Abstract
Communication between the nervous and immune systems is required for the body to regulate physiological homeostasis. Beta-adrenergic receptors expressed on immune cells mediate the modulation of immune response by neural activity. Activation of Beta-adrenergic signaling results in suppression of anti-tumor immune response and limits the efficacy of cancer immunotherapy. Beta-adrenergic signaling is also involved in regulation of hematopoietic reconstitution, which is critical to Graft-versus-Tumor (GvT) effect and Graft-versus-Host disease (GvHD) following allogeneic hematopoietic cell transplantation (HCT). In this review, the function of Beta-adrenergic signaling in mediating tumor immunosuppression will be highlighted. We will also discuss the implication of targeting Beta-adrenergic signaling to improve the efficacy of cancer immunotherapy including the GvT effect, and to diminish the adverse effects including GvHD.
Keywords: Cancer immunotherapy, beta-adrenergic receptor, graft-versus-host disease, graft-versus-tumor effect, allogeneic hematopoietic cell transplantation
I. Introduction
Cancer immunotherapy is a new modality of therapies that exploit the immune system to recognize and destroy cancer. It is based on improved understanding of the intricate cellular and molecular mechanisms controlling immune responses and a growing explication of oncology including the discovery of mutation instigated neoantigens.1 Generally, cancer immunotherapy has been categorized as active immunotherapy, which boosts activation of the immune system to attack cancer cells, such as tumor vaccines2–3 and dendritic cell (DC) based-immunotherapy,4 and passive immunotherapy, which harnesses existing components of immune responses that include using monoclonal antibodies,5–7 cytokines,8 and adoptive transfer of T cells9–10 and natural killer (NK) cells11–12 to treat cancer patients. Most recently immune checkpoint inhibitors, which are monoclonal antibodies against the inhibitory signaling pathways of programmed cell death protein 1 (PD-1) and cytotoxic T lymphocytes antigen 4 (CTLA-4), have shown promising efficacy in clinical trials of a broad range of solid and hematological cancers, and therefore led to FDA approvals for clinical use to treat a rapidly rising list of cancers.13–14 On the other hand, genetically engineered T lymphocytes that express chimeric antigen receptors (CARs) or T cell receptors (TCRs) have emerged as another effective approach of caner immunotherapy.15 In 2017, two of CAR-T cell therapies, marked as KYMRIAH and YESCARTA, have been approved by FDA for treatment of certain types of B-cell precursor acute lymphoblastic leukemia and certain types of large B-cell lymphoma and non-Hodgkin lymphoma. Furthermore, personalized therapeutic cancer vaccines have also been demonstrated as a potent treatment strategy for cancer patients recently.16–18 However, there are still limitations in these immunotherapies. Both CAR-T cell therapy and immune checkpoint blockade therapy are restricted to certain types of cancer and a small population of cancer patients.19–20 Resistance to immune checkpoint blockade and CD19-targeted CAR-T cell therapy was also found in cancer patients.21–22 Severe and even lethal adverse side effects have recently been reported in many patients treated with PD-1 and CTLA-4 blockade.23–24 For example, severe diarrhea, colitis, increased alanine aminotransferase levels, inflammation pneumonitis, and interstitial nephritis have been reported in patients with various type of cancer.25–27 Therefore, to improve the efficacy and safety of cancer immunotherapy, enormous and challenging research work still needs to be performed to further elucidate the complex mechanisms underlying the dysfunction of immune response to cancer, which includes immunosuppression in tumor microenvironment, host systemic immunosuppression, tumor heterogeneity including gene mutations and neoantigens in tumor cells.
The regulation of immunity by nervous system has been identified in various conditions including inflammatory and autoimmune diseases.28–32 Activated neurons release neurotransmitters and other regulatory molecules, which engage corresponding receptors, including adrenergic receptors expressed on macrophages, dendritic cells, T cells and other immune cells, and facilitate neural regulation of immune responses.33–35 In the tumor context, nervous system is also involved in the tumor microenvironment.36–37 In addition to directly promoting tumor cell growth, invasion and tumor angiogenesis,38–39 neuronal input also mediates immunosuppression in the tumor microenvironment.36, 40 Furthermore, sympathetic nervous system (SNS) is also involved in regulation of hematopoiesis.41 Here, we summarize the mechanistic insights in the suppressive role of Beta-adrenergic signaling in immune response to cancer and highlight the implication of Beta-adrenergic signaling blockade in the improvement of cancer immunotherapy. The function of Beta-adrenergic signaling in GvT effect and GvHD will also be discussed.
II. Beta-adrenergic signaling in tumor immunosuppression
Several studies have shown that sympathetic nervous system (SNS) activation, which stimulates Beta-adrenergic signaling, is able to regulate cancer cell metastasis and tumor growth by recruiting macrophages into tumor parenchyma.42 In a restraint mouse model, chronic stress was able to enhance mammary adenocarcinoma cell metastasis to distant tissues including lung and lymph node but had a negligible effect on the growth of the primary tumor.42 The stress-enhanced metastasis was not depending on T lymphocytes, but mediated by promoting infiltration of macrophages, which could induce expression of prometastatic gene such as Tgfb, Arg1 and Csf1, in primary tumor microenvironment.42 The Beta-adrenergic antagonist propranolol was able to largely abrogate the stress-induced increase of macrophage infiltration, suggesting that Beta-adrenergic signaling is involved in stress-mediated promotion of tumor metastasis. In another restraint stress mouse model, activation of Beta-adrenergic signaling led to ovarian carcinoma growth by promoting monocytes and macrophages infiltration into tumor tissue. These myeloid cells were recruited by stress-enhanced production of monocyte chemotactic protein 1 from ovarian cancer cells.43 A study analyzing tumors from socially isolated patients demonstrated upregulated expression of genes involved in M2 macrophage polarization and epithelial-mesenchymal transition (EMT) as well as increased density of lymphatic vessels.44 Activation of CREB (cAMP response element-binding protein) family transcription factors, which mediate the gene regulatory effects of Beta-adrenergic signaling, was also shown by a TELiS promoter-based bioinformatics analyses.44 Bioinformatics analysis of Beta-adrenergic signaling stimulated macrophages showed a transcriptome that locates on the M2 side of the M1-M2 spectrum, but not fit entirely into any pre-defined category of M1 or M2 spectrum.45 Beta2-adrenergic receptor activation by social isolation-induced stress also promoted 4T1 breast cancer progression by upregulating macrophage number and enhancing the M2 polarization in the tumor microenvironment.46 Given the critical role of macrophages in remodeling tumor microenvironment and facilitating angiogenesis and extracellular matrix breakdown to enhance primary tumor progression and tumor metastasis,47–49 blockade of macrophage infiltration into tumor microenvironment implies an important strategy for improving cancer immunotherapy.
Beta-adrenergic signaling was also demonstrated to mediate the suppression of anti-tumor immune response. In murine studies, a striking decrease of tumor formation, growth, and metastasis in B16-F10, 4T1, CT26 and pan02 tumor models was observed in mice housed at thermoneutral temperature compared to mildly cold temperature that stimulates Beta2-adrenergic signaling. The enhanced control of tumor growth was dependent on the increased numbers of antigen-specific CD8+ T cells and effector phenotype of the CD8+ T cells in the tumor microenvironment and a significant reduction of immunosuppressive myeloid-derive suppressive cells (MDSCs) and regulatory T cells in the spleen.50 Chronic cold induced a stress response that caused tumor immunosuppression mediated by Beta2-adrenergic signaling in host immune cells.51 Using physiologic, pharmacologic and genetic blockade of Beta2-adrenergic signaling, the authors demonstrated that reduction of Beta2-adrenergic signaling facilitated an increased frequency of effector CD8+ T cells and an elevated effector ratio of CD8+ T cells to CD4+ regulatory T cells, and a decreased frequency of PD-1 expressing CD8+ T cells in the B16 tumor microenvironment.52 The accumulation of MDSCs in the spleen of 4T1 tumor-bearing mice was also decreased by blockade of Beta2-adrenergic signaling.52 In a B-cell lymphoma mouse model, chronic elevated Beta-adrenergic signaling resulted in less effective control of lymphoma growth, which was mediated by the reduced proliferation, decreased production of IFN-γ and cytotoxic capacity of antigen-specific CD8+ T cells.53 The suppressive effect on anti-lymphoma response by chronic Beta-adrenergic signaling was shown to be selective to T cells and independent of innate lymphocyte response to an experimental NKT cell-targeting vaccine.53 In a triple negative breast cancer mouse model, social isolation-induced stress promoted 4T1 tumor progression and caused a reduction of survival in tumor-bearing mice, which was mediated by reducing CD8+ and CD3+CD69+ T cells in the spleen.54 Acute and chronic restraint stress also increased the gene expression of granzyme B, a serine protease in cytotoxic T cells and NK cells and CXCL 10, a T cell chemoattractant, in the tumor microenvironment, which were reduced by propranolol, a Beta-adrenergic receptor antagonist.54 Beta-adrenergic signaling was involved in suppression of antitumor cytotoxic T cell generation by inhibiting Tumor necrosis factor-α (TNF-α) gene expression.55 Recently, a study demonstrated that Beta-adrenergic signaling inhibited CD8+ T cells activation by suppressing the required metabolic reprogramming.56 Activation of the reward system, which is the dopaminergic neurons in the ventral tegmental area that constitutes a key neuronal network mediating positive emotions, expectations and motivation,57 improved the control of B16 melanoma and Lewis lung carcinoma growth in mice, manifested by reduced noradrenergic input to the bone marrow, attenuated the immunosuppressive effect of MDSCs and enhanced Granzyme B expression by CD8+ T cells in the tumor.58 Similar to the effect of the decreased noradrenergic input, treatment with propranolol, a Beta-adrenergic receptor antagonist, delayed primary tumor growth and development of metastasis in a murine melanoma model, which was mediated by suppressing the infiltration of myeloid cells and prompting cytotoxic lymphocytes including NK and CD8+ T cells into the primary and metastatic tumor.59
The function of Beta1 and Beta3-adrenergic signaling in immunity still need to be investigated. There are very few reports reveal the role of Beta1 and Beta3-adrenergic receptors in modulating immune response. The Beta1-adrenergic receptor was demonstrated mediating acute cold/restraint stress inhibition of host resistance to Listeria monocytogenes by modifying T cells activation or subsequent T cell function involved in adaptive immunity60 and by suppressing cellular immune response.61
Chronic restraint stress was also able to impair antitumor T cell response, which was mediated by thyroid hormones instead of noradrenaline and corticosterone.62 Fluoxetine, an anti-depressant for cancer patients, was shown to inhibit lymphoma growth by modulating antitumor immunity, specifically by enhancing mitogen-induced T-cell proliferation and expression of Tumor Necrosis Factor-α (TNF-α) and Interferon-γ (IFN-γ).63–64 These studies imply other mechanisms, working in parallel to or in concert with Beta-adrenergic signaling, are also involved in the stress-induced suppression of antitumor immunity.
III. Blockade of Beta-adrenergic signaling in cancer immunotherapy
Given the critical role of Beta-adrenergic signaling in suppressing antitumor immune response and regulating immune cells in the tumor microenvironment, blockade of the Beta-adrenergic signaling has broad implication for improving cancer immunotherapy. Indeed, pan-Beta-adrenergic receptor blocker propranolol was able to reverse the immunosuppression caused by cold induced stress, resulting in increased antitumor effector T cells and improved anti-PD-1 efficacy.52 A retrospective analysis showed that Beta-adrenergic blocker improved overall survival of metastatic melanoma patients who received immunotherapy (IL-2, anti-CTLA4, or anti-PD1).65 This enhanced immunotherapy was further reinforced in a preclinical murine melanoma model showing that Beta-adrenergic blocker combined with anti-PD-1 blockade led to a better control of melanoma growth.65
Blockade of Beta-adrenergic signaling was also shown to reduce the immunosuppression caused by surgical excision of primary tumor and may improve immunotherapy when combined with surgery.66 Catecholamine and prostaglandin, which are produced abundantly by tumor cells and stromal cells in the tumor microenvironment and host physiologic systems due to tissue trauma of surgery and perioperative stress, are involved in promoting cancer metastasis following tumor surgical resection by suppressing cellular immune response, increasing prometastatic cytokines and inducing inflammation. Therefore, pharmacologic inhibition of Beta-adrenergic receptor signaling and/or prostaglandin synthesis is able to reduce the prometastatic and immunosuppressive effects of tumor surgery and physiologic stress in clinical trials and preclinical studies.67 NK cells play an important anti-metastasis role that was suppressed by activation of Beta-adrenergic signaling in animal tumor models,68–73 whereas this function was able to be recovered by Beta-adrenergic antagonist.74–75 Recently, administration of the Beta-adrenergic antagonist propranolol and the COX-2 inhibitor etodolac led to multiple favorable effects in a phase-2 randomized trial of breast cancer patients.76–77 In addition to reducing epithelial-mesenchymal transition (EMT) and decreasing the activity of proinflammatory and prometastatic transcription factors (GATA-1, GATA-2 and STAT-3) and Ki-67 in tumor cells, Beta-adrenergic antagonist treatment suppressed monocytes while promoted B cells infiltrating into the tumor, abrogated postoperative mobilization of CD16− ‘classical’ monocytes and enhanced CD11a expression on circulating NK cells.76–77 In animal studies, adrenergic nerve input was also shown to control lymphocyte egress from lymph nodes (LNs), but not entry to, through Beta2-adrenergic receptor.78–79 The retention of lymphocytes in LNs was mediated by physical interaction between Beta2-adrenergic receptors and chemokine receptors CCR7 and CXCR4, and consequently inhibited antigen-primed T cell migration into peripheral tissues in an experimental autoimmune encephalomyelitis mouse model78 and enhanced humoral immune response in the LNs.79 Therefore, using Beta2-adrenergic blockade to enhance lymphocyte mobilization has substantial significance because infiltration of effector immune cells into tumor microenvironment is critical for improving cancer immunotherapy inlcluding CAT-T cell therapy80–82 and immune checkpoint blockade therapy.83–84
IV. Beta-adrenergic signaling in Graft-vs-Tumor effect and Graft-vs-Host disease
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative adoptive immunotherapy for a variety of hematologic malignancies.85 Allogenic HCT is a complicated treatment because of its incorporation of major elements of immunology, oncology, radiobiology and infectious disease. A successfully allogeneic HCT largely depends on GvT effect, which is typically attributed to donor T cells and also likely involves a complex interaction of multiple cell types and cytokines.86–87 However, the GvT effect is closely connected with GvHD, which is caused by the mismatched major and minor histocompatibility antigens expressed between host and donor.88–89
Recently, several studies demonstrated that Beta-adrenergic signaling is involved in the GvT effect and GvHD. In a mouse allogeneic HCT study, host Beta2-adrenergic signaling was shown to inhibit GvT activity by suppressing donor T cell reconstitution.90 Blockade of Beta2-adrenergic signaling was able to induce increased CD11c+ DC development and render DC more immunogenic, thereby improving donor T cell reconstitution.90 The improved reconstitution of T cells, including CD8+, CD4+, and CD4+Foxp3+ regulatory T cells led to an enhanced GvT effect without increasing GvHD. In another study, GvHD was suppressed by cold temperature-induced stress through Beta2-adrenergic signaling.91 Host-derived, but not donor T cell-derived, Beta2-adrenergic receptor signaling is essential for inhibiting GvHD.91 Through MLR-based in vitro T cell activation and tumor cell-killing experiments, it was demonstrated that Beta2-adrenergic receptor signaling deficiency in DCs enhances the alloreactive CD8+ T cell response in the tumor setting. However, the other study showed that Beta2-adrenergic receptor inhibition exacerbated GvHD induced by total T cells, in which CD4+ T cells presumably play a dominant role. Overall, these two studies may appear to contradict each other. However, these findings suggest that Beta2-adrenergic signaling plays more complicated and differential roles in GvT and GvHD involving CD4+ versus CD8+ T cell responses and possibly other immune cells including DCs and MDSCs. Further studies will be required to delineate the underlying mechanisms.
After performing total body irradiation and allogeneic HCT, the recovery of hematopoietic homeostasis is critical to the generation of GvT and GvHD. Hematopoietic stem cells (HSCs) are the only cell type in the bone marrow (BM) that is able to differentiate to all blood cell lineages, and their behavior is tightly regulated by the HSC niche in the BM microenvironment.92–93 The function and regenerative capacity of HSC can be diminished by the damaging effects of radiation injury or chemotherapy, and their recovery to homeostasis is highly dependent on the BM microenvironment.94–95 Neural regulation of hematopoiesis has been reported recently.41 The sympathetic nervous system is a critical regulatory component of the BM microenvironment, and sympathetic nerve fibers and neural crest-derived cells serve as major niche constituents and are essential to maintain HSC homeostasis and restore normal function from stress.96–97 Sympathetic neuronal activation was revealed to promote HSC proliferation by modulating Beta-adrenergic signaling. Starting from the observation of increased leukocytes, including neutrophils, monocytes and lymphocytes in patient blood, the authors further found that chronic variable stress activated proliferation of primitive hematopoietic progenitors, and led to decreased CXCL12 levels through Beta3-adrenergic signaling in BM niche cells, which was signaled by surplus noradrenaline released from nerve fibers.98 Most recently, myeloid cell numbers in blood of diabetic patients were shown strongly correlated with concentration of norepinephrine in the plasma.99 Consistent with the observation in diabetic patients, myeloid cell number was increased in blood and spleen of experimental diabetic mice, in which proliferation and differentiation of splenic granulocyte macrophage progenitors (GMPs) are activated by sympathetic neuronal activity through Beta2-adrenergic receptors expressed on the splenic granulocyte macrophage progenitors (GMPs).99 Beta-adrenergic signaling also plays an important role in modulating mobilization of HSCs from BM. In UDP-galactose ceramide galactosyltransferase-deficient mice, which exhibit aberrant nerve conduction, hematopoietic stem and progenitor cell (HSPC) egress from BM was significantly decreased when induced by granulocyte colony-stimulating factor (G-CSF).96 Specifically, Beta2-adrenergic receptor signaling is required for granulocyte colony-stimulating factor (G-CSF)-induced HSPC mobilization, osteoblast suppression and bone CXCL12 downregulation.96 Further study also showed that cooperation of Beta2- and Beta3-adrenergic receptor signaling is essential for granulocyte colony-stimulating factor (G-CSF)-induced HSPC egress from BM.100 The recruitment of leukocytes to tissues under homeostasis exhibited circadian oscillation, which is orchestrated by molecular clock via adrenergic nerves.101 Beta2- and Beta3-adrenergic signaling is impaired in sympathetic denervation that causes decreased expression of HSC-regulating genes in osteoblasts,93, 95 which is an important HSPC niche and mediator of HSPC mobilization.102
V. Conclusion
Effective immunotherapy is not only dependent on activating antitumor immune response, but also needs to reverse tumor-induced immunosuppression. Beta-adrenergic receptors expressed on immune cells engage with neurotransmitters released by sympathetic nervous system and mediate immunosuppression in the tumor microenvironment by recruiting tumor-associated macrophage and dampening cytotoxic T cells and NK cells (Fig. 1). Therefore, blockade of the Beta-adrenergic signaling implies a promising strategy to improve current immunotherapy.
Figure 1.
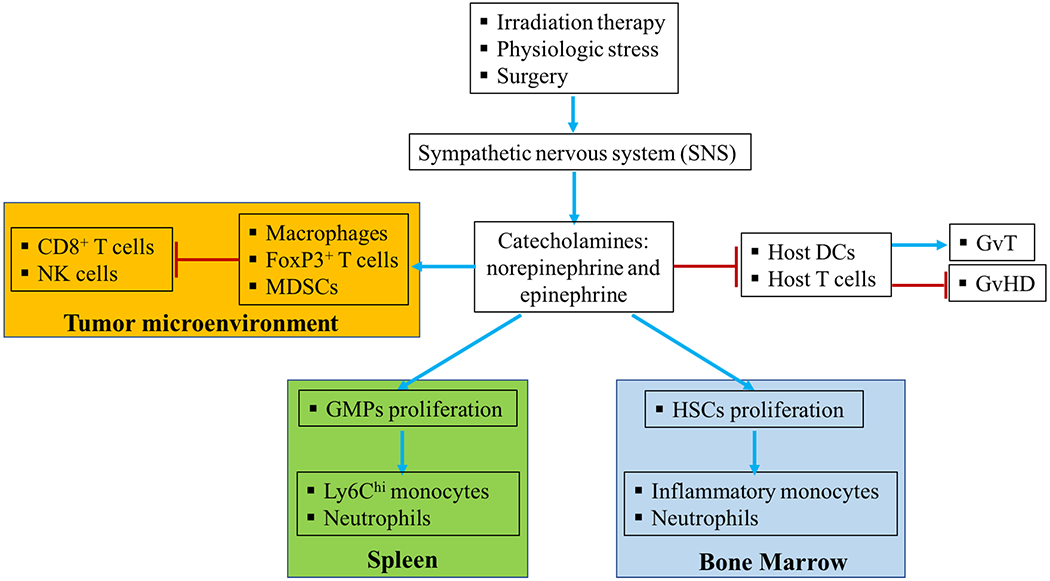
The complex role of Beta-adrenergic signaling in immunosuppression in tumor microenvironment and hematopoiesis. Stress caused by surgery and irradiation can activate sympathetic nervous system (SNS) and induce production of neurotransmitters including norepinephrine and epinephrine. These neurotransmitters engage Beta-adrenergic receptors expressed on various innate and adaptive immune cells and may differentially regulate their function. Beta-adrenergic signaling can also stimulate hematopoietic stem cells (HSCs) and granulocyte macrophage progenitors (GMPs) and promote their proliferation in bone marrow and spleen, respectively. The neurotransmitters can enter the tumor microenvironment and induce immunosuppressive cells including macrophages, FoxP3+ T cells and myeloid-derived suppressive cells (MDSCs) to suppress the function of cytotoxic CD8+ T cells and natural killer (NK) cells. Beta-adrenergic signaling may also be manipulated to improve the Graft-versus-Tumor effect (GvT) and inhibit Graft-versus Host Disease (GvHD) via modulating T cell reconstitution and dendritic cell (DC) function during allogeneic hematopoietic cell transplantation.
Given the limitation of current immunotherapies, combination immunotherapy or combination therapy between immunotherapy and chemotherapy, surgical therapy or radiotherapy, would be considered prospective treatment strategies for cancer patients in the near future.103–105 Immunotherapy combining with hematopoietic cell transplantation (HCT) also potentially provides synergistic effect for treating cancer patients.106–108 Physical and psychosocial stress caused by these treatment strategies is involved in promoting tumor progression and reducing life quality and overall survival of cancer patients.109–112 Beta-adrenergic signaling is one of the suppressive mechanisms induced by stress (Fig. 1). Tumor cells expressing Beta-adrenergic receptors are also able to be regulated by activated neural system and thereby increase tumorigenesis, angiogenesis, proliferation and metastasis.113 Indeed, some cancer patients are shown to benefit from taking the Beta-blocker propranolol.114–117 Propranolol has also been shown as beneficial for treating multiple myeloma patients during HCT.118 However, the complete mechanisms underlying the suppressive function of Beta-adrenergic signaling still need to be fully understood. As discussed in this review, combination immunotherapy with Beta-adrenergic signaling blockade would be promising treatment strategy for cancer patients.
Acknowledgments
This work attempts to summarize most studies of the beta-adrenergic signaling relevant to cancer immunology and immunotherapy. However, we apologize for not being able to be comprehensive in including all publications on this topic due to limitation of space. This work was supported by NIH Grant R21 CA202358 (to XC).
Abbreviations:
- GvHD
Graft-versus-Host disease
- GvT
Graft-versus-Tumor
- HCT
Hematopoietic cell transplantation
- PD-1
Programmed cell death protein 1
- CTLA-4
Cytotoxic T lymphocytes antigen 4
- MDSCs
Immunosuppressive myeloid-derive suppressive cells
- NK
Natural killer cell
- DC
Dendritic cell
- BM
Bone marrow
- HSPC
Hematopoietic stem and progenitor cell
References
- 1.Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nature Rev Cancer. 2017; 17(9): 569. [DOI] [PubMed] [Google Scholar]
- 2.Finn OJ. The dawn of vaccines for cancer prevention. Nature Rev Immunol. 2018;18(3):183–194. [DOI] [PubMed] [Google Scholar]
- 3.Wong KK, Li WA, Mooney DJ, Dranoff G. Advances in therapeutic cancer vaccines. Adv Immunol. 2016;130:191–249. [DOI] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010; 363 (5): 411–22. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015; 161(2): 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009; 373 (9668): 1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Rev Immunol. 2010;10(5): 317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dranoff G Cytokines in cancer pathogenesis and cancer therapy. Nature Rev Cancer. 2004;4 (1): 11–22. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science (New York, NY). 2015; 348 (6230): 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013; 39 (1): 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daher M, Rezvani K. Next generation natural killer cells for cancer immunotherapy: the promise of genetic engineering. Curr Op Immunol. 2018;51:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther. 2017; 25 (8): 1769–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteside TL, Demaria S, Rodriguez-Ruiz ME, Zarour HM, Melero I Emerging opportunities and challenges in cancer immunotherapy. Clin Cancer Res. 2016; 22 (8): 1845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, Lin Y. Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nature Rev Drug Discov. 2018; 17 (12): 854–855. [DOI] [PubMed] [Google Scholar]
- 15.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nature Rev Cancer. 2016; 16 (9): 566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Z, Ott PA, Wu CJ. Towards personalized tumour-specific therapeutic vaccines for cancer. Nature Rev Immunol. 2018;18 (3): 168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Muller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Bruck AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Holler C, Utikal J, Huber C, Loquai C, Tureci O. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017; 547 (7662): 222–226. [DOI] [PubMed] [Google Scholar]
- 18.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES Fritsch EF, Hacohen N, Wu CJ. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017; 547 (7662): 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin J, Lao CD, Urba WJ, McDermott DF, Horak C, Jiang J, Wolchok JD. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015; 1 (4): 433–40. [DOI] [PubMed] [Google Scholar]
- 20.Long GV, Weber JS, Larkin J, Atkinson V, Grob JJ, Schadendorf D, Dummer R, Robert C, Marquez-Rodas I, McNeil C, Schmidt H, Briscoe K, Baurain JF, Hodi FS, Wolchok JD. Nivolumab for patients with advanced melanoma treated beyond progression: analysis of 2 phase 3 clinical trials. JAMA Oncol. 2017; 3 (11): 1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, Klichinsky M, Shestova O, Patel PR, Kulikovskaya I, Nazimuddin F, Bhoj VG, Orlando EJ, Fry TJ, Bitter H, Maude SL, Levine BL, Nobles CL, Bushman FD, Young RM, Scholler J, Gill SI, June CH, Grupp SA, Lacey SF, Melenhorst JJ. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nature Med. 2018; 24 (10): 1499–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. British J Cancer. 2018; 118 (1): 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action efficacy and limitations. Frontiers Oncol. 2018;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018; 4 (12): 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Annals Oncol. 2015; 26 (12): 2375–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semper H, Muehlberg F, Schulz-Menger J, Allewelt M, Grohe C. Drug-induced myocarditis after nivolumab treatment in a patient with PDL1- negative squamous cell carcinoma of the lung. Lung Cancer. 2016;99:117–9. [DOI] [PubMed] [Google Scholar]
- 27.Tadokoro T, Keshino E, Makiyama A, Sasaguri T, Ohshima K, Katano H, Mohri M. Acute lymphocytic myocarditis with anti-PD-1 antibody nivolumab. Circ Heart Failure. 2016;9(10):e003514. [DOI] [PubMed] [Google Scholar]
- 28.Pavlov VA, Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci. 2017; 20 (2): 156–166. [DOI] [PubMed] [Google Scholar]
- 29.Pavlov VA, Chavan SS, Tracey KJ. Molecular and functional neuroscience in immunity. Annual Rev Immunol. 2018; 36 783–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chavan SS, Tracey KJ. Essential neuroscience in immunology. J Immunol (Baltimore MD : 1950). 2017; 198 (9): 3389–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bucsek MJ, Giridharan T, MacDonald CR, Hylander BL, Repasky EA. An overview of the role of sympathetic regulation of immune responses in infectious disease and autoimmunity. Int J Hyperthermia. 2018; 34 (2): 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity. 2017; 46 (6): 927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao G, Chen M, Bucsek MJ, Repasky EA, Hylander BL. Adrenergic signaling: a targetable checkpoint limiting development of the antitumor immune response. Front Immunol. 2018;9:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mani SK. Neuroendocrine regulation of reproduction stress inflammation and energy homeostasis. J Neuroendocrinol. 2018; 30 (10): e12648. [DOI] [PubMed] [Google Scholar]
- 35.Padro CJ, Sanders VM. Neuroendocrine regulation of inflammation. Semin Immunol. 2014; 26 (5): 357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eng JW, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA. A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells immunosuppression and immunotherapeutic response. Cancer Immunol Immunother. 2014; 63 (11): 1115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Z, Shioda S, Masahisa J, Kawakami Y, Ohtaki H, Calista Lim H, Wang S, Zhao X, Liu Y, Zhou D, Guo Y. Role of the autonomic nervous system in the tumor micro-environment and its therapeutic potential. Curr Pharm Design. 2017;23(11):1687–92. [DOI] [PubMed] [Google Scholar]
- 38.Arese M, Bussolino F, Pergolizzi M, Bizzozero L, Pascal D. Tumor progression: the neuronal input. Annals Trans Med. 2018; 6 (5): 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature Med. 2006; 12 (8): 939–44. [DOI] [PubMed] [Google Scholar]
- 40.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nature Rev Cancer. 2015; 15 (9): 563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanoun M, Maryanovich M, Arnal-Estapé A, Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron. 2015. April 22;86(2):360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Resh. 2010. September 15;70(18):7042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armaiz-Pena GN, Gonzalez-Villasana V, Nagaraja AS, Rodriguez-Aguayo C, Sadaoui NC, Stone RL, Matsuo K, Dalton HJ, Previs RA, Jennings NB, Dorniak P. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget. 2015. February;6(6):4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bower JE, Shiao SL, Sullivan P, Lamkin DM, Atienza R, Mercado F, Arevalo J, Asher A, Ganz PA, Cole SW. Prometastatic molecular profiles in breast tumors from socially isolated women. JNCI Cancer Spectrum. 2018. July 19;2(3):pky029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamkin DM, Ho HY, Ong TH, Kawanishi CK, Stoffers VL, Ahlawat N, Ma JCY, Arevalo JMG, Cole SW, Sloan EK. beta-Adrenergic-stimulated macrophages: Comprehensive localization in the M1-M2 spectrum. Brain Behav Immun. 2016;57:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin JF, Jin FJ, Li N, Guan HT, Lan L, Ni H, Wang Y. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015. May;48(5):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006. January 27;124(2):263–6. [DOI] [PubMed] [Google Scholar]
- 48.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature Rev Cancer. 2004. January;4(1):71. [DOI] [PubMed] [Google Scholar]
- 49.Yang M, McKay D, Pollard JW, Lewis CE. Diverse functions of macrophages in different tumor microenvironments. Cancer Res. 2018. October 1;78(19):5492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, Repasky EA. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci. 2013. December 10;110(50):20176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eng JW, Reed CB, Kokolus KM, Pitoniak R, Utley A, Bucsek MJ, Ma WW, Repasky EA, Hylander BL. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β 2-adrenergic receptor activation. Nature Commun. 2015. March 10;6:6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B, Liu H, Kokolus KM, Eng JW, Messmer MN, Attwood K. β-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017. October 15;77(20):5639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nissen MD, Sloan EK, Mattarollo SR. β-adrenergic signaling impairs antitumor CD8+ T-cell responses to B-cell lymphoma immunotherapy. Cancer Immunol Res. 2018. January 1;6(1):98–109. [DOI] [PubMed] [Google Scholar]
- 54.Budiu RA, Vlad AM, Nazario L, Bathula C, Cooper KL, Edmed J, Thaker PH, Urban J, Kalinski P, Lee AV, Elishaev EL. Restraint and social isolation stressors differentially regulate adaptive immunity and tumor angiogenesis in a breast cancer mouse model. Cancer Clin Oncol. 2017. May;6(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalinichenko VV, Mokyr MB, Graf LH Jr, Cohen RL, Chambers DA. Norepinephrine-mediated inhibition of antitumor cytotoxic T lymphocyte generation involves a beta-adrenergic receptor mechanism and decreased TNF-alpha gene expression. J Immunol (Baltimore MD : 1950). 1999; 163 (5): 2492–9. [PubMed] [Google Scholar]
- 56.Qiao G, Bucsek MJ, Winder NM, Chen M, Giridharan T, Olejniczak SH, Hylander BL, Repasky EA. β-Adrenergic signaling blocks murine CD8+ T-cell metabolic reprogramming during activation: a mechanism for immunosuppression by adrenergic stress. Cancer Immunol Immunother. 2019. January 25;68(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature Rev Neurosci. 2013. September;14(9):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ben-Shaanan TL, Schiller M, Azulay-Debby H, Korin B, Boshnak N, Koren T, Krot M, Shakya J, Rahat MA, Hakim F, Rolls A. Modulation of anti-tumor immunity by the brain’s reward system. Nature Commun. 2018. July 13;9(1):2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wrobel LJ, Bod L, Lengagne R, Kato M, Prévost-Blondel A, Le Gal FA. Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget. 2016. November 22;7(47):77825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao L, Hudson CA, Lawrence DA. Acute cold/restraint stress inhibits host resistance to Listeria monocytogenes via β1-adrenergic receptors. Brain Behavior Immunity. 2003. April 1;17(2):121–33. [DOI] [PubMed] [Google Scholar]
- 61.Emeny RT, Gao D, Lawrence DA. β1-adrenergic receptors on immune cells impair innate defenses against Listeria. J Immunol. 2007. April 15;178(8):4876–84. [DOI] [PubMed] [Google Scholar]
- 62.Frick LR, Rapanelli M, Bussmann UA, Klecha AJ, Arcos ML, Genaro AM, Cremaschi GA. Involvement of thyroid hormones in the alterations of T-cell immunity and tumor progression induced by chronic stress. Biol Psych. 2009. June 1;65(11):935–42. [DOI] [PubMed] [Google Scholar]
- 63.Frick LR, Palumbo ML, Zappia MP, Brocco MA, Cremaschi GA, Genaro AM. Inhibitory effect of fluoxetine on lymphoma growth through the modulation of antitumor T-cell response by serotonin-dependent and independent mechanisms. Biochem Pharmacol. 2008. May 1;75(9):1817–26. [DOI] [PubMed] [Google Scholar]
- 64.Rosso D, Emilia M, Sterle HA, Cremaschi GA, Genaro AM. Beneficial effect of fluoxetine and sertraline on chronic stress-induced tumor growth and cell dissemination in a mouse model of lymphoma: crucial role of antitumor immunity. Frontiers Immunol. 2018. June 19;9:1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kokolus KM, Zhang Y, Sivik JM, Schmeck C, Zhu J, Repasky EA, Drabick JJ, Schell TD. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. OncoImmunology. 2018. March 4;7(3):e1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bakos O, Lawson C, Rouleau S, Tai LH. Combining surgery and immunotherapy: turning an immunosuppressive effect into a therapeutic opportunity. J Immunother Cancer. 2018. December;6(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, Meiboom H, Ben-Eliyahu S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010; 184 (5): 2449–57. [DOI] [PubMed] [Google Scholar]
- 68.Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and β-adrenoceptors. Neuroimmunomodulation. 2000;8(3):154–64. [DOI] [PubMed] [Google Scholar]
- 69.Shakhar G, Ben-Eliyahu S. In vivo β-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998. April 1;160(7):3251–8. [PubMed] [Google Scholar]
- 70.Katafuchi TO, Take SA, Hori TE. Roles of sympathetic nervous system in the suppression of cytotoxicity of splenic natural killer cells in the rat. J Physiol. 1993. June 1;465(1):343–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One. 2011. April 29;6(4):e19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ben-Eliyahu S, Shakhar G, Shakhar K, Melamed R. Timing within the oestrous cycle modulates adrenergic suppression of NK activity and resistance to metastasis: possible clinical implications. British J Cancer. 2000. December;83(12):1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenne E, Sorski L, Shaashua L, Neeman E, Matzner P, Levi B, Ben-Eliyahu S. In vivo suppression of NK cell cytotoxicity by stress and surgery: glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behavior Immunity. 2014. March 1;37:207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a β-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behavior Immunity. 2005. March 1;19(2):114–26. [DOI] [PubMed] [Google Scholar]
- 75.Sorski L, Melamed R, Matzner P, Lavon H, Shaashua L, Rosenne E, Ben-Eliyahu S. Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through beta-adrenoceptors blockade and COX2 inhibition. Brain Behav Immun. 2016;58:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaashua L, Shabat-Simon M, Haldar R, Matzner P, Zmora O, Shabtai M, Sharon E, Allweis T, Barshack I, Hayman L, Arevalo J. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res. 2017. August 15;23(16):4651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haldar R, Shaashua L, Lavon H, Lyons YA, Zmora O, Sharon E, Birnbaum Y, Allweis T, Sood AK, Barshack I, Cole S. Perioperative inhibition of β-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain Behavior Immunity. 2018. October 1;73:294–309. [DOI] [PubMed] [Google Scholar]
- 78.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J Exper Med. 2014. December 15;211(13):2583–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki K, Hayano Y, Nakai A, Furuta F, Noda M. Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J Exper Med. 2016. November 14;213(12):2567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mirzaei HR, Rodriguez A, Shepphird J, Brown CE, Badie B. Chimeric antigen receptors T cell therapy in solid tumor: challenges and clinical applications. Frontiers Immunol. 2017. December 22;8:1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Idorn M Chemokine receptors and exercise to tackle the inadequacy of T cell homing to the tumor site. Cells. 2018. August;7(8):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maus MV, June CH. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer Res. 2016;22(8):1875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, Kirkwood JM, Gajewski TF, Chen L, Gorski KS, Anderson AA, Diede SJ, Lassman ME, Gansert J, Hodi FS, Long GV. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018; 174 (4): 1031–1032. [DOI] [PubMed] [Google Scholar]
- 84.Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guérin M, Biton J, Ouakrim H, Régnier F. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti–PD-1 treatment. Proc Natl Acad Sci. 2018. April 24;115(17):E4041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, Martin PJ, Sandmaier BM, Marr KA, Appelbaum FR, Storb R. Reduced mortality after allogeneic hematopoietic-cell transplantation. New England J Med. 2010. November 25;363(22):2091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.González-Vicent M, Perez MA. Allogeneic hematopoietic stem-cell transplantation from haploidentical donors using ‘ex-vivo’T-cell depletion in pediatric patients with hematological malignancies: state of the art review. Curr Op Oncol. 2018. November 1;30(6):396–401. [DOI] [PubMed] [Google Scholar]
- 87.Porter DL. Allogeneic immunotherapy to optimize the graft-versus-tumor effect: concepts and controversies. ASH Education Program Book. 2011. December 10;2011(1):292–8. [DOI] [PubMed] [Google Scholar]
- 88.Szyska M, Na IK. Bone marrow GvHD after allogeneic hematopoietic stem cell transplantation. Frontiers Immunol. 2016. March 30;7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rezvani AR, Storb RF. Separation of graft-vs.-tumor effects from graft-vs.-host disease in allogeneic hematopoietic cell transplantation. J Autoimmun. 2008. May 1;30(3):172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohammadpour H, O’Neil R, Qiu J, McCarthy PL, Repasky EA, Cao X. Blockade of Host β2-Adrenergic Receptor Enhances Graft-versus-Tumor Effect through Modulating APCs. J Immunol. 2018. April 1;200(7):2479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leigh ND, Kokolus KM, O’Neill RE, Du W, Eng JW, Qiu J, Chen GL, McCarthy PL, Farrar JD, Cao X, Repasky EA. Housing temperature–induced stress is suppressing murine graft-versus-host disease through β2-adrenergic receptor signaling. J Immunol. 2015. November 15;195(10):5045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014. January;505(7483):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, MacArthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010. August;466(7308):829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao X, Wu X, Frassica D, Yu B, Pang L, Xian L, Wan M, Lei W, Armour M, Tryggestad E, Wong J. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc Natl Acad Sci. 2011. January 25;108(4):1609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lucas D, Scheiermann C, Chow A, Kunisaki Y, Bruns I, Barrick C, Tessarollo L, Frenette PS. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat Med. 2013;19(6): 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006. January 27;124(2):407–21. [DOI] [PubMed] [Google Scholar]
- 97.Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011. November 23;147(5):1146–58. [DOI] [PubMed] [Google Scholar]
- 98.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, Von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP. Chronic variable stress activates hematopoietic stem cells. Nature Med. 2014. July;20(7):754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vasamsetti SB, Florentin J, Coppin E, Stiekema LC, Zheng KH, Nisar MU, Sembrat J, Levinthal DJ, Rojas M, Stroes ES, Kim K. Sympathetic neuronal activation triggers myeloid progenitor proliferation and differentiation. Immunity. 2018. July 17;49(1):93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Méndez-Ferrer S, Battista M, Frenette PS. Cooperation of β2-and β3-adrenergic receptors in hematopoietic progenitor cell mobilization. Annals New York Acad Sci. 2010. March 1;1192(1):139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012. August 24;37(2):290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richter R, Forssmann W, Henschler R. Current developments in mobilization of hematopoietic stem and progenitor cells and their interaction with niches in bone marrow. Transfusion Med Hemother. 2017;44(3):151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karachaliou N, Gonzalez-Cao M, Sosa A, Berenguer J, Bracht JW, Ito M, Rosell R. The combination of checkpoint immunotherapy and targeted therapy in cancer. Annals Trans Med. 2017. October;5(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Melichar B, Spisarova M. Combined regimens in immunotherapy. Klin Onkol. 2017;30(Suppl 3):45–49. [DOI] [PubMed] [Google Scholar]
- 105.Moya-Horno I, Viteri S, Karachaliou N, Rosell R. Combination of immunotherapy with targeted therapies in advanced non-small cell lung cancer (NSCLC). Therap Adv Med Oncol. 2018. January 8;10:1758834017745012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maeda Y New immunotherapy-based approach in allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2018. February 1;107(2):129-. [DOI] [PubMed] [Google Scholar]
- 107.Bouchlaka MN, Redelman D, Murphy WJ. Immunotherapy following hematopoietic stem cell transplantation: potential for synergistic effects. Immunotherapy. 2010. May;2(3):399–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gschweng E, De Oliveira S, Kohn DB. Hematopoietic stem cells for cancer immunotherapy. Immunol Rev. 2014. January;257(1):237–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barre PV, Padmaja G, Suvashisa Rana T. Stress and quality of life in cancer patients: Medical and psychological intervention. Indian J Psych Med. 2018. May;40(3):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sehlen S, Hollenhorst H, Schymura B, Herschbach P, Aydemir U, Firsching M, Dühmke E. Psychosocial stress in cancer patients during and after radiotherapy. Strahlentherapie Onkologie. 2003. March 1;179(3):175–80. [DOI] [PubMed] [Google Scholar]
- 111.Chen H, Liu D, Guo L, Cheng X, Guo N, Shi M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating beta-adrenergic signaling. J Pathol. 2018; 244 (1): 49–60. [DOI] [PubMed] [Google Scholar]
- 112.Goyal NG, Maddocks KJ, Johnson AJ, Byrd JC, Westbrook TD, Andersen BL. Cancer-specific stress and trajectories of psychological and physical functioning in patients with relapsed/refractory chronic lymphocytic leukemia. Ann Behav Med. 2018; 52 (4): 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tang J, Li Z, Lu L, Cho CH. beta-Adrenergic system a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin Cancer Biol. 2013;23(6 Pt B):533–42. [DOI] [PubMed] [Google Scholar]
- 114.Na Z, Qiao X, Hao X, Fan L, Xiao Y, Shao Y, Sun M, Feng Z, Guo W, Li J, Li J. The effects of beta-blocker use on cancer prognosis: a meta-analysis based on 319,006 patients. OncoTargets Ther. 2018;11:4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Giorgi V, Grazzini M, Benemei S, Marchionni N, Botteri E, Pennacchioli E, Geppetti P, Gandini S. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 2018;4(2): e172908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hwa YL, Shi Q, Kumar SK, Lacy MQ, Gertz MA, Kapoor P, Buadi FK, Leung N, Dingli D, Go RS, Hayman SR, Gonsalves WI, Russell S, Lust JA, Lin Y, Rajkumar SV, Dispenzieri A. Beta-blockers improve survival outcomes in patients with multiple myeloma: a retrospective evaluation. Am J Hematol. 2017; 92 (1): 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amaya CN, Perkins M, Belmont A, Herrera C, Nasrazadani A, Vargas A, Khayou T, Montoya A, Ballou Y, Galvan D, Rivas A, Rains S, Patel L, Ortega V, Lopez C, Chow W, Dickerson EB Bryan B. A Non-selective beta blockers inhibit angiosarcoma cell viability and increase progression free- and overall-survival in patients diagnosed with metastatic angiosarcoma. Oncoscience. 2018; 5 (3–4): 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Knight JM, Kerswill SA, Hari P, Cole SW, Logan BR, D’Souza A, Shah NN, Horowitz MM, Stolley MR, Sloan EK, Giles KE, Costanzo ES, Hamadani M, Chhabra S, Dhakal B, Rizzo JD. Repurposing existing medications as cancer therapy: design and feasibility of a randomized pilot investigating propranolol administration in patients receiving hematopoietic cell transplantation. BMC Cancer. 2018; 18 (1): 593. [DOI] [PMC free article] [PubMed] [Google Scholar]


