Abstract
Bacterial infection of the kidney leads to a rapid cascade of host protective responses, many of which are still poorly understood. We have previously shown that following kidney infection with uropathogenicEscherichia coli (UPEC), vascular coagulation is quickly initiated in local perivascular capillaries that protects the host from progressing from a local infection to systemic sepsis. The signaling mechanisms behind this response have not however been described. In this study, we use a number ofin vitro andin vivo techniques, including intravital microscopy, to identify two previously unrecognized components influencing this protective coagulation response. The acylation state of the Lipid A of UPEC lipopolysaccharide (LPS) is shown to alter the kinetics of local coagulation onsetin vivo. We also identify epithelial CD147 as a potential host factor influencing infection-mediated coagulation. CD147 is expressed by renal proximal epithelial cells infected with UPEC, contingent to bacterial expression of the α-hemolysin toxin. The epithelial CD147 subsequently can activate tissue factor on endothelial cells, a primary step in the coagulation cascade. This study emphasizes the rapid, multifaceted response of the kidney tissue to bacterial infection and the interplay between host and pathogen during the early hours of renal infection.
Keywords: coagulation, UTI, UPEC, kidney, tissue microbiology, CD147, lipid A, Escherichia coli
Kidney response to infection is influenced by both host and pathogen. The structure of bacterial LPS effects the kinetics of protective coagulation, while infected epithelia releases CD147, activating tissue factor.
INTRODUCTION
Bacterial infection of the kidney leads to an immediate and complex ‘dialogue’ between host and pathogen that is influenced by the bacterial repertoire of virulence factors, the host immune response and the microenvironment of the tissue. Understanding the sometimes-subtle interplay between these factors is crucial to understanding the complete picture of kidney infectionin vivo. We previously developed a ‘Tissue Microbiology’in vivo model of uropathogenicEscherichia coli (UPEC) infection to study this interaction (Manssonet al.2007; Melicanet al.2008; Melicanet al.2011). Combining the spatial and temporal control of microinfusion with multiphoton intravital microscopy, this model allowed us to study UPEC infection in ‘real-time’ within living kidneys. Visualizing the ongoing infection process, with subcellular resolution, revealed a remarkable complexity. Differential expression of bacterial virulence factors was shown to alter the course of infection in terms of bacterial adhesion and bacterial lifestyles (Manssonet al.2007; Melicanet al.2008; Melicanet al.2011). We also demonstrated the effect of these bacterial virulence factors in influencing the kinetics of the host inflammatory response (Manssonet al.2007; Melicanet al.2008; Melicanet al.2011). A major finding from this previous work was that UPEC infection of kidney tubules induces a rapid vascular coagulation in local peritubular capillaries (Melicanet al.2008). This coagulation leads to local tissue hypoxia and vascular isolation of the infection site, preventing a progression to sepsis and facilitating bacterial clearance (Melicanet al.2008).
The next step has been to find the signaling mechanisms that initiate this infection-mediated vascular coagulation. A key point is that at the onset of this coagulation, bacteria are still located at the epithelial barrier and not in direct contact with the blood vessels. The importance of understanding the complex associations between bacterial infection and coagulation has been highlighted in recent reviews (Davis, Miller-Dorey and Jenne2016; Peetermanset al.2016). Coagulation in response to bacterial sepsis and blood-borne bacteria has been comprehensively studied (McDonaldet al.2017). Sepsis can be considered, however, a ‘late stage’ of infection, the result of a local infection that the host has failed to control. Our previous work showed that if coagulation can be initiated before bacteria came into direct contact with the blood vessels, sepsis might be avoidable (Melicanet al.2008). This indicates that there is some form of cell-cell communication from the infected epithelia, initiating the protective coagulation response, prior to the onset of sepsis. An important aspect of clinical treatment of sepsis is the use of anticoagulants. We have shown that anticoagulation may actually enhance the progression from local to systemic infection by disrupting this important protective response (Melicanet al.2008). It is becoming more apparent through both basic research and clinical trials that there is a need to be able to functionally uncouple essential physiological hemostasis and infection-mediated coagulation so that we can pharmacologically target only the latter when necessary (Davis, Miller-Dorey and Jenne2016). It may also be that by understanding the mechanism of this protective response that we may be able to harness or enhance this as an immunotherapeutic alternative to antibiotic treatment of early stage infections.
Deciphering the mechanisms initiating infection-mediated coagulation during the early hours of bacterial infection is the focus of this work. Our hypothesis is that signaling events occurring at the bacterial-epithelial interface initiates tissue signaling which leads to a coagulation response in the local vasculature. To address this, we analyze both host and bacterial elements of epithelial infection that affect the kinetics of coagulationin vitro andin vivo.
METHODS
Bacterial strains
Bacterial strains, plasmids and oligonucleotides are presented in Table 1. LT004 ΔmsbB, was created using the one step allelic knockout method (Datsenko and Wanner2000), a kanamycin resistance cassette was inserted into themsbB gene of LT004 between nt 281,464–281,858. No significant alterations in growth rates, capsule morphology or expression of α-hemolysin were observed. Bacteria were grown in Luria broth aerated over night at 37°C. On the day of infection, bacteria were diluted 1:50 or 1:100 and grown to OD600nm 0.5, corresponding to ≈108 CFU/ml.
Table 1.
Bacterial strains, plasmids and oligonucleotides.
| Strain | Genotype | Reference |
|---|---|---|
| CFT073 | O6:K2:H1 | (Mobleyet al.1990; Manssonet al.2007) |
| LT002 | CFT073hlyA::kmr | (Manssonet al.2007) |
| LT004 | CFT073 cobS:: Φ (PLtetO-1-gfp+), cmr | (Manssonet al.2007) |
| LT005 | LT004hlyA::kmr | (Manssonet al.2007) |
| LT004 ΔmsbB | LT004msbB::kmr | This study |
| Plasmids | ||
| pKD4 | Kanamycin resistance cassette source | (Datsenko and Wanner2000) |
| pKD46 | Lambda red recombinase | (Datsenko and Wanner2000) |
| Oligonucleotides | ||
| MsbBKO_F1 | ATAATAGCGAATACATTCCTGAGTTTGATAAATCCTTTCGTGTGTAGGCTGGAGCTGCTTC | This study |
| MsbBKO_R2 | AATTTTAATATCCAGGTGTATTGTTCTGGTCGCGGACCAACATATGAATATCCTCCTTAG | This study |
Human cells
RPTEC/TERT1 (ATCC® CRL-4031) cells were plated at 2 × 106 cells per well and grown for 11 days at 37°C, 5% CO2, 95% humidity in complete DMEM-F12 media containing DMEM-F12 (ATCC, Sweden cat #30–2006), 5pM T3 (#T6397), 10 ng/ml recombinant human EGF (#PMG8041, Life Technologies), 3.5 μg/ml ascorbic acid (#A4403), 5 μg/ml human transferrin (#T1147), 5 μg/ml bovine insulin (#I6634), 25 ng/ml prostaglandin E1 (#P8908), 25 ng/ml hydrocortisone (#H0888), 8.65 ng/ml sodium selenite (#S5261) and 100 μg/ml G418 (#G418-RO) (Sigma, Sweden). Monolayer formation, was evaluated using a mouse-anti human-ZO1 antibody (Invitrogen, Sweden cat #339 100), Cy3- donkey anti-mouse IgG (Jackson Immunoresearch Europe cat#715–1650-150) Phalloidin-Alexa Fluor633 (Life Technologies, Sweden, cat #A22284) and Hoechst 33 258 pentahydrate (bis-benzimide) (Life Technologies, Sweden cat # H1398). LIVE/DEAD® Viability/Cytotoxicity kit (Invitrogen, Sweden cat #L3224) for eukaryotic cells was used according to the manufacturer's instructions.
HMEC-1 ATCC® CRL-3243™ was grown in MCDB 131 medium (#10 372 019, Invitrogen, Sweden) supplemented with 10% of FBS (Lot # 111M3397 Sigma, Sweden), 10 mM GlutaMAX (#35 050 061, Gibco), 10 ng/ml recombinant mouse epidermal growth factor (mEGF # PMG804, Life Technologies) and 1 μg/ml of hydrocortisone (# H0888 Sigma) at 37°C and 5% CO2.
Epithelial cell infection
Cells were infected at an MOI of 1:20 or stimulated with 5μg/ml lipopolysaccharide (LPS). LPS was either prepared as previously described (Backhedet al.2001) or Ultrapure K-12 LPS (Invitogen, France, cat #tlrl-peklps). Where indicated, bacteria or media were pre-treated with Polymyxin B (PMB) (Sigma–Aldrich cat #P4932), 8U/ml, for 30 min at 8°C.
Endothelial cell stimulation
HMEC-1 cells were plated at 8 × 105 cells per well. Cells were stimulated for 4 h at 37°C with aliquots of supernatant collected from the ‘Epithelial cell infection’ described above, (diluted 1:5), LPS (5μg/ml) or PMB (8 U/ml) in complete RPTEC/TERT1 medium (see above). The complete DMEM-F12 medium did not activate HMEC-1 cytokine/chemokine secretion. Cell supernatants were collected, centrifuged and stored at −80°C.
Cytokine and chemokine determinations
ELISA kits for IL-6 (#550 799), IL-8 (#555 244 BD Bioscience), EMMPRIN (#DY972) and DPPVI/CD26 (#DY1180 R&D Systems, Minneapolis, MN) were used according to the manufacturer's instructions. IL-6 (# 558 276), IL-8 (# 558 277), IL-1β (# 558 279) and TNF-α (# 560 112) were analyzed simultaneously using the Cytometric Bead Array (CBA) Human Master Buffer Kit (#558 264 BD Biosciences, Sweden), using a FACS Canto II flow cytometer operated with the FACSDiva software. Data were analyzed with FCAP Array software, following the manufacturer's tutorial.
Tissue factor determination
Cell lysates were prepared by lysis using 150 μl 15mM octyl-ß-D-glycopyranoside (#O8001 Sigma, Sweden) for 15 min at 37°C or by 3 repeated cycles of freeze-thaws in 150 μl of buffer of 50 mM Tris-HCl, 100 mM NaCl, 0.1% Triton X-100, pH 7.4. Tissue factor (TF) was extracted for 18 h at 4°C –8°C. TF-procoagulant activity was measured using either the chromogenic assay ACTICHROME® TF kit (Sekisui Diagnostics, # 846) or TF Human Chromogenic Activity Assay Kit (ABCAM, #ab108906) according to the manufacturer's instructions.
Animals
Studies were performed in accordance with the National Institutes of Health'sGuide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (Indianapolis, Indiana, USA). Male Munich–Wistar rats (255 ± 22 g body weight) (Harlan, USA), with free access to chow and water, were used. Rats were anesthetized with 130–150 mg/kg thiobutabarbital (Inactin) (#T133 Sigma, St. Louis, MO and Sigma, Sweden). Animals underwent tracheostomy, cannulation of a femoral artery, femoral vein and jugular vein. Infection by tubular microperfusion was performed as previously described (Manssonet al.2007). Fresh LT004 ΔmsbB was cultivated and concentrated to 109 CFU/ml in PBS+ (PBS with 1 mM CaCl2 and 2 mM MgCl2) containing 1 mg/ml Fast Green FCF (Fisher, Fair Lawn, NJ) and 0.2 mg/ml cascade blue-conjugated 10 kDa dextran (#D1976 Molecular Probes, Eugene, OR). The left kidney was exposed via a subcostal flank incision and supported in a kidney cup. The bacterial suspension was infused over 10 min into the lumen of superficial proximal tubules using a micromanipulator (Leitz or World Precision Instruments, UK) and mercury leveling bulb. Bacteria or PBS were infused at an average rate of 54 nl/min.
Multiphoton microscopy
All multiphoton imaging was performed using the set-up previously optimized and described (Manssonet al.2007; Melicanet al.2008). Images were collected using a Bio-Rad MRC 1024 confocal/2-photon system (Bio-Rad, Hercules, CA) attached to a Nikon Diaphot inverted microscope (Fryer Co, Huntley, IL) with a Nikon 20x water-immersion objective. Images were collected using an excitation wavelength of 810 nm and neutral density filters set to 25%–40%. Fluorescent probes were injected as a single bolus via a jugular vein access line. Tetramethylrhodamine-conjugated 500 kDa dextran (∼2.5 mg/400 μl 0.9% saline, Molecular Probes, Eugene, OR) and Hoechst 33 342 (∼600 μg/0.4 ml of 0.9% saline, Molecular Probes, Eugene, OR). Line scan measurements were performed as previously described (Kleinfeldet al.1998), and as illustrated in Fig. 3. For line scanning, a capillary adjacent to the infected tubule, which lay parallel to scan head direction, was required. Typically, only the single most appropriate capillary per infection site (n = 4) was selected for scanning. Images and data-volumes were processed using Metamorph Image Processing Software (Universal Imaging-Molecular Devices, PA, USA), and Image J (U. S. National Institutes of Health, MD,http://rsb.info.nih.gov/ij/). Final figures were prepared with Adobe Photoshop (Adobe, CA, USA).
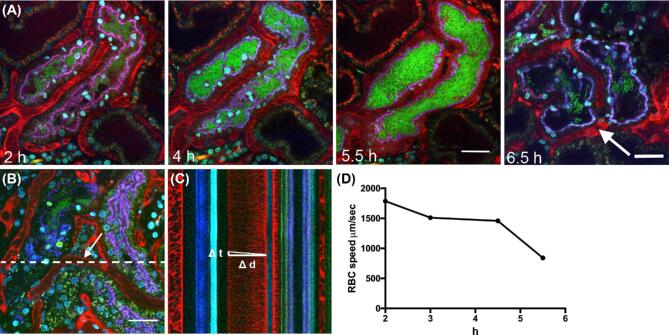
Figure 3.
Penta-acylated Lipid A delays coagulationin vivo. Representative intravital microscopy showing the infection kinetics over time of (A) LT004 ΔmsbB microinfused into a single renal proximal tubule of a live rat (n = 4). Bacteria express GFP (green), blood plasma is visualized by i.v. delivered 500 kDa tetramethylrhodamine conjugated dextran (red) and cell nuclei with i.v. delivered Hoechst 33 342 (cyan). The infected tubule is outlined with a 10 kDa cascade blue conjugated dextran co-infused with the bacteria and selectively endocytosed by the proximal tubule cells (blue). Scale bars = 30 μm. (B) Reference image showing the location of the line scan (dashed line). The arrow shows the location of the vessel of interest shown in (C). (C) Part of the cross-section of the line scan from (B) showing the calculation of RBC velocity, calculated by determining the distance Δd a RBC has travelled over time Δt. (D) Representative RBC velocity in the selected vessel adjacent to an LT004 ΔmsbB infected tubule (vessel shown in B) over time.
Proteome Array
The human XL Cytokine array kit (Proteome Profiler™ array) (R&D Systems, # ARY022B) was used according to manufacturer's protocols. The chemiluminescence was measured using a imageQuant LAS 4000 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Spot size and pixel intensity were determined using ImageJ software Fiji (NIH, Bethesda, MD, USA).
EMMPRIN and CD26 stimulation
Endothelial cells were stimulated with homo-dimer human recombinant EMMPRIN (Abcam, Sweden #AB155636) or human CD26/DPP4 recombinant protein (Sino Biological #10 688-HNCH-10) for 4 h before TF activation determination at the noted concentrations.
Statistical analysis
Data are shown as mean ± standard deviation (SD). Statistics were determined using ANOVA with Tukeys correction for multiple comparisons. Independent samples were analyzed using an unpairedt-test with Welches correction. The proteome array was analyzed with an uncorrected multiplet-test. Significance is considered = P ≤ 0.05 and annotated by* unless otherwise stated. All data were analyzed using the GraphPad inStat version 6.0 (GraphPad software, San Diego, CA, USA).
RESULTS
Epithelial cell inflammatory responses to UPEC infection
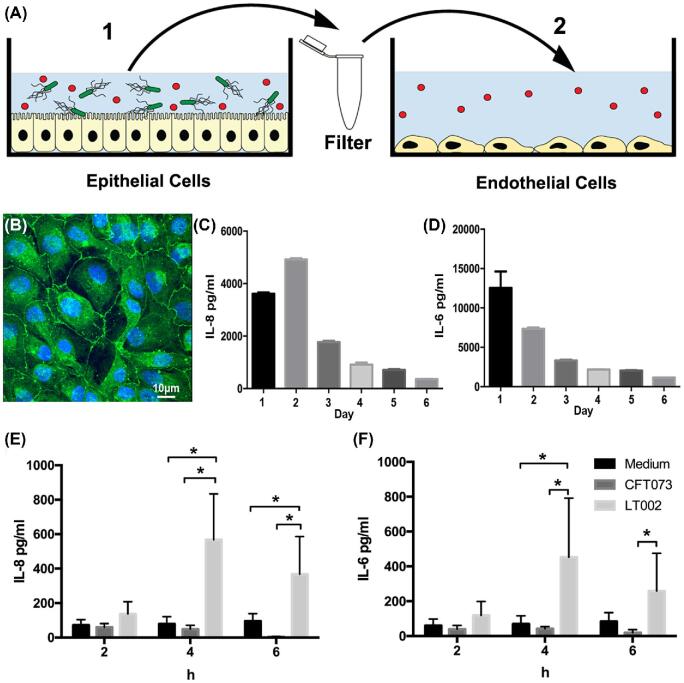
To study the communication between infected renal proximal tubule epithelial cells and vascular endothelial cells we used a controlled 2-stepin vitro cell culture model (Fig. 1A). In an effort to faithfully replicate thein vivo microenvironment of the kidney, we sought an epithelial cell line that closely replicated the morphological and functional characteristics of renal proximal tubules. We used RPTEC/TERT1 (ATCC® CRL-4031), a human renal epithelial cell line that has been shown to reproduce renal proximal tubular characteristics in both 2D and 3D culture (Wieseret al.2008; Simon-Friedtet al.2015; Seckeret al.2018). We optimized growth conditions, with extended (10 day) culturing, high-density seeding and frequent media changes, which resulted in a confluent monolayer that displayed tight junction formation (Fig. 1B), dome formation indicative of fluid transport and a reduced basal cytokine secretion over time (Fig. 1C, D). Tubular infection was modeled by infecting RPTEC/TERT1 with the prototypic UPEC strain CFT073 (Table1) at an MOI of 1:20. As the response of RPTEC/TERT1 to bacterial infection had not been previously reported, we started by evaluating the epithelial inflammatory profile of these cells following UPEC infection. Supernatants from infected RPTEC/TERT1 were collected at 2, 4 and 6 h post infection and the epithelial inflammatory profile evaluated using a cytometric bead array (CBA). CFT073 infection led to no increase in epithelial IL-8 or IL-6 (Fig. 1E, F). We have shown previously that expression of the UPEC protein toxin α-hemolysin affects the kinetics of infection-mediated coagulationin vivo (Manssonet al.2007). To analyze the epithelial inflammatory response in the absence of α-hemolysin, we infected RPTEC/TERT1 with LT002, an isogenic mutant of CFT073 that lacks expression of the α-hemolysin toxin (Δhly) (Table1). Following LT002 infection, epithelial IL-8 (Fig. 1E) and IL-6 (Fig. 1E) production was increased at 4 and 6 h. The α-hemolysin toxin expressed by UPEC has a concentration-dependent bi-phasic action either modulating cell signaling at sub-lytic concentrations or as a cytotoxic pore-forming toxin at higher concentrations (Uhlenet al.2000; Dhakal and Mulvey2012). We evaluated the cytotoxic action of α-hemolysin on RPTEC/TERT1 using a live dead assay (Supplementary Fig.S1), which showed that infection with the α-hemolysin expressing CFT073 strain caused increased epithelial cell death/detachment after 6 h of infection when compared to the LT002 (Δhly) strain.
Figure 1.
Epithelial cell response to UPEC infection. (A) Schematic of thein vitro infection model system. (B) Z0–1 staining (green) showing tight junction formation in RPTEC/TERT1 cells at cultivation day 10. Cell nuclei are seen in blue. (C) ELISA measurements of basal IL-8 and (D) IL-6 expression in RPTEC/TERT1 cells on days 1 to 6 of cultivation,n = 3. (E) IL-8 and (F) IL-6 expression in renal RPTEC/TERT1 epithelial cells infected with an MOI 1:20 of CFT073 (dark grey) or LT002 (light grey) for 2, 4 and 6 h. Uninfected cell culture medium (black) was used as the comparative control. Expression was measured using the cytometric bead array (CBA),n = 5. All bars represent mean ± SD. Significance was tested with a 2-way ANOVA with Turkeys post-test for multiple comparisons.
Together this data indicated that the RPTEC/TERT1 cell line could respond to UPEC infection, and that bacterial expression of the toxin HlyA influenced the renal epithelial inflammatory response, a phenomenon that has been previously described on bladder epithelial cells (Dhakal and Mulvey2012; Hilbertet al.2012; Ristow and Welch2016).
Endothelial cell activation following epithelial cell infection
We then investigated how endothelial cells may respond to the multitude of factors produced by infected renal epithelia. Working from our previousin vivo data showing initiation of infection-mediated coagulation at 4 h (Melicanet al.2008), we focused on signaling components produced up to 4 h post infection. Supernatant from a 4 h epithelial infection (step 1, Fig. 1A) was collected, filtered (0.2um) and then used to stimulate a human microvascular endothelial cell line (HMEC-1) for 4 h (Step 2, Fig. 1A).
HMEC-1 cells stimulated for 4 h with supernatant from CFT073 infected epithelial cells showed an increased production of IL-8, IL-6 and IL-1β, and a moderate increase of TNF-α (Fig. 2A-D). Endothelial stimulation with supernatant from LT002 (Δhly) infected epithelial cells showed a similar pattern, with significant up-regulation of IL-8, IL-6, and an increase of TNF-α and IL-1β (Fig. 2A-D). The similarity of the endothelial response to the supernatants from the different epithelial infections (Fig. 1E) demonstrated that epithelial production of IL-8 and IL-6 are not decisive factors in this cell-cell signaling and that other factors must be involved.
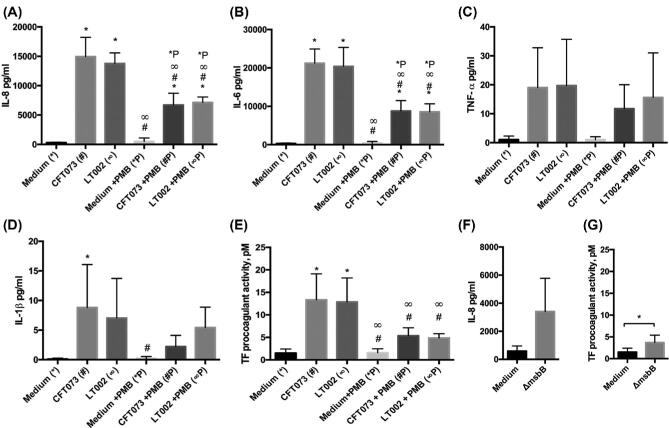
Figure 2.
Activation of endothelial cells by supernatant from infected epithelia. (A) IL-8, (B) IL-6, (C) TNF-α and (D) IL-1β expression measured by CBA from HMEC-1 endothelial cells stimulated for 4 h with supernatant from RPTEC/TERT1 epithelial cells infected for 4 h with either CFT073 or LT002n = 5. Supernatant from epithelial cultures treated with uninfected cell culture medium was used as a comparative control. Epithelial supernatants were pre-treated with 8 U/ml PMB prior to endothelial stimulation (+PMB),n = 5. (E) TF pro-coagulant activity, pM, measured using chromogenic assays, in HMEC-1 cells treated with supernatant from infected RPTEC/TERT1 epithelial cells as described above,n = 5 (F) IL-8 expression measured by ELISA of HMEC-1 cells stimulated for 4 h with supernatant from RPTEC/TERT1 epithelial cells infected with LT004 ΔmsbB for 4 h,n = 3 (G) TF pro-coagulant activity, pM, in HMEC-1 cells treated for 4 h with supernatant from RPTEC/TERT1 infected with LT004 ΔmsbB for 4 h,n = 5. All bars represent mean ± SD. In A-E statistical significance (P = < 0.05) is shown by the use of symbols corresponding to the comparative experiment. i.e. significance to the medium control is shown with* and significance to the CFT073 culture is shown by #.
We were also interested in the ability of the epithelial factors to induce coagulation. Tissue factor (TF) is a central regulator of the coagulation cascade. We therefore measured the activation of endothelial TF following stimulation with infected epithelial supernatants. Stimulation of endothelial cells with supernatant from epithelial cells infected with both CFT073 and LT002 led to significant endothelial TF activation (Fig. 2E). This data supports our hypothesis that factors originating from the infected epithelium can mediate endothelial activation and the initiation of coagulation.
Role of LPS Lipid A in endothelial activation
We then investigated what factors may be mediating TF activation. A major component of the filtered supernatants is the bacterial endotoxin LPS. As bacteria grow, they shed significant amounts of LPS into their environment (Mattsby-Baltzeret al.1991). Control experiments with purified LPS showed that LPS alone could stimulate endothelial production of IL-8, IL-6 and IL-1β (Supplementary Fig.2A-D) and also induced significant TF activation (Supplementary Fig.2E).
To study the extent of the role played by LPS in endothelial activation in our model, we used the antibiotic Polymyxin B (PMB), which binds to the lipid A portion of LPS, antagonizing LPS- TLR4 signaling (Backhedet al.2003). Control experiments confirmed that PMB treatment (8 U/ml) of purified LPS reduced endothelial IL-8, IL-6 and IL-1β production as well as TF activation (Supplementary Fig.2A-E). PMB treatment of the supernatant from epithelial cells infected with both CFT073 and LT002 prior to endothelial cell stimulation (Step 2, Fig. 1A), resulted in a reduction in endothelial IL-8 and IL-6 production (Fig. 2A,B, +PMB). Endothelial TF activation was significantly reduced following PMB treatment of the supernatant from CFT073 and LT002 infected epithelial cells (Fig. 2E, +PMB).
The reduction in both cytokine signaling (Fig. 2A-D) and TF activation (Fig. 2E) was, however, not complete abrogation. PMB treated supernatant from both CFT073 and LT002 still stimulated a significant IL-8 and IL-6 response when compared to its non-infected controls (Fig. 2A-B, +PMB). PMB treated epithelial supernatants also showed an increase in TF activation (Fig. 2E, +PMB). These results indicate that lipid A mediated LPS signaling is an important element in ourin vitro model, and blocking it reduces, but does not eliminate, inflammation and TF activation.
LPS lipid A mediated signaling affects coagulation kineticsin vivo
Testing the relevance of this lipid A mediated LPS signalingin vivo is more challenging. PMB is nephrotoxic and cannot be used in animal studies (Falagas and Kasiakou2006). To overcome this, we used an isogenicmsbB mutant of the GFP expressing CFT073 strain LT004, LT004 ΔmsbB (Table1), which produces a penta-acylated lipid A that is poorly recognized by the TLR4 signalling complex (Somervilleet al.1996; Backhedet al.2003). Epithelial infectionin vitro with LT004 ΔmsbB for 4 h showed lower IL-8 (Fig. 2F) and TF activation (Fig. 2G) in stimulated endothelial cells, as compared to its CFT073 counterpart (Fig. 2A,2E). In agreement with the PMB results, neither of these reductions were complete abrogation (Fig. 2F, G), with LT004 ΔmsbB epithelial infection still demonstrating a significant effect on endothelial TF activation. This confirmed the reduced inflammatory profile of LT004 ΔmsbB and a reduced, though not eliminated, ability of this strain to induce TF activationin vitro.
To test the role of lipid A in infection-mediated coagulationin vivo LT004 ΔmsbB was introduced into our renal infection model (Melicanet al.2008). LT004 ΔmsbB was microinfused (105 CFU in 40μl over 10 min) into a single proximal tubule of an exposed kidney in a live rat and the progress of infection followed by intravital multiphoton microscopy. The LT004 ΔmsbB strain (green in Fig. 3A) adhered to and colonized the renal epithelium (Fig. 3A, 2 h – 8 h). Within 4–5 h, the bacteria filled the tubular lumen in the majority of infected tubules (Fig. 3A, 4–5.5 h). Bacterial adherence and colonization patterns were comparable to that previously described for the LT004 (Manssonet al.2007; Melicanet al.2008). The first signs of vascular perturbation and coagulation (assessed by the red plasma marker in Fig. 3A) were seen approximately 6.5 h after infection (Fig. 3A, 6.5 h arrow). This represented a delay compared to the 4–5 h time frame seen in LT004 infection (Manssonet al.2007; Melicanet al.2008). To measure the velocity of red blood cells (RBC) in the peritubular capillaries, line scanning (Kleinfeldet al.1998) was performed (Fig. 3B-D). A peritubular capillary adjacent to the infected tubule was scanned along its central axis at 2ms per line for 4 s (Fig. 3B dotted line). Individual RBC velocity was determined by calculating the distance it travelled through the vessel over time (Fig. 3C). Line scanning demonstrated a gradual slowing in RBC flow following LT004 ΔmsbB infection (Fig. 3D). In wild-type LT004 infections the RBC rate has been shown to drop to 0 μm/sec by 4.5 h (Melicanet al.2008). This data confirmed a slowing of infection-mediated coagulation kinetics in response to the penta-acylated lipid A of LT004 ΔmsbB, but not complete abrogation of coagulation. This demonstrates a role for lipid A mediated signaling in modulating the kinetics of infection-mediated coagulationin vivo.
Host epithelial signaling factors induce endothelial tissue factor activation.
The acylation state of lipid A can now join α-hemolysin as bacterial factors affecting the kinetics of infection-mediated coagulationin vivo, however, neither of these bacterial factors act in isolation (Amori, Lau and Pittas2007; Melicanet al.2008). Elimination of either of these factors is not enough to completely abrogate infection-mediated coagulation, but rather delay its onset. Our data also indicated that other factors may be involved in this response as themsbB mutation could not completely eliminate the TF activation response (Fig. 2G). Our hypothesis is thatin vivo the expression of these bacterial virulence factors affects the epithelial response to infection rather than acting directly on the endothelium as occurs in thein vitro models.
We therefore screened for candidate host derived molecules involved in infection- mediated coagulation by performing a proteome array study on supernatant from RPTEC/TERT1 cells infected with the wild-type CFT073 strain. The array covered 106 human cytokines (see methods). Following 4 h of epithelial infection we could detect signal for 15 proteins (Fig. 4A). Of these, ICAM-1, CD26 and CD147 were significantly up-regulated compared to non-infected controls. ICAM-1 up-regulation in UPEC infection has already been reported, and is believed to be involved in neutrophil recruitment (Manssonet al.2007). CD26 (also known as DPPIV) and CD147 (also known as EMMPRIN) represent new candidate proteins in epithelial UPEC infection.
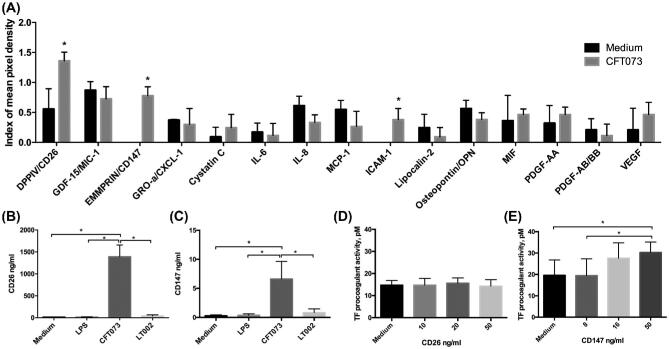
Figure 4.
Epithelial signaling factors induce endothelial TF activation. (A) Proteome array of supernatant of epithelial cells infected with CFT073 for 4 h. Supernatant from uninfected cells was used as the comparative control. Bars represent index of mean pixel density from the nitrocellulose membranes,n = 3. (B) CD26 and (C) CD147 expression in the supernatant of RPTEC/TERT1 cells stimulated for 4 h with cell culture medium, 5 mg/ml LPS or infected at an MOI 1:20 with CFT073 or LT002. Measurements were performed by ELISA,n = 5. (D) TF pro-coagulant activity, pM, measured using chromogenic assays in endothelial cells treated with the noted concentrations of (D) CD26, or (E) CD147,n = 3. All bars represent mean ± SD.
To confirm the infection-induced production of CD26 and CD147 we performed ELISA on supernatant of RPTEC/TERT1 cells infected with CFT073 for 4 h. Infection induced significant levels of both CD26 and CD147 in epithelial supernatant (Fig. 4B,C). As we had seen that LPS played a role in infection kinetics, we tested whether LPS alone could stimulate CD26 and CD147. Stimulation of epithelial cells with purified LPS (5mg/ml) for 4 h did not induce release of either CD26 or CD147 into the supernatant (Fig. 4B, C), demonstrating a limited role for the Lipid A pathway in this response. To evaluate the role of α-hemolysin in this signaling, we tested supernatant from epithelial cells infected with the α-hemolysin deficient LT002 strain. No CD26 or CD147 was released from epithelial cells infected with LT002 (Fig. 4B, C). This suggests that their release appears to be specific to infection with the wild-type CFT073 bacteria, the strain that shows the most rapid coagulatory responsein vivo (Manssonet al.2007).
To investigate whether CD26 and CD147 may play a direct role in infection-mediated coagulation caused by CFT073, we stimulated HMEC-1 endothelial cells with purified CD26 and CD147 and tested for TF activation. Concentrations of active human CD26 from 10–50 ng/ml showed no significant up-regulation of endothelial TF activation following 4 h of stimulation (Fig. 4E). CD147, however, revealed an increase in endothelial TF activation at 4 h at higher concentrations (50mg/ml) (Fig. 4E). This data implies that high local concentrations of CD147, expressed by CFT073 infected epithelia, could play a role in epithelial-endothelial signaling and the initiation of infection-mediated coagulation during renal infection. This suggests CD147 as a possible host-derived factor in infection-mediated coagulation.
DISCUSSION
This work highlights two new players in host-pathogen cross-talk that influences local infection-mediated coagulation: (1) the acylation state of bacterial LPS and (2) host derived CD147. The importance of infection-mediated coagulation has already been shown; it plays a protective role in renal UPEC infection, preventing the transition to urosepsis (Melicanet al.2008). Our present work adds new knowledge by showing that both bacterial virulence determinants and host cell factors are involved in the activation of TF.
The role of Lipid A and effect of themsbB mutation on TLR4 mediated IL-8 production has been reported previously (Backhedet al.2001) but the exclusivity of this interaction in UPEC urinary tract infection has been less clear (Hedlundet al.1999; Backhedet al.2003). Ambiguity in previous results using the common renal epithelial cell line A498 (Backhedet al.2001; Demirelet al.2015), led us to seek out an alternativein vitro model that more closely represented the morphology of thein vivo microenvironment. The RPTEC/TERT1 cell line originates from a healthy male donor and is immortalized by telomerase overexpression (Wieseret al.2008). This line is being extensively used as a model for nephrotoxicity (Simon-Friedtet al.2015). Our data demonstrated that this cell line did respond to UPEC infection. The lack of IL-6 and IL-8 signaling in response to the hemolysin expressing CFT073 strain suggests that hemolysin may influence the regulation of pro-inflammatory signaling, as has been well described in bladder epithelium (Dhakal and Mulvey2012; Hilbertet al.2012) and even for the non-relatedStaphylococcus aureus beta-hemolysin toxin (Tajimaet al.2009).
Antagonizing Lipid A mediated signalingin vitro with polymyxin B demonstrated a significant role for this pathway in the activation of the endothelial cell line. A drawback of ourin vitro model is that the bacterial products come into direct contact with the endothelia, a situation we do not believe represents thein vivo situation. We see coagulation occurringin vivo when bacteria and seemingly their products are still maintained at the epithelial barrier (Melicanet al.2008; Melicanet al.2011).In vivo infection with the penta-acylated lipid A UPEC strain demonstrated slower kinetics, though not elimination, of coagulation as compared with the WT strain. While a strain may have a less immunogenic LPS, it can still express α-hemolysin, and, as each of these factors appears to play a role in the onset of coagulation, the result is a nuanced change in host response kinetics, not an on-or-off switch. LPS plays an enormous role in the pathogenesis of many gram-negative infections, our data suggests a role for LPS Lipid A triggered cell-cell communication as a factor in renal coagulation following tubule infection.
Our data also introduces CD147 as the first host epithelial factor to be directly implicated in infection-mediated coagulation. CD147, also known as EMMPRIN and Basigin (Grass and Toole2015), is perhaps best recognized for its role in cancer, where it acts to regulate the expression of VEGF and matrix metalloproteinase, contributing to malignancies (Grass and Toole2015). Targeting of CD147 has been suggested as a novel cancer treatment strategy (Haoet al.2010). In terms of bacterial infection, CD147 has been implicated as an adhesion factor forNeisseria meningitidis (Bernardet al.2014) andListeria monocytogenes (Tillet al.2008). We show here that CD147 may play a role in infectious cell signaling and the initiation of local coagulation during renal infection. In support of our findings, CD147 has been shown to be released from epithelial cells in vesicles and to alter the angiogenic state of endothelial cells (Millimaggiet al.2007). We show that epithelial cells infected with the α-hemolysin expressing CFT073 strain secrete CD147. CD147 may be an effector explaining the enhancedin vivo kinetics of infection-mediated coagulation for the wild-type UPEC vs the α-hemolysin deficient LT005 strain (Manssonet al.2007). Further investigation of the role of CD147 during renal infectionin vivo is of huge interest and the focus of ongoing work. Human CD147 however exhibits only 65% homology with the rodent protein (Watanabeet al.2010), makingin vivo experimentation challenging at this point.
While CD26, also known as dipeptidyl peptidase-4 (DPP4), did not show a direct action on TF in our study, its high expression during epithelial infection is also of interest. The DPP4 inhibitor sitagliptin, an oral glucose-lowering agent, has been shown to inhibit platelet aggregation (Guptaet al.2012). Clinical trials with sitagliptin also demonstrated an increased risk for urinary tract infections (Amori, Lau and Pittas2007). This suggests that CD26 may play a role in infection-mediated coagulation, just not via TF activation as measured here.
Our work has revealed roles for bacterial LPS lipid A and host CD147 in the modulation of infection-mediated coagulation kinetics. The picture that is emerging highlights the complexity, cross talk and dynamics of renal infection. Understanding this cross-talk will be important to evaluate the possible detrimental influence that seemingly unrelated treatments such as anti-coagulation, cancer treatment (anti-CD147) and diabetes treatment (anti-CD26) may have on infection progression in UTI. A single molecular mechanism cannot control the multifaceted tissue response to bacterial infection; evolution has ensured that multiple pathways are involved, ensuring that fail-safes are in place to prevent bacterial spread and life-threatening sepsis. Learning to harness and enhance this host response is an attractive possibility in the search for new treatments in the post-antibiotic era.
Supplementary Material
Acknowledgements
The authors would like to thank Dr SB Campos for valuable surgical assistance. Live Imaging data was generated at the Indiana Center for Biological Microscopy.
Author Contributions: Conceptualization, A.R.D and K.M; Methodology, R.M.S.; B.A.M; G.A.T; A.R.D.; O.D.C.; H.A.; A.S.; and K.M. Investigation, A.S.; O.D.C.; H.A.; S.E.S.; G.A.T.; R.M.S and K.M. Writing—Original Draft, K.M.; Writing—Review & Editing, All authors. Funding Acquisition, B.A.M.; A.R.D and K.M.; Resources, G.A.T.; B.A.M. and A.R.D.; Supervision; A.R.D. and K.M.
FUNDING
The work was supported by: Stiftelsen för Strategisk Forskning (SSF), Vetenskåpsrådet, Carl Bennet AB, Vinnova, Familjen Erling-Perssons Stiftelse (ARD); National Institute of Health (NIH) PO1 DK-53 465 and DK069408 (BAM), Jeanssons Stiftelse, Åke Wiberg Stiftelse, Clas Groschinskys Stiftelse and Karolinska Institutet (KM). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The authors declare no conflicts of interest.
Conflict of interest. None declared.
REFERENCES
- Amori RE,Lau J,Pittas AG. Efficacy and Safety of Incretin Therapy in Type 2 Diabetes.JAMA 2007;298:194–206. [DOI] [PubMed] [Google Scholar]
- Backhed F,Soderhall M,Ekman P et al. Induction of innate immune responses by Escherichia coli and purified lipopolysaccharide correlate with organ- and cell-specific expression of Toll-like receptors within the human urinary tract.Cell Microbiol 2001;3:153–8. [DOI] [PubMed] [Google Scholar]
- Backhed F,Normark S,Schweda EK et al. Structural requirements for TLR4-mediated LPS signalling: a biological role for LPS modifications.Microbes Infect 2003;5:1057–63. [DOI] [PubMed] [Google Scholar]
- Bernard SC,Simpson N,Join-Lambert O et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization.Nat Med 2014;20:725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA,Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products.Proc Natl Acad Sci 2000;97:6640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RP,Miller-Dorey S,Jenne CN. Platelets and coagulation in infection.Clin Trans Immunol 2016;5:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirel I,Kruse R,Onnberg A et al. Ceftibuten-induced filamentation of extended spectrum beta lactamase (ESBL)-producing uropathogenic Escherichia coli alters host cell responses during an in vitro infection.Microb Pathog 2015;78:52–62. [DOI] [PubMed] [Google Scholar]
- Dhakal BK,Mulvey MA. The UPEC pore-forming toxin alpha-hemolysin triggers proteolysis of host proteins to disrupt cell adhesion, inflammatory, and survival pathways.Cell Host Microbe 2012;11:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME,Kasiakou SK. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies.Crit Care 2006;10:R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass GD,Toole BP. How, with whom and when: an overview of CD147-mediated regulatory networks influencing matrix metalloproteinase activity.Biosci Rep 2016;36:e00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK,Verma AK,Kailashiya J et al. Sitagliptin: anti-platelet effect in diabetes and healthy volunteers.Platelets 2012;23:565–70. [DOI] [PubMed] [Google Scholar]
- Hao JL,Cozzi PJ,Khatri A et al. CD147/EMMPRIN and CD44 are potential therapeutic targets for metastatic prostate cancer.Curr Cancer Drug Targets 2010;10:287–306. [DOI] [PubMed] [Google Scholar]
- Hedlund M,Wachtler C,Johansson E et al. P fimbriae-dependent, lipopolysaccharide-independent activation of epithelial cytokine responses.Mol Microbiol 1999;33:693–703. [DOI] [PubMed] [Google Scholar]
- Hilbert DW,Paulish-Miller TE,Tan CK et al. Clinical Escherichia coli isolates utilize alpha-hemolysin to inhibit in vitro epithelial cytokine production.Microbes Infect 2012;14:628–38. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D,Mitra PP,Helmchen F et al. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex.Proc Natl Acad Sci U S A 1998;95:15741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansson LE,Melican K,Boekel J et al. Real-time studies of the progression of bacterial infections and immediate tissue responses in live animals.Cell Microbiol 2007;9:413–24. [DOI] [PubMed] [Google Scholar]
- Mattsby-Baltzer I,Lindgren K,Lindholm B et al. Endotoxin shedding by enterobacteria: free and cell-bound endotoxin differ in Limulus activity.Infect Immun 1991;59:689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B,Davis RP,Kim S-J et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice.Blood 2017;129:1357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melican K,Sandoval RM,Kader A et al. Uropathogenic Escherichia coli P and Type 1 fimbriae act in synergy in a living host to facilitate renal colonization leading to nephron obstruction.PLoS Pathog 2011;7:e1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melican K,Boekel J,Mansson LE et al. Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis.Cell Microbiol 2008;10:1987–98. [DOI] [PubMed] [Google Scholar]
- Millimaggi D,Mari M,D’Ascenzo S et al. Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells.Neoplasia 2007;9:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley HL,Green DM,Trifillis AL et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains.Infect Immun 1990;58:1281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peetermans M,Vanassche T,Liesenborghs L et al. Bacterial pathogens activate plasminogen to breach tissue barriers and escape from innate immunity.Crit Rev Microbiol 2016;42:866–82. [DOI] [PubMed] [Google Scholar]
- Ristow LC,Welch RA. Hemolysin of uropathogenic Escherichia coli: A cloak or a dagger?Biochim Biophys Acta 2016;1858:538–45. [DOI] [PubMed] [Google Scholar]
- Secker PF,Luks L,Schlichenmaier N et al. RPTEC/TERT1 cells form highly differentiated tubules when cultured in a 3D matrix.ALTEX 2018;35:223–34. [DOI] [PubMed] [Google Scholar]
- Simon-Friedt BR,Wilson MJ,Blake DA et al. The RPTEC/TERT1 cell line as an improved tool orin vitro Nephrotoxicity assessments.Biol Trace Elem Res 2015;166:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville JE Jr.,Cassiano L,Bainbridge B et al. A novel Escherichia coli lipid A mutant that produces an anti-inflammatory lipopolysaccharide.J Clin Invest 1996;97:359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima A,Iwase T,Shinji H et al. Inhibition of endothelial interleukin-8 production and neutrophil transmigration by Staphylococcus aureus beta-hemolysin.Infect Immun 2009;77:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till A,Rosenstiel P,Brautigam K et al. A role for membrane-bound CD147 in NOD2-mediated recognition of bacterial cytoinvasion.J Cell Sci 2008;121:487–95. [DOI] [PubMed] [Google Scholar]
- Uhlen P,Laestadius A,Jahnukainen T et al. Alpha-Haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells.Nature 2000;405:694–7. [DOI] [PubMed] [Google Scholar]
- Watanabe A,Yoneda M,Ikeda F et al. CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells.J Virol 2010;84:4183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser M,Stadler G,Jennings P et al. hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics.Am J Physiol Renal Physiol 2008;295:F1365–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.