Dear Editor,
The novel coronavirus SARS-CoV2 that causes coronavirus disease 2019 (COVID-19), has approximately afflicted over 2 million people worldwide,1 including approximately a million cases with over 50,000 deaths in the United States2 as of 27 April 2020. Pulmonary arterial hypertension (PAH), a chronic, progressively fatal condition, requires complicated medical management.3 Patients with PAH are very sensitive to the changes in their cardiopulmonary status and any disruption in treatment or development of additional cardiac or pulmonary pathology can trigger a rapid course of decline leading to death. Furthermore, all-cause hospitalization itself is a risk factor for disease progression in PAH.4 A recently published small case series suggested that 86% of critically ill COVID-19-infected patients had heart failure and chronic kidney disease as the most common underlying medical conditions.5 These conditions are commonly seen in patients with PAH and increase the risk for PAH patients to develop severe disease. Many PAH patients are on complicated regimens, including oral, inhaled or continuous intravenous therapies, some of which may be difficult to deliver during an acute illness. In this brief communication, we will highlight the in-patient management of COVID-19-infected PAH patients.
Management of non-critically ill hospitalized PAH patients
The most common factor responsible for admission of COVID-19-infected patients to a hospital is hypoxia and respiratory distress. Respiratory distress should be carefully evaluated in patients with PAH to ascertain its etiology. PAH therapy6 or the disease itself does not increase the risk of developing lower respiratory tract infections except into case of patients with associated PAH treated with immune suppressive therapy. A careful history and examination should be performed to assess the cause of underlying respiratory distress. New onset fever, cough or myalgias might suggest COVID-19 infection; however, PAH patients receiving intravenous continuous prostacyclin infusions can present with high grade fever due to catheter infection. We suggest, in the current pandemic, until the etiology of infection is clearly diagnosed, consider all patients as presumed COVID-19 infections (PUI); as such, personal protective equipment should be used when evaluating these patients. Worsening PAH itself can contribute to respiratory distress and signs of right heart failure; elevated jugular venous pulse, pulsatile liver or leg edema may suggest this etiology. However, one should keep in mind that a lower respiratory tract infection, such as COVID-19 infection, may lead to hypoxemia leading to hypoxic pulmonary vasoconstriction, which increases pulmonary vascular resistance (PVR) resulting in decompensation of a high-risk PAH patient who was previously stable. We suggest performing computed tomography (CT) of the chest and echocardiography at the earliest per institutional policy keeping the testing staff safe. A crazy paving or patchy ground glass opacities on a CT are suggestive of COVID-19 infection even in the absence of positive polymerase chain reaction (PCR) testing for COVID-19 infection. In addition to supplemental oxygenation, to keep saturation above 92%, we recommend continuing oral and parenteral therapies, like phosphodiesterase-5 inhibitors (PDE5i), soluble guanylate cyclase stimulator (sGC), endothelin receptor antagonist (ERA), oral prostacyclin, oral prostaglandin I2 receptor agonist (PGI2) and intravenous or subcutaneous prostacyclins while treating with concurrent therapies for COVID-19 infection. Many institutions treat COVID-19 infection with hydroxychloroquine, azithromycin, convalescent plasma, anti-interleukin-6 like tocilizumab and sarilumab with antiviral agents like remdesivir or ribavirin. There are no known concerning major interactions between the PAH medications and these experimental therapies targeting COVID 19 infection. In patients who are mildly hypoxemic, providers could consider a pulsed inhaled nitric oxide delivery system (currently approved by FDA for expanded access use as a part of clinical trial for COVID -19 infected patients, NCT04305457). Inhaled nitric oxide (iNO) could have beneficial effects on COVID-19 infection7 by inhibiting cell entry and viral replication. Additionally, inhaled NO is a potent pulmonary vascular vasodilator which can help with hypoxemia and reduction of mean pulmonary artery pressure (mPAP) and PVR.8 Patients using inhaled treprostinil therapy should be admitted to a negative pressure room as there is a potential risk of aerosolization; although, the aerosolization risk is extremely low, many patients cough (54% in TRIUMPH study in the treprostinil arm)9 post inhalation, which may increase the risk for health care providers. We suggest in high-risk patients, consider transition to parenteral treprostinil or prostacyclin. In low-risk patients with mild illness, brief interruption of inhaled treprostinil therapy should be tolerable; however, this should be assessed on an individual case basis and must be done at an expert center which is capable of initiating parenteral prostacyclin emergently. There are some anecdotal data suggesting that ERA can block angiotensin II receptors, which are needed for viral entry into the cells, thus offering some potential protection in PAH patients.10,11 However, such hypotheses require robust evaluation. Many PAH patients are receiving systemic anticoagulation; we recommend continuing it as there are increasing data from the non-PAH COVID-19 infected patients that microthrombi and complement-mediated vascular injury are a large component of the disease.12
Management of critically ill PAH patients with COVID infection
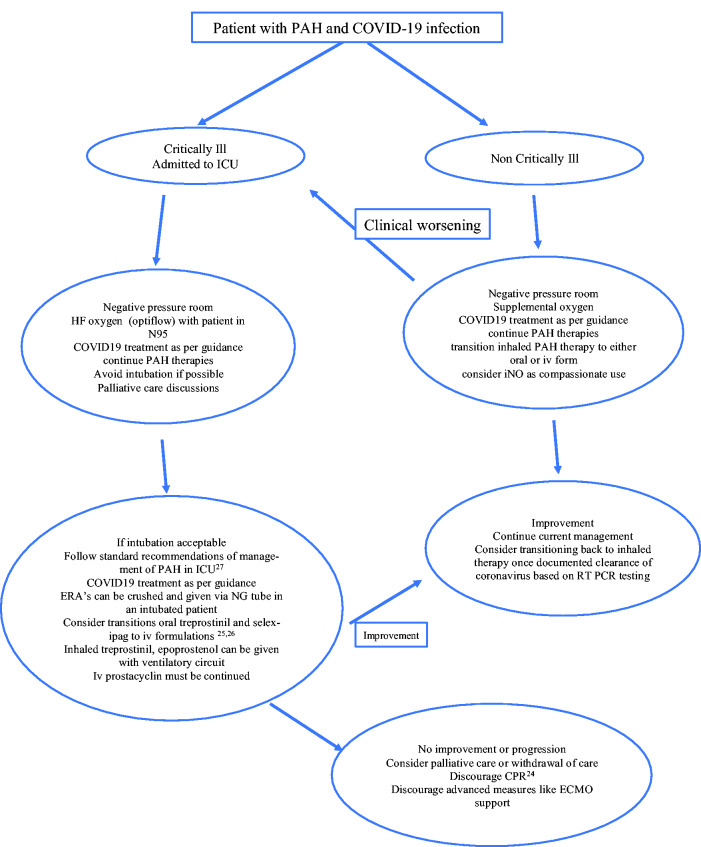
Management of PAH patients in intensive care unit (ICU) is challenging regardless of COVID-19 infection. COVID-19 infection has further complicated the management of this patient population. Sepsis in non COVID-9 PAH carries poor prognosis and leads to higher mortality.13 COVID-19 infection primarily affects the respiratory system; the most common reason for ICU admission is acute respiratory failure, in most cases acute respiratory distress syndrome (ARDS), needing ventilatory support.14 However, circulatory shock due to viral myocarditis, or cytokine release syndrome also occurs in COVID-19 infection.15 In PAH patient population, we generally suggest avoiding invasive positive pressure ventilation due to adverse effect on cardiopulmonary hemodynamics.16 Unfortunately, this is often impossible during respiratory failure from COVID-19 infection. As the respiratory status of the patient deteriorates, higher amounts of oxygen are needed. Options at this point in non-COVID-19 PAH patients are high-flow oxygen via nasal cannula (HFNC) or the initiation of noninvasive ventilation (NIV). However, in patients with COVID-19 because of the potential of aerosolization of particles and the rapidity and the severity of the deterioration, this decision is controversial and subject to ongoing debate.17 Despite this controversy, both modalities have been used variably. In retrospective cohorts, rates for HFNC use ranged from 14 to 63%, while 11 to 56% were treated with NIV.14,18 However, there are no data describing whether these modalities were successful at avoiding intubation. Intubation of PAH patients is often problematic due to the effects of sedatives on cardiac function and nonselective vasodilation leading to systemic hypotension and hemodynamic collapse.19 We suggest admission of PAH patients to negative pressure room in ICU and a trial of noninvasive ventilation or high flow oxygen (opti flow) as a reasonable approach (we often place a N95 mask on the patient using these modalities). At centers which have facility for inhaled nitric oxide or inhaled epoprostenol, it should be considered and can be delivered with these modalities. If respiratory failure progresses (the most common scenario), and need for intubation is inevitable, goals of care should be established (preferably, goals of care should have been established before reaching this point). Palliative care team involvement is also very helpful and recommended in this context. These discussions should be based on the factors like patient age, functional capacity, risk status of PAH prior to infection and the likely impact of this current hospitalization on the quality of life. However, if intubation and mechanical ventilation are acceptable options, intubation must be carefully planned. Hypotension and loss of RV contractility must be prevented as much as possible and the administration of catecholamines and/or fluids should be considered before anesthesia. Despite the lack of controlled clinical trials, etomidate is the preferred drug for induction of general anesthesia as it has little effect on cardiac contractility and vascular tone.20,21 Maintenance of anesthesia is usually achieved with low-dose opioids or ketamine together with benzodiazepines or propofol. Airway pressures should be kept to a minimum while at the same time hypercapnia should be avoided, if possible, because of its deleterious effects on pulmonary hemodynamics.22,23 Discussion of code status should not be overlooked as cardiopulmonary resuscitation remains largely unsuccessful in patients with PAH and RV failure.24 In a critically ill PAH patient with COVID-19 infection, we emphasize continuing PAH-specific therapies. Inhaled epoprostenol or iloprost can be used as a substitute for inhale treprostinil or to correct hypoxemia. Endothelin receptor antagonists can be crushed and delivered via nasogastric tube. Patients on oral treprostinil should be transitioned to iv treprostinil25 as the oral form is in extended release tablet and should not be crushed. Similarly, patients on oral selexipag can be briefly transitioned to iv selexipag (or iv epoprostenol) for the duration of mechanical ventilation26. Management of right ventricular failure in PAH should follow previously published literature.19,27 We recommend continuing treatment with investigational therapies for COVID-19 infection as per institutional guidelines along with the PAH treatment. Systemic anticoagulation in patients with PAH along with experimental COVID-19 treatments could be an option, considering growing evidence of thrombotic events in COVID-19 patients;28 however, at this time it is still controversial due to lack of evidence. In an unfortunate circumstance, if no clinical improvement is noticed, we discourage consideration for extracorporeal membrane oxygenation (ECMO) support in a PAH patient with COVID-19 infection. Outcome for a PAH patient on ECMO will likely be unfavorable unless there is an exit strategy in place, such as lung transplant. At this time, organ transplant is not considered an option for a COVID-19 infected patient, thus ECMO for a PAH patient with COVID 19 will be an unjustifiable use of resources. As such, we encourage active discussion with family or care provider to consider comfort measures. Fig 1 describes a flow chart to guide the management of these patients.
Fig. 1.
Flowchart highlighting the management of a PAH patient with COVID-19 infection.
COVID-19 infection poses an unprecedented threat to our vulnerable patient population with PAH. So far, nationally, we have not seen many PAH patients with COVID-19 infections, and most of them have had favorable outcomes. This may reflect the ability of these patients to isolate and quarantine themselves. However, we believe, it is early in this pandemic. Based on our current epidemiological data, we are far from having a successful treatment option or a vaccination. Thus, we are likely to see more PAH patients with COVID-19 infection and hope this review provides assistance to the clinicians involved in managing these patients in this critical situation.
Conflict of interest
SS: Dr. Sahay reports personal fees and non-financial support from Bayer Pharmaceuticals, personal fees and non-financial support from United Therapeutics, personal fees and non-financial support from Actelion Pharmaceuticals, grants and personal fees from American College of Chest Physicians.
HWF: Dr Farber reports personal fees from Actelion, personal fees from Bayer, personal fees from United Therapeutics, personal fees from Boehringer-Ingelheim, personal fees from Bristol-Myers Squibb, personal fees from Altavant, personal fees from Acceleron, outside the submitted work.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Sandeep Sahay https://orcid.org/0000-0002-0672-1680
References
- 1.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323(11): 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 – COVID-NET, 14 States, March 1–30, 2020 | MMWR. MMWR Morb Mortal Wkly Rep 2020; 69: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahay S, Humbert M, Sitbon O. Medical treatment of pulmonary arterial hypertension. Semin Respir Crit Care Med 2017; 38: 686–700. [DOI] [PubMed] [Google Scholar]
- 4.Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension. Chest 2019; 156: 323–337. [DOI] [PubMed] [Google Scholar]
- 5.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA 2020; 323: 1612–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu Z, Zhang C, Wei A, et al. Incidence and risk of respiratory tract infection associated with specific drug therapy in pulmonary arterial hypertension: a systematic review. Sci Rep 2017; 7: 16218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keyaerts E, Vijgen L, Chen L, et al. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis 2004; 8: 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Channick RN, Newhart JW, Johnson FW, et al. Pulsed delivery of inhaled nitric oxide to patients with primary pulmonary hypertension: an ambulatory delivery system and initial clinical tests. Chest 1996; 109: 1545–1549. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol 2010; 55: 1915–1922. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181(2): 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenzel RR, Ruethemann J, Bruck H, et al. Endothelin-A receptor antagonist inhibits angiotensin II and noradrenaline in man. Br J Clin Pharmacol 2001; 52: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020. DOI: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reyes A, Frost AE, Safdar Z, et al. Outcomes of pulmonary hypertension patients presenting with sepsis in a single large hospital system. AJRCCM 2019; 199: A6505. [Google Scholar]
- 14.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myles PS, Hall JL, Berry CB, et al. Primary pulmonary hyper- tension: prolonged cardiac arrest and successful resuscitation following induction of anesthesia for heart–lung transplantation. J Cardiothorac Vasc Anesth 1994; 8: 678–681. [DOI] [PubMed] [Google Scholar]
- 17.Cheung JC, Ho LT, Cheng JV, et al. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med 2020; 8: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. COVID-19 Lombardy ICU Network. JAMA 2020; 323(16): 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoeper MM, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med 2011; 184: 1114–1124. [DOI] [PubMed] [Google Scholar]
- 20.Gordon C, Collard CD, Pan W. Intraoperative management of pulmonary hypertension and associated right heart failure. Curr Opin Anaesthesiol 2010; 23: 49–56. [DOI] [PubMed] [Google Scholar]
- 21.Pritts CD, Pearl RG. Anaesthesia for patients with pulmonary hypertension. Curr Opin Anaesthesiol 2010; 23: 6. [DOI] [PubMed] [Google Scholar]
- 22.Viitanen A, Salmenpera M, Heinonen J. Right ventricular response to hypercarbia after cardiac surgery. Anesthesiology 1990; 73: 393–400. [DOI] [PubMed] [Google Scholar]
- 23.Viitanen A, Salmenpera M, Heinonen J, et al. Pulmonary vascular resistance before and after cardiopulmonary bypass: the effect of PaCO2. Chest 1989; 95: 773–778. [DOI] [PubMed] [Google Scholar]
- 24.Hoeper MM, Galie N, Murali S, et al. Outcome after cardiopulmonary resuscitation in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2002; 165: 341–344. [DOI] [PubMed] [Google Scholar]
- 25.Chakinala MM, Feldman JP, Rischard F, et al. Transition from parentral to oral treprostinil in pulmonary arterial hypertension. J Heart Lung Transplant 2017; 36: 193–201. [DOI] [PubMed] [Google Scholar]
- 26.Klose H, Chin K, Ewert R, et al. Safety, tolerability and pharmacokinetics study in patients with pulmonary arterial hypertension (PAH) temporarily switching from oral to IV selexipag. J Heart Lung Transplant 2019; 38: S490. [Google Scholar]
- 27.Hoeper MM, Benza RL, Corris P, et al. Intensive care, right ventricular support and lung transplantation in patients with pulmonary hypertension. Eur Respir J 2019; 53: 1801906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020. 15: S0735-1097(20)35008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]