Abstract
Following the outbreak of Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) last December 2019 in China, Italy was the first European country to be severely affected, with the first local case diagnosed on 20 February 2020. The virus spread quickly, particularly in the North of Italy, with three regions (Lombardy, Veneto and Emilia-Romagna) being the most severely affected. These three regions accounted for >80% of SARS-CoV-2 positive cases when the tight lockdown was established (March 8). These regions include one of Europe's areas of heaviest air pollution, the Po valley. Air pollution has been recently proposed as a possible risk factor of SARS-CoV-2 infection, due to its adverse effect on immunity and to the possibility that polluted air may even carry the virus. We investigated the association between air pollution and subsequent spread of the SARS-CoV-2 infection within these regions. We collected NO2 tropospheric levels using satellite data available at the European Space Agency before the lockdown. Using a multivariable restricted cubic spline regression model, we compared NO2 levels with SARS-CoV-2 infection prevalence rate at different time points after the lockdown, namely March 8, 22 and April 5, in the 28 provinces of Lombardy, Veneto and Emilia-Romagna. We found little association of NO2 levels with SARS-CoV-2 prevalence up to about 130 μmol/m2, while a positive association was evident at higher levels at each time point. Notwithstanding the limitations of the use of aggregated data, these findings lend some support to the hypothesis that high levels of air pollution may favor the spread of the SARS-CoV-2 infection.
Keywords: Covid-19, Coronavirus, Air pollution, Nitrogen dioxide, Sentinel-5P, Public health
Graphical abstract
Highlights
-
•
Air pollution might increase susceptibility to SARS-CoV-2 infection.
-
•
We examined infection prevalence in the most affected regions in Northern Italy.
-
•
Satellite-detected tropospheric nitrogen dioxide was used to assess air pollution.
-
•
High NO2 levels were associated with spread of the infection.
-
•
A causal role remains speculative, given ecologic biases and uncontrolled confounding.
1. Introduction
The first indigenous case of COVID-19 in Italy was diagnosed on February 20, 2020. Despite the isolation of potential hotspots in eleven affected municipalities (‘red areas’) and two national lockdowns established on February 23 and March 8–9, the outbreak quickly spread throughout Northern Italy and particularly in the Lombardy, Veneto and Emilia-Romagna regions (Gabutti et al., 2020; Ministry of Health, 2020). The later and more intensive lockdown, which started on March 8 in almost the entire territory of these three regions and on March 9 over all of Italy, was effective in slowing and curbing the infection, with a flattening and then a reversal of the outbreak beginning 9 days later. Further restrictions were established on March 22, including closure of all non-essential activities and prohibition of movements out of the municipality of residence.
Northern Italy is the most heavily industrialized and polluted area of Italy (EEA, 2019). The rapid and more intense spread of COVID-19 in Northern Italy, following the initial outbreak in densely populated and polluted Wuhan, China, prompted the hypothesis that the spread of the SARS-CoV-2 infection and possibly the severity of the associated COVID-19 disease was related to high air pollution levels (Coccia, 2020; Conticini et al., 2020; RIAS, 2020; SIMA, 2020; Watts and Kommenda, 2020).
Though there is some evidence that air pollution may enhance the susceptibility to viral infections including that by SARS-CoV-2 (Barcelo, 2020; Chen et al., 2010; Domingo and Rovira, 2020; Peng et al., 2020; Tsatsakis et al., 2020), the connection is still speculative. Transmission of airborne infections such as that due to SARS-CoV-2 occurs mainly through droplets and aerosols in the vicinity of infected individuals (Gabutti et al., 2020), but airborne particles might serve as carriers for pathogens (Setti et al., 2020a; SIMA, 2020; Zhao et al., 2019). Another possible mechanism linking air pollution to SARS-CoV-2 infection and COVID-19 might be higher susceptibility to respiratory viral diseases induced by air pollutants, through damage to respiratory tissue, immune dysregulation, overexpression of inflammatory cytokines and chemokines leading to innate immune system hyper-activation (Conticini et al., 2020) or ACE-2 receptor overexpression (Frontera et al., 2020).
Among the most commonly investigated air contaminants, nitrogen dioxide (NO2) is a secondary pollutant formed by combustion processes, primarily from road transport (41%), energy production (22%), energy use from households and commercial activities (13%), and from industry (13%) (EEA, 2020). NO2 is used for air pollution monitoring, with a threshold yearly average of 40 μg/m3 according to both European and WHO standards (EEA, 2019). In 2017 around 7% of the European urban population was exposed to concentrations above the annual EU limit (EEA, 2019). Traffic-related NO2 exposure has been shown to be associated with increased risk of asthma and decreased lung function (Bowatte et al., 2017; Cai et al., 2017; McCreanor et al., 2007) and also rhinitis in adult population (Cesaroni et al., 2008; de Marco et al., 2002). In addition, high NO2 levels has been associated with increased mortality for all causes, cardiovascular and respiratory mortality (Brunekreef et al., 2009; Eum et al., 2019; Hoek et al., 2013), and also pneumonia in older adults (Eum et al., 2019). With reference to SARS-CoV-2 infection, a recent spatial analysis has highlighted that up to 78% of COVID-19 deaths occurred in the five European areas located in Italy and Spain also showing the highest NO2 levels, thus indicating a possible contribution to fatality by NO2 long-term exposure (Ogen, 2020). In addition, a recent Chinese study showed a positive association between short-term exposure to air pollutants (i.e. particulate matter, sulfur dioxide, carbon monoxide, ozone and nitrogen dioxide) and newly diagnosed COVID-19 confirmed cases (Zhu et al., 2020), and other studies suggested a positive association with poor Air Quality Index (Li et al., 2020; Zoran et al., 2020).
The aim of our study was to investigate the relation between levels of air pollution in Northern Italy, assessed through remote-sensing satellite information on tropospheric NO2, and subsequent spread of the SARS-CoV-2 infection using publicly available data on newly-diagnosed infected cases.
2. Methods
2.1. Study area
We studied the Lombardy, Veneto and Emilia-Romagna regions of Northern Italy, which have a population of 19.4 million people, around 30% of Italian population, and accounted for 82% of Italian cases of SARS-CoV-2 infection on March 8, 2020 (Ministry of Health, 2020) with more severe forms of the disease requiring intensive care unit admission (Frontera et al., 2020). These regions contribute about 40% of the gross national product (ISTAT, 2020b), and are also characterized by the highest number of workers in the international export business sector (ISTAT, 2020a).
2.2. Health endpoints
The number of new daily SARS-CoV-2 positive cases was available by region and province of diagnosis from the national governmental website (CPD, 2020). From these data we computed prevalence rates, using population data for the study provinces from the Italian National Institute of Statistic website (ISTAT, 2020b).
2.3. Environmental exposure assessment
To assess the environmental pollution characterizing the study area before onset of the outbreak, we used daily information for the 1–23 February period from the Copernicus Open Access Hub-Sentinel-5P mission. Through this source, the European Space Agency (ESA) makes satellite data publicly available for the entire Europe (ESA, 2020). We focused on the geolocated (with a spatial resolution of 7 × 7 km2) tropospheric column of nitrogen dioxide reported by ESA Sentinel-5P, made available after Near Real Time (NRT) processing (typically 3 h from sensing), and, after addition, Offline (OFFL) processing (5 days after sensing time) (Eskes et al., 2019).
We collected all satellite images related to the area of interest (the provinces of the three study regions) for each day of the analysis period, and we computed population-weighted average of NO2 values (in μmol/m2) for each province. Data about NO2 were considered missing for one or more days when satellite coverage in those days decreased below 30% of the spatial units (provinces) for cloud-covered scenes or snow/ice on the surface influencing satellite image reliability (Eskes et al., 2019). We also validated satellite data through measured ground-level NO2 concentration by monitoring stations, finding a high correlation between satellite and measured provincial average NO2, with Pearson correlation coefficients ranging from 0.34 (Sondrio) to 0.81 (Cremona).
To control for population mobility, a possible confounding factor, we collected data on mobile phone daily movements through anonymous data of the SIM cards of residents (approximately 27 million people) processed and made available by Teralytics (Polzer, 2020). Information on the position of mobile telephones is available through the Call Detail Records (CDR), from which transport models can describe daily trip chains. We used daily movements in Italy available from February 1 through March 27 divided by the population in each province. We also retrieved temperature data publicly available from the European Centre for Medium-Range Weather Forecasts website (ECMWF, 2020), and relative humidity that has been calculated using environmental temperature and dewpoint temperature according previous method (Lawrence, 2005). We also considered as a potential confounder the presence of international airports in the study provinces for which the traffic flow was higher than 100,000 passengers in January 2020 (ASSAEROPORTI, 2020). This criterion included airports in Milan, Bergamo, Varese in Lombardy, Venice, Verona and Treviso in Veneto, and Bologna in Emilia-Romagna.
2.4. Data analysis
To assess the average amount of air pollution preceding the onset of the SARS-CoV-2 outbreak, we modeled the time-series of daily NO2 tropospheric levels from February 1 to 24 using a linear regression taking into account heteroskedasticity and autocorrelation up to 7 days using a Newey–West estimator (Newey and West, 1987). We also modeled these values after the lockdowns. We then examined the relation between NO2 levels before February 24, using the estimated NO2 levels on February 12, which is the midpoint of the period before the lockdown, and SARS-CoV-2 infection prevalence rates at several time points: 14 days (corresponding to the period between outbreak onset and the establishment of the tight lockdown), 28 and 42 days, corresponding to March 8, March 22, and April 5. We used restricted cubic spline regression analysis fitting a model with three fixed knots (10th, 50th and 90th percentiles) according to Harrel's method (Harrell, 2001) to assess the shape of the relation.
We also collected information on other possible confounding factors, including population density and age distribution in 2019, percentage of population commuting daily for work or school, percentage of single-member families, and percentage of dwellings with one resident only based on 2011 census data, the latest available at provincial level (ISTAT, 2020b).
We performed the analysis within a multivariable model adjusting for population density (number of inhabitants per km2), an index indicating age of the population (ratio between resident population aged ≥65 years and those aged ≤14 years), people mobility (measured using number of movements of mobile telephones before the lockdown), temperature (°C), relative humidity in the three subsequent periods (in percentage), and airport presence (yes/no). We alternatively further added to the model the percentage of population commuting daily outside the municipality of residence for work or study, single member families, or dwellings occupied by only one resident.
3. Results
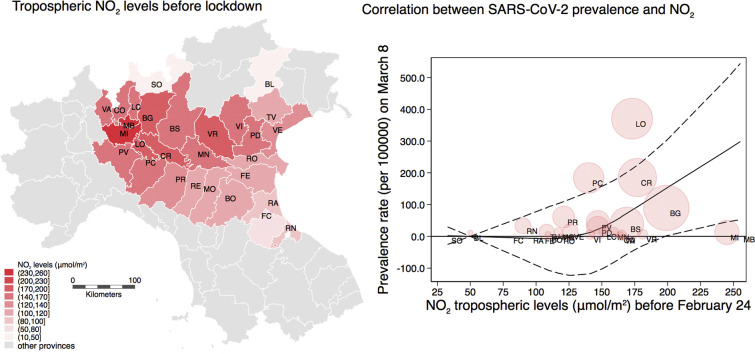
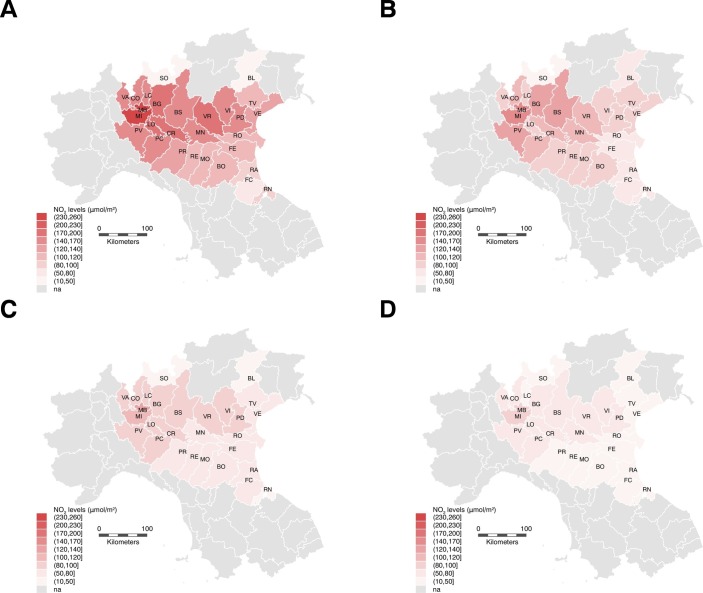
Fig. 1 shows the average tropospheric levels of NO2 in study provinces before the disease outbreak, and in the subsequent periods during the spread of SARS-CoV-2 infection, when NO2 levels steadily decreased. In particular, before the outbreak the average NO2 levels were high in Lombardy (193 μmol/m2), Milan (238 μmol/m2) and Monza/Brianza (243 μmol/m2), and they fell below 100 μmol/m2 in all investigated provinces in the period following the tightest mobility restrictions.
Fig. 1.
Northern Italy study area showing levels of NO2 tropospheric levels (μmol/m2) before the first lockdown (A), and in the subsequent periods February 24–March 8 (B), March 8–March 22 (C), and after March 22 (D).
Table 1 reports the province-specific prevalence rates of the SARS-CoV-2 infection in the three follow-up time points considered in the study, i.e. the one corresponding to the institution of the tight lockdown (March 8), and the next two 2-week periods, along with corresponding NO2 pollution levels before and after the detection of the outbreak (February 24).
Table 1.
Number of total SARS-CoV-2 positive cases and prevalence rate on March 8, March 22, and April 5 and predicted NO2 tropospheric levels (μmol/m2) before the lockdown, in the subsequent periods after partial lockdown (February 24–March 8), after full lockdown (March 8–March 22), and after March 22.
| Total cases |
Prevalence rate (per 100,000) |
NO2 levels (μmol/m2) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population at Jan 1, 2019a | Mar 8 | Mar 22 | Apr 5 | Mar 8 | Mar 22 | Apr 5 | Before Feb 24 | After Feb 24 |
|||
| Feb 24–Mar 8 | Mar 8–Mar 22 | After Mar 22 | |||||||||
| Lombardy | 10,060,574 | 4189 | 27,206 | 50,455 | 41.6 | 270.4 | 501.5 | 198 | 126 | 98 | 72 |
| Bergamo (BG) | 1,114,590 | 997 | 6296 | 9712 | 89.5 | 557.7 | 871.4 | 199 | 124 | 93 | 59 |
| Brescia (BS) | 1,265,954 | 501 | 5317 | 9340 | 39.6 | 420.0 | 737.8 | 169 | 120 | 91 | 61 |
| Como (CO) | 599,204 | 27 | 512 | 1384 | 4.5 | 85.4 | 231 | 163 | 101 | 95 | 64 |
| Cremona (CR) | 358,955 | 665 | 2895 | 4233 | 185.3 | 806.5 | 1179.3 | 177 | 107 | 84 | 60 |
| Lecco (LC) | 337,380 | 53 | 872 | 1678 | 15.7 | 258.5 | 497.4 | 151 | 92 | 81 | 48 |
| Lodi (LO) | 230,198 | 853 | 1772 | 2255 | 370.6 | 769.8 | 979.6 | 173 | 98 | 83 | 74 |
| Mantua (MN) | 412,292 | 56 | 905 | 3046 | 13.6 | 219.5 | 348.5 | 159 | 105 | 74 | 53 |
| Milan (MI) | 3,250,315 | 406 | 5096 | 11,230 | 12.5 | 156.9 | 345.5 | 245 | 157 | 111 | 98 |
| Monza/Brianza (MB) | 873,935 | 59 | 1108 | 3046 | 6.8 | 126.8 | 348.5 | 255 | 153 | 138 | 80 |
| Pavia (PV) | 545,888 | 243 | 1306 | 2619 | 44.5 | 239.2 | 479.8 | 147 | 98 | 72 | 50 |
| Sondrio (SO) | 181,095 | 6 | 205 | 591 | 3.3 | 113.2 | 326.3 | 33 | 25 | 22 | 29 |
| Varese (VA) | 890,768 | 32 | 386 | 1191 | 3.6 | 43.3 | 133.7 | 166 | 99 | 92 | 60 |
| Veneto | 4,905,854 | 670 | 5122 | 11,226 | 13.7 | 104.4 | 228.8 | 136 | 94 | 75 | 50 |
| Belluno (BL) | 202,950 | 23 | 226 | 538 | 11.3 | 111.4 | 265.1 | 50 | 56 | 31 | 29 |
| Padua (PD) | 937,908 | 255 | 1277 | 2744 | 27.2 | 136.2 | 292.6 | 147 | 88 | 84 | 55 |
| Rovigo (RO) | 234,937 | 5 | 76 | 186 | 2.1 | 32.3 | 79.2 | 118 | 68 | 56 | 42 |
| Treviso (TV) | 887,806 | 126 | 935 | 1712 | 14.2 | 105.3 | 192.8 | 108 | 96 | 59 | 42 |
| Venezia (VE) | 853,338 | 126 | 732 | 1425 | 14.8 | 85.8 | 167 | 126 | 94 | 66 | 46 |
| Verona (VR) | 926,497 | 63 | 1046 | 2688 | 6.8 | 112.9 | 290.1 | 181 | 114 | 97 | 59 |
| Vicenza (VI) | 862,418 | 50 | 631 | 1647 | 5.8 | 73.2 | 191 | 141 | 97 | 84 | 54 |
| Emilia-Romagna | 4,459,477 | 1180 | 7555 | 17,089 | 26.5 | 168.4 | 383.2 | 109 | 81 | 66 | 38 |
| Bologna (BO) | 1,014,619 | 62 | 674 | 2521 | 6.1 | 66.4 | 248.5 | 109 | 86 | 71 | 37 |
| Ferrara (FE) | 345,691 | 6 | 150 | 488 | 1.7 | 43.4 | 141.2 | 104 | 71 | 55 | 39 |
| Forlì-Cesena (FC) | 394,627 | 15 | 329 | 977 | 3.8 | 83.4 | 247.6 | 80 | 61 | 51 | 29 |
| Modena (MO) | 705,393 | 97 | 1010 | 2609 | 13.8 | 143.2 | 369.9 | 117 | 95 | 70 | 42 |
| Parma (PR) | 451,631 | 276 | 1209 | 2275 | 61.1 | 267.7 | 503.7 | 121 | 87 | 72 | 42 |
| Piacenza (PC) | 287,152 | 528 | 1765 | 2892 | 183.9 | 614.7 | 1007.1 | 140 | 113 | 90 | 58 |
| Ravenna (RA) | 389,456 | 13 | 309 | 708 | 3.3 | 79.3 | 181.8 | 95 | 67 | 60 | 31 |
| Reggio Emilia (RE) | 531,891 | 70 | 1167 | 3066 | 13.2 | 219.4 | 576.4 | 118 | 83 | 68 | 43 |
| Rimini (RN) | 339,017 | 113 | 942 | 1553 | 33.3 | 277.9 | 458.1 | 90 | 53 | 40 | 23 |
| Italy | 60,359,546 | 7375 | 59,138 | 128,948 | 12.2 | 98.0 | 213.6 | ||||
Most recent data available from Italian National Institute of Statistic (ISTAT, 2020b).
We first fit a spline curve to the crude data. There was an increase in prevalence rates can be noted at low levels of NO2, with a plateau above 150 μmol/m2 (Fig. S1). In multivariable analysis adjusting for population density, elderly population, airport presence, people mobility before February 24, and temperature and relative humidity in the two weeks before prevalence assessment (Fig. 2 ), NO2 levels in the pre-outbreak period were not meaningfully associated with COVID-19 prevalence rates at any of the three follow-up time points, up to approximately 130 μmol/m2 of NO2 levels. Above 130 μmol/m2 of NO2 levels, however, there was a higher prevalence of COVID-19 with increasing levels of air pollution, with a similar curve relating air pollution to prevalence for each of the three time periods. Further addition of possible confounding factors, such as percentage of daily commuters outside the municipality of residence (Fig. S2), percentage of single-member families (Fig. S3), and percentage of dwellings occupied by only one resident (Fig. S4), did not appreciably change the results. In these analyses, the limited precision inherent in these ecological data are reflected in the broad confidence bands.
Fig. 2.
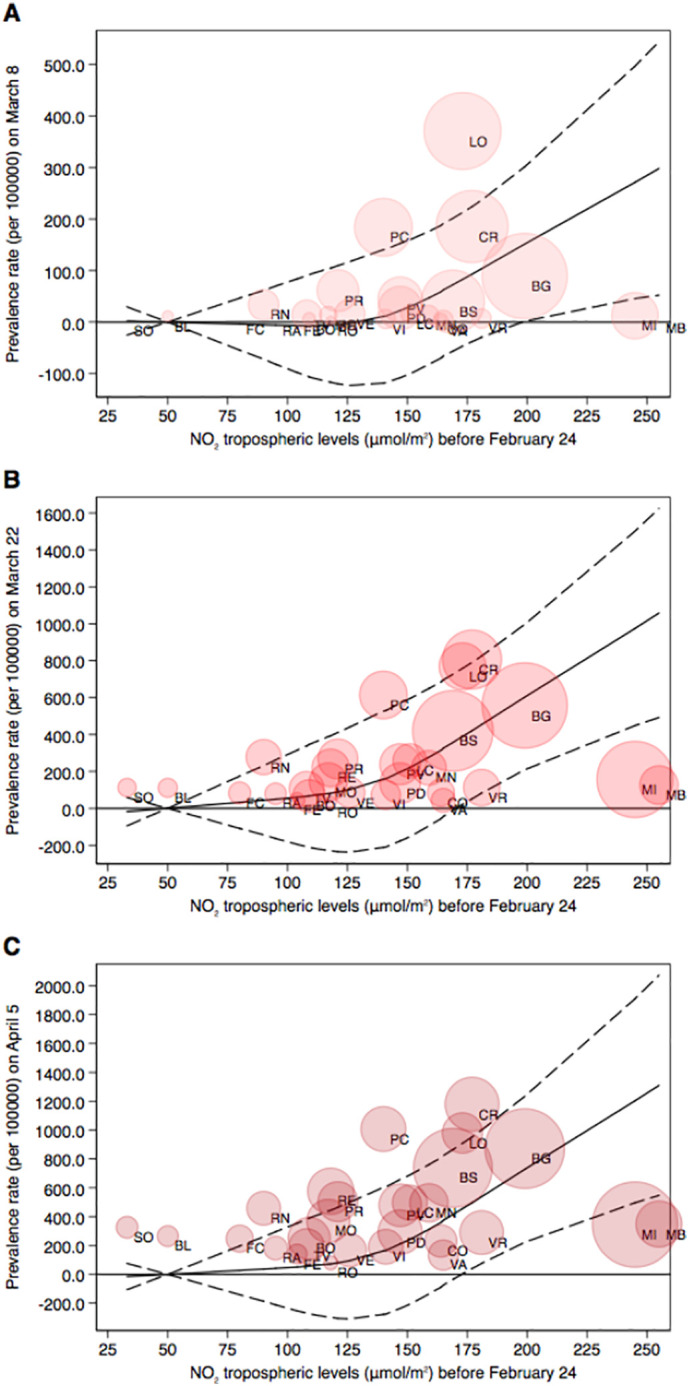
Restricted cubic spline regression analysis between NO2 tropospheric levels (μmol/m2) before the spread of the outbreak and SARS-CoV-2 positivity prevalence (cases per 100,000) in the three periods after the lockdown dates (A: February 24–March 8; B: March 8–March22; C: March 22–April 5). Results presented SARS-CoV-2 infection prevalence rate (solid line) with 95% confidence interval (dash lines) in a multivariable model adjusted for population density, an index indicating age of the population, people mobility measured from telephone movements before the lockdown, temperature (°C) and relative humidity in the three subsequent periods, and airport presence. Shaded circles are weighted on number of cases corresponding to the prevalence rates at each time point.
4. Discussion
In this study, we found a positive association between levels of NO2 levels and subsequence prevalence of SARS-CoV-2 positivity in Northern Italy, though this occurred only at high levels of NO2. This relation is consistent with severe outdoor air pollution enhancing the spread of the SARS-CoV-2 virus, possibly by altering the immunological status and thus increasing individual susceptibility to infectious diseases (Ibironke et al., 2019; Marchini et al., 2020; Rivas-Santiago et al., 2015; Williams et al., 2011). The area of the study comprises the densest industrial, trading and agricultural zone in Italy, with an orography that favors the stagnation of pollutants. This region has seen some of the worst air pollution in Europe (EEA, 2019; Mazzola et al., 2010). Within this area, high levels of air pollution have been associated with increased mortality and hospitalization for both cardiovascular and respiratory diseases (Carugno et al., 2016; Fattore et al., 2011).
A positive association between spread of the SARS-CoV-2 outbreak in Italy and excesses of particulate matter at monitoring stations has also been reported (CPD, 2020; Setti et al., 2020b), and in the US an increase of 1 μg/m3 in particular matter (PM2.5) has been associated with a 15% increase in mortality from COVID-19 (Wu et al., 2020). High air pollution levels could also have favored the spread of another coronavirus-induced disease, SARS, based on the positive correlation between air pollution and its lethality in Chinese provinces (Cui et al., 2003). In addition, a study carried out in Milan metropolitan area (Lombardy region) found a positive association between COVID-19 new daily cases and particulate matter levels, with Pearson correlation coefficient between average and maximum PM10 levels and daily new cases of 0.35 and 0.51, respectively (Zoran et al., 2020). We used tropospheric NO2 levels as a proxy for overall air pollution. This metric was closely associated with ground levels of this contaminant in a validation study in the study area as well as in other European urban areas (Ialongo et al., 2020; Lorente et al., 2019).
In vitro studies have shown that NO2 can alter expression and synthesis of pro-inflammatory mediators in human bronchial cells (Bayram et al., 2001; Blomberg et al., 1997; Blomberg et al., 1999; Devalia et al., 1993; Mirowsky et al., 2016), thus increasing inflammation and hyperresponsiveness of epithelial cells. Also, both direct cytotoxicity and cytokine-mediated has been reported after NO2 exposure of epithelial cells in the lung (Ayyagari et al., 2004; Persinger et al., 2002). Overall, these observations provide some biological plausibility for the association we and other detected. In addition, NO2 levels are likely to mirror the air levels of other contaminants, such as particulate matter (Bigi et al., 2012), which also has deleterious effects on the immunity (Blomberg, 2000; Glencross et al., 2020).
Despite biologic plausibility, this study has important limitations. Most important is the potential for ecologic bias, because we used aggregated data. With aggregated data we could not take into account individual risk factors that affected the spread of infection, nor control directly for confounding at the individual level. Factors such as working in health care delivery, travel to China or other infected countries, residence in nursing homes, and individual contact with infected relatives, friends, or workmates were not controlled and differences in the distributions of these factors may have influenced the results. A further limitation was the inability to examine other clinical endpoints of COVID-19, but information on hospitalizations, deaths, and other outcomes were not publicly available at the provincial level. Finally, the effect estimates we computed were imprecise, as reflected in broad confidence intervals. Although these limitations preclude strong inferences from these ecologic data, our findings appear to confirm an association between heavy air pollution and spread of the SARS-CoV-2 virus.
Abbreviations
CRediT authorship contribution statement
Tommaso Filippini: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Kenneth J. Rothman: Formal analysis, Investigation, Writing - review & editing. Alessia Goffi: Data curation, Resources, Writing - review & editing. Fabrizio Ferrari: Data curation, Resources, Writing - review & editing. Giuseppe Maffeis: Data curation, Methodology, Resources, Writing - review & editing. Nicola Orsini: Formal analysis, Methodology, Writing - review & editing. Marco Vinceti: Conceptualization, Formal analysis, Funding acquisition, Investigation, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Supported by grant 'UNIMORE FAR 2019 Interdisciplinare Linea FCRMO - Fondazione Cassa di Risparmio di Modena'.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.140278.
Appendix A. Supplementary data
Supplemental figures
References
- ASSAEROPORTI . Associazione Italiana Gestori di Aeroporti; 2020. January 2020 Statistics.http://assaeroporti.com/statistiche_202001/ [Google Scholar]
- Ayyagari V.N., Januszkiewicz A., Nath J. Pro-inflammatory responses of human bronchial epithelial cells to acute nitrogen dioxide exposure. Toxicology. 2004;197:149–164. doi: 10.1016/j.tox.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Barcelo D. An environmental and health perspective for COVID-19 outbreak: Meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. J. Environ. Chem. Eng. 2020;8 doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram H., Sapsford R.J., Abdelaziz M.M., Khair O.A. Effect of ozone and nitrogen dioxide on the release of proinflammatory mediators from bronchial epithelial cells of nonatopic nonasthmatic subjects and atopic asthmatic patients in vitro. J. Allergy Clin. Immunol. 2001;107:287–294. doi: 10.1067/mai.2001.111141. [DOI] [PubMed] [Google Scholar]
- Bigi A., Ghermandi G., Harrison R.M. Analysis of the air pollution climate at a background site in the Po valley. J. Environ. Monit. 2012;14:552–563. doi: 10.1039/c1em10728c. [DOI] [PubMed] [Google Scholar]
- Blomberg A. Airway inflammatory and antioxidant responses to oxidative and particulate air pollutants - experimental exposure studies in humans. Clin. Exp. Allergy. 2000;30:310–317. doi: 10.1046/j.1365-2222.2000.00814.x. [DOI] [PubMed] [Google Scholar]
- Blomberg A., Krishna M.T., Bocchino V., Biscione G.L., Shute J.K., Kelly F.J. The inflammatory effects of 2 ppm NO2 on the airways of healthy subjects. Am. J. Respir. Crit. Care Med. 1997;156:418–424. doi: 10.1164/ajrccm.156.2.9612042. [DOI] [PubMed] [Google Scholar]
- Blomberg A., Krishna M.T., Helleday R., Soderberg M., Ledin M.C., Kelly F.J. Persistent airway inflammation but accommodated antioxidant and lung function responses after repeated daily exposure to nitrogen dioxide. Am. J. Respir. Crit. Care Med. 1999;159:536–543. doi: 10.1164/ajrccm.159.2.9711068. [DOI] [PubMed] [Google Scholar]
- Bowatte G., Erbas B., Lodge C.J., Knibbs L.D., Gurrin L.C., Marks G.B. Traffic-related air pollution exposure over a 5-year period is associated with increased risk of asthma and poor lung function in middle age. Eur. Respir. J. 2017;50:1602357. doi: 10.1183/13993003.02357-2016. [DOI] [PubMed] [Google Scholar]
- Brunekreef B., Beelen R., Hoek G., Schouten L., Bausch-Goldbohm S., Fischer P. Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: the NLCS-AIR study. Res. Rep. Health Eff. Inst. 2009:5–71. (discussion 73-89) [PubMed] [Google Scholar]
- Cai Y., Zijlema W.L., Doiron D., Blangiardo M., Burton P.R., Fortier I. Ambient air pollution, traffic noise and adult asthma prevalence: a BioSHaRE approach. Eur. Respir. J. 2017;49 doi: 10.1183/13993003.02127-2015. [DOI] [PubMed] [Google Scholar]
- Carugno M., Consonni D., Randi G., Catelan D., Grisotto L., Bertazzi P.A. Air pollution exposure, cause-specific deaths and hospitalizations in a highly polluted Italian region. Environ. Res. 2016;147:415–424. doi: 10.1016/j.envres.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Cesaroni G., Badaloni C., Porta D., Forastiere F., Perucci C.A. Comparison between various indices of exposure to traffic-related air pollution and their impact on respiratory health in adults. Occup. Environ. Med. 2008;65:683–690. doi: 10.1136/oem.2007.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.S., Tsai F.T., Lin C.K., Yang C.Y., Chan C.C., Young C.Y. Ambient influenza and avian influenza virus during dust storm days and background days. Environ. Health Perspect. 2010;118:1211–1216. doi: 10.1289/ehp.0901782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020;261 doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CPD Italian civil protection department: COVID-19 data. 2020. https://github.com/pcm-dpc/COVID-19 [DOI] [PMC free article] [PubMed]
- Cui Y., Zhang Z.F., Froines J., Zhao J., Wang H., Yu S.Z. Air pollution and case fatality of SARS in the People’s Republic of China: An ecologic study. Environ. Health. 2003;2:15. doi: 10.1186/1476-069X-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marco R., Poli A., Ferrari M., Accordini S., Giammanco G., Bugiani M. The impact of climate and traffic-related NO2 on the prevalence of asthma and allergic rhinitis in Italy. Clin. Exp. Allergy. 2002;32:1405–1412. doi: 10.1046/j.1365-2745.2002.01466.x. [DOI] [PubMed] [Google Scholar]
- Devalia J.L., Campbell A.M., Sapsford R.J., Rusznak C., Quint D., Godard P. Effect of nitrogen dioxide on synthesis of inflammatory cytokines expressed by human bronchial epithelial cells in vitro. Am. J. Respir. Cell Mol. Biol. 1993;9:271–278. doi: 10.1165/ajrcmb/9.3.271. [DOI] [PubMed] [Google Scholar]
- Domingo J.L., Rovira J. Effects of air pollutants on the transmission and severity of respiratory viral infections. Environ. Res. 2020;187 doi: 10.1016/j.envres.2020.109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECMWF . 2020. ERA5 dataset European Centre for Medium-Range Weather Forecasts.https://www.ecmwf.int/en/forecasts/datasets/reanalysis-datasets/era5 [Google Scholar]
- EEA . In: EEA Report No 10/2019 - Air Quality in Europe 2019. European Environment Agency, editor. 2019. https://www.eea.europa.eu/publications/air-quality-in-europe-2019 Copenhagen, Denmark. [Google Scholar]
- EEA . In: The European Environment State and Outlook 2020. European Environment Agency, editor. EEA; Luxembourg: 2020. https://www.eea.europa.eu/publications/soer-2020 [Google Scholar]
- ESA . European Space Agency; 2020. Sentinel-5P Data.https://www.esa.int/Applications/Observing_the_Earth/Copernicus/Sentinel-5P [Google Scholar]
- Eskes H., van Geffen J., Boersma F., Eichmann K.-U., Apituley A., Pedergnana M. In: Sentinel-5 Precursor/TROPOMI Level 2 Product User Manual Nitrogendioxide. Royal Netherlands Meteorological Institute, editor. Royal Netherlands Meteorological Institute; 2019. https://sentinel.esa.int/documents/247904/2474726/Sentinel-5P-Level-2-Product-User-Manual-Nitrogen-Dioxide [Google Scholar]
- Eum K.D., Kazemiparkouhi F., Wang B., Manjourides J., Pun V., Pavlu V. Long-term NO2 exposures and cause-specific mortality in American older adults. Environ. Int. 2019;124:10–15. doi: 10.1016/j.envint.2018.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore E., Paiano V., Borgini A., Tittarelli A., Bertoldi M., Crosignani P. Human health risk in relation to air quality in two municipalities in an industrialized area of Northern Italy. Environ. Res. 2011;111:1321–1327. doi: 10.1016/j.envres.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Frontera A., Cianfanelli L., Vlachos K., Landoni G., Cremona G. Severe air pollution links to higher mortality in COVID-19 patients: The “double-hit” hypothesis. J. Inf. Secur. 2020 doi: 10.1016/j.jinf.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabutti G., d’Anchera E., Sandri F., Savio M., Stefanati A. Coronavirus: update related to the current outbreak of COVID-19. Infect. Dis. Ther. 2020;9:241–253. doi: 10.1007/s40121-020-00295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glencross D.A., Ho T.R., Camina N., Hawrylowicz C.M., Pfeffer P.E. Air pollution and its effects on the immune system. Free Radic. Biol. Med. 2020;151:56–68. doi: 10.1016/j.freeradbiomed.2020.01.179. [DOI] [PubMed] [Google Scholar]
- Harrell F.E. Springer-Verlag; New York: 2001. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. [Google Scholar]
- Hoek G., Krishnan R.M., Beelen R., Peters A., Ostro B., Brunekreef B. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ. Health. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ialongo I., Virta H., Eskes H., Hovila J., Douros J. Comparison of TROPOMI/Sentinel-5 Precursor NO2 observations with ground-based measurements in Helsinki. Atmos. Meas. Tech. 2020;13:205–218. doi: 10.5194/amt-13-205-2020. [DOI] [Google Scholar]
- Ibironke O., Carranza C., Sarkar S., Torres M., Choi H.T., Nwoko J. Urban air pollution particulates suppress human T-cell responses to mycobacterium tuberculosis. Int. J. Environ. Res. Public Health. 2019;16:4112. doi: 10.3390/ijerph16214112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISTAT . Italian National Institut of Statistic; 2020. Coeweb - Statistiche del commercio estero.https://www.coeweb.istat.it [Google Scholar]
- ISTAT . 2020. Data from the Italian National Institute of Statistic.https://www.istat.it/it/popolazione-e-famiglie [Google Scholar]
- Lawrence M.G. The relationship between relative humidity and the dewpoint temperature in moist air: a simple conversion and applications. Bull. Am. Meteorol. Soc. 2005;86:225–234. doi: 10.1175/BAMS-86-2-225. [DOI] [Google Scholar]
- Li H., Xu X.L., Dai D.W., Huang Z.Y., Ma Z., Guan Y.J. Air pollution and temperature are associated with increased COVID-19 incidence: a time series study. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente A., Boersma K.F., Eskes H.J., Veefkind J.P., van Geffen J., de Zeeuw M.B. Quantification of nitrogen oxides emissions from build-up of pollution over Paris with TROPOMI. Sci. Rep. 2019;9:20033. doi: 10.1038/s41598-019-56428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini T., Zirlik A., Wolf D. Pathogenic role of air pollution particulate matter in cardiometabolic disease: Evidence from mice and humans. Antioxid. Redox Signal. 2020 doi: 10.1089/ars.2020.8096. [DOI] [PubMed] [Google Scholar]
- Mazzola M., Lanconelli C., Lupi A., Busetto M., Vitale V., Tomasi C. Columnar aerosol optical properties in the Po Valley, Italy, from MFRSR data. J. Geophys. Res. Atmos. 2010;115:D17206. doi: 10.1029/2009JD013310. [DOI] [Google Scholar]
- McCreanor J., Cullinan P., Nieuwenhuijsen M.J., Stewart-Evans J., Malliarou E., Jarup L. Respiratory effects of exposure to diesel traffic in persons with asthma. N. Engl. J. Med. 2007;357:2348–2358. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- Ministry of Health Official Italian statistics on SARS-CoV-2, March 8, 2020. 2020. http://www.salute.gov.it/imgs/C_17_pagineAree_5351_0_file.pdf
- Mirowsky J.E., Dailey L.A., Devlin R.B. Differential expression of pro-inflammatory and oxidative stress mediators induced by nitrogen dioxide and ozone in primary human bronchial epithelial cells. Inhal. Toxicol. 2016;28:374–382. doi: 10.1080/08958378.2016.1185199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey W.K., West K.D. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica. 1987;55:703–708. doi: 10.2307/1913610. [DOI] [Google Scholar]
- Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Zhao X., Tao Y., Mi S., Huang J., Zhang Q. The effects of air pollution and meteorological factors on measles cases in Lanzhou, China. Environ. Sci. Pollut. Res. Int. 2020;27:13524–13533. doi: 10.1007/s11356-020-07903-4. [DOI] [PubMed] [Google Scholar]
- Persinger R.L., Poynter M.E., Ckless K., Janssen-Heininger Y.M. Molecular mechanisms of nitrogen dioxide induced epithelial injury in the lung. Mol. Cell. Biochem. 2002;234–235:71–80. doi: 10.1023/A:1015973530559. [DOI] [PubMed] [Google Scholar]
- Polzer G. La Repubblica, Italy. 2020. La mappa della nostra era glaciale: così il coronavirus ha congelato l’Italia.https://lab.gedidigital.it/repubblica/2020/cronaca/coronavirus-mappa-italia-impatto-sulla-mobilita/?ref=RHPPLF-BH-I252606083-C8-P3-S1.8-T1 [Google Scholar]
- RIAS . SCIRE; 2020. Inquinamento atmosferico e COVID-19, Rete Italiana Ambiente e Salute.https://www.scienzainrete.it/articolo/inquinamento-atmosferico-e-covid-19/rete-italiana-ambiente-e-salute/2020-04-13 [Google Scholar]
- Rivas-Santiago C.E., Sarkar S., Cantarella Pt, Osornio-Vargas A., Quintana-Belmares R., Meng Q. Air pollution particulate matter alters antimycobacterial respiratory epithelium innate immunity. Infect. Immun. 2015;83:2507–2517. doi: 10.1128/IAI.03018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Borelli M. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: first evidence. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Piazzalunga A. The potential role of particulate matter in the spreading of COVID-19 in northern Italy: first evidence-based research hypotheses. medRxiv. 2020 doi: 10.1101/2020.04.11.20061713. 2020.04.11.20061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMA Position paper particulate matter and COVID-19 Italian Society of Environmental Medicine (SIMA) 2020. http://www.simaonlus.it/wpsima/wp-content/uploads/2020/03/COVID_19_position-paper_ENG.pdf
- Tsatsakis A., Petrakis D., Nikolouzakis T.K., Docea A.O., Calina D., Vinceti M. COVID-19, an opportunity to reevaluate the correlation between long-term effects of anthropogenic pollutants on viral epidemic/pandemic events and prevalence. Food Chem. Toxicol. 2020;141 doi: 10.1016/j.fct.2020.111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J., Kommenda N. The Guardian. 2020. Coronavirus pandemic leading to huge drop in air pollution.https://www.theguardian.com/environment/2020/mar/23/coronavirus-pandemic-leading-to-huge-drop-in-air-pollution?CMP=Share_iOSApp_Other [Google Scholar]
- Williams L., Ulrich C.M., Larson T., Wener M.H., Wood B., Chen-Levy Z. Fine particulate matter (PM2.5) air pollution and immune status among women in the Seattle area. Arch. Environ. Occup. Health. 2011;66:155–165. doi: 10.1080/19338244.2010.539636. [DOI] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. Exposure to air pollution and COVID-19 mortality in the United States. medRxiv. 2020 doi: 10.1101/2020.04.05.20054502. 2020.04.05.20054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Richardson B., Takle E., Chai L., Schmitt D., Xin H. Airborne transmission may have played a role in the spread of 2015 highly pathogenic avian influenza outbreaks in the United States. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-47788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran M.A., Savastru R.S., Savastru D.M., Tautan M.N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan, Italy. Sci. Total Environ. 2020;738 doi: 10.1016/j.scitotenv.2020.139825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figures