Abstract
Clinical descriptions about influenza-like illnesses (ILI) in COVID-19 seem non-specific. We aimed to compare the clinical features of COVID-19 and influenza. We retrospectively investigated the clinical features and outcomes of confirmed cases of COVID-19 and influenza in Nord Franche-Comté Hospital between February 26th and March 14th 2020. We used SARS-CoV-2 RT-PCR and influenza virus A/B RT-PCR in respiratory samples to confirm the diagnosis. We included 124 patients. The mean age was 59 (±19 [19–98]) years with 69% female. 70 patients with COVID-19 and 54 patients with influenza A/B. Regarding age, sex and comorbidities, no differences were found between the two groups except a lower Charlson index in COVID-19 group (2 [±2.5] vs 3 [±2.4],p = 0.003). Anosmia (53% vs 17%,p < 0.001), dysgeusia (49% vs 20%,p = 0.001), diarrhea (40% vs 20%,p = 0.021), frontal headache (26% vs 9%,p = 0.021) and bilateral cracklings sounds (24% vs 9%,p = 0.034) were statistically more frequent in COVID-19. Sputum production (52% vs 29%,p = 0.010), dyspnea (59% vs 34%,p = 0.007), sore throat (44% vs 20%,p = 0.006), conjunctival hyperhemia (30% vs 4%,p < 0.001), tearing (24% vs 6%,p = 0.004), vomiting (22% vs 3%,p = 0.001) and rhonchi sounds (17% vs 1%,p = 0.002) were more frequent with influenza infection. We described several clinical differences which can help the clinicians during the co-circulation of influenza and SARS-CoV-2.
Keywords: COVID- 19, SARS-CoV-2, Influenza, Clinical feature, Influenza-like-illness
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) represents the causative agent of coronavirus disease 2019 (COVID-19), a potentially fatal disease that is of great global public health concern [1]. The early detection of suspected cases is a major health issue. This can help health care workers to use rapidly preventive measures and specific precautions to reduce transmission, appropriate treatments and supportive care for COVID-19 patients. Early detection also may help to differentiate COVID-19 from other illnesses such that the appropriate International Classification of Diseases-10 (ICD-10) code can be made to identify all deaths due to COVID-19 [2].
The epidemiological arguments as contact with a confirmed SARS-CoV-2 infected patient or return from an “at-risk area” with SARS-CoV-2 circulation are crucial to suspect a case of COVID-19. The main symptoms of COVID-19 and influenza A/B virus are similar. During influenza season in an area without any known circulation of SARS-CoV-2, without epidemiological arguments, it appears difficult to suspect COVID-19.
Until now, functional signs in COVID-19 are incompletely described, for example, neurological, otorhinolaryngological and gastrointestinal symptoms seems to be underestimated [[3], [4], [5], [6]]. Furthermore, no study compared the functional signs between COVID-19 and influenza. In this study, we aimed to describe clinical features of patients with confirmed COVID-19, and to compare the clinical features between these patients and those with confirmed influenza A/B.
1. Method
We conducted a retrospective and observational study in NFC (Nord Franche-Comté) Hospital as a major French cluster of COVID-19 began on February, the 26th in the near-by Grand-Est region. Between February, 26th and March, 14th 2020, we enrolled all adult patients (≥18 years) with confirmed COVID- 19 or confirmed influenza A/B who consulted or were hospitalized in this hospital. Pregnant women, children (<18 years) and patients with dementia (unable to report functional symptoms) were excluded.
We collected demographic characteristics, comorbidities, and characteristics of the current COVID-19 or influenza infection: incubation period (defined by the time between the presumed contact and the symptoms onset), clinical features, evolution of the symptoms and outcome. Due to the beginning of the outbreak of COVID-19 we prepared a standardized questionnaire for each patient suspect of COVID-19 (also suspected of influenza) to help us screen their functional symptoms and the onset and duration of their symptoms. A home follow-up was recommended in our national guidelines, for patients who were not hospitalized, until they are asymptomatic for more than 48 h [7]. Consequently, outpatients and patients who were discharged after their hospitalization were called 14 days ± 7 days after the beginning of the symptoms; in case of persistent symptoms, we contacted them again 7 days later, until they were asymptomatic to ascertain epidemiological and clinical data. For each patient, we followed the clinical evolution and outcomes at least until recovery plus 48 h. We stopped the follow-up of the study on March, 24th 2020.
Diagnosis was confirmed by real-time RT-PCR on respiratory samples, mainly nasopharyngeal swabs, sputum, bronchial aspirates or bronchoalveolar lavage fluids for SARS-CoV-2 and influenza virus A and B. Viral RNA was extracted using the NucleoSpin® RNA Virus kit (Macherey–Nagel) according to the manufacturers’ instructions and amplified by RT-PCR protocols developed by the Charité (E gene) [8] and the Institut Pasteur (RdRp gene) [9], and by the R-DiaFlu kit (Diagenode) for SARS-CoV-2 and influenza virus, respectively. Quantified positive controls of SARS-CoV-2 were kindly provided by the French National Reference Center for Respiratory Viruses, Institut Pasteur, Paris.
Concerning the statistical analysis, continuous variables were expressed as mean and standard deviation (SD) and compared with ANOVA test. Categorical variables were expressed as number (%) ans and compared by χ2 test or Fisher’s exact test between the two groups (patients with confirmed COVID-19 and patients with confirmed influenza A/B). A p-value < 0.05 was considered significant. The nonparametric bootstrap method was used to obtain 95% pointwise confidence intervals (95% CI). We used the SPSS v24.0 software (IBM, Armonk, NY, USA).
2. Results
During the study period 139 patients had COVID-19 or influenza A/B confirmed in our hospital. Four patients with COVID-19 and 11 patients with influenza A/B were excluded (under 18 years old, pregnancy or dementia). Finally, one hundred and twenty-four patients were included in this study: 70 patients with confirmed COVID-19 and 54 with confirmed influenza A/B. The mean age was 59 (±19, [19–98]) years with 69% female. No case of co-infection by the 2 viruses was observed.
2.1. Clinical description of patients with COVID-19
In the group COVID-19, the mean age of patients was 57 (±19) years and 29 (41%) were male. Half of the patients (51%) were between 41 and 70 years-old. Charlson comorbidity index mean was 1.7 (±2.5). Thirty-six patients (51%) had underlying comorbidities, main comorbidities were: cardiovascular disease (41%, n = 29), chronic obstructive pulmonary disease (COPD) or asthma (16%, n = 11) and diabetes mellitus (14%, n = 10) (Table 1 ).
Table 1.
Demographics and baseline characteristics of patients infected with SARS-CoV-2 and Influenza virus A/B in Nord Franche-Comte Hospital.
| Characteristics | SARS-CoV-2 (n = 70) | Influenza A/B (n = 54) | p-(value) |
|---|---|---|---|
| Age (y) (mean, extremes, SD) | 56.7 [19–96] ± 19.3 | 61.3 [25–98] ± 18.8 | 0.176 |
| [18–30] | 9 (12.9%) | 4 (7.4%) | 0.387 |
| [31–40] | 7 (10%) | 5 (9.3%) | 1 |
| [41–50] | 12 (17.2%) | 6 (11.1%) | 0.444 |
| [51–60] | 13 (18.6%) | 10 (18.5%) | 1 |
| [61–70] | 11 (15.7%) | 9 (16.7%) | 1 |
| [71–80] | 10 (14.3%) | 14 (25.9%) | 0.115 |
| [81–90] | 7 (10%) | 4 (7.4%) | 0.755 |
| >90 | 1 (1.4%) | 2 (3.7%) | 0.579 |
| Sex (Number, %) | |||
| Male | 29 (41.4) | 17 (31.5) | 0.192 |
| Female | 41 (58.6) | 37 (68.5) | 0.268 |
| Health care worker (Number, %) | 22 (31.4) | 3 (5.6) | <0.001 |
| Current smoking (Number, %) | 10 (14.3) | 11 (20.4) | 0.359 |
| Comorbidities (Number, %) | |||
| No | 34 (48.6) | 17 (24.3) | 0.067 |
| Cardio-vascular diseases | |||
| Heart failure | 4 (5.7) | 2 (3.7) | 0.696 |
| Othersa | 25 (35.7) | 23 (42.6) | 0.199 |
| COPDbor asthma | 11 (15.7) | 9 (16.7) | 0.714 |
| Immunosuppressionc | 3 (4.3) | 3 (5.6) | 0.206 |
| Diabetes mellitus | 10 (14.3) | 15 (27.8) | 0.074 |
| Malignancy | 3 (4.3) | 7 (13) | 0.101 |
| Charlson comorbidity index (mean, extremes, SD) | 1.7 [0–10] ±2.5 | 3 [0–8] ±2.4 | 0.003 |
| Incubation period: time from contact symptomatic case to illness onset (days) (mean, extremes, SD) | 6 [1–12] ±2.1 | 4.4 [1–10] ±2.9 | 0.010 |
| Interval between illness onset and seeing a doctor (days) (mean, extremes, SD) | 3.4 [1–10] ±2.3 | 2.6 [1–11] | 0.100 ±2.2 |
Bold value signifies if p < 0.05.
Defined by: cardiac failure, cardiac arrhythmia, coronary heart disease, stroke, peripheral arterial obstructive disease and thromboembolic disease.
COPD: chronic obstructive pulmonary disease.
Defined by: transplantation, cirrhosis, long-term steroids therapy, immunomodulators treatments and human immunodeficiency virus (HIV).
The most frequent symptoms (>50% of cases) were fatigue (93%, n = 65), cough (80%, n = 56), fever (76%, n = 53), headache (73%, n = 51), myalgia (59%, n = 41), arthralgia (54%, n = 38) and anosmia (53%, n = 37) (Table 2 ). About anosmia, the mean duration was 7.3 days (±5). Only one patient had not recovered at the end of the follow-up (after 28 days). Only 8/37 cases of anosmia (22%) were associated with nasal obstruction but 31/37 (84%) were associated with dysgeusia. Other symptoms present in roughly half of patients were myalgia (59%, n = 41), dysgeusia (49%, n = 34) and rhinorrhea (49%, n = 34). Diarrhea (40%, n = 28) and dyspnea (34% n = 24) were present in at least a third of patients. Other symptoms were present in less than 30% of cases ( Table 2 ). We noticed that one of the patients had a febrile confusion. Patients were symptomatic on average for 10 days (±5).
Table 2.
Differences in symptoms between SARS-CoV-2 and Influenza virus A/B infected patients in Nord Franche-Comte Hospital.
| Functional Signs | SARS-CoV-2 (n = 70) | Influenza A/B (n = 54) | p-(value) |
|---|---|---|---|
| General symptoms | |||
| Fever (Number, (%)) | |||
| Fever ≥ 38 (objective) | 53 (75.7) | 50 (92.6) | 0.042 |
| Feeling of fever | 13 (18.6) | 3 (5.6) | 0.056 |
| No fever neither feeling of fever | 4 (5.7) | 1 (1.8) | 0.386 |
| Highest temperature (mean, extremes, SD) | 38.7 [36.7–41.5] ±0.91 | 39 [36.2–41.4] ± 0.97 | 0.064 |
|
Fatigue (Number, (%)) |
65 (92.9) |
47 (87) |
0.362 |
| Pain symptoms | |||
| Myalgia (Number, (%)) | 41 (58.6) | 38 (70.4) | 0.192 |
| Arthralgia (Number, (%)) | 38 (54.3) | 36 (66.7) | 0.198 |
| Headache (Number, (%)) | |||
| Total | 51 (72.9) | 31 (57.4) | 0.086 |
| Diffuse | 20 (28.6) | 24 (44.4) | 0.088 |
| Frontal | 18 (25.7) | 5 (9.3) | 0.021 |
| Othersa |
13 (17.2) |
2 (3.7) |
0.013 |
| Respiratory symptoms | |||
| Cough (Number, (%)) | 56 (80) | 44 (81.5) | 1 |
| Sputum production (Number, (%)) | 20 (28.6) | 28 (51.9) | 0.010 |
| Sneezing (Number, (%)) | 13 (18.6) | 25 (46.3) | 0.001 |
| Chest pain (Number, (%)) | 18 (25.7) | 10 (18.5) | 0.391 |
| Hemoptysis (Number, (%)) | 6 (8.6) | 3 (5.6) | 0.730 |
|
Dyspnea (Number, (%)) |
24 (34.3) |
32 (59.3) |
0.007 |
| Otorhinolaryngologicalsymptoms | |||
| Tinnitus (Number, (%)) | 7 (10) | 4 (4.7) | 0.755 |
| Sore throat (Number, (%)) | 14 (20) | 24 (44.4) | 0.006 |
| Hearing loss (Number, (%)) | 4 (5.7) | 4 (7.4) | 0.727 |
| Dysgeusia (Number, (%)) | 34 (48.6) | 11 (20.4) | 0.001 |
| Anosmia (Number, (%)) | 37 (52.9) | 9 (16.7) | <0.001 |
| Rhinorrhea (Number, (%)) | 34 (48.6) | 30 (55.6) | 0.4 |
| Nasal obstruction (Number, (%)) | 13 (18.6) | 19 (35.2) | 0.08 |
|
Epistaxis (Number, (%)) |
3 (4.3) |
3 (5.6) |
1 |
| Ocular symptoms | |||
| Conjunctival hyperemia (Number, (%)) | 3 (4.3) | 16 (29.6) | <0.001 |
| Tearing (Number, (%)) | 4 (5.7) | 13 (24.1) | 0.004 |
| Dry eyes (Number, (%)) | 3 (4.3) | 2 (3.7) | 1 |
|
Blurred vision (Number, (%)) |
3 (4.3) |
1 (1.8) |
0.6 |
| Gastro-intestinal symptoms | |||
| Nausea (Number, (%)) | 22 (31.4) | 11 (20.4) | 0.219 |
| Vomiting (Number, (%)) | 2 (2.8) | 12 (22.2) | 0.001 |
| Diarrhea (Number, (%)) | 28 (40) | 11 (20.4) | 0.021 |
|
Abdominal pain (Number, (%)) |
14 (20) |
9 (16.7) |
0.816 |
| Physical examination | |||
| Respiratory rate > 22 (Number, (%)) | 15 (21.4) | 14 (25.9) | 0.669 |
| Sounds heard on pulmonary auscultation (Number, (%)) | |||
| Total | 29 (41.4) | 21 (38.9) | 0.845 |
| Crackling (total) | 27 (38.6) | 11 (20.4) | 0.032 |
| Crackling (unilateral) | 10 (14.3) | 6 (11.1) | 0.788 |
| Crackling (bilateral) | 17 (24.3) | 5 (9.2) | 0.034 |
| Sibilant | 1 (1.4) | 1 (1.8) | 1 |
| Rhonchib | 1 (1.4) | 9 (16.7) | 0.002 |
|
Sat 02 at admission (%) (mean, extremes, SD) |
93.16 [85–98] ± 3.46 |
93.46 [70–99] ± 5.45 |
0.784 |
| Duration of symptoms | |||
| Duration of all symptomsc (days) (mean, extremes, SD) | 10 [3–27] ±4.9 | 8.4 [2–15] ± 3.7 | 0.077 |
| Duration of fever (days) (mean, extremes, SD) | 5.5 [1–19] ± 4.4 | 3.1 [1–13] ±2.7 | 0.002 |
| Duration of cough (days) (mean, extremes, SD) | 7.7 [1–18] ± 4.3 | 7.7 [2–21] ±4.7 | 0.974 |
| Duration of anosmia (days) (mean, extremes, SD) | 7.3 [1–19] ± 5 | 2.6 [2–3] ± 0.5 | 0.046 |
Bold value signifies if p < 0.05.
In the group of patients with COVID-19: twelve patients had retro-orbital headache and 1 patient has temporal headache/In the group of Influenza A/B: two patients had retro-orbital headache.
The only patient who has rhonchi sounds heard has a past history of COPD.
Defined by: pain syndrome, fever, cough, diarrhea and anosmia.
Concerning the outcome, 33 patients (47%) were hospitalized for a mean duration of 7 days (±6). During hospitalization, 23 patients (33%) required oxygen therapy and 11 patients (16%) were admitted in Intensive Care Unit (UCI) for acute respiratory failure and needed artificial ventilation for 8 days (±7). By March, the 24th, 57 patients of the COVID 19 group (81%) had been discharged from hospital and 4 (6%) patients had died; the other patients (9%, n = 6) were still in hospital.
2.2. Comparison between patients with COVID-19 and patients with influenza A/B
In the group influenza A/B, 51 patients (94%) were diagnosed with influenza A, and 3 (6%) with influenza B. No significant differences were found between the two groups (COVID 19 and influenza A/B) with regard to age, sex and the different comorbidities: cardiovascular diseases, COPD or asthma, immunosuppression, diabetes and malignancy. However, patients with COVID-19 had a lower Charlson comorbidity index than patients with influenza A/B (2 (±2.5) vs 3 (±2.4); p = 0.003).
The mean incubation period was longer in patients with COVID-19 than patients with influenza (6 days (±2) vs 4 days (±3) respectively, p = 0.01). Fever or feeling of fever, fatigue, cough and pain symptoms (myalgia, arthralgia and headache) were the fourth most prevalent symptoms for both diseases (COVID-19 and influenza A/B) without significant statistical differences except for frontal headache and other localization of headache. In two groups patients without fever or a feeling of fever were scarce: 4 patients in COVID-19 group and 1 patient in influenza group (p = 0,386). However, fever ≥38 °C was significantly higher in influenza group than COVID-19 group (93% vs 76% respectively, p = 0.042) but the mean highest temperature was not significantly different (39 °C vs 38.7 °C respectively; p = 0.064). Apart from the fourth main symptoms, most of the other symptoms differed with significant differences. The third table (Table 3 ) summarized significant diagnostic criteria based on symptoms between infection with SARS-CoV-2 and influenza.
Table 3.
Significant diagnostic criteria (on clinical features) between infection with SARS-CoV-2 and influenza A/B (summary table with p < 0.05).
| Significant diagnostic criteria | SARS-CoV-2 (n = 70) | Influenza A/B (n = 54) | p-(value) |
|---|---|---|---|
|
Symptoms statistically more frequents in the group COVID-19 | |||
| Headache Number, (%, [95% CI]a) | |||
| Frontal | 18 (25.7, [15.8–37.1]) | 5 (9.3, [1.9–16.7]) | 0.021 |
| Retro-orbital or temporal | 13 (18.6, [10–27]) | 2 (3.7, [0–9.3]) | 0.013 |
| Dysgeusia Number, (%, [95% CI]) | 34 (48.6, [37.1–61.4]) | 11 (20.4, [11.1–31.5]) | 0.001 |
| Anosmia Number, (%, [95% CI]) | 37 (52.9, [40.4–64.3]) | 9 (16.7, [7.4–27.8]) | <0.001 |
| Diarrhea Number, (%, [95% CI]) | 28 (40, [28.6–51.4]) | 11 (20.4, [11.1–31.5]) | 0.021 |
|
Crackling sounds heard on pulmonary auscultation Number, (%, [95% CI]) |
27 (38.6, [27.1–50]) |
11 (20.4, [9.3–31.5]) |
0.032 |
|
Symptoms statistically more frequents in the group influenza A/B | |||
| Fever ≥ 38 (objective) Number, (%, [95% CI]) | 53 (75.7, [65.8–85.7]) | 50 (92.6, [85.2–98.1]) | 0.042 |
| Sputum production Number, (%, [95% CI]) | 20 (28.6, [18.6–38.6]) | 28 (51.9, [38.9–64.8]) | 0.010 |
| Sneezing Number, (%, [95% CI]) | 13 (18.6, [10–28.6]) | 25 (46.3, [33.3–59.3]) | 0.001 |
| Dyspnea Number, (%, [95% CI]) | 24 (34.3, [22.9–45.7]) | 32 (59.3, [46.3–72.2]) | 0.007 |
| Sore throat Number, (%, [95% CI]) | 14 (20, [11.4–30]) | 24 (44.4, [29.6–57.4]) | 0.006 |
| Conjunctival hyperemia Number, (%, [95% CI]) | 3 (4.3, [0–10]) | 16 (29.6, [18.5–42.6]) | <0.001 |
| Tearing Number, (%, [95% CI]) | 4 (5.7, [1.4–11.4]) | 13 (24.1, [13–37]) | 0.004 |
| Vomiting Number, (%, [95% CI]) | 2 (2.8, [0–7.1]) | 12 (22.2, [13–33.3]) | 0.001 |
| Rhonchi sounds heard on pulmonary auscultation Number, (%, [95% CI]) | 1 (1.4, [0–4.3]) | 9 (16.7, [5.6–27.8]) | 0.002 |
Bold value signifies if p < 0.05.
95% CI: Confidence Intervals.
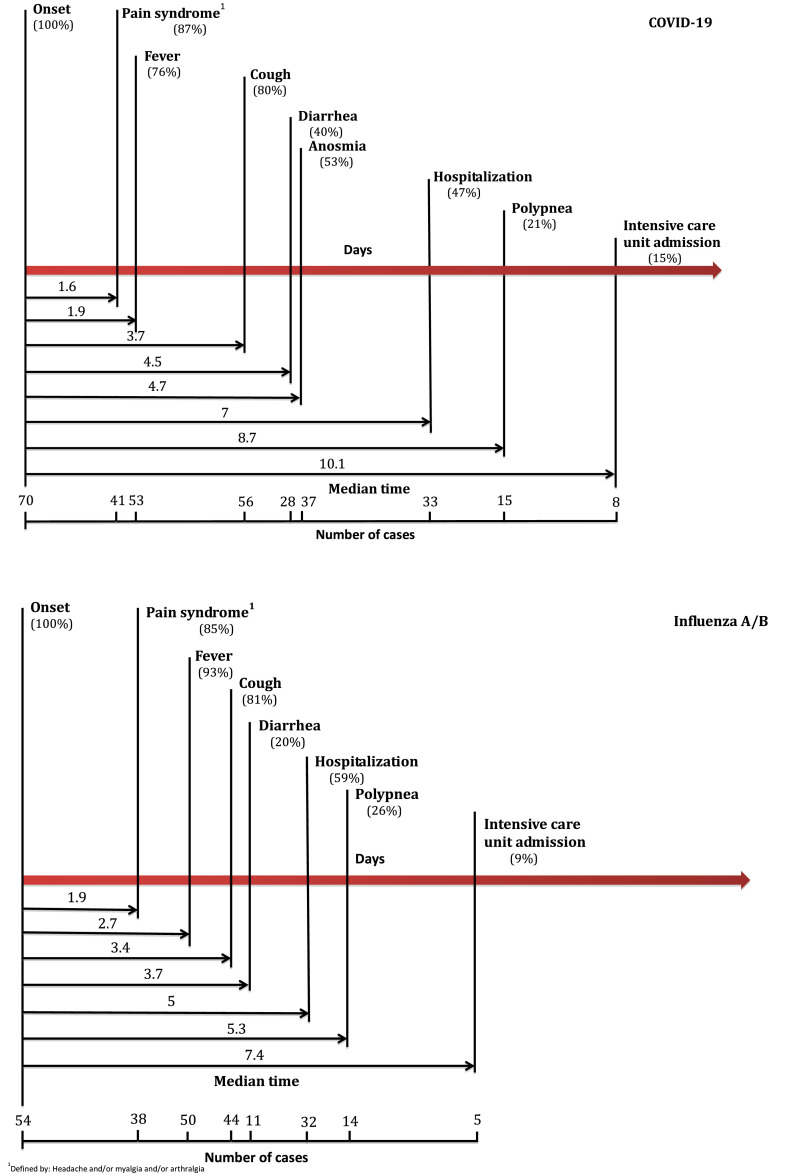
We reconstituted the history of evolution and onset of the main symptoms in COVID-19 and influenza (Fig. 1 ). We noticed the same natural evolution of symptoms in COVID-19 and influenza: firstly, pain syndrome appeared, secondly, fever, thirdly, cough and fourthly diarrhea. The onset of these symptoms (from illness onset) didn’t differ between the two groups except for fever which appeared earlier in COVID-19 than in influenza (respectively 1.9 days [±1.5] vs 2.7 days [±1.5], p = 0.045). We also reconstituted the history of clinical aggravation for both groups (Fig. 1). Hospitalization and clinical aggravation appeared later in COVID-19 than in influenza: respectively, patients were hospitalized at day 7 (±3) vs day 5 (±2) (p = 0.038), patients had a respiratory rate ≥22/min at day 9 (±0.8) vs day 5 (±1.3) (p < 0.001) and patients were admitted in an ICU at day 10 (±2.7) vs day 7 (±2.4) (p < 0.004).
Fig. 1.
Timeline of COVID-19 and influenza A/B after onset of illness.
There was no significant difference in the evolution of the two diseases (mean number of hospitalized patients, duration, oxygen therapy, hospitalization in the ICU, Invasive mechanical ventilation and outcome) ( Table 4 ).
Table 4.
Clinical outcomes of patients infected with SARS-CoV-2 and Influenza virus A/B in Nord Franche-Comte Hospital.
| Characteristics | SARS-CoV-2 (n = 70) | Influenza A/B (n = 54) | p-(value) | |
|---|---|---|---|---|
| Hospitalization (Number, %) | 33 (47.1) | 32 (59.3) | 0.207 | |
| Duration of hospitalization (days) (mean, extremes, SD) | 6.9 [1–21] ±5.8 | 7.6 [1–22] ±6.9 | 0.667 | |
| Oxygen therapy (Number, %) | 23 (32.9) | 20 (37) | 0.705 | |
| Days from illness onset to oxygenation (days) (mean, extremes, SD) | 6.7 [1–13] ±4.1 | 4.9 [1–8] ±2.2 | 0.477 | |
| Patients admitted or transferred to ICU (Number, %) | 11 (15.7) | 5 (9.3) | 0.499 | |
| Days from conventional hospitalization to ICU (mean, extremes, SD) | 3.1 [1–13] ±1.7 | 2.4 [1–6] ±2.4 | 0.458 | |
| IMV (Number, %) | 11 (15.7) | 5 (9.3) | 0.499 | |
|
Duration of hospitalization in ICU (mean, extremes, SD) |
7.9 [2–21] ±6.6 |
8.2 [2–12] ± 3.8 |
0.924 |
|
| Outcome (Number, %) | Discharge | 57 (81.4) | 47 (87) | 0.466 |
| Still hospitalized | 6 (8.6) | 2 (3.7) | 0.464 | |
| Death | 4 (5.7) | 5 (9.3) | 1 | |
IMV: invasive mechanical ventilation; ICU: intensive care unit.
3. Discussion
3.1. Clinical description of patients with COVID-19
This study described a population of 70 symptomatic adults (53% outpatient and 47% inpatients), infected with SARS-CoV-2 between February, the 26th and March, the 14th, 2020. The mean age of patients was 57 years (±19), 59% were female. In the literature, the mean age of patients with COVID-19 was 46 years without predominance for sex [10]. Fifty one percent had comorbidities, including cardiovascular disease, COPD and diabetes mellitus in the COVID-19 group. These comorbidities had a prevalence ≥10% in our study as in other studies [[10], [11], [12]].
In our study, the most common symptoms in COVID-19 group were fever, cough, fatigue and myalgia as in medical literature. In a systematic review and meta-analysis (including 46248 COVID-19-infected patients) by Jing Yang et al. [10], the most common symptoms were fever, followed by cough, fatigue and dyspnea. Fever was reported as the main symptom in most cohorts with clinical description of COVID-19, followed by cough (mostly dry) [13]. Myalgias and fatigue were present in 44–60% of cases in China’s series [12]. However, we noticed two otorhinolaryngological symptoms recently described with SARS-CoV-2: anosmia and dysgeusia, present in half of our patients. Knowing that anosmia and dysgeusia are part of COVID-19 clinical features is a major point for the medical community in order to suspect this diagnostic in case of a COVID-19 with a predominance of otorhinolaryngological symptoms. Anosmia appeared on average 5 days after the onset of the first other symptoms and may persisted for up to 28 days with a mean duration of 7 days, while usually the duration of anosmia with viral rhinitis with nasal obstruction were less than 3 days [14]. Furthermore, in our patients anosmia was rarely associated with nasal obstruction, but often with dysgeusia. These notions led to suspect another pathogenesis than a nasal obstruction for anosmia, as it is described in post-viral olfactory loss (POL) which can also be associated with dysgeusia [15]. The pathogenesis of POL involves probably a damage of the sensory receptors [16] or a lesion of the neural system (olfactory cranial nerve or central lesion) [17].
In our study, among patients with COVID-19, 40% had diarrhea and 31 had nausea. In a study with 73 patients with confirmed COVID-19, 36% (26/73) had diarrhea [18]. It is known that the entry of SARS-CoV-2 into human host cells is mediated mainly by a cellular receptor angiotensin-converting enzyme 2 (ACE2), which is expressed in human airway epithelia, lung parenchyma, but also in small intestine cells, which may explain this clinical features [19]. One of our patients in the COVID-19 group presented with confusion without any respiratory symptoms. There is increasing evidence that coronaviruses are not always confined to the respiratory tract and that they may also invade the central nervous system inducing neurological diseases [20].
3.2. Comparison of patients with COVID-19 and patients with influenza
Our populations of COVID-19 and influenza didn’t differ in age, sex, different comorbidities, rate of hospitalization and ICU admission. The only difference was about the number of comorbidities with a Charlson comorbidity index higher in the group influenza than COVID-19. Possibly the high number of health care workers (which is a younger population able to work) in the group COVID-19 explain this. There was a significant difference between the two groups in terms of health care workers infected (31.4% vs 5.6%, p < 0.001). Reports describes physical and mental exhaustion in this community, the torment of difficult triage decisions, and the pain of losing patients and colleagues, all in addition to the infection risk. Early detection will contribute to breaking the cycle of SARS-CoV-2 transmission in community hospital [21]. Fever (or feeling of fever), fatigue, cough, myalgia and arthralgia were the most prevalent symptoms (>50% of cases) for both diseases (COVID-19 and influenza) without statistical differences about the frequency found for each symptom between the two groups.
However, we noticed some interesting differences about other functional symptoms. Patients with COVID-19 presented more frequently, a dry cough, anosmia and dysgeusia, diarrhea, frontal and retro-orbital headache and bilateral crackling sound at pulmonary auscultation compared to the patients of influenza group. After an influenza infection, anosmia is possible as with many different viruses [22]. However, anosmia and dysgeusia are uncommon after influenza infection: in medical literature anosmia and dysgeusia were not described among the clinical feature of influenza [23,24]. In our study, anosmia and dysgeusia were present, in 17% and 20% in influenza group vs 53% and 49% in COVID-19 group respectively (p < 0,001 and p = 0.001 respectively) which confirms that these symptoms were clearly more associated with COVID-19 than with influenza. Furthermore, the mean duration of anosmia was shorter in influenza than in COVID-19, respectively 3 days vs 7 days (p = 0.046). This short duration of anosmia conducts to suspect a banal viral rhinitis with nasal obstruction during influenza [14] in contrast with the mechanism suspected for the pathogenesis of anosmia in patients with COVID-19. As described in the first part of the discussion, diarrhea was present in 40% of patients with COVID-19. On the contrary, in a cohort from an emergency department including 119 patients with confirmed influenza no one had diarrhea. Pedersen et al. conclude that the absence of gastrointestinal symptoms was one of the factors that can help distinguish influenza from other acute respiratory illnesses in the ambulatory population [25]. During pulmonary auscultation, cracklings sounds were statistically more frequents in COVID-19 group than influenza group (39% vs 20%, p = 0,032), especially bilateral cracklings (24% vs 9%, p = 0.034). In patients with COVID-19, lung parenchymal involvement is bilateral, hence the bilateral character of the crackling sound [26].
On the other hand, sore throat, conjunctival hyperemia, tearing, sneezing, sputum production, dyspnea, vomiting and rhonchi at pulmonary auscultation were more frequently described in influenza group than COVID-19 group with statistically significant differences. Sore throat was a well-known symptom in influenza, found in about 30–40% of the patients in the literature as in our study [25,27,28]. Conjunctival hyperemia seems to be rare with COVID-19, less than 5% of cases in our study vs 30% of cases in influenza group (p < 0.001). Ocular symptoms as conjunctival hyperemia and tearing were described in influenza. Souty et al. proved that conjunctivitis was associated with influenza compared to others respiratory viruses (OR 1.27, 95% CI 1.08–1.50) [29]. About respiratory symptoms, sputum production and dyspnea were statistically more frequents in influenza group than in COVID-19 group (respectively 52% vs 29%, p = 0.010 and 59% vs 34%, p = 0.007). In a study with 470 patients with confirmed influenza, 286 (64%) had a productive cough [27]. Dyspnea was also described in more than half of patients with influenza A [28,30]. In our study, 17% of patients had rhonchi in influenza group vs 1% in COVID-19 group (p = 0.002). In the study of Erçen Diken, rhonchi sounds on pulmonary auscultation were found in more than a third of 91 patients with confirmed influenza [30]. In our study, the only patient who had rhonchi sounds in COVID-19 group had a past history of COPD. In our study, patients with COVID-19 never had rhonchi sounds on pulmonary auscultation without a past of COPD.
On recently published articles since December 2019, symptoms of SARS-CoV-2 infection appeared after a mean incubation period of 5.2 days [31]. In our study, the mean incubation period was 6 days (±2.1), longer than the mean influenza’s period, which was 4.4 days (±2.9) (p = 0.010). About the natural history of both diseases it’s interesting to notice that the 5 first days don’t differ, the three main symptoms appeared in the same order for both diseases: firstly, pain syndrome, followed by fever and cough. In the literature, the period from the onset of COVID-19 symptoms to conventional hospitalization ranged from 1 to 13 days with a mean of 6.7 days [32]; in our study we have also a mean of 7 days. Patients with COVID-19 were hospitalized later than patients with influenza (7 days vs 5 days, p = 0,038). In the same way, patients with COVID-19 became critically ill later than patients with influenza (considering the period after which a respiratory rate ≥ 22/min appeared, or the period after which the patients had to be admitted in ICU).
The distribution of virus entry receptors may at least partially explain the differences of symptoms between the 2 infections. Human influenza A virus binds to α2,6-linked sialic acid receptors, which were widely expressed on the respiratory tract, from the nasopharynx to the bronchi, except the alveoli (predominant expression of α2,3-linked sialic acid receptors on alveolar cells) [33]. This distribution correlates well with the short incubation period of influenza infection and with the predominant manifestations caused by the virus (sore throat, sneezing, sputum production, rhonchi on pulmonary auscultation). By contrast, ACE2 protein, the functional receptor of SARS-CoV-2, was largely expressed on alveolar epithelial cells, enterocytes, and endothelial cells (including in the central nervous system) but poorly found on the surface of nasopharyngeal cells [34]. This distribution of ACE2 might reflect the longer incubation period of COVID-19 infection, during which the virus has to bind and replicate in available ACE2-expressing cells, and the observed symptoms of dyspnea, dry cough, diarrhea, and bilateral crackling sound on pulmonary auscultation. Since a high expression of ACE2 receptor has recently been reported in the oral mucosa [35], the role of these receptors in the observation of frequent dysgeusia during COVID-19 infection should be explored. Symptoms related to the systemic inflammatory response, such as fever, fatigue, myalgia and arthralgia were not directly linked to the distribution of viral receptors and may be equally prevalent for the 2 viruses, as observed in our study population.
The fatality of COVID-19 infection was higher in our hospital as compared with the mortality described in China (5.7% vs 2.4%). New ICD-10 codes were attributed for COVID-19. Both categories U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified; clinically-epidemiologically diagnosed COVID-19) may be used for mortality coding as cause of death. This higher case fatality rate in our cluster might impact the diagnostic criteria seen based on clinical features. Symptoms reported by critically ill patients may be underestimated due to the severity of illness. However, most patients had positive outcome in both groups; the rate of patients discharged from hospital was 81% (in COVID-19 group) and 87% (in influenza group).
One of the limitations of our study was the limited number of patients; a bigger study would be interesting to confirm and support our results. Knowing the differences between COVID 19 and influenza A/B symptoms seems essential and may help clinicians in the diagnosis of these diseases.
To conclude, natural evolution of symptoms with COVID-19 and influenza were similar during the first 5 days, however, the evolution differed afterwards; indeed, patients with COVID-19 may became critically ill during the second week, around day 10, later than patients with influenza, in the end of the first week, around day 7. ILI was the main presentation of COVID-19 and influenza A/B. We described several differences among other symptoms than fever, cough and pain syndrome. The distribution of virus entry receptors may partially support the differences in symptoms between the 2 infections. These clinical differences can help the clinicians in front of cases with influenza like illness during the co-circulation of influenza and SARS-CoV-2.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
All authors declare no competing interests.
Acknowledgements
Contributors: SZ, TK and JN collected the epidemiological and clinical data and processed statistical data. SZ and TK drafted the manuscript. LT, PYR, QL and VG revised the final manuscript.
We thank all patients involved in the study and especially Dr Zahra Hajer for her help.
References
- 1.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020 Mar;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . WHO. World Health Organization; 2020. Emergency use ICD codes for COVID-19 disease outbreak.http://www.who.int/classifications/icd/covid19/en/ [Internet] [cited 2020 May 26]. Available from: [Google Scholar]
- 3.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 Apr 15;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2020 Apr 6:1–11. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klopfenstein T., Kadiane-Oussou N.J., Toko L., Royer P.-Y., Lepiller Q., Gendrin V. Features of anosmia in COVID-19. Med Maladies Infect. 2020 Apr 16 doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zayet S., N’dri Juliette K.-O., Royer P.-Y., Toko L., Gendrin V., Klopfenstein T. Coronavirus disease 2019: new things to know! J Med Virol. 2020 Apr 13 doi: 10.1002/jmv.25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DICOM_Lisa C. Ministère des Solidarités et de la Santé; 2020. DICOM_Lisa.C. En ambulatoire : recommandations COVID-19 et prise en charge.http://solidarites-sante.gouv.fr/soins-et-maladies/maladies/maladies-infectieuses/coronavirus/professionnels-de-sante/article/en-ambulatoire-recommandations-covid-19-et-prise-en-charge [Internet] [cited 2020 Apr 3]. Available from: [Google Scholar]
- 8.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard Stoecklin S., Rolland P., Silue Y., Mailles A., Campese C., Simondon A. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25(6) doi: 10.2807/1560-7917.ES.2020.25.6.2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020 Mar 12;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupia T., Scabini S., Mornese Pinna S., Di Perri G., De Rosa F.G., Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020 Mar 7;21:22–27. doi: 10.1016/j.jgar.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akerlund A., Bende M., Murphy C. Olfactory threshold and nasal mucosal changes in experimentally induced common cold. Acta Otolaryngol. 1995 Jan;115(1):88–92. doi: 10.3109/00016489509133353. [DOI] [PubMed] [Google Scholar]
- 15.Rawal S., Hoffman H.J., Bainbridge K.E., Huedo-Medina T.B., Duffy V.B. Prevalence and risk factors of self-reported smell and taste alterations: results from the 2011-2012 US national health and nutrition examination survey (NHANES) Chem Senses. 2016 Jan;41(1):69–76. doi: 10.1093/chemse/bjv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamagishi M., Fujiwara M., Nakamura H. Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection. Rhinology. 1994 Sep;32(3):113–118. [PubMed] [Google Scholar]
- 17.Kim Y.K., Hong S.-L., Yoon E.J., Kim S.E., Kim J.-W. Central presentation of postviral olfactory loss evaluated by positron emission tomography scan: a pilot study. Am J Rhinol Allergy. 2012 Jun;26(3):204–208. doi: 10.2500/ajra.2012.26.3759. [DOI] [PubMed] [Google Scholar]
- 18.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 Mar 3;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with covid-19. N Engl J Med. 2020 Mar 30;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y.-C., Bai W.-Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 Feb 27 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz J., King C.-C., Yen M.-Y. Protecting health care workers during the COVID-19 coronavirus outbreak -lessons from taiwan’s SARS response. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 Mar 12 doi: 10.1093/cid/ciaa255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki M., Saito K., Min W.-P., Vladau C., Toida K., Itoh H. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007 Feb;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paules C., Subbarao K. Influenza. Lancet Lond Engl. 2017 Aug 12;390(10095):697–708. doi: 10.1016/S0140-6736(17)30129-0. [DOI] [PubMed] [Google Scholar]
- 24.Cavallazzi R., Ramirez J.A. Influenza and viral pneumonia. Clin Chest Med. 2018;39(4):703–721. doi: 10.1016/j.ccm.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen C.J., Quinn J.V., Rogan D.T., Yang S. Factors associated with influenza in an emergency department setting. J Emerg Med. 2019 May;56(5):478–483. doi: 10.1016/j.jemermed.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 Feb 26:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong P.L., Sii H.L., P’ng C.K., Ee S.S., Yong Oong X., Ng K.T. The effects of age on clinical characteristics, hospitalization and mortality of patients with influenza-related illness at a tertiary care centre in Malaysia. Influenza Other Respir Viruses. 2020 Feb 5;14:286–293. doi: 10.1111/irv.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nateghian A., Gouya M.M., Nabavi M., Soltani H., Mousavi S.V., Agah E. Demographic, clinical, and virological characteristics of patients with a laboratory-confirmed diagnosis of influenza during three consecutive seasons, 2015/2016-2017/18, in the Islamic Republic of Iran. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2020 Mar;124:104281. doi: 10.1016/j.jcv.2020.104281. [DOI] [PubMed] [Google Scholar]
- 29.Souty C., Masse S., Valette M., Behillil S., Bonmarin I., Pino C. Baseline characteristics and clinical symptoms related to respiratory viruses identified among patients presenting with influenza-like illness in primary care. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2019 Sep;25(9):1147–1153. doi: 10.1016/j.cmi.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erçen Diken Ö., Arslan S., Akdoğan Ö., Yapar D., Ünal Ö., Demir E. Clinical, radiological and prognostic features of influenza cases in the influenza epidemic during years 2016-2017. Tuberk Ve Toraks. 2018 Jun;66(2):144–149. doi: 10.5578/tt.66122. [DOI] [PubMed] [Google Scholar]
- 31.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 26;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92(4):441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumlin U., Olofsson S., Dimock K., Arnberg N. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respir Viruses. 2008 Sep;2(5):147–154. doi: 10.1111/j.1750-2659.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004 Jun;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020 24;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]