Abstract
We discovered a rare phenomenon wherein a thieno-pyrrole fused BODIPY dye (SBDPiR690) generates singlet oxygen without heavy halogen atom substituents. SBDPiR690 generates both singlet oxygen and fluorescence. To our knowledge, this is the first example of such a finding. To establish a structure–photophysical property relationship, we prepared SBDPiR analogs with electron-withdrawing groups at the para-position of the phenyl groups. The electron-withdrawing groups increased the HOMO–LUMO energy gap and singlet oxygen generation. Among the analogs, SBDPiR688, a CF3 analog, had an excellent dual functionality of brightness (82290m−1cm−1) and phototoxic power (99170m−1cm−1) comparable to those of Pc 4, due to a high extinction coefficient (211000m−1cm−1) and balanced decay (Φflu=0.39 and ΦΔ=0.47). The dual functionality of the lead compound SBDPiR690 was successfully applied to preclinical optical imaging and for PDT to effectively control a subcutaneous tumor.
Keywords: BODIPY, fluorescence imaging, photodynamic therapy, singlet oxygen generation, theranostics
Introduction
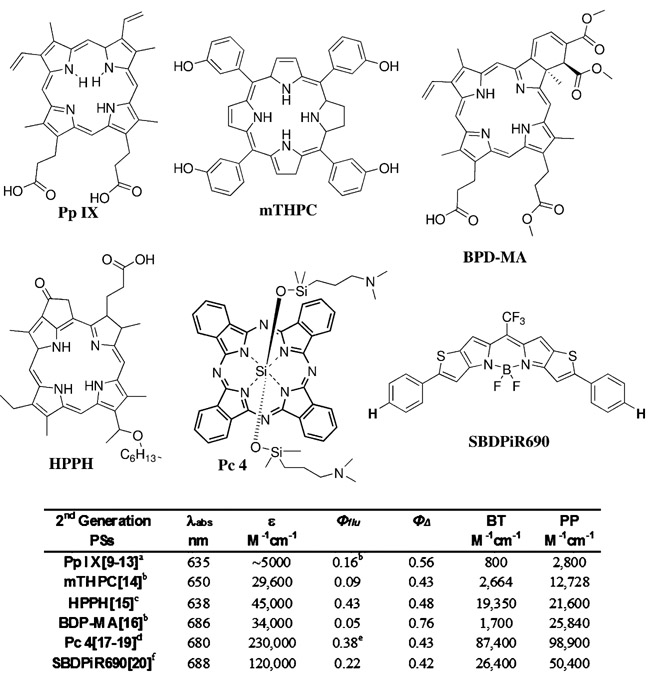
Photodynamic therapy (PDT) is an FDA-approved regimen for the treatment of non-malignant macular degeneration, acne, and neoplastic diseases, including lung, bladder, brain, ovarian, and esophagus cancers[1]. PDT is a minimally invasive three-component regimen using light, a photosensitizer (PS), and molecular oxygen. While independently non-toxic, the interaction of light and PS initiates a photochemical reaction with oxygen to produce singlet oxygen, thereby invoking tumor ablation via direct cell killing, vascular damage, and immune stimulation.[2] In addition to PDT, PSs have also been used for tumor detection due to their selective accumulation and fluorescence emission. Commonly known as PS fluorescence detection (PFD), this technique has been used to more effectively visualize tumors during surgery or PDT (image-guided surgery or PDT), enabling precise ablation at a minimal cost to normal surrounding tissues.[3] PFD has served as a reporter of the photodynamic response in near real time by monitoring PDT outcomes.[4] However, the low brightness (BT=ε×Φflu) of porphyrin-based PSs limits their application to superficial imaging (Figure 1).[5]
Figure 1.
Structures of second-generation PSs, SBDPiR690, and their photophysical data. Extinction coefficients correspond to the longest Q band in the respective solvents: [a] Triton X-100, [b] methanol, [c] benzene, [d] ethanol, [e] dimethyl sulfoxide, and [f] chloroform.
The development of efficient and effective dual-functioning PSs (i.e., fluorescent PSs) is our primary interest, as combining the two modalities stands to offer a more personalized image-guided treatment approach. Unfortunately, designing and synthesizing a PS with high BT and phototoxic power (PP=ε×ΦΔ) is challenging, as fluorescence emission and singlet oxygen generation are competing decay processes of the excited state. Second-generation PSs have improved PP, but BT remained low and not optimal for imaging deep-seated malignancies (Figure 1).[3] With the exception of HPPH and Pc4, the decay of second-generation PS excited states is heavily biased toward intersystem crossing (ISC) to generate singlet oxygen. For effective dual applications, PSs need to be balanced in decaying. In addition, BT and PP, along with a high extinction coefficient (>100000m−1cm−1) at near-infrared absorption maxima (650–800 nm) are essential for maximizing the therapeutic outcome.
BODIPY (4,4-difluoro-4-bora-3a, 4a-diaza-s-indacene) dyes are attractive as PSs due to their excellent optical properties, high molar extinction coefficient, and fluorescence quantum yield, and have thus been applied as imaging/detection agents.[6] Conventional BODIPY PSs have been synthesized by increasing spin orbit coupling through the heavy atom effect.[7] However, previous reports indicate that the inclusion of heavy atoms, such as bromine and iodine, results in cytotoxicity in the absence of light and compromises the fluorescence yield due to increased intersystem crossing efficiency.[8] Thus, fluorescent BODIPY PSs without heavy halogen atoms are preferred for effective dual clinical applications.
Recently, we reported an unprecedented case that a thieno-pyrrole fused BODIPY PS, SBDPiR690, had the ability to generate singlet oxygen (ΦΔ=0.42) without heavy halogen atom inclusion (Figure 1).[9] More interestingly, SBDPiR690 maintains a moderate fluorescence quantum yield (Φflu=0.22). The rare coexistence of these photophysical properties in conjunction with an excellent extinction coefficient (120000m−1cm−1) afforded appreciable BT (26400m−1cm−1) and PP (50400m−1cm−1), deeming this mono-chromophore a dual-functioning fluorescent PS for theranostics. Herein, we examined the peripheral substituent effects on the photophysical properties to establish a BODIPY structure–photophysical property relationship. As a result, the key factors for singlet oxygen generation in non-heavy halogen-atom-assisted BODIPYs were validated and mechanistic insights concerning the decay of excited states were obtained through the use of transient absorption spectra. Theranostic application was successful via SBDPiR690 preclinical optical imaging and effective PDT using a BALB/c mouse model of subcutaneous (S.C.) colon-26 tumors.
Results and Discussion
Design and synthesis of new BODIPY analogs
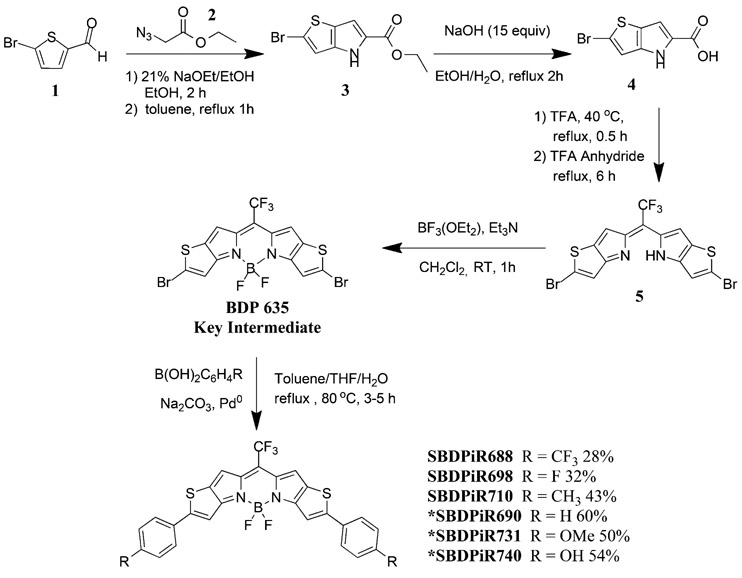
In our previous study, BODIPY with hydrogen at the para-position (SBDPiR690, Scheme 1) possessed an singlet oxygen quantum yield (ΦΔ) of 0.42, but hydroxyl- and methoxy- para-substituted BODIPYs did not exhibit any detectable singlet oxygen generation (Table 1).[9] We hypothesized that the electronic effects of the para-substituents make a key contribution to singlet oxygen generation. Thus, to systemically investigate the electronic effects with respect to singlet oxygen generation, we designed and prepared RODIPY analogs with electron-withdrawing groups (F and CF3) and mild electron-donating groups (i.e., without oxygen (CH3)).
Scheme 1.
Synthesis of SBDPiRs. BDP635 was obtained through a four-step reaction following conditions published previously.[9] The asterisk denotes previously reported analogs.
Table 1.
Photophysical parameters of SBDPiRs
| Compound | R |
λabs [nm] |
λflu [nm] |
ε[a] [M−1 cm−1] |
Φflu[b]] | ΦΔ[c] | BT[e] [M−1 cm−1 |
PP[f] ] [M−1 cm−1] |
|---|---|---|---|---|---|---|---|---|
| SBDPiR690 | H | 688 | 700 | 120000 | 0.22 | 0.42 | 26400 | 50400 |
| SBDPiR688 | CF3 | 688 | 695 | 211000 | 0.39 | 0.47 | 82290 | 99170 |
| SBDPiR698 | F | 698 | 705 | 146000 | 0.38 | 0.20 | 55480 | 29200 |
| SBDPiR710 | CH3 | 709 | 712 | 287000 | 0.67 | ND[d] | 192290 | ND |
| SBDPiR731 | OCH3 | 731 | 755 | 185000 | 0.38 | ND | 70300 | ND |
| SBDPiR740 | OH | 738 | 763 | 160000 | 0.10 | ND | 16000 | ND |
Molar absorptivity measurements were carried out in CHCl3 except for BDP740-OH which was performed in EtOH.
The fluorescence quantum yield was determined in CHCl3 (MeOH for BDP740-OH) at 298 K with Aza BODIPY (Φflu = 0.34).
SO quantum yields were determined in CHCl3 with [Ru(bipy)3]Cl2 as a reference (ΦΔ = 0.77 in MeOH).
Not determined under our experimental conditions.
Brightness (BT = ε × Φflu).
Phototoxic power (PP = ε × ΦΔ).
Following the synthetic scheme reported in our previous paper, we prepared the thieno-pyrrole-fused key intermediate BDP635 in four steps.[9] The new compounds SBDPiR688, SBDPiR698, and SBDPiR710 (the numbers represent their absorption maxima) were synthesized according to Suzuki–Miyaura reaction conditions (Scheme 1). In a one-pot reaction, BDP635 and the respective commercially available aryl-boronic acid were dissolved in a biphasic mixture under reflux at 80°C to achieve analogs. The reaction times varied between 2 and 4 h, presumably due to the deactivation of the aryl rings by electron-withdrawing groups. An essential benefit of our synthetic route is the use of the BDP635 platform. The distinct color shift from the blue BDP635 to the primarily green SBDPiR products was used to monitor reaction progression and completion.
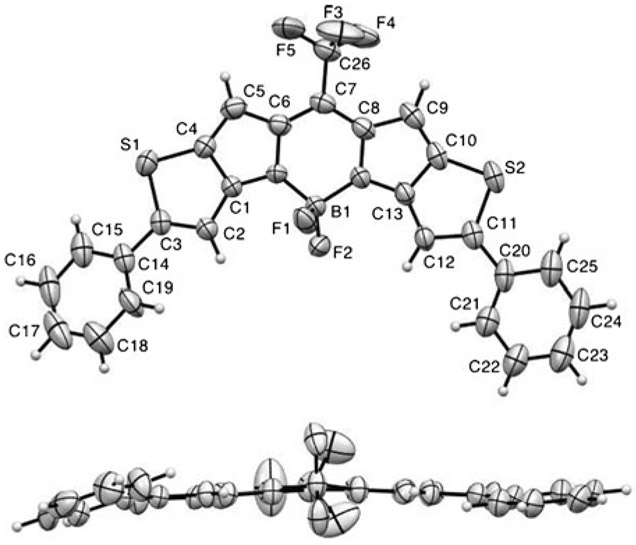
X-ray structural analysis of SBDPiR690
The X-ray crystal structure of SBDPiR690 (Figure 2) confirmed its structural similarity with that of the dibromo-intermediate (BDP635).[9] The molecule crystallized in the monoclinic crystal system with the space group P21/c.[10] The five core-fused rings were almost planar. The conjugated nature of the middle ring was confirmed by the comparable bond lengths between C7─C6 (1.396 Å) and C7─C8 (1.393 Å); between C6─N1 (1.398 Å) and C8─N2 (1.392 Å); and between N1─B1 (1.509 Å) and N2─B1 (1.526 Å). The boron center also maintained a tetrahedral geometry with an N1-B1-N2 angle of 105.7° and an F-B-F angle of 108.5°. Two phenyl groups, although rotatable in solution, were also close to the same plane with the main core. The dihedral angles of S1-C3-C14-C15 and S2-C11-C20-C25 were 11.6° and 15.4°, respectively. The crystal packing diagram (Figure S1) and anisotropic displacement parameters, bond lengths, angles, and H coordinates are given in the Supporting Information.
Figure 2.
ORTEP view of the X-ray crystal structure of SBDPiR690. Displacement ellipsoids are drawn at the 50% probability level.
Photophysical Properties
Spectroscopic properties.
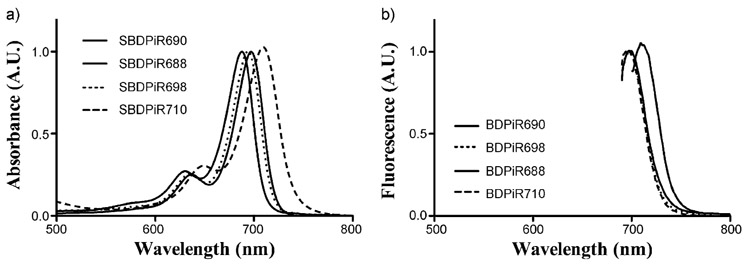
The absorption and emission spectroscopic data are presented in Table 1. SBDPiRs exhibit sharp absorption characteristic of BODIPY dyes indicative of the S0-S1 transition (Figure 3). The varying substituents impacted the absorption and emission maxima. SBDPiRs with electron-donating groups (CH3, OMe, OH) have red-shifted maxima compared with SBDPiRs carrying electron-withdrawing groups (F and CF3; absorbance=709–738 vs. 688 and 698 nm and fluorescence=712–763 vs. 695 and 705 nm). The bathochromic shifts by electron-donating groups were evident—21–50 nm shift in absorbance and 12–63 nm shift in fluorescence from those of SBDPiR690. This is perhaps due to a better push/pull system between the pull group (CF3) at the meso position and the push group (OH, OCH3, and CH3 groups) at the para-position of the phenyl groups.[6a] The values of the Stokes shift of SBDPiRs with electron-withdrawing groups (F and CF3) are smaller than those of SBDPiRs with electron-donating groups (OMe and OH): 7 nm versus approximately 24 nm (Table 1). The impact of solvent polarity on fluorescence and Stokes shifts was minor, presumably due to the non-polar nature of SBDPiR analogs.[11]
Figure 3.
a) UV/Vis and b) fluorescence spectra of SBDPiRs observed in CH2Cl2. The fluorescence spectral excitation wavelength corresponds to the absorption peak maximum of each analog.
The singlet oxygen quantum yield (ΦΔ) was determined by measuring the phosphorescence of singlet oxygen after the excitation of the BODIPYs.[9] The electronic properties of the para-substituents demonstrated positive and negative effects with respect to singlet oxygen generation. While SBDPiRs with electron-donating groups (CH3, OH, and OMe) did not show any detectable ΦΔ, SBDPiRs with electron-withdrawing groups (F and CF3) show moderate ΦΔ (0.20 and 0.47). The extraordinarily high molar extinction coefficient and BT of SBDPiR710 are noteworthy: 287 000 and 192840m−1cm−1, respectively. Thus, this derivative has a good potential as a near-IR fluorescence dye, although the small Stokes shift of 3 nm could be a problem. Further investigation is needed to determine the key structural feature for maximizing the extinction coefficient.
Compared with Φfiu and ΦΔ, BT and PP are better parameters for evaluating the potential for fluorescence imaging and PDT; furthermore, this takes into account the extinction coefficient of PSs: BT=ε×Φflu and PP=ε×ΦΔ.[9] Light harvesting is especially critical under in vivo conditions in which the excitation light is extremely limited due to the light attenuation by tissues. The BT and PP of electron-withdrawing analogs SBDPiR690, 688, and 698 were higher than those of all second-generation PSs in Figure 1 except Pc 4. These values are a good indication of their potential as dual functioning fPSs. SBDPiR688 showed excellent BT and PP comparable to those of Pc 4.
Singlet oxygen detection by chemical trapping.
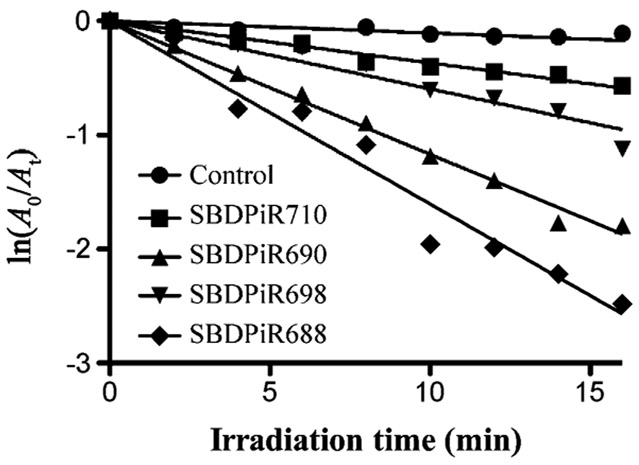
Singlet oxygen generation by SBDPiRs was also determined by using a chemical method (Figure 4). Diphenylisobenzofuran (DPBF) is a known singlet oxygen scavenger and essentialy traps the production of singlet oxygen through its oxidation. The rate of DPBF oxidation was determined during the illumination of a solution of respective SBDPiRs and DPBF with broadband light (400–850 nm at 0.5 mWcm−2). Oxidation and ultimately singlet oxygen production was determined by the evident decrease in the DPBF absorbance band at 410 nm.[12] The order of the rates of oxidation was consistent with that of ΦΔ: SBDPiR 688>690>698>710. The DPBF plots of SBDPiR731 and −740 were negative for singlet oxygen generation, as shown in our earlier publication.[7a]
Figure 4.
Time-dependent decrease of absorbance (410 nm) by oxidation of DPBF (90×10−6m) with SBDPiR (5×10−6m) under illumination with broadband light (400–850 nm at 0.5 mW cm−2).
Electrochemical potentials.
The electrochemical potentials were measured in CH2Cl2 containing 0.1 m TBAP with Ag/AgCl as a reference electrode and ferrocene/ferrocenium (Fc/Fc+) as an internal standard (Table 2). Excluding SBDPiR740, all SBDPiRs underwent two completely reversible redox potentials forming the respective stable anion(s) and cation(s) (Figure S4, Supporting Information). The third oxidations reported previously of SBDPiRs-731 and 690 were primarily quasi-reversible, presumably due to the instability of the trication.[9] Cyclic voltammograms (Figure S4) of the new analogs showed the oxidation potential range from E1/2=0.78 to 1.22 and the reduction potentials between E1/2=−0.76 and −1.51 eV.
Table 2.
Summary of redox potentials and HOMO/LUMO calculations.
| Compound | R |
E1/2ox[a] (CV) [V] |
E1/2red[a] (CV) [V] |
HOMO[b] (CV) [eV] |
LUMO[b] (CV) [eV] |
Egap[c] (CV) [eV] |
|---|---|---|---|---|---|---|
| SBDPiR690 | H | 0.79 | −0.93 | −5.17 | −3.45 | −1.72 |
| SBDPiR688 | CF3 | 0.94 | −0.76 | −5.32 | −3.62 | −1.70 |
| SBDPiR698 | F | 0.78 | −0.80 | −5.16 | −3.50 | −1.66 |
| SBDPiR710 | CH3 | 0.69 | −0.84 | −5.07 | −3.54 | −1.53 |
| SBDPiR731 | OCH3 | 0.98 | −0.45 | −5.36 | −3.93 | −1.43 |
| SBDPiR740 | OH | 1.02 | −0.32 | −5.40 | −4.06 | −1.34 |
We discovered a natural relationship between the energy gap of the first oxidation and reduction potentials with ΦΔ. With values of 1.77 and 1.72 V, respectively, BDP635 (dibrominated intermediate) and SBDPiR 690 had the largest energy gaps between the redox potentials. These two compounds exemplify appreciable singlet oxygen generation (ΦΔ>0.2) among the 12 analogs. The energy gaps of the remaining analogs were smaller, ranging from 1.57 to 0.92 V. Interestingly, a large energy gap, 1.58 and 1.70 V, is persistent in the two new analogs (SBDPiRs688 and 698) containing electron-withdrawing groups.
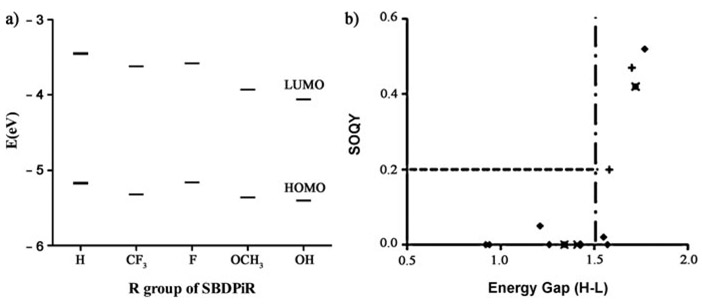
We estimated the HOMO and LUMO energy levels of the two groups of SBDPiRs containing electron-withdrawing groups (SBDPiR688 and 698) or electron-donating groups (SBDPiR710, 731, 740) from their first oxidation and reduction potentials (Table 2), and the corresponding values are shown in Figure 5a. The HOMO energy levels of the analogs with electron-donating groups decreased by −0.10, −0.19, and −0.23 eV, while the decrease of the analogs with electron-withdrawing groups was much smaller, by −0.01 and −0.15 eV, compared with the HOMO energy level of SBDPiR690. The HOMO–LUMO energy gaps of SBDPiRs with electron-withdrawing groups were also larger (1.70 and 1.66 eV) than those of SBDPiRs with electron-donating groups (1.34–1.53 eV). The relation between the HOMO–LUMO energy gap and ΦΔ of all BODIPY analogs (including analogs in our previous report[9]) is plotted in Figure 5b. Even though we could not identify a single equation covering the relationship for all of the compounds of our previous and current studies, there was a noticeable trend. It appears that a HOMO–LUMO gap of ≥ 1.5 eV is necessary for the SBDPiRs to exhibit appreciable singlet oxygen generation (ΦΔ≥0.2). However, such a gap alone does not suffice for the observed singlet oxygen generation, as two analogs with >1.5 eV gaps did not generate singlet oxygen.
Figure 5.
a) Mapping of HOMO and LUMO energy levels. b) Critical HOMO–LUMO energy difference threshold required for singlet oxygen generation.
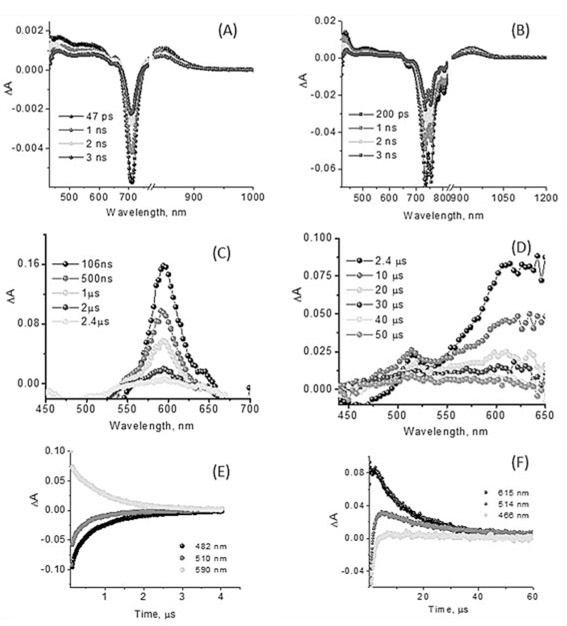
Time-resolved transient absorption spectra of SBDPiRs 690 and 731.
In order to study the excited states of SBDPiRs, we recorded time-resolved transient spectra of SBDPiRs 690 and 731 (Figure 6).[15] Simple BODIPY dyes are known to efficiently populate the singlet excited states exhibiting fluorescence. However, intersystem crossing to populate the triplet excited states is difficult to observe by direct excitation.[6c] Often, this is accomplished by first forming a charge-separated state in BODIPY–acceptor dyad systems in which the charge-separated state populates the triplet state of BODIPY prior to returning to the ground state.[16] Contrary to these observations of simple BODIPY derivatives, the present thieno-pyrrole fused BODIPY dyes seem to populate the triplet states via the intersystem crossing process.
Figure 6.
(A, B) Femtosecond (400 nm excitation, 100 fs laser pulses) and (C, D) nanosecond transient absorption spectra (532 nm excitation, 7 ns laser pulses) of SBDPiR690 and 731 at the indicated delay times. (E, F) Decay profiles after nanosecond pulsed excitation at the indicated wavelength in oxygen-free benzonitrile.
The fluorescence lifetimes of SBDPiR690 and SBDPiR731 in CHCl3, determined from time-correlated single photon counting (TCSPC), were found to be 1.55 and 3.22 nm, respectively.[9] The femtosecond transient absorption spectra (Figure 6A and B) of both compounds revealed significant bleaching at 708 nm and 726–754 nm, respectively, due to the depletion of ground-state SBDPiRs with any contributions from stimulated emission. Positive peaks at 464, 541, and 850 nm for SBDPiR690 and 433, 509, and 938 nm for SBDPiR731 were observed. With time, recovery of these bands was observed similar to that reported for BODIPY compounds,[17] however, with some positive absorbance around 594 nm for SBDPiR690 and around 610 nm for SBDPiR731, likely due to triplet formation. Further nanosecond transient spectra were recorded to confirm triplet formation.
As shown in Figure 6C and D, in the nanosecond transient absorption spectra a positive absorption peak was observed at 594 and 550–650 nm for SBRPiRs 690 and 731, respectively, due to the absorption of triplet species of these dyes. Interestingly, transient bands of the triplet excited state were persistent for nearly 50 μs in the case of SBDPiR731, compared with about 3 μs of 690. Since a longer triplet lifetime is preferred for singlet oxygen generation, we hypothesize that other factors such as quantum yield of triplet states could also contribute to the overall quantum yields of singlet oxygen generation. Detailed photophysical studies are underway to explore this hypothesis, and the corresponding data will be presented in due course.
Biological Evaluation
Phototoxicity activity.
The assay was performed using colon-26 cells. Varying concentrations of SBDPiR690 were incubated with the cells in the dark for 24 h. Subsequently, the culture medium was removed and fresh clear culture medium was added to each well. The plates were irradiated with a laser light source of 690 nm wavelength at a delivered light dose of 5.6 mWcm−2 for 30 min (~ 10 Jcm−2). After illumination, the cells were returned to normal medium and incubated for an additional 24 h at 37 °C, and the cell viability was determined using the MTT assay.[18] The dark toxicity of SBDPiRs was determined in a similar fashion as described above, with the exception of illumination. All assay experiments were carried out in triplicate, and the average of three individual runs are presented.
Following our assay protocol, no observable dark toxicity was observed for cells incubated with SBDPiR690, up to a concentration of 5.0×10−6m. By contrast, illumination with 10 Jcm−2 light dose resulted in a significant phototoxicity, with an IC50 value of 3.0×10−6m (Figure 7). The significant phototoxicity of SBDPiR690 supports our photophysical data and further establishes the unprecedented non-heavy atom-assisted NIR BODIPY structures as phototoxic agents. SBDPiR690 was used due to solubility issues with SBDPiR698 and SBDPiR688.
Figure 7.
Cellular phototoxicity of SBDPiR690.
Optical imaging of live mice.
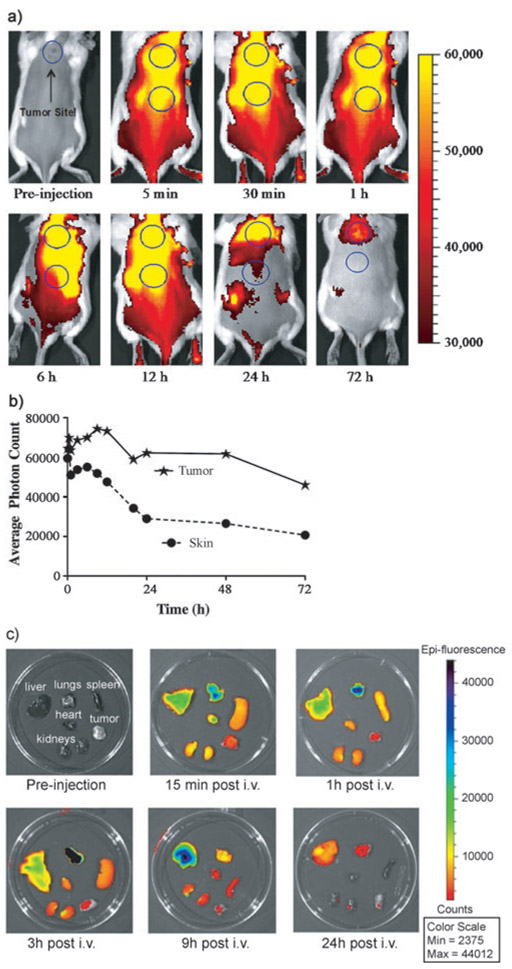
Time-dependent in vivo imaging was conducted to monitor the distribution of intraveneously injected SBDPiR690 in a live mouse and in excised organs (Figure 8). Whole-body imaging with SBDPiR690 showed bright fluorescence signals with which we could monitor its distribution in the tumor and skin (Figure 8A). Interestingly, the emission intensity of the skin decreased faster than that from the tumor, particularly at 9 h post injection (Figure 8B). Thus, the tumor-to-skin ratio gradually increased from 1 at 5 min to 2.3 at 48 h. In the ex vivo imaging, lung, liver, and heart showed relatively high emission intensity. The lung and liver showed the highest emission at 3 and 9 h post injection.
Figure 8.
a) Time-dependent in vivo images using SBDPiR690 (2 μmole kg−1) at 0.5 s exposure time. b) Time-dependent tumor-to-skin fluorescence ratio. c) Time-dependent ex vivo fluorescence imaging.
Antitumor effects of PDT treatment with SBDPiR690.
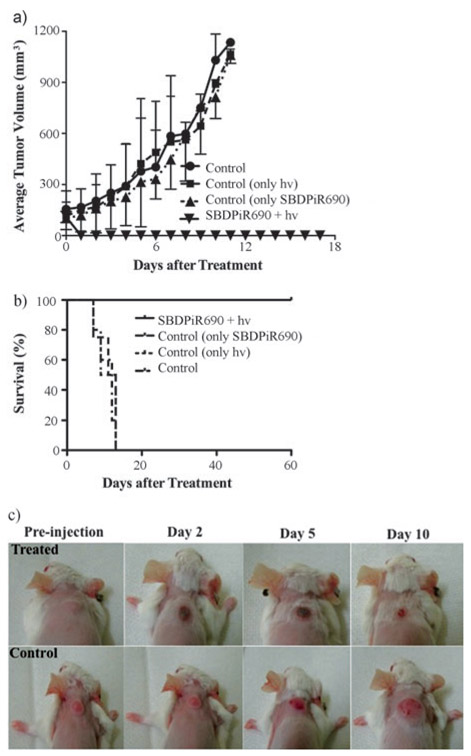
Balb/c tumor-bearing mice were used to demonstrate therapeutic potential in vivo. Mice (n=24) were divided into 4 groups: control (negative control), dark control (SBDPiR690 only), light control (light only), and SBDPiR690-PDT. We intravenously injected the mice with 200 μL of SBDPiR690 (5 μmole kg−1), followed by light illumination [100 mWcm−2, 30 min (150 Jcm−2)] 30 min post IV-injection using a 690 nm diode laser. We chose this time point to damage the tumor by vascular-targeted PDT. While the two positive control groups (light alone or SBPiR690) did not show any significant tumor growth delay compared with the negative control group, the PDT-treated group showed a dramatic reduction in tumor size (Figure 9A). Mass necrosis localized at the tumor region was evident 24–72 h after the illumination (Figure 9C). An associated edema and inflammation were observed after 24 h. This healed after a few days. Mice were pronounced cured after tissue remodeling with no palpable tumor observed at 60 days post-treatment (Figure 9B).
Figure 9.
Antitumor effect of PDT with BDPiR690 (5 μmolekg−1) in Balb/c mice with colon-26 tumor model (n = 4): a) Tumor growth curves, b) Kaplan–Meier plots, and c) Mouse images post-PDT treatment.
Experimental Section
General Methods:
Chemical reagents and solvents of analytical grade were purchased from commercial suppliers (Sigma–Aldrich, USA; VWR, USA) and were used without purification. Air-sensitive reactions were performed under a nitrogen atmosphere. NMR spectra were recorded in CDCl3, CD2Cl2, or [D6]DMSO using a Varian 300 MHz spectrometer. Chemical shifts are given in parts per million relative to TMS or 7.23 for residual CHCl3. Coupling constants were measured in Hz. High-resolution mass spectrometry was performed using electron impact at an ionized voltage of 70 eV and electrospray (ESI) ionization mass spectrometry was done with a QTOF-1 analyzer.
2,8-dibromo-5,5-difluoro-11-(trifluoromethyl)-5H-thieno[2’,3’:4,5]pyrrolo[1,2-c]thieno[2’,3’4,5]pyrrolo[2,1-f][1,3,2]diazaborinin-4-ium-5-uide (BDP635):
2-Bromo-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 4 (2.0 g, 8.13 mmol) was dissolved in trifluoroacetic acid (25 mL) and stirred at 40°C for 30 min. Trifluoroacetic anhydride (15 mL) was added and the mixture stirred at 80°C for 6 h, forming an intense blue color. After cooling, the reaction was poured into saturated aqueous NaHCO3 solution to neutralize. The formed precipitate was filtered, washed with water, and dried in vacuo. The resulting compound was dissolved in dichloromethane (500 mL), before boron trifluoride diethyl ether complex (7 mL) and triethylamine (5 mL) were added and stirred for 1 h. The reaction mixture was directly purified by column chromatography on silica gel (CH2Cl2) to afford 2,8-dibromo-5,5-difluoro-11-(trifluoromethyl)-5H-thieno[2’,3’:4,5] pyrrolo [1,2-c]thieno[2’,3’:4,5] pyrrolo[2,1-f] [1,3,2]diaza borinin-4-ium-5-uide (BDP635) as a bluish-green metallic solid (0.215 g, 5%). 1H NMR (CD2Cl2, 300 MHz): δ=7.3 (s, 2H), 7.2 ppm (s, 2H); HRMS EI (m/z): calculated for C14H4BBr2F5N2S2: 529.8200; found 529.8170 [M]+. We should note that compound 4 (Scheme 1) and BDP635 have been previously reported and were synthesized according to the literature procedure,[9] with few modifications.
5,5-Difluoro-11-(trifluoromethyl)-2,8-bis(4-(trifluoromethyl) phenyl)-5H-thieno[2’,3’:4,5]pyrrolo[1,2-c]thieno [2’,3’:4,5]pyrrolo [2,1f] [1,3,2] diazaborinin-4-ium-5-uide (SBDPiR688):
The method described for SBDP698 was followed using BDP635 (0.100 g, 0.19 mmol), 4-(trifluoromethyl)phenyl) boronic acid (0.100 g, 0.56 mmol) and Na2CO3 (0.060 g, 0.57 mmol). The dried mixture was purified by column chromatography on silica gel (hexanes/ethyl acetate 9:1) to afford SBDPiR688 as a dark green solid (0.103 g, 28%). 1HNMR (CD2Cl2, 300 MHz): δ=7.9 (d, J=8.1, 4H), 7.6 (d, J=3.6, 4H), 7.5 (s, 2H), 7.4 ppm (s, 2H); HRMS EI (m/z): calculated for C28H12BF11N2S2: 660.0359; found 660.0355 [M]+.
5,5-Difluoro-2,8-diphenyl-11-(trifluoromethyl)-5H-thieno [2’,3’:4,5]pyrrolo[1,2-c]thieno[2’,3’:4,5]pyrrolo[2,1-f][1,3,2] diazaborinin-4-ium-5-uide (SBDPiR690):
SBDiR690 was synthesized according to the reported procedure.[9] 1H NMR (CD2Cl2, 300 MHz): δ 7.8 (s, 1H), 7.7 (s, 1H), 7.6 (d, J=8.0 Hz, 4H), 7.5 (s, 1H), 7.4 ppm (peaks overlap, 7H). HRMS EI (m/z): Calculated for C26H14BF5N2S2: 524.0612; found: 524.0599 [M]+.
5,5-Difluoro-2,8-bis(4-fluorophenyl)-11-(trifluoromethyl)-5H-thieno[2’,3’:4,5]pyrrolo[1,2-c]thieno[2’,3’:4,5]pyrrolo[2,1-f][1,3,2] diazaborinin-4-ium-5-uide (SBDPiR698):
To a solution of BDP635 (0.100 g, 0.19 mmol) in toluene, tetrahydrofuran, and water (30 mL, 1:1:1), 4-fluorophenyl boronic acid (0.079 g, 0.57 mmol) and Na2CO3 (0.060 g, 0.57 mmol) were added. The reaction was purged with nitrogen gas for 10 min. A catalytic amount of [Pd(PPh3)4] (0.033 mg, ~5 mol%) was added and the reaction was heated to 80°C for 2–3 h. After completion of the reaction as judged by TLC, the reaction was diluted with water (10 mL) and extracted with toluene (10 mL). The combined organic layer was washed with water and brine (100 mL each) and dried over anhydrous Na2SO4. The dried mixture was purified by column chromatography on silica gel (hexanes/toluene 4:1) to afford SBDPiR698 as a dark green solid (0.034 g, 32%). 1H NMR (CD2Cl2, 300 MHz): δ=7.8 (t, J=7.6, 4H), 7.4 (s, 4H), 7.2 ppm (t, J=8.3, 4H); HRMS EI (m/z): calculated for C26H12BF7N2S2: 560.0423; found 560.0416 [M]+.
5,5-Difluoro-2,8-di-p-tolyl-11-(trifluoromethyl)-5H-thieno[2’,3’:4,5]pyrrolo[1,2-c]thieno[2’,3’:4,5]pyrrolo[2,1-f][1,3,2]diazaborinin-4-ium-5-uide (SBDPiR710):
The method described for SBDPiR698 was followed using BDP635 (0.100 g, 0.19 mmol), p-tolylboronic acid (0.076 g, 0.56 mmol), and Na2CO3 (0.060 g, 0.57 mmol). The dried mixture was purified by column chromatography on silica gel (hexanes/toluene 7:3) to afford SBDPiR710 as a dark green solid (0.024 g, 43%). 1H NMR ([D6]acetone, 300 MHz): δ=7.67(d, J=7.2 Hz, 4H), 7.4 (s, 2H), 7.3 (m, 6H), 2.4 ppm (s, 6H); HRMS EI (m/z): calculated for C28H18BF5N2S2: 552.1; found 552.0 [M]+.
5,5-Difluoro-2,8-bis(4-methoxyphenyl)-11-(trifluoromethyl)-5H-thieno[2’,3’:4,5]pyrrolo[1,2-c]thieno[2’,3’:4,5]pyrrolo[2,1-f][1,3,2] diazaborinin-4-ium-5-uide (SBDPiR731):
SBDPiR731 was synthesized according to the reported procedure.[9] 1H NMR (CD2Cl2, 400 MHz): δ=7.8 (d, J=8.0 Hz, 4H), 7.3 (s, 2H), 7.3 (s, 2H), 7.0 (d, J=8.0 Hz, 4H), 3.9 ppm (s, 6H); HRMS EI (m/z): Calculated for C28H18BF5N2O2S2: 584.0823; found: 584.0825 [M]+.
5,5-Difluoro-2,8-bis(4-hydroxyphenyl)-11-(trifluoromethyl)-5H-thieno[2’,3’:4,5]pyrrolo[1,2-c]thieno[2’,3’:4,5]pyrrolo[2,1-f][1,3,2] diazaborinin-4-ium-5-uide (SBDPiR740):
SBDPiR740 was synthesized according to the reported procedure.[9] 1H NMR ([D6]acetone, 300 MHz): δ=7.8 (d, J=9.0 Hz, 4H), 7.5 (s, 2H), 7.4 (s, 2H) 7.0 ppm (d, J=9.0 Hz, 4H); HRMS EI (m/z): Calculated for C26H14BF5N2O2S2: 556.0510; found: 556.0520 [M]+.
Photophysical measurements:
We acquired the photophysical properties of the novel SBDPiRs, including absorption, fluorescence spectra, singlet oxygen and fluorescence quantum yields, fluorescence lifetimes, molar extinction coefficients, CV and DPV, according to our previous study.[9] Details of femto- and nanosecond transient absorption spectroscopy setups are given in our previous publication.[17]
Photosensitizer formulation:
To obtain aqueous solutions of SBDPiR690 for in vitro and in vivo assays, the nonionic surfactant and emulsifier Polysorbate 80 (Tween 80) was used. The formulation procedure required dissolving SBDPiR690 in DMSO and subsequent treatment with a mixture of 1% Tween 80 and 5% dextrose. The final DMSO concentration was up to 5% in the injection solution for the in vivo study and less than 0.5% in the final culture medium.
Cells:
Colon-26 cells were obtained from the National Cancer Institute (NCI) and cultured in Dulbecco’s minimum essential medium (DMEM) supplemented with 10% (v/v) fetal calf serum, 1 % (v/v) l-glutamine, 50 Uml−1 penicillin, and 50 μgmL−1 streptomycin. All cells were maintained in 5% CO2 (v/v) and 21 % O2 (v/v) at 37 °C.
Photocytotoxicity assay:
Colon-26 cells were seeded at 1×104 cells per well in the complete medium (200 μL) using 96-well plates and were incubated for 24h at 37°C under 5% CO2. SBDPiR690 was dissolved in DMSO. The stock solution was diluted with 1% Tween 80–5% Dextrose solution and complete media to appropriate concentrations and added to the cells. The cells were then incubated for 24h at 37°C under 5% CO2. After this, the medium was removed and cells were rinsed with phosphate-buffered saline (PBS), resuspended with 100 μL of clear medium, and illuminated with a laser light source of 690 nm wavelength at a delivered light dose of 5.6 mW cm−2 for 30 min (~ 10 Jcm−2).
Animal model and antitumor effect study:
Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Oklahoma Health Sciences Center. Four-to-six-week-old Balb/c mice (18–20 g, Charles River Laboratories, Inc.) were used for the murine tumor model. The mice were inoculated subcutaneously with 2×106 colon-26 cells in PBS (100 μL) on the lower back-neck region. Tumors grew predictably in all mice, reached a size of 5–6 mm diameter within 10–14 days after the inoculation, and were used for PDT. Tumor growth was monitored with a digital caliper. Two dimensions, l and w (l, the longest axis of tumor; w, the axis perpendicular to l), were used to calculate tumor volume (lw2/2). Stock solutions in DMSO (4 mM) and further dilutions in 1% Tween 80 and 5% dextrose solution to achieve final doses were as follows: Control, SBDPiR690 only, light only, 5 μmolkg−1 SBDPiR690+100 mWcm−2 light for 30 min. Samples were filtered using a 0.2 μm sterile syringe filter. To each mouse, 200 μL of sample was injected IV (via intraocular injection). After 15 min of drug administration, mice were anesthetized by IP injection of ketamine 80 mgkg−1 and xylazine 6 mgkg−1. Tumors were irradiated with a 690 nm diode laser at 100 mWcm−2 for 30 min (180 Jcm−2). Tumor size was measured with the digital caliper every day after the treatment. Mice recovered after light irradiation and returned to normal activity.
Fluorescence optical imaging:
Small animal, whole-body fluorescence imaging was performed with an IVIS Spectrum In Vivo Imaging System (PerkinElmer, Inc.), and the acquired images and fluorescence signals were analyzed using Living Image Software v4.0 (PerkinElmer, Inc.). Excitation and emission wavelengths of 675 and 720 nm, respectively, were used for acquiring in vivo SBDPiRs fluorescent images. All images were obtained using an exposure time of 0.5 or 2 s and an f/stop of 2. Mice were injected with 2 μmole kg−1 SBDPiR690 through the retro-orbital vein and were anesthetized before imaging, using the excitation and emission wavelength of SBDPiR690 at various time points. For ex vivo biodistribution studies, mice were euthanized, and the tumor, liver, lungs, heart, spleen and kidneys were excised. Fluorescent images were taken at different time points.
Supplementary Material
Acknowledgements
This work was supported by the OCAST (HR11-58) and the American Cancer Society (RSG-13-102-01-CCE). The authors thank the NSF (grant CHE-1401188 to FD). Special thanks to Dr. Wai Tak Yip and Dr. Gregory Nkepang of the University of Oklahoma, and Venugopal Bandi of University of North Texas for their insightful discussions and experimental foundation. We thank the Peggy and Charles Stephenson Cancer Center at the University of Oklahoma, Oklahoma City, OK and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institute of Health under grant number P20 GM103639 for the use of Molecular Imaging Core, which provided optical in vivo imaging service.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/asia.201500140.
References
- [1]a).Dougherty TJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q, J. Natl. Cancer Inst 1998, 90, 889–905; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J, CA-Cancer J. Clin 2011, 61, 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]a).Awuah SG, You Y, RSC Adv. 2012, 2, 11169–11183; [Google Scholar]; b) Oleinick NL, Morris RL, Belichenko I, Photochem. Photobiol. Sci 2002, 1, 1–21; [DOI] [PubMed] [Google Scholar]; c) Dougherty TJ, Semin. Surg. Oncol 1986, 2, 24–37. [DOI] [PubMed] [Google Scholar]
- [3].Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T, Chem. Rev 2010, 110, 2795–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ethirajan M, Chen Y, Joshi P, Pandey RK, Chem. Soc. Rev 2011, 40, 340–362. [DOI] [PubMed] [Google Scholar]
- [5].Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y, Chem. Rev. 2010, 110, 2620–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]a).Loudet A, Burgess K, Chem. Rev 2007, 107, 4891–4932; [DOI] [PubMed] [Google Scholar]; b) Lu H, Mack J, Yang YC, Shen Z, Chem. Soc. Rev 2014, 43, 4778–4823; [DOI] [PubMed] [Google Scholar]; c) Kamkaew A, Lim SH, Lee HB, Kiew LV, Chung LY, Burgess K, Chem. Soc. Rev 2013, 42, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]a).Awuah SG, Polreis J, Biradar V, You Y, Org. Lett 2011, 13, 3884–3887; [DOI] [PubMed] [Google Scholar]; b) Yogo T, Urano Y, Ishitsuka Y, Maniwa F, Nagano T, J. Am. Chem. Soc 2005, 127, 12162–12163; [DOI] [PubMed] [Google Scholar]; c) Yang YC, Guo QL, Chen HC, Zhou ZK, Guo ZJ, Shen Z, Chem. Commun 2013, 49, 3940–3942. [DOI] [PubMed] [Google Scholar]
- [8].Cakmak Y, Kolemen S, Duman S, Dede Y, Dolen Y, Kilic B, Kostereli Z, Yildirim LT, Dogan AL, Guc D, Akkaya EU, Angew. Chem. Int. Ed 2011, 50, 11937–11941; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2011, 123, 12143–12147. [Google Scholar]
- [9].Awuah SG, Das SK, D′Souza F, You Y, Chem. Asian J. 2013, 8, 3123–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].CCDC 1053388 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Crystal data and structure refinement for SBDPiR690. Empirical formula C26H14BF5N2S2, Formula weight 524.32, Temperature 200(2) K, Wavelength 0.71073 Å, Crystal system Monoclinic, Space group P21/c, Unit cell dimensions a = 13.976(3) Å, α = 90°, b = 11.672(3) Å, β = 111.368(4)°, c = 14.646(4) Å, γ = 90°, Volume 2224.9(9) Å3, Z 4, Density (calculated) 1.565 Mg/m3, Absorption coefficient 0.301 mm−1, F(000) 1064, Crystal size 0.20×0.16×0.03 mm3, Theta range for data collection 1.56 to 26.02°, Index ranges −17 < = h < = 17, −14 < = k < = 14, −18 < = l < = 18, Reflections collected 19787, Independent reflections 4387 [R(int) = 0.1118], Completeness to θ = 26.02° 100.0%, Absorption correction Semi-empirical from equivalents, Max. and min. transmission 0.9916 and 0.9417, Refinement method Full-matrix least-squares on F2, Data/restraints/parameters 4387/0/325, Goodness-of-fit on F2 1.025, Final R indices [I > 2σ(I)], R1 = 0.0657, wR2 = 0.1128, R indices (all data) R1 = 0.1606, wR2 = 0.1407, Largest diff. peak and hole 0.428 and −0.339 e Å−3.
- [11].Lakowicz J, Pinciples of Fluorescence Spectroscopy, 3rd ed., Springer, Heidelberg, 2006. [Google Scholar]
- [12].Adarsh N, Avirah RR, Ramaiah D, Org. Lett 2010, 12, 5720–5723. [DOI] [PubMed] [Google Scholar]
- [13].Gagne RR, Koval CA, Lisensky GC, Inorg. Chem 1980, 19, 2854–2855. [Google Scholar]
- [14].Awuah SG, Polreis J, Prakash J, Qiao QQ, You YJ, J. Photochem. Photobiol. A 2011, 224, 116–122. [Google Scholar]
- [15].Bandi V, Das SK, Awuah SG, You Y, D′Souza F, J. Am. Chem. Soc 2014, 136, 7571–7574. [DOI] [PubMed] [Google Scholar]
- [16].Amin AN, El-Khouly ME, Subbaiyan NK, Zandler ME, Fukuzumi S, D′Souza F, Chem. Commun 2012, 48, 206–208; [DOI] [PubMed] [Google Scholar]; El-Khouly ME, Fukuzumi S, D′Souza F, ChemPhysChem 2014, 15, 30–47. [DOI] [PubMed] [Google Scholar]
- [17].Kc CB, Lim GN, Nesterov VN, Karr PA, D′Souza F, Chem. Eur. J 2014, 20, 17100–17112; [DOI] [PubMed] [Google Scholar]; Bandi V, D′Souza FP, Gobeze HB, D′Souza F, Chem. Eur. J 2015, 21, 2669–2679. [DOI] [PubMed] [Google Scholar]
- [18].Berridge MV, Tan AS, Arch. Biochem. Biophys 1993, 303, 474–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.