A logrank test-based method for sizing clinical trials with two co-primary time-to-event endpoints (10.1093/biostatistics/kxs057)
In the article by Tomoyuki Sugimotoet al. (’A logrank Test-Based Method for Sizing Clinical Trials With Two Co-Primary Time-to-Event Endpoints'), there were errors on pages409–421 of issue 14(3) ofBiostatistics.
In Section 4.2 on p. 418, the article described “the total sample size is 928 commonly for
the three copulas when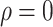 . When
. When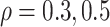 , and 0.8, they are 928, 926,
and 924 for the Clayton copula; 926, 922, and 920 for the Gumbel copula; and 926, 924, and 920
for the Frank copula.” However, these numbers were calculated with the two hazard ratios
, and 0.8, they are 928, 926,
and 924 for the Clayton copula; 926, 922, and 920 for the Gumbel copula; and 926, 924, and 920
for the Frank copula.” However, these numbers were calculated with the two hazard ratios
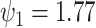 and
and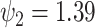 , not
, not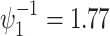 and
and
 . The corrected numbers are as follows:
. The corrected numbers are as follows:
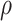
|
Clayton | Gumbel | Frank |
| 0.0 | 1220 | 1220 | 1220 |
| 0.3 | 1218 | 1210 | 1212 |
| 0.5 | 1216 | 1202 | 1204 |
| 0.8 | 1210 | 1188 | 1188 |
Also Figure 1 on p. 419 is corrected as below. The authors apologize for these errors.
Fig. 1.
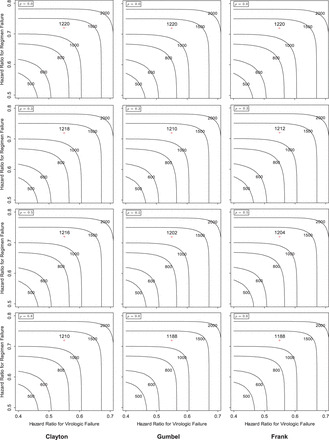
Contour plots of the required total sample size with the hazard ratios of time-to-events
of virologic and regimen failures, and correlation for the three copulas. The sample size
was calculated to detect the joint reduction for both time-to-event outcomes with the
overall power of 0.90 at the one-sided significance level of 0.0125, where
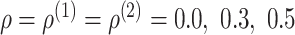 and 0.8;
and 0.8;
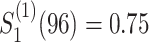 and
and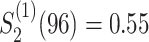 ;
;
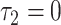 , and
, and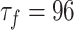 ;
; .
.
Contributor Information
Tomoyuki Sugimoto, Department of Mathematical Sciences, Hirosaki University Graduate School of Science and Technology, 3 Bunkyocho, Hirosaki, Aomori 036-8561, Japan , tomoyuki@cc.hirosaki-u.ac.jp .
Takashi Sozu, Department of Biostatistics, Kyoto University School of Public Heath, Kyoto 606-8501, Japan .
Toshimitsu Hamasaki, Department of Biomedical Statistics, Osaka University Graduate School of Medicine, Suita 565-0871, Japan .
Scott R. Evans, Department of Biostatistics, Harvard University School of Public Heath, Boston, MA 02115-6018, USA


