Abstract
Background:
High frequency chest wall oscillation (HFCWO) has long been used for airway clearance for patients with cystic fibrosis. Only limited research has evaluated this therapy in adult patients with non-cystic fibrosis bronchiectasis (NCFB).
Methods:
Data from 2596 patients from a registry of adult bronchiectasis patients using HFCWO therapy was used to evaluate hospitalization patterns before and after initiation of HFCWO therapy, as well as antibiotic use and self-reported metrics of quality of life. Self-reported outcomes were also reviewed by cross-checking with sampled patient charts and found to be consistent.
Results:
The number of patients who had at least one respiratory-related hospitalization decreased from 49.1% (192/391) in the year before to 24.0% (94/391) in the year after starting HFCWO therapy (p-value < 0.001). At the same time, the number of patients who required three or more hospitalizations dropped from 14.3% (56/391) to 5.6% (22/391). Patients currently taking oral antibiotics for respiratory conditions decreased from 57.7% upon initiation of therapy to 29.9% within 1 year (p < 0.001). Patients who subjectively rated their “overall respiratory health” as good to excellent increased from 13.6% upon initiation of therapy to 60.5% in 1 year (p < 0.001) and those who rated their “ability to clear your lungs” as good to excellent increased from 13.9% to 76.6% (p < 0.001).
Conclusion:
NCFB patients showed improved self-reported outcomes associated with the initiation of HFCWO therapy as measured by number of hospitalizations, antibiotic use, and the subjective experience of airway clearance. The improvement was observed early on after initiation of therapy and sustained for at least 1 year.
The reviews of this paper are available via the supplemental material section.
Keywords: airway clearance, high-frequency chest wall oscillation, non-cystic fibrosis bronchiectasis
Introduction
Bronchiectasis is a complex progressive pulmonary disease characterized by irreversible dilation of the airways and is diagnosed by high resolution computed tomography.1,2 It is the end result of a number of conditions including, but not limited to, prior infection, interstitial lung disease, immunodeficiency, chronic obstructive pulmonary disease (COPD) and rheumatic lung disease; in addition, there is a sizable fraction of cases in which no cause is identified.3 The prevalence of non-cystic fibrosis bronchiectasis (NCFB) has been growing at a rate of 8.7% per year since 2001 and potentially may affect more than 500,000 people in the United States currently.4,5
The dilation and subsequent change in the architecture of the airways is thought to be caused by an acute or chronic insult that leads to inflammation.3 Dilated airways result in impaired mucociliary clearance and airway obstruction, and in combination with dysregulated immune responses, increase the propensity for infection.3 These mechanisms can interact and self-perpetuate, leading over time to impaired lung function. In some patients with NCFB, this leads to recurrent respiratory infections, which has been associated with a decline in pulmonary function and progression of the disease.2,6,7 Therefore, bronchiectasis treatment centers around addressing the underlying cause, airway clearance, and suppression of chronic infection.7
Patients with bronchiectasis may have exacerbations that lead to an increase in healthcare utilization and poor health-related quality of life. These outcomes are particularly important because they have been linked to worse severity scores, such as the bronchiectasis severity index, and increased mortality.8 Ideally, effective therapies would reduce exacerbations and the need for hospitalizations, as well as improve quality of life and respiratory symptoms. A number of pharmacological interventions have been used for NCFB with mixed results, including expectorants, mucolytics and mucokinetics, and antibiotics.9,10 Chronic antibiotic use has resulted in reductions in exacerbation rates in NCFB, but this therapy is associated with significant side effects.11 Non-pharmacological modalities of treatment include chest physiotherapy, postural drainage, chest wall percussion and vibration, forced expiration, positive expiratory pressure devices, and high frequency chest wall oscillation (HFCWO).12 Typically, HFCWO therapy is utilized if other airway clearance techniques or devices have been ineffective.
HFCWO, delivered via a percussive vest, is a commonly accepted means for airway clearance in cystic fibrosis (CF) and NCFB. Several studies in CF patients found HFCWO therapy as effective as or superior to other airway clearance methods.13–21 While there are numerous studies that compare the effectiveness of various devices in treating adults with CF, studies that address airway clearance methods in NCFB are small, short in duration, and do not test similar outcomes.22–26 For these reasons, studies are still needed to determine the best airway clearance therapy in NCFB.27 Despite the paucity of information regarding the outcomes on the use of HFCWO in NCFB, the therapy has gained some level of acceptance.28,29 This study was designed to explore the effectiveness of HFCWO in patients with NCFB in the real-world setting using a large data set from a prospectively collected outcomes registry.
Methods
Study population
The High Frequency Chest Wall Oscillation Outcomes Registry (HFCWO-OR) was designed to collect demographic and clinical data from NCFB patients, aged 21 years and older, before and after prescription of HFCWO therapy using the inCourage system (RespirTech, a Philips company, St. Paul, MN, USA). Data are housed and managed by an independent actuarial firm (Cirdan Health Systems, St. Paul, MN, USA) and results are used for quality control purposes by prescribing physicians. Only patients with bronchiectasis were included in this study, as indicated by any of the following diagnosis codes: 494.0, 494.1 (ICD-9), or J47.9, J47.1 (ICD-10), and the diagnosis was confirmed using chest computed tomography (CT) by the prescribing physician. For this study, all successive records from September 2013 to November 2015 were extracted, de-identified, and delivered to NAMSA (Minneapolis, MN, USA) for statistical analysis. Western Institutional Review Board’s (WIRB) IRB Affairs Department has confirmed that this study meets the conditions for IRB exemption under 45 CFR 46.101(b)(4). In addition, informed consent was obtained from all patients.
The HFCWO-OR comprises phone surveys of patients at structured intervals of zero months (the baseline HFCWO training date), 1 month, 3 months, 6 months, 12 months, and at 6 month intervals thereafter. During each survey, patients were asked how many hospitalizations for respiratory causes had occurred since starting therapy. For the baseline interview, the question applied to the previous year. In addition, patients were asked about the number of antibiotic treatments received for respiratory purposes and also rated their respiratory health and ability to clear secretions on a five-point Likert scale (Table 1). In addition, for a subset of patients, a detailed chart review was performed.
Table 1.
Questions asked by the outcomes survey.
| Likert scale | How would you rate your overall respiratory health? | How would you rate your ability to clear your lungs? |
|---|---|---|
| 1 | Poor | Poor |
| 2 | Fair | Fair |
| 3 | Good | Good |
| 4 | Very good | Very good |
| 5 | Excellent | Excellent |
| Are you currently taking any antibiotics for your breathing problems? | Y or N | |
| Since starting vest therapy, how many times have you been in the hospital for breathing problems (as an admission)? | Zero | |
| One | ||
| Two | ||
| Three or more | ||
Analyses
Data were sometimes unavailable for either the pre- or post-training intervals, Accordingly, two complementary strategies were utilized to analyze the results. The “pooled” approach included all available data regardless of where the subjects were in their follow-up schedule. This approach maximized the number of patients in the study because it considered pre and post data in an aggregate, regardless of the presence of missing records. This was more consistent with an approach that is intended to be compared against an outside benchmark. The disadvantage of this approach was that it did not account for potential biases due to inclusion of differing patient groups at different times during the course of treatment. The “pairwise” approach focused only on those patients with both baseline and a specific follow-up interval in the survey registry. While this method resulted in fewer subjects compared with the pooled cohort, it allowed for a direct comparison of change from baseline to follow-up for individual pre and post data pairs.
In all methods, services were limited to those that occurred within the defined study period. The post-training therapy period used in the study was limited to the date a device was returned regardless of the chart review end date. The device was deemed returned if a patient was deceased, and the return date acted as a proxy for a date of death where necessary.
Confidence limits were calculated at a 95% level for all measures. p-values for pairwise data with a binary outcome were calculated using McNemar’s test and Bowker’s test for categorical outcomes with more than two levels. p-values for the change in score on a continuous scale were based on a two-sided Wilcoxon test. p-values for the pooled quality of life data were calculated using a repeated measures model. Statistical analysis was performed on SAS Version 9.3 (SAS Institute, Cary, NC, USA) or later.
Results
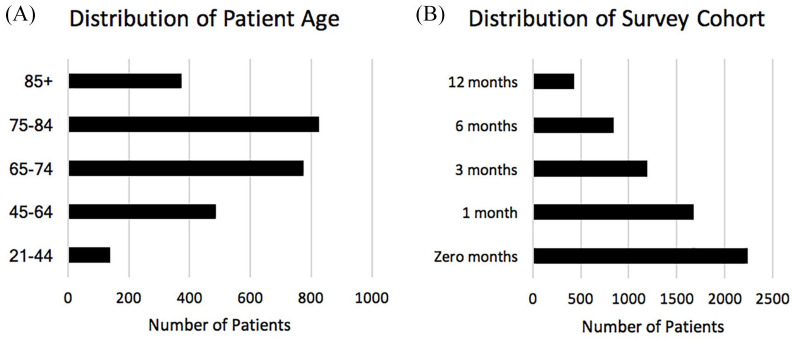
The data extract included 2596 NCFB patients with an average age of 70 years, age range 21–101 years, 63% female, 37% male. The patient data represented services provided by 1374 different medical practitioners. The largest concentration of patients associated with a single National Provider Identifier was 1.9%. Because the HFCWO-OR is constantly growing, there are more data available for the first months after initiating HFCWO therapy than for later survey intervals (Figure 1). Every effort was made to reach patients in the HFCWO-OR at the designated time intervals, with a success rate of 78.0%.
Figure 1.
Characteristics of the patient population used for this study. Included is the distribution of patient age and the number of patients found at each survey cohort time interval.
One hundred and ninety-eight patients were included in the subset for detailed chart review. The average age of the subset was 70 years, age range 23–92 years, 69% female, 31% male. Data were extracted from the charts regarding hospitalizations and antibiotic use and correlated with self-reported data.
The results of both pairwise and pooled approaches for hospitalization and quality of life produced remarkably similar results despite the differing methodology. In the subsequent discussions, paired data were used unless otherwise indicated.
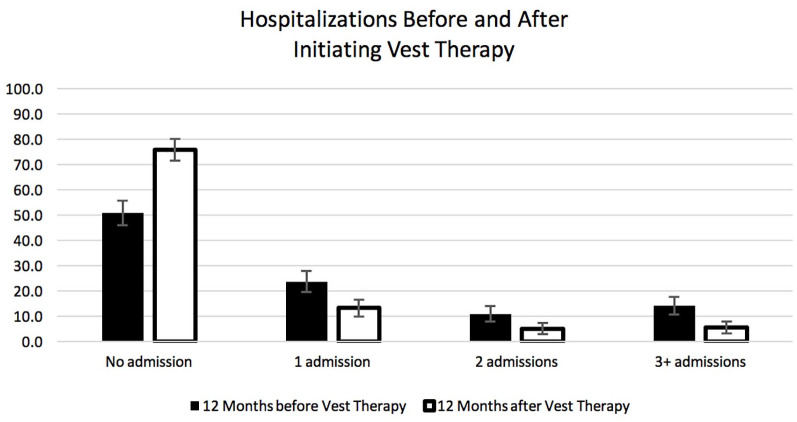
Comparison of hospitalizations before and after initiation of HFCWO therapy shows a substantial reduction in the number of hospitalizations (Table 2). The number of patients who had at least one respiratory-related hospitalization decreased from 49.1% (192/391) in the year before to 24.0% (94/391) in the year after starting HFCWO therapy (p-value < 0.001). In addition, the percentage of patients who required three or more hospitalizations (frequent exacerbators) decreased from 14.3% (56/391) in the year prior to HFCWO therapy to 5.6% (22/391) in the year after starting HFCWO therapy (p-value < 0.001) (Figure 2). In addition, the rate of hospitalizations fell from 0.887 to 0.404 admits/patient in the year before and after initiating HFCWO therapy, respectively: a reduction of 54.5% (p-value < 0.001). In the frequent exacerbators, based on the prior year data, 50.0% had no admissions in the year after initiating HFCWO therapy, while only 17.9% continued to fall in the 3+ category.
Table 2.
Self-reported hospitalizations for patients in the 12 months before and the 12 months after initiating high frequency chest wall oscillation therapy.
| A. Paired data | 12 months prior |
12 months after |
||||||
|---|---|---|---|---|---|---|---|---|
| n | % | Lower 95% confidence limit (%) | Upper 95% confidence limit (%) | n | % | Lower 95% confidence limit (%) | Upper 95% confidence limit (%) | |
| None | 199 | 50.9 | 45.9 | 55.9 | 297 | 76 | 71.7 | 80.2 |
| 1 admission | 93 | 23.8 | 19.6 | 28.0 | 52 | 13.3 | 9.9 | 16.7 |
| 2 admissions | 43 | 11.0 | 7.9 | 14.1 | 20 | 5.1 | 2.9 | 7.3 |
| 3+ admissions | 56 | 14.3 | 10.9 | 17.8 | 22 | 5.6 | 3.3 | 7.9 |
| Total | 391 | 100 | 391 | 100 | ||||
| B. Pooled data | 12 months prior |
12 months after |
||||||
| n | % | Lower 95% confidence limit (%) | Upper 95% confidence limit (%) | n | % | Lower 95% confidence limit (%) | Upper 95% confidence limit (%) | |
| None | 988 | 45.3 | 43.2 | 47.4 | 313 | 75.1 | 70.9 | 79.2 |
| 1 admission | 548 | 25.1 | 23.3 | 26.9 | 56 | 13.4 | 10.2 | 16.7 |
| 2 admissions | 265 | 12.1 | 10.8 | 13.5 | 22 | 5.3 | 3.1 | 7.4 |
| 3+ admissions | 381 | 17.5 | 15.9 | 19.1 | 26 | 6.2 | 3.9 | 8.6 |
| Total | 2182 | 100 | 417 | 100 | ||||
The count and proportion is shown for patients who had zero, 1, 2, or 3+ admissions. Lower and upper 95% confidence limits are also shown. A shows results for subjects for which paired before and after data were available. B shows the results for pooled data.
Figure 2.
The proportion of patients who had zero, 1, 2, or 3+ hospital admissions in the year before and the year after initiating high frequency chest wall oscillation therapy. Data were from the paired data set. Error bars are 95% confidence limits.
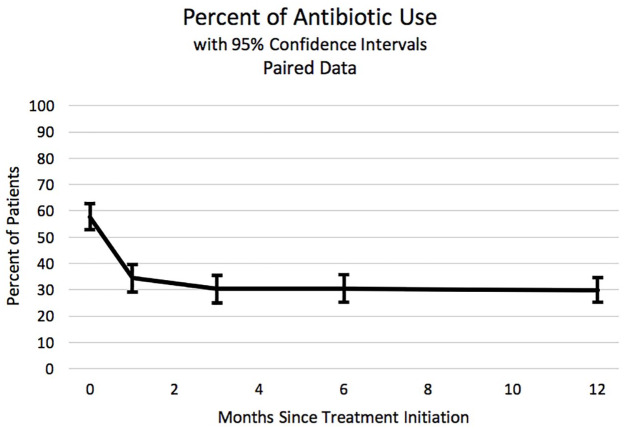
In parallel to this improvement, the proportion of patients who answered positively to the question “Are you currently taking oral antibiotics for breathing problems?” dropped sharply after initiating HFCWO use, from 57.7% initially to 29.9% after 1 year (p-value < 0.001). It is notable that the drop was evident after the first month and sustained for at least 1 year (Figure 3).
Figure 3.
The proportion of patients who answered “yes” to the question: “Are you presently using antibiotics for breathing problems?” The x-axis indicates months since initiating high frequency chest wall oscillation therapy. Data were from the paired data set. Error bars are 95% confidence limits.
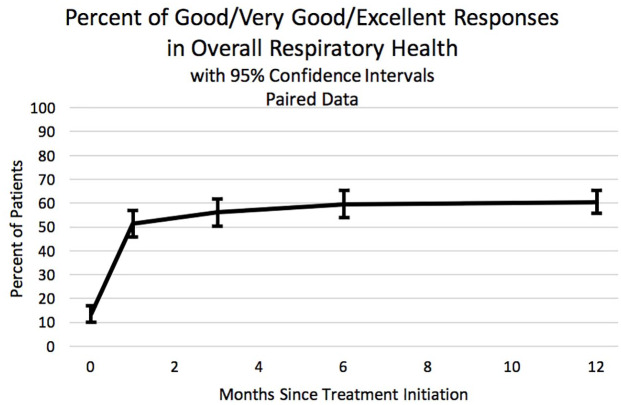
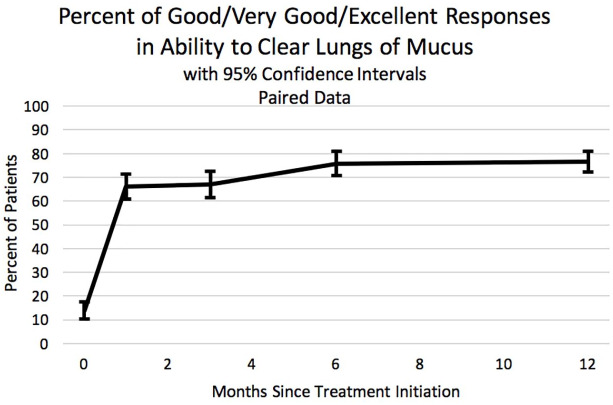
The patient-reported quality of life questions track the general improvement that occurred after starting HFCWO therapy. In answering the question “How would you rate your overall respiratory health?”, the number of patients who answered positively (“good,” “very good” or “excellent”) increased from 13.6% to 60.5% after 1 year (p-value < 0.001) (Figure 4). Similarly, in answering the question “How would you rate your ability to clear your lungs?”, the number of patients who answered positively increased from 13.9% to 76.6% after 1 year of HFCWO therapy (p-value < 0.001) (Figure 5). Again, the majority of the improvement for both questions was seen within the first month and sustained for at least 1 year.
Figure 4.
The proportion of patients who answered positively (“good,” “very good,” or “excellent”) to the question: “How would you rate your overall respiratory health?” The x-axis indicates months since initiating high frequency chest wall oscillation therapy. Data were from the paired data set. Error bars are 95% confidence limits.
Figure 5.
The proportion of patients who answered positively (“good,” “very good,” or “excellent”) to the question: “How would you rate your ability to clear your lungs?” The x-axis indicates months since initiating high frequency chest wall oscillation therapy. Data were from the paired data set. Error bars are 95% confidence limits.
In the phone survey, respondents indicate whether they have been admitted to an inpatient facility for worsening respiratory symptoms. Data from the medical chart review were compared against the data captured in the phone survey in order to assess whether the self-reported data were consistent with the information in the medical charts. Table 3 presents the results from the phone survey against corresponding hospitalization data from the medical charts. It is evident that, while there are differences between the two sources, the results are reasonably consistent. For example, for the post-therapy period based on paired data, 76.0% of the population self-reported that they had no hospital admissions for breathing difficulty, 13.3% reported one hospital admission, and 10.7% reported two or more in the year following the start of therapy. Based upon data from the chart reviews, 69.7% incurred no admissions, 18.2% experienced one, and 12.1% experienced two or more in the year following the start of therapy.
Table 3.
A comparison of the number of self-reported hospitalizations (paired data) compared with the number of hospitalizations found in the chart review.
| # of admits | Self-reported |
Chart review |
||||
|---|---|---|---|---|---|---|
| n | 12 months prior | 12 months after | n | 12 months prior | 12 months after | |
| None | (199/297) | 51% | 76% | (112/138) | 57% | 70% |
| 1 admission | (93/52) | 24% | 13% | (55/36) | 28% | 18% |
| 2 admissions | (43/20) | 11% | 5% | (15/15) | 8% | 8% |
| 3+ admissions | (56/22) | 14% | 6% | (16/9) | 8% | 5% |
Data are segmented by the number of hospitalizations and whether they occurred in the 12 months prior to or after initiation of high frequency chest wall oscillation therapy. n is shown as (number records prior to treatment/number of records after treatment).
Paired spirometry data forced expiratory volume at 1 s (FEV1) and forced vital capacity (FVC) were available in chart reviews for 41 patients. In comparison with the self-reported improvements in respiratory health and the ability to clear their lungs, improvements in pulmonary function were also found. Patients reported favorable responses (⩾3) to the “overall respiratory health” and “ability to clear their lungs” questions of 60.5% and 76.6%, respectively after 1 year of HFCWO treatment. This trend is generally consistent with percentage of patients showing an improvement in lung function during the same period; in the year after initiating HFCWO treatment, the percentage of patients showing at least a 4% increase in mean FEV1 and FVC was 39% and 48%, respectively.
Discussion
This study showed that in patients with NCFB, the initiation of HFCWO was associated with reductions in patient-reported exacerbation rates, hospitalizations, antibiotic use, and improvements in respiratory symptoms and quality of life. Importantly these benefits were also observed in the frequent exacerbator subgroup. Moreover, the improvements in hospitalization rates, consistent with self-reported quality of life measures, suggest that patient health improvement is sustained for at least a year after initiating therapy.
Exacerbations of NCFB result in significant health-care utilization, as the costs for a single exacerbation can exceed US$7827.30 In addition, hospitalizations may result in prolonged periods of immobility and loss of muscle mass, as well as exposure to nosocomial pathogens.31,32 Also, the reduction in oral antibiotic usage for respiratory conditions after initiation of HFCWO therapy is in agreement with the decrease in hospitalization rates. Although chronic macrolide therapy has been linked to improved outcomes regarding exacerbation rates in NCFB, data on the benefits of HFCWO therapy are a welcome addition as a non-pharmacologic alternative for this group of at-risk patients. These findings have important implications, because patients with frequent exacerbations may have a more pronounced lung function decline.33,34
Changes in patients’ perceived improvements in their lung health correlated with reduced hospitalization rates. The “respiratory health” question was intended to include symptoms like cough, chest pain, and dyspnea along with airway clearance. The response shows an important, sustained improvement, but not as marked as the “ability to clear lungs” question, which is more specific to airway clearance. This further supports the HFCWO mechanism of action. Both questions exhibit a similar pattern: a significant improvement within the first month, followed by continued improvement of a lesser degree out to 12 months. It remains unclear whether these improvements will continue to improve or are sustained beyond this specified time.
Self-reported findings were successfully validated against objective chart data within a reasonable degree of accuracy. The reasons for the difference may be the patients’ recall bias or that chart data may not include all available records. The different spans of time covered by the two distinct sources created complexity in comparing the self-reported results with those from the chart reviews. Furthermore, the phone survey only asked about hospitalizations relating to breathing difficulty while the chart reviews captured all admissions. Thus, a complete correspondence between self-reported and objective data was neither possible nor expected.
The present study evaluated only one mode of HFCWO (i.e. triangle wave percussive pulse). Although smaller studies have shown that different modes of HFCWO improve clinical outcomes in patients with bronchiectasis, including the need for antibiotics and hospitalization rates in patients with bronchiectasis, additional studies are needed to explore the differences between these modes of airway clearance.23–25
This study has inherent limitations that are common to registry studies that deserve mention. First, it is possible that other interventions were occurring at the same time as initiation of HFCWO therapy, potentially reflecting in an improvement in the various outcomes. For example, data on the different medication regimens and the time of initiation of these therapies were not available for comparison. In addition, mild exacerbations that did not require a hospital were not captured in the survey. This reflects a risk in attributing effectiveness to results that may be due to regression to the mean. However, the improvement in health status was seen across a number of measures, hospitalizations and quality of life. Importantly, the improvement was rapid, corresponded closely in time to initiation, and was sustained for at least a year. This makes regression to the mean unlikely. Despite the diverse range of real-world practice patterns, the response to HFCWO overall remained consistent.
A second limitation was that patients in the study were not randomly selected. It is possible that use of the device is correlated to an exacerbation or associated with an alternative treatment protocol which could artificially increase hospitalizations prior to initiating HFCWO therapy, produce an improvement in health status post-use not related to the device, or produce other effects. A variety of techniques were used to evaluate whether a bias was introduced when identifying patients for the quality of life survey or if other factors were influencing results. In addition to comparing results before and after initiating therapy, a cohort of patients with experience both before and after beginning therapy was separately evaluated. Study periods of 12 and 6 months before and after initiating therapy were evaluated to assist in determining whether results reflected a “crisis event” or other surge in service utilization prior to initiating therapy, and if apparent improvement was simply reflecting a return to an average health status. A review of service utilization patterns found that healthcare usage appeared modestly higher around the initiation of therapy. Possible explanations could include missing chart data prior to the initiation of therapy, a general deterioration of health status driving the new therapy, or possibly other factors.
A third limitation was that the diagnosis or severity of bronchiectasis was not independently confirmed by the investigators. The diagnosis was reported by the prescribing physician based on findings from a chest CT during the initial request for HFCWO therapy. Another limitation is that the survey utilized was not previously validated and future studies are still required to test these questions, in this and other clinical settings. It is significant that previous severity scores have used similar outcomes, such as hospitalizations and antibiotic use.8 Finally, it is possible that the responders inaccurately recalled their exacerbations in the previous 12 months. Patient-reported outcomes certainly have this potential limitation and studies have mixed results regarding the accuracy of the report of significant events, in particular exacerbations in chronic lung disease.35,36
Despite these limitations, the present study has several strengths. It is the largest series reported to date regarding the use of HFCWO therapy in NCFB patients. Prior studies have recruited a small number of patients and have had a limited follow-up period. Also, the present study evaluated various clinically relevant outcomes, including quality of life. Ideally, a randomized, sham-controlled, double-blind study would answer more accurately the efficacy of HFCWO therapy in NCFB. Nevertheless, the sham component would be difficult to design given the nature of the therapy (i.e. chest wall oscillation) and therefore would be impossible to blind. In addition, other sham studies have shown a considerable placebo effect, which decreases the validity of the findings.37 Similarly, comparing different modalities of airway clearance in a blinded fashion would be problematic given the considerable differences among therapies. For these reasons, real-life or pragmatic studies are an alternative to address these important questions.
More research is necessary to further clarify the impact of HFCWO on NCFB, particularly for important outcomes like hospitalization rates and when best to initiate this therapy in the course of the disease. The question of long-term effectiveness is partially unanswered, and long-term outcomes studies are needed to elucidate whether there are sustained benefits with this treatment modality. A comparison between our highly targeted quality of life questions and broader surveys such as the QOL-B remains to be done.38 In addition, it is not clear whether subgroups of patients with NCFB (i.e. COPD bronchiectasis overlap) may or may not have a higher likelihood of benefit.39 The results presented here may have implications for health economics, particularly in regard to exacerbations and hospitalizations, but studies are required to estimate cost savings associated with HFCWO therapy.
Conclusion
Adult NCFB patients showed improved outcomes associated with the initiation of HFCWO therapy as measured by number of hospitalizations, antibiotic use, and respiratory-specific quality of life questions. The improvement, correlated with chart reviews, was associated with the initiation of therapy and sustained for 1 year.
Supplemental Material
Supplemental material, Author_Response_1 for Real-life experience with high-frequency chest wall oscillation vest therapy in adults with non-cystic fibrosis bronchiectasis by Tara Lynn Barto, Diego Jose Maselli, Sarah Daignault, John Stiglich, Jared Porter, Carlye Kraemer and Gary Hansen in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Real-life experience with high-frequency chest wall oscillation vest therapy in adults with non-cystic fibrosis bronchiectasis by Tara Lynn Barto, Diego Jose Maselli, Sarah Daignault, John Stiglich, Jared Porter, Carlye Kraemer and Gary Hansen in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Real-life experience with high-frequency chest wall oscillation vest therapy in adults with non-cystic fibrosis bronchiectasis by Tara Lynn Barto, Diego Jose Maselli, Sarah Daignault, John Stiglich, Jared Porter, Carlye Kraemer and Gary Hansen in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.1 for Real-life experience with high-frequency chest wall oscillation vest therapy in adults with non-cystic fibrosis bronchiectasis by Tara Lynn Barto, Diego Jose Maselli, Sarah Daignault, John Stiglich, Jared Porter, Carlye Kraemer and Gary Hansen in Therapeutic Advances in Respiratory Disease
Footnotes
Author contribution(s): Tara Lynn Barto: Conceptualization; Methodology; Supervision; Validation; Writing-original draft; Writing-review & editing.
Diego Jose Maselli: Investigation; Methodology; Writing-original draft; Writing-review & editing.
Sarah Daignault: Data curation; Formal analysis; Methodology; Writing-original draft.
John Stiglich: Data curation; Formal analysis; Funding acquisition; Writing-original draft.
Jared Porter: Formal analysis; Investigation; Methodology; Writing-original draft.
Carlye Kraemer: Formal analysis; Investigation; Writing-original draft.
Gary Hansen: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Writing-original draft; Writing-review & editing.
Conflict of interest: TLB: consultant for Respirtech, a Philips company. Grant funding: Cystic Fibrosis Foundation.
DJM: consultant for Novartis, GSK, AZ, Sanofi/Regeneron.
SD: employee at RespirTech, a Philips company.
JS: no conflicts of interest.
JP: employee of Cirdan Health Systems
CK: employee of NAMSA.
GH: employee at RespirTech, a Philips company.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: provided by a research grant from RespirTech, a Philips company, through funding to Cirdan and NAMSA.
ORCID iD: Diego Jose Maselli  https://orcid.org/0000-0001-8010-0260
https://orcid.org/0000-0001-8010-0260
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Tara Lynn Barto, Section of Pulmonary, Critical Care and Sleep Medicine, Baylor College of Medicine, 7200 Cambridge St., 8th Floor, Suite 8A, Houston, TX 77030, USA.
Diego Jose Maselli, Division of Pulmonary Diseases & Critical Care Medicine, UT Health, San Antonio, TX, USA.
Sarah Daignault, RespirTech, A Philips Company, St. Paul, MN, USA.
John Stiglich, Cirdan Health Systems, St. Paul, MN, USA.
Jared Porter, Cirdan Health Systems, St. Paul, MN, USA.
Carlye Kraemer, North American Science Associates, Inc., Minneapolis, MN, USA.
Gary Hansen, RespirTech, A Philips Company, St. Paul, MN, USA.
References
- 1. Barker CF. Bronchiectasis. N Engl J Med 2002; 346: 1383–1393. [DOI] [PubMed] [Google Scholar]
- 2. McShane PJ, Tino G. Bronchiectasis. Chest 2019; 155: 825–833. [DOI] [PubMed] [Google Scholar]
- 3. Chalmers JD, Chang AB, Chotirmall SH, et al. Bronchiectasis. Nat Rev Dis Primers 2018; 4: 45. [DOI] [PubMed] [Google Scholar]
- 4. Seitz AE, Olivier KN, Adjemian J, et al. Trends in bronchiectasis among medicare beneficiaries in the United States, 2000 to 2007. Chest 2012; 142: 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis 2017; 14: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018; 392: 880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Neill K, O’Donnell AE, Bradley JM. Airway clearance, mucoactive therapies and pulmonary rehabilitation in bronchiectasis. Respirology 2019; 24: 227–237. [DOI] [PubMed] [Google Scholar]
- 8. Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balsamo R, Lanata L, Egan CG. Mucoactive drugs. Eur Respir Rev 2010; 19: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Metersky ML. New treatment options for bronchiectasis. Ther Adv Respir Dis 2010; 4: 93–99. [DOI] [PubMed] [Google Scholar]
- 11. Polverino E, Goeminne PC, McDonnell MJ, et al. European respiratory society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. [DOI] [PubMed] [Google Scholar]
- 12. Welsh EJ, Evans DJ, Fowler SJ, et al. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2015; 7: CD010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arens R, Gozal D, Omlin KJ, et al. Comparison of high frequency chest compression and conventional chest physiotherapy in hospitalized patients with cystic fibrosis. Am J Respir Crit Care Med 1994; 150: 1154–1157. [DOI] [PubMed] [Google Scholar]
- 14. Bauer ML, McDougal J, Schoumacher RA. Comparison of manual and mechanical chest percussion in hospitalized patients with cystic fibrosis. J Pediatr 1994; 124: 250–254. [DOI] [PubMed] [Google Scholar]
- 15. Braggion C, Cappelletti LM, Cornacchia M, et al. Short-term effects of three chest physiotherapy regimens in patients hospitalized for pulmonary exacerbations of cystic fibrosis: a cross-over randomized study. Pediatr Pulmonol 1995; 19: 16–22. [DOI] [PubMed] [Google Scholar]
- 16. Darbee JC, Kanga JF, Ohtake PJ. Physiologic evidence for high-frequency chest wall oscillation and positive expiratory pressure breathing in hospitalized subjects with cystic fibrosis. Phys Ther 2005; 85: 1278–1289. [PubMed] [Google Scholar]
- 17. Hansen LG, Warwick WJ. High-frequency chest compression system to aid in clearance of mucus from the lung. Biomed Instrum Technol 1990; 24: 289–294. [PubMed] [Google Scholar]
- 18. Kluft J, Beker L, Castagnino M, et al. A comparison of bronchial drainage treatments in cystic fibrosis. Pediatr Pulmonol 1996; 22: 271–274. [DOI] [PubMed] [Google Scholar]
- 19. McIlwaine MP, Alarie N, Davidson GF, et al. Long-term multicentre randomised controlled study of high frequency chest wall oscillation versus positive expiratory pressure mask in cystic fibrosis. Thorax 2013; 68: 746–751. [DOI] [PubMed] [Google Scholar]
- 20. Oermann CM, Swank PR, Sockrider MM. Validation of an instrument measuring patient satisfaction with chest physiotherapy techniques in cystic fibrosis. Chest 2000; 118: 92–97. [DOI] [PubMed] [Google Scholar]
- 21. Varekojis SM, Douce FH, Flucke RL, et al. A comparison of the therapeutic effectiveness of and preference for postural drainage and percussion, intrapulmonary percussive ventilation, and high-frequency chest wall compression in hospitalized cystic fibrosis patients. Respir Care 2003; 48: 24–28. [PubMed] [Google Scholar]
- 22. Chakravorty I, Chahal K, Austin G. A pilot study of the impact of high-frequency chest wall oscillation in chronic obstructive pulmonary disease patients with mucus hypersecretion. Int J Chron Obstruct Pulmon Dis 2011; 6: 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray MP, Pentland JL, Hill AT. A randomised crossover trial of chest physiotherapy in non-cystic fibrosis bronchiectasis. Eur Respir J 2009; 34: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 24. Nicolini A, Cardini F, Landucci N, et al. Effectiveness of treatment with high-frequency chest wall oscillation in patients with bronchiectasis. BMC Pulm Med 2013; 13: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powner J, Nesmith A, Kirkpatrick DP, et al. Employment of an algorithm of care including chest physiotherapy results in reduced hospitalizations and stability of lung function in bronchiectasis. BMC Pulm Med 2019; 19: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gokdemir Y, Karadag-Saygi E, Erdem E, et al. Comparison of conventional pulmonary rehabilitation and high-frequency chest wall oscillation in primary ciliary dyskinesia. Pediatr Pulmonol 2014; 49: 611–616. [DOI] [PubMed] [Google Scholar]
- 27. Lee AL, Burge AT, Holland AE. Airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev 2015; 11: CD008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hill AT, Barker AF, Bolser DC, et al. Treating cough due to non-CF and CF bronchiectasis with nonpharmacological airway clearance: CHEST expert panel report. Chest 2018; 153: 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hill AT, Sullivan AL, Chalmers JD, et al. British thoracic society guideline for bronchiectasis in adults. Thorax 2019; 74(Suppl. 1): 1–69. [DOI] [PubMed] [Google Scholar]
- 30. Seitz AE, Olivier KN, Steiner CA, et al. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993–2006. Chest 2010; 138: 944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 2010; 13: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Umscheid CA, Mitchell MD, Doshi JA, et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol 2011; 32: 101–114. [DOI] [PubMed] [Google Scholar]
- 33. Brill SE, Patel AR, Singh R, et al. Lung function, symptoms and inflammation during exacerbations of non-cystic fibrosis bronchiectasis: a prospective observational cohort study. Respir Res 2015; 16: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martínez-García MA, Soler-Cataluña JJ, Perpiñá-Tordera M, et al. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007; 132: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 35. Frei A, Siebeling L, Wolters C, et al. The inaccuracy of patient recall for COPD exacerbation rate estimation and its implications: results from central adjudication. Chest 2016; 150: 860–868. [DOI] [PubMed] [Google Scholar]
- 36. Quint JK, Donaldson GC, Hurst JR, et al. Predictive accuracy of patient-reported exacerbation frequency in COPD. Eur Respir J 2011; 37: 501–507. [DOI] [PubMed] [Google Scholar]
- 37. Brim RL, Miller FG. The potential benefit of the placebo effect in sham-controlled trials: implications for risk-benefit assessments and informed consent. J Med Ethics 2013; 39: 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quittner AL, Marciel KK, Salathe MA, et al. A preliminary quality of life questionnaire-bronchiectasis: a patient-reported outcome measure for bronchiectasis. Chest 2014; 146: 437–448. [DOI] [PubMed] [Google Scholar]
- 39. Polverino E, Dimakou K, Hurst J, et al. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J 2018; 52: 3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Real-life experience with high-frequency chest wall oscillation vest therapy in adults with non-cystic fibrosis bronchiectasis by Tara Lynn Barto, Diego Jose Maselli, Sarah Daignault, John Stiglich, Jared Porter, Carlye Kraemer and Gary Hansen in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Real-life experience with high-frequency chest wall oscillation vest therapy in adults with non-cystic fibrosis bronchiectasis by Tara Lynn Barto, Diego Jose Maselli, Sarah Daignault, John Stiglich, Jared Porter, Carlye Kraemer and Gary Hansen in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Real-life experience with high-frequency chest wall oscillation vest therapy in adults with non-cystic fibrosis bronchiectasis by Tara Lynn Barto, Diego Jose Maselli, Sarah Daignault, John Stiglich, Jared Porter, Carlye Kraemer and Gary Hansen in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.1 for Real-life experience with high-frequency chest wall oscillation vest therapy in adults with non-cystic fibrosis bronchiectasis by Tara Lynn Barto, Diego Jose Maselli, Sarah Daignault, John Stiglich, Jared Porter, Carlye Kraemer and Gary Hansen in Therapeutic Advances in Respiratory Disease