indicate a higher level of nicotine intake or operant responding in the LgA group than in the ShA group. Asterisks indicate an increase or decrease in nicotine intake or operant responding compared to day 1. In the LgA rats, nicotine intake during the light phase increased over time (d). Asterisks indicate that a higher percentage of nicotine (light phase intake as percentage of total intake) is self-administered during the light phase compared to day 1. The plus sign indicates a higher percentage of nicotine intake during the light phase compared to day 4. *, + p < .05, **, ++ p < .01. IVSA, intravenous self-administration; RL, right lever; LL, left lever. N = 8–10/group. Data are expressed as means ± SEM.
Figure 2.
Effect of intermittent short access (ShA) and long access (LgA) to nicotine on brain reward thresholds and latencies. One group of animals had intermittent LgA (23 hours) to nicotine and the reward thresholds (a) and latencies (b) were assessed immediately and 1 day after nicotine self-administration. The other group of animals had intermittent ShA (1 hour) to nicotine and the reward thresholds (c) and response latencies (d) were also assessed immediately and 1 day after nicotine self-administration. *p < .05, **p < .01. IVSA, intravenous self-administration. N = 8–10/group. Data are expressed as means ± SEM.
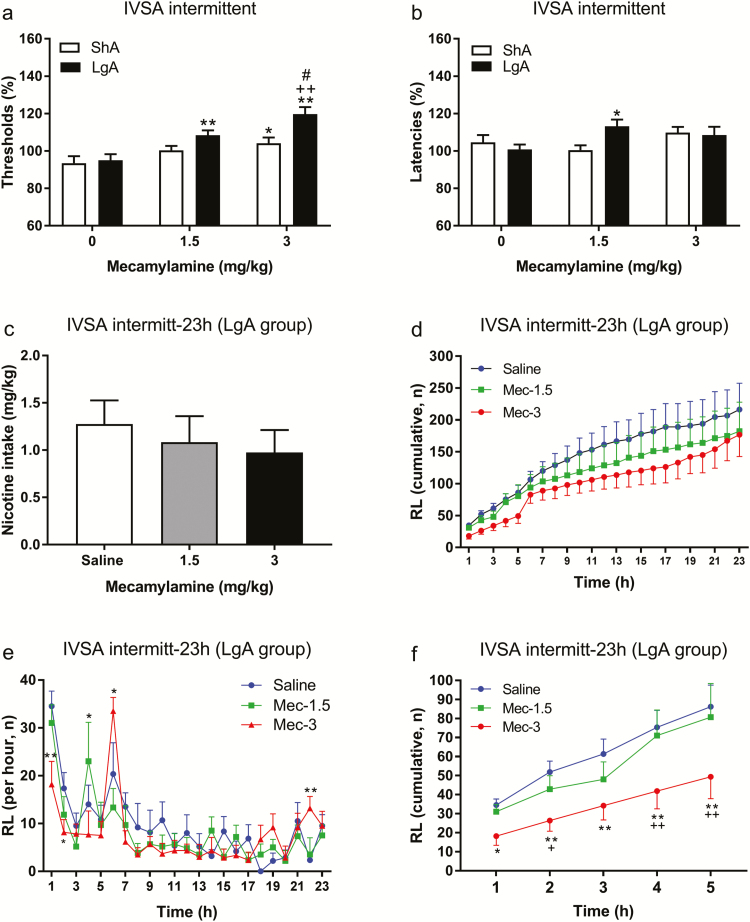
After the 5-week LgA period, we investigated if the nAChR antagonist mecamylamine precipitates affective withdrawal signs. To compare the effects of mecamylamine in intermittent ShA and LgA rats, the data from these two groups were analyzed together. Mecamylamine elevated the reward thresholds (mecamylamine dose: F2,30 = 19.53, p < .0001; Figure 3a) and the effect of mecamylamine was larger in the LgA rats than in the ShA rats (Access schedule: F1,15 = 7.559, p < .05). The post hoc analysis indicated that the low and high dose of mecamylamine elevated the reward thresholds in the LgA rats. The highest dose of mecamylamine also elevated the reward thresholds in the ShA rats but this dose induced a larger increase in the reward thresholds of the LgA rats. Mecamylamine differently affected the response latencies of the ShA and LgA rats (mecamylamine dose × Access schedule: F2,30 = 4.201, p < .05; Figure 3b). The post hoc analyses revealed that the low dose of mecamylamine increased the response latencies of the LgA rats but not those of the ShA rats.
Figure 3.
Mecamylamine precipitates nicotine withdrawal in long access (LgA) rats and briefly reduces nicotine intake. The nicotinic acetylcholine receptor (nAChR) mecamylamine induced a larger increase in the reward thresholds (a) and response latencies (b) in the LgA rats than in the short access (ShA) rats. Asterisks indicate an increase in reward thresholds or response latencies compared to rats that self-administered nicotine under the same schedule (ShA or LgA) and received vehicle. Plus signs indicate an increase in reward thresholds compared to rats that had ShA to nicotine and received the same dose of mecamylamine. Pound signs indicate an increase in reward thresholds compared to rats that had LgA to nicotine and received 1.5 mg/kg of mecamylamine. There is a rebound increase in nicotine intake after brief period of nAChR blockade in the LgA rats. Pretreatment with mecamylamine did not affect nicotine intake over a 23-hour period (c, d). Mecamylamine briefly decreased nicotine intake and this was followed by an increase in nicotine intake (e, f). Asterisks indicate an increase or decrease in responding on the right lever (RL; active lever) compared to the saline-treated animals. Plus signs indicate a decrease in responding on the RL compared to animals treated with 1.5 mg/kg of mecamylamine. *, +, # p < .05, **, ++ p < .01. IVSA, intravenous self-administration. N = 8–10/group. Data are expressed as means ± SEM.
After the withdrawal studies, it was investigated if mecamylamine affected the self-administration of nicotine under the LgA schedule. Mecamylamine decreased the self-administration of nicotine at the beginning of the session and this was followed by a rebound increase in nicotine intake. Pretreatment with mecamylamine did not affect total nicotine intake (Figure 3c) and responding on the RL (Figure 3d) during the 23-hour self-administration period. A close look at the response pattern shows that the effect of mecamylamine differs over the 23-hour period (time: F22,110 = 18.22, p < .0001; time × mecamylamine: F44,220 = 2.482, p < .0001; Figure 3e). The post hoc analysis indicated that 3 mg/kg mecamylamine significantly decreased nicotine self-administration during the first 2 hours of the self-administration session (Figure 3e). The mecamylamine-induced decrease in responding was followed by a very strong increase in nicotine self-administration at the 6-hour timepoint and a small increase at the end of the 23-hour period. A similar, but less pronounced effect was observed after the administration of a lower dose of mecamylamine (1.5 mg/kg). After the administration of 1.5 kg/kg of mecamylamine, nicotine intake was slightly, but not significantly, decreased during the first 3 hours, and this was followed by a significant increase in nicotine self-administration at the 4-hour timepoint. A separate analysis was conducted over the first 5 hours of nicotine self-administration. During this period, mecamylamine decreased nicotine self-administration (time: F4,20 = 25.15, p < .0001; mecamylamine: F2,10 = 6.034, p < .05; Figure 3f). The post hoc analysis showed that the mecamylamine-induced decrease in nicotine intake was mainly because of the effect of the 3 mg/kg of mecamylamine dose (Figure 3f). Overall, this indicates that mecamylamine decreases nicotine intake at the beginning of the self-administration session and this is followed by a robust rebound increase in nicotine intake.
Intermittent ShA to Nicotine and Reward Function
The rats self-administered nicotine for 10 daily, 1 hour, sessions, and the reward thresholds and latencies were assessed before and after the self-administration sessions. Nicotine intake (F9,81 = 5.935, p < .0001; Supplementary Figure 2a) and responding on the RL (F9,81 = 3.964, p < .003; Supplementary Figure 2c) was relatively high during the first session and then decreased and stabilized. Responding on the LL did not change over time (Supplementary Figure 2c). Nicotine self-administration decreased the brain reward thresholds (F1,9 = 30, p < .001; Supplementary Figure S3c) and the response latencies (F1,9 = 19.72, p < .01; Supplementary Figure 3d). The post hoc analysis indicated that the brain reward thresholds were significantly lower after than before nicotine self-administration on days 4, 5, 8, and 10. A correlational analysis was conducted to determine if there was a relationship between nicotine intake and changes in the reward thresholds and response latencies. There was no significant relationship between nicotine intake and changes in reward thresholds or response latencies. The relationship between these variables was not observed when the analysis included days 1–10 or when days 1–2 were excluded.
After 10 days of daily ShA to nicotine, the rats were switched to the intermittent ShA schedule. When the rats were on the intermittent ShA schedule, nicotine intake (F9,81 = 2.358, p < .05; Figure 1a) and responding on the RL (F9,81 = 2.632, p < .05; Figure 1b) slightly increased and then decreased. Responding on the LL did not change over time (Figure 1c). The brain reward thresholds and response latencies were assessed immediately before, immediately after, and 1 day after 1 hour of nicotine self-administration. The reward thresholds (F2,18 = 16.9, p < .0001; Figure 2c) and response latencies (F2,18 = 23.49, p < .0001; Figure 2d) were decreased after nicotine self-administration. The post hoc analysis indicated that the thresholds and latencies were decreased immediately after nicotine self-administration but not 1 day later.
Integration, ShA, and LgA to Nicotine and Reward Function
Animals with intermittent LgA to nicotine had a higher level of nicotine intake than animals with intermittent ShA to nicotine (F1,16 = 101.9, p < .0001; Figure 1a). Furthermore, responding for nicotine increased over time in the LgA group but not in the ShA group (time: F9,144 = 4.0, p < .001; Access schedule × time: F9,144 = 3.57, p < .001). Animals that had intermittent LgA to nicotine also responded more on the RL than animals that had intermittent ShA to nicotine (F1,16 = 101.7, p < .0001; Figure 1b). Responding on the RL increased over time in the LgA group but did not change in the ShA group (time: F9,144 = 3.727, p < .001; Access schedule × time: F9,144 = 3.618, p < .001). The LgA rats responded more on the LL than the ShA rats (Access schedule: F1,16 = 21.49, p < .001; Figure 1c). Responding on the LL decreased over time in the LgA rats and did not change in the ShA rats (time: F9,144 = 3.771, p < .001; time × Access schedule: F9,144 = 2.59, p < .01).
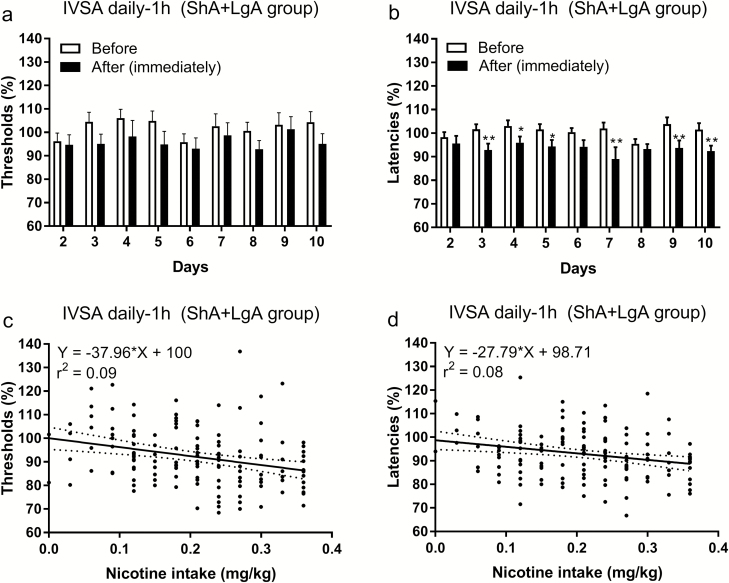
Daily ShA (1 hour, days 1–10) nicotine self-administration lowered the brain reward thresholds (F1,17 = 8.098, p < .05; Figure 4a) and decreased the response latencies (F1,17 = 17.19, p < .001; Figure 4b). A correlational analysis using data from days 1–10 showed that there was a negative correlation between nicotine intake and brain reward thresholds (r = –.17, p < .05), but not between nicotine intake and the response latencies. A correlation analysis using data from days 3 to 10 showed that there was a negative correlation between nicotine intake and brain reward thresholds (r = –.29, p < .001) and between nicotine intake and the response latencies (r = –.26, p < .01). Thus, a higher level of nicotine intake leads to a greater decrease in reward thresholds and shorter response latencies. To further explore the relationship between nicotine intake and changes in reward thresholds and response latencies a linear regression analysis was conducted on nicotine intake and ICSS data from days 3 to 10. This analysis also indicated that a higher level of nicotine intake leads to a greater decrease in reward thresholds (F1,142 = 13.49, p < .001; Figure 4c) and response latencies (F1,142 = 10.61, p < .01; Figure 4d).
Figure 4.
Nicotine self-administration decreases brain reward thresholds and response latencies. The reward thresholds (a) and response latencies (b) were assessed immediately before and after 1 hour of daily nicotine self-administration. A linear regression analysis showed that high levels of nicotine intake were associated with larger decreases in the reward thresholds (c) and response latencies (d). In c and d, the brain reward threshold and response latencies after nicotine self-administration were expressed as a percentage of the reward thresholds and latencies before nicotine self-administration. Asterisks indicate shorter response latencies after nicotine self-administration. N = 18/group. *, + p < .05, **, ++ p < .01. Abbreviations: IVSA, intravenous self-administration; ShA, short access; LgA, long access. N = 18/group. Data are expressed as means ± SEM.
Discussion
The goal of these studies was to determine if there is a relationship between nicotine self-administration and the state of the brain reward system. ShA to nicotine led to a decrease in the brain reward thresholds and response latencies. This indicates that a brief period of nicotine self-administration enhances reward function and improves psychomotor performance. Nicotine self-administration under the LgA schedule did not affect the brain reward thresholds but decreased the response latencies. The nAChR antagonist mecamylamine induced a larger increase in the brain reward thresholds of the LgA rats then the ShA rats. Thus, prolonged, but not brief, access to nicotine leads to the development of dependence. LgA to nicotine also led to an increase in nicotine intake during the light period and decreased circadian stability of nicotine intake. It was then investigated if the administration of mecamylamine before the LgA self-administration session affects the self-administration of nicotine. Mecamylamine decreased nicotine intake for a brief period and this was followed by a large rebound increase in nicotine intake. Because of this rebound increase in nicotine intake, mecamylamine did not affect total nicotine intake over the 23-hour period. These studies indicate that a brief period of nicotine self-administration enhances brain reward function and that LgA to nicotine disrupts circadian rhythmicity and leads to the development of dependence.
In this study, we found that daily, 1-hour, nicotine self-administration decreased the brain reward thresholds and the response latencies. The effect of nicotine self-administration on the reward thresholds was relatively small. Nicotine self-administration lowered the brain reward thresholds but the post hoc tests did not reveal significant differences at specific timepoints. Nicotine self-administration had a more pronounced effect on the response latencies. The post hoc tests revealed significant differences in the response latencies before and after nicotine intake on several test days. There was a strong correlation between the self-administration of nicotine and changes in brain reward thresholds and response latencies. Higher levels of nicotine intake led to a greater decrease in brain reward thresholds and response latencies. A frequency analysis was conducted to gain a better understanding of the effects of nicotine self-administration on changes in reward thresholds. The most common occurrence was a 10% decrease in reward thresholds after the daily 1-hour nicotine self-administration sessions. It is interesting to note that the noncontingent administration of 0.3 mg/kg of nicotine also induces a 10% decrease in ICSS thresholds.5 The 0.3 mg/kg dose of nicotine is the most rewarding dose and lower or higher doses induce smaller decreases or increases in reward thresholds. Kenny and Markou34 also compared the brain reward thresholds before and after nicotine self-administration (latencies were not reported). They reported that 1 hour of nicotine self-administration (0.03 mg/kg/infusion) leads to a 10% decrease in brain reward thresholds. However, a difference between our study and the study by Kenny and Markou is that they observed a gradual decrease in the reward thresholds before the 1-hour self-administration sessions and the thresholds remained below baseline after the discontinuation of nicotine self-administration. At this point, it is not clear under what conditions nicotine self-administration induces this long-term enhancement of reward function (decrease in reward thresholds). There are no apparent methodological differences that could account for the difference between this study and the study by Kenny and Markou. Furthermore, in other studies in which rats received nicotine contingently or noncontingently this long-term enhancement of reward function was not observed.5,35
After 10 days of daily nicotine self-administration, the rats were switched to an intermittent ShA or LgA schedule. ShA to nicotine led to a decrease in the brain reward thresholds and the response latencies. LgA to nicotine did not affect the brain reward thresholds but decreased the response latencies. This indicates that psychomotor performance is enhanced immediately after the 23-hour self-administration session but reward function is not affected at this timepoint. Harris et al. investigated the effects of daily, 22 hour, nicotine self-administration on reward thresholds and response latencies.35 They found no effect of LgA to nicotine on reward thresholds or response latencies. Taken together, these findings indicate that neither daily nor intermittent LgA to nicotine affects the brain reward thresholds immediately at the end of the sessions. However, intermittent LgA leads to a decrease in response latencies, which is not observed after daily access.35 It might be possible that rats with daily LgA have more tolerance to the effects of nicotine, which diminishes the psychomotor effects of nicotine.
To further investigate the development of dependence in rats with intermittent ShA and LgA to nicotine, we investigated the effects of the nAChR antagonist mecamylamine on the reward thresholds and the response latencies. Mecamylamine induced a large increase in the brain reward thresholds and response latencies in the LgA rats compared to the ShA rats. This indicates that LgA to nicotine induces adaptations in the brain cholinergic system that are indicative of the development of dependence.36 In this study, the LgA rats had elevated reward thresholds after the administration of mecamylamine but did not have elevated reward thresholds between self-administration sessions (spontaneous withdrawal). Previous studies have shown that precipitated withdrawal is more readily observed than spontaneous withdrawal.37 A more prolonged nicotine exposure period is needed to observe spontaneous withdrawal signs compared to precipitated withdrawal signs.20,37 The upregulation of nAChRs plays a critical role in the development of dependence and longer nicotine exposure periods or higher doses lead to a greater upregulation of nAChRs.38–40 Therefore, we might have been able to measure spontaneous withdrawal signs in the LgA rats after a more prolonged exposure period or after a higher dose of nicotine. Increasing the nicotine dose from 0.03 to 0.06 mg/kg leads a decrease in operant responding but an overall increase in nicotine intake.41,42 This might explain why a small (10%), but significant, increase in reward thresholds is observed during abstinence in animals that self-administered 0.06 mg/kg/infusion of nicotine (22 hours/day, 7 days/week).43
Mecamylamine decreases the self-administration of nicotine in a daily ShA paradigm.13 In this study, we found that 3 mg/kg of mecamylamine decreased the self-administration of nicotine during the first few hours of the LgA session and that 1.5 mg/kg of mecamylamine did not significantly decrease the self-administration of nicotine. This observation is in line with studies that showed that 3 mg/kg, but not 1.5 mg/kg, of mecamylamine, decreases nicotine self-administration in animals that have ShA (1 hour) to nicotine.13,44 We also found that pretreatment with mecamylamine did not affect total nicotine intake over the 23-hour period. Interestingly, the highest dose of mecamylamine induced a decrease in nicotine intake during the first 2 hours of the 23-hour session and this was followed by a large rebound increase in nicotine intake. Mecamylamine blocks the rewarding effects of nicotine and the half-life of nicotine in rats is about 1.2 hour.45,46 Therefore, our finding suggests that the rats displayed a rebound increase in nicotine intake after the effects of mecamylamine subsided. This is supported by the observation that the increase in nicotine intake was observed 2 hour earlier (3-hour timepoint) in the rats that received 1.5 mg/kg of mecamylamine. Using standard half-life calculations,47 it can be determined how much mecamylamine is left 3 hours after the administration of 1.5 mg/kg of mecamylamine and 5 hours after the administration of 3 mg/kg of nicotine. At the 3-hour timepoint, 18% (0.27 mg/kg) mecamylamine is left and at the 5-hour timepoint, only 6% is left (0.18 mg/kg). Thus, this suggests that rats increase their nicotine intake when the mecamylamine levels have decreased to 0.2–0.3 mg/kg. This is in line with the observation that doses up to 0.3 mg/kg of mecamylamine do no decrease the self-administration of nicotine.13
Although little is known about the effects of mecamylamine on nicotine self-administration in dependent animals, several studies have investigated the effects of mecamylamine in human smokers. In smokers, mecamylamine increases the number of cigarettes smoked, increases the number of puffs per cigarette, and increases nicotine levels.48–50 In contrast, pretreatment with nicotine decreases smoking.51 Therefore, these findings suggest that blockade of nAChRs with a nonselective nAChR antagonist is not an effective treatment to decrease smoking.
In conclusion, the present studies indicate that daily (1 hour) and intermittent ShA (1 hour) to nicotine enhances brain reward function and psychomotor function. Furthermore, animals in the intermittent LgA groups had more severe affective withdrawal signs than animals in the intermittent ShA group. Nicotine receptor blockade temporarily decreased nicotine intake in the LgA group, but this was followed by a large rebound increase in nicotine intake. Overall, these findings indicate that daily and intermittent ShA to nicotine has mildly rewarding effects and intermittent LgA leads to the development of dependence. Therefore, people who smoke several days per week are at a high risk for becoming nicotine dependent. The intermittent LgA model will be a valuable animal model to explore the role of mood states in smoking in dependent subjects, and aid in the development of new treatments to reduce smoking.
Funding
This work was supported by a National Institute on Drug Abuse (NIDA)/National Institutes of Health (NIH) grant (DA023575 to LO), a NIDA/NIH grant (DA038009 to MF), and a NIDA/NIH and FDA Center for Tobacco Products (CTP) grant (DA042530 to AB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Declaration of Interests
None declared.
Supplementary Material
References
- 1. Panday S, Reddy SP, Ruiter RA, Bergström E, de Vries H. Nicotine dependence and withdrawal symptoms among occasional smokers. J Adolesc Health. 2007;40(2):144–150. [DOI] [PubMed] [Google Scholar]
- 2. Leventhal AM, Piper ME, Japuntich SJ, Baker TB, Cook JW. Anhedonia, depressed mood, and smoking cessation outcome. J Consult Clin Psychol. 2014;82(1):122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. Neurosci Biobehav Rev. 2012;36(5):1418–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacol Biochem Behav. 2009;91(4):481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Igari M, Alexander JC, Ji Y, Qi X, Papke RL, Bruijnzeel AW. Varenicline and cytisine diminish the dysphoric-like state associated with spontaneous nicotine withdrawal in rats. Neuropsychopharmacology. 2014;39(2):455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl). 2002;160(1):56–66. [DOI] [PubMed] [Google Scholar]
- 7. Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–79. [DOI] [PubMed] [Google Scholar]
- 8. Malin DH, Lake JR, Newlin-Maultsby P, et al. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43(3):779–784. [DOI] [PubMed] [Google Scholar]
- 9. Bruijnzeel AW. Neuropeptide systems and new treatments for nicotine addiction. Psychopharmacology (Berl). 2017;234(9-10):1419–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24(11):566–573. [DOI] [PubMed] [Google Scholar]
- 11. Markou A, Arroyo M, Everitt BJ. Effects of contingent and non-contingent cocaine on drug-seeking behavior measured using a second-order schedule of cocaine reinforcement in rats. Neuropsychopharmacology. 1999;20(6):542–555. [DOI] [PubMed] [Google Scholar]
- 12. Orrù A, Caffino L, Moro F, et al. Contingent and non-contingent recreational-like exposure to ethanol alters BDNF expression and signaling in the cortico-accumbal network differently. Psychopharmacology (Berl). 2016;233(17):3149–3160. [DOI] [PubMed] [Google Scholar]
- 13. Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl). 1989;99(4):473–478. [DOI] [PubMed] [Google Scholar]
- 14. O’Dell LE, Chen SA, Smith RT, et al. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320(1):180–193. [DOI] [PubMed] [Google Scholar]
- 15. George O, Lloyd A, Carroll FI, Damaj MI, Koob GF. Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology (Berl). 2011;213(4):715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scharf DM, Dunbar MS, Shiffman S. Smoking during the night: prevalence and smoker characteristics. Nicotine Tob Res. 2008;10(1):167–178. [DOI] [PubMed] [Google Scholar]
- 17. Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. [DOI] [PubMed] [Google Scholar]
- 18. Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22(4):413–421. [DOI] [PubMed] [Google Scholar]
- 19. Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl). 2006;186(1):48–53. [DOI] [PubMed] [Google Scholar]
- 20. Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl). 2004;173(1–2):64–72. [DOI] [PubMed] [Google Scholar]
- 21. Cohen A, Koob GF, George O. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology. 2012;37(9):2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clin Pharmacol Ther. 1994;56(1):86–99. [DOI] [PubMed] [Google Scholar]
- 23. Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34(7):1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamada H, Bruijnzeel AW. Stimulation of α2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology. 2011;60(2–3):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qi X, Shan Z, Ji Y, et al. Sustained AAV-mediated overexpression of CRF in the central amygdala diminishes the depressive-like state associated with nicotine withdrawal. Transl Psychiatry. 2014;4:e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qi X, Guzhva L, Yang Z, et al. Overexpression of CRF in the BNST diminishes dysphoria but not anxiety-like behavior in nicotine withdrawing rats. Eur Neuropsychopharmacol. 2016;26(9):1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barr AM, Markou A, Phillips AG. A ‘crash’ course on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci. 2002;23(10):475–482. [DOI] [PubMed] [Google Scholar]
- 28. Liebman JM. Anxiety, anxiolytics and brain stimulation reinforcement. Neurosci Biobehav Rev. 1985;9(1):75–86. [DOI] [PubMed] [Google Scholar]
- 29. Qi X, Yamada H, Corrie LW, et al. A critical role for the melanocortin 4 receptor in stress-induced relapse to nicotine seeking in rats. Addict Biol. 2015;20(2):324–335. [DOI] [PubMed] [Google Scholar]
- 30. Fernández-Galaz C, Fernández-Agulló T, Campoy F, et al. Decreased leptin uptake in hypothalamic nuclei with ageing in Wistar rats. J Endocrinol. 2001;171(1):23–32. [DOI] [PubMed] [Google Scholar]
- 31. Hughes ME, Hogenesch JB, Kornacker K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms. 2010;25(5):372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harfmann BD, Schroder EA, England JH, et al. Temperature as a circadian marker in older human subjects: relationship to metabolic syndrome and diabetes. J Endocr Soc. 2017;1(7):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tranel HR, Schroder EA, England J, et al. Physical activity, and not fat mass is a primary predictor of circadian parameters in young men. Chronobiol Int. 2015;32(6):832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31(6):1203–1211. [DOI] [PubMed] [Google Scholar]
- 35. Harris AC, Pentel PR, Burroughs D, Staley MD, Lesage MG. A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology (Berl). 2011;217(2):153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology. 2008;54(8):1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kutlu MG, Oliver C, Huang P, Liu-Chen LY, Gould TJ. Impairment of contextual fear extinction by chronic nicotine and withdrawal from chronic nicotine is associated with hippocampal nAChR upregulation. Neuropharmacology. 2016;109:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16(5):905–908. [DOI] [PubMed] [Google Scholar]
- 40. Marks MJ, Rowell PP, Cao JZ, Grady SR, McCallum SE, Collins AC. Subsets of acetylcholine-stimulated 86Rb+ efflux and [125I]-epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology. 2004;46(8):1141–1157. [DOI] [PubMed] [Google Scholar]
- 41. Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl). 1997;129(1):35–43. [DOI] [PubMed] [Google Scholar]
- 42. Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl.). 2006;184(3–4):353–366. [DOI] [PubMed] [Google Scholar]
- 43. Harris AC, Burroughs D, Pentel PR, LeSage MG. Compensatory nicotine self-administration in rats during reduced access to nicotine: an animal model of smoking reduction. Exp Clin Psychopharmacol. 2008;16(1):86–97. [DOI] [PubMed] [Google Scholar]
- 44. Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62(4):743–751. [DOI] [PubMed] [Google Scholar]
- 45. Debruyne D, Sobrio F, Hinschberger A, Camsonne R, Coquerel A, Barré L. Short-term pharmacokinetics and brain distribution of mecamylamine as a preliminary to carbon-11 labeling for nicotinic receptor investigation. J Pharm Sci. 2003;92(5):1051–1057. [DOI] [PubMed] [Google Scholar]
- 46. Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1985;22(2):237–241. [DOI] [PubMed] [Google Scholar]
- 47. Mahato RI, Narang AS. Pharmaceutical Dosage Forms and Drug Delivery. 3rd ed. CRC Press; 2017. [Google Scholar]
- 48. Pomerleau CS, Pomerleau OF, Majchrzak MJ. Mecamylamine pretreatment increases subsequent nicotine self-administration as indicated by changes in plasma nicotine level. Psychopharmacology (Berl). 1987;91(3):391–393. [DOI] [PubMed] [Google Scholar]
- 49. Nemeth-Coslett R, Henningfield JE, O’Keeffe MK, Griffiths RR. Effects of mecamylamine on human cigarette smoking and subjective ratings. Psychopharmacology (Berl). 1986;88(4):420–425. [DOI] [PubMed] [Google Scholar]
- 50. Stolerman IP, Goldfarb T, Fink R, Jarvik ME. Influencing cigarette smoking with nicotine antagonists. Psychopharmacologia. 1973;28(3):247–259. [DOI] [PubMed] [Google Scholar]
- 51. Lucchesi BR, Schuster CR, Emley GS. The role of nicotine as a determinant of cigarette smoking frequency in man with observations of certain cardiovascular effects associated with the tobacco alkaloid. Clin Pharmacol Ther. 1967;8(6):789–796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.