Abstract
The clinical signs of coronavirus disease-19 (COVID-19) can be heterogenous because of the diversity of potential organ involvement. We describe a 58-year-old woman who developed new-onset dysarthria and hemiplegia and was found to be COVID-19-positive. This is among the first cases of COVID-19 presenting solely with focal neurologic deficits.
Keywords: Coagulopathy, Coronavirus disease-19, COVID-19, Ischemic stroke, SARS-CoV-2
Highlights
-
•
Neurologic signs and symptoms may occur early in the course of COVID-19.
-
•
Ischemic stroke may occur in 0.9 to 2.3% of patients with COVID-19.
-
•
The mechanism of pathogenesis may involve hypercoagulability and/or inflammation.
-
•
Recent data show increased production of antiphospholipid antibodies in COVID-19.
-
•
Early prophylactic anticoagulation may reduce the risk of ischemic stroke.
On March 11, 2020, the World Health Organization declared coronavirus disease-19 (COVID-19) a pandemic. As of this writing, the global number of cases exceeds 6.5 million. However, despite the rapidly-increasing prevalence of COVID-19, many questions remain regarding this unusual and highly-lethal disease. The clinical presentation is heterogeneous and may include fever, dyspnea, cough, diarrhea, and skin changes. We report a case of COVID-19 manifesting with dysarthria and hemiplegia.
A 58-year-old woman presented on April 3, 2020 for evaluation of acute-onset dysarthria, left-sided facial droop, and left-sided hemiparesis of one-hour duration. The patient's past medical history was significant for a right middle cerebral artery ischemic stroke in 2019, for which she was receiving dual antiplatelet therapy (DAPT) with clopidogrel and aspirin. In addition, she was status-post renal transplant and was on chronic immunosuppressive therapy with tacrolimus and mycophenolate mofetil.
Review of systems was positive only for the aforementioned focal neurologic deficits. The patient denied fevers, cough, dyspnea, and gastrointestinal symptoms. There was no recent contact with any individuals known to be positive for COVID-19. Vital signs were within normal limits. Physical examination revealed left homonymous hemianopia, right gaze preference, left-sided facial droop, and left-sided hemiplegia affecting the upper and lower extremities. Sensation was intact. Deep tendon reflexes were normal. Babinski sign was positive on the left side. National Institutes of Health Stroke Scale (NIHSS) score was 15. Laboratory studies – including complete blood count (CBC) and complete metabolic panel (CMP) – were within normal limits.
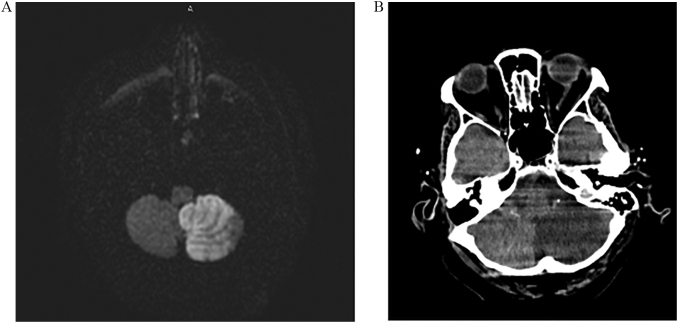
An emergent computed tomography (CT) scan of the head showed no acute intracranial abnormality. CT angiography demonstrated patent vasculature. Thrombolytics were administered for management of a suspected ischemic stroke. Magnetic resonance imaging was subsequently obtained and revealed a left cerebellar infarction in the territory of the posterior inferior cerebellar artery (PICA) (Fig. 1). The patient was started on aspirin 325 mg daily as well as a high-intensity statin. Neurologic symptoms gradually began to improve over the ensuing days.
Fig. 1.
(A) Axial view of a magnetic resonance image of the brain, diffusion-weighted imaging sequence, demonstrates increased signal consistent with acute ischemic infarction in the territory of the posterior inferior cerebellar artery. (B) Axial view of a computed tomography scan without contrast obtained four days after the initial magnetic resonance image shows evolution of the left cerebellar infarction with increased edema.
On April 8, 2020, the patient was noted to have worsening focal neurologic deficits, including right-sided gaze preference, left lower extremity weakness, and dysarthria. Emergent CT scan of the head revealed marked edema affecting the left cerebellum with worsening effacement of the fourth ventricle and displacement of the midbrain. The patient was transferred to the intensive care unit (ICU) for osmotic therapy with hypertonic saline and close monitoring of her neurologic status.
A routine chest radiograph was obtained upon transfer to the ICU and showed ill-defined bilateral opacities in the peripheral lung fields. CBC was significant for lymphopenia; absolute lymphocyte count was <0.1 × 109 cells/L as compared to 0.9 × 109 cells/L on admission (reference range: 1.0–4.8 × 109 cells/L). Correlation of the imaging and laboratory findings prompted testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Results returned 24 h later and were positive.
The patient's ICU stay was complicated by worsening hypoxic respiratory failure that failed to improve with high-flow nasal cannula oxygen therapy. Hydroxychloroquine was not administered due to underlying QT prolongation. On the evening of April 13, 2020, the patient spontaneously became hypotensive and bradycardic. Endotracheal intubation was performed for airway protection. However, the patient's respiratory status continued to decline, and care was transitioned to comfort measures only per her advance directive. The patient was pronounced dead the following morning.
This case illustrates the diverse initial manifestations of COVID-19. Our patient developed a new ischemic stroke despite consistent use of DAPT; this occurs in only 2.4% of individuals taking clopidogrel and aspirin therapy for secondary prevention [1]. We hypothesize that SARS-CoV-2-induced inflammation and hypercoagulability contributed to an increased risk for arterial thrombosis in our patient.
Emerging evidence suggests that neurologic manifestations of COVID-19 are more common than previously recognized, occurring in over one-third of affected individuals [2]. A wide range of symptoms have been described, including dizziness, headache, ataxia, seizure, and taste impairment. Ischemic stroke may occur in 0.9% to 2.3% of patients with COVID-19 [2,3]. Acute necrotizing hemorrhagic encephalopathy (ANE) – a rare and potentially lethal intracranial inflammatory disease – has also been reported [4]. Focal neurologic deficits typically occur early in the course of infection; as in our patient, respiratory symptoms may not appear for several days or more.
The pathogenesis of COVID-19-associated neurologic injury remains to be established. SARS-CoV-2 has been shown to induce a hypercoagulable state, thus increasing the risk of arterial thrombosis with acute ischemic stroke [5]. However, direct virus- and immune-mediated neuronal damage may also occur. Similar to other coronaviridae, it is thought that SARS-CoV-2 can enter the central nervous system via the hematogenous or retrograde neuronal route [6]. Activation of the neuroimmune system may generate a cytokine storm with consequent inflammation and necrosis. It is also conceivable that SARS-CoV-2 induces a viral vasculopathy; indeed, this has been described in varicella zoster virus, cytomegalovirus, and human immunodeficiency virus [7].
The pathogenetic linkage between infection and ischemic stroke has been explored by other authors. In a case-control study by Grau et al., investigators found that recent viral or bacterial infection significantly increased the risk of cardioembolism and arterioarterial embolism [8]. The Grau group hypothesized that pro-coagulant and inflammatory pathways are activated in infection due to a decrease in the anticoagulant protein C and increase in interleukin-1, respectively. SARS-CoV-2 may also stimulate production of antiphospholipid antibodies; therefore, antibody screening and immediate prophylactic anticoagulation may reduce the risk of ischemic stroke in the setting of COVID-19 [9].
Management of neurologic symptoms in COVID-19 should be based on the specific disease manifestation. Our patient was treated with thrombolytics, as she presented within three hours of the development of focal neurologic deficits. However, had COVID-19 been suspected, she may have benefited from early administration of antiviral and/or anti-inflammatory agents. Although there is no definitive, evidence-based treatment for COVID-19, preliminary data suggest that remdesivir may improve outcomes in critically-ill patients [10]. Further carefully designed studies are needed to confirm the role of these agents in the treatment of COVID-19.
In summary, our case provides further evidence that COVID-19 may present with neurologic deficits with or without typical respiratory symptoms. Testing for SARS-CoV-2 infection should be considered for patients who develop neurologic symptoms during the height of this pandemic.
Declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
All authors have seen and approved of the final version of this manuscript.
All authors have made significant intellectual contributions to this manuscript.
The content of this manuscript is not under consideration elsewhere.
No funding was received for the publication of this manuscript.
Informed consent was obtained from the patient prior to submission of the case details.
Acknowledgements
None.
References
- 1.Gouya G., Arrich J., Wolzt M., Huber K., Verheugt F.W.A. Antiplatelet treatment for prevention of cerebrovascular events in patients with vascular diseases: a systematic review and meta-analysis. Stroke. 2014;45(2):492–503. doi: 10.1161/STROKEAHA.113.002590. [DOI] [PubMed] [Google Scholar]
- 2.Mao L., Jin H., Wang M., Hu Y., Chen S. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;10 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020 doi: 10.1161/STROKEAHA.120.030335. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020:201187. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arabi Y.M., Balkhy H.H., Hayden F.G., Bouchama A., Luke T., Baillie J.K. Middle east respiratory syndrome. N. Engl. J. Med. 2017;376(6):584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagel M.A., Mahalingam R., Cohrs R.J., Gilden D. Virus vasculopathy and stroke: an under-recognized cause and treatment target. Infect. Disord. Drug Targets. 2010;10:105–111. doi: 10.2174/187152610790963537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grau A.J., Buggle F., Becher H., Zimmerman E., Spiel M. Recent bacterial and viral infection is a risk factor for cerebrovascular ischemia. Neurology. 1998;50(1):196–203. doi: 10.1212/WNL.50.1.196. [DOI] [PubMed] [Google Scholar]
- 9.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry. 2020 doi: 10.1136/jnnp-2020-323586. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe COVID-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]