Abstract
Background:
Previous studies have described echocardiographic indices of right ventricular (RV) diastolic function in patients with tetralogy of Fallot (TOF) but these indices have not been validated against invasive hemodynamic data. The purpose of this study was to determine echocardiographic predictors of severe RV diastolic dysfunction, and the impact of severe RV diastolic dysfunction on transplant-free survival.
Methods:
Cohort study of TOF patients that underwent non-simultaneous cardiac catheterization and echocardiogram at Mayo Clinic. Based on prior studies we selected these indices for assessment: tricuspid E/A, E/e’, deceleration time, pulmonary artery forward flow, dilated inferior vena cava (IVC), and hepatic vein diastolic flow reversal (HVDFR). RV diastolic function classes (normal, mild/moderate and severe dysfunction) were created using arbitrary cut-off points of the median values of right ventricular end-diastolic pressure (RVEDP) and right atrial pressure (RAP) for the cohort.
Results:
Among 173 patients (age 40 ± 13 years), 68 patients were classified as normal (RVEDP≤14 and RAP≤10), 37 as mild/moderate dysfunction (either RVEDPN14 or RAPN10), and 69 as severe dysfunction (RVEDP>14 and RAP>10). Of the indices assessed, dilated IVC had the best sensitivity of 95% (area under the curve [AUC] 0.689) while HVDFR had the best specificity of 69% (AUC 0.648) for detecting severe RV diastolic dysfunction. Severe RV diastolic dysfunction was an independent risk factor for death/transplant (hazard ratio 2.83, p = 0.009).
Conclusion:
Severe RV diastolic dysfunction, as defined by invasive hemodynamic indices, was associated with poor prognosis. Echocardiographic indices can identify these high risk patients, and hence improve risk stratification in clinical practice.
Keywords: Tetralogy of Fallot, Right ventricular compliance, Restrictive physiology
1. Introduction
Patients with tetralogy of Fallot (TOF) sustain myocardial injury due to cyanosis and pressure overload prior to surgical repair, hypoxic injury during cardiopulmonary bypass, and volume/pressure overload due to recurrent hemodynamic lesions after repair [1,2]. Although the prevalence of right ventricular (RV) diastolic dysfunction has not been systematically studied in TOF patients, we expect that the cumulative effect of these insults will result in diastolic dysfunction in these patients.
The American Society of Echocardiography endorsed the use of tricuspid Doppler indices for assessment of RV diastolic function [3]. However these indices have not been validated in the TOF population. Studies conducted in TOF patients have described some echocardiographic markers of RV diastolic dysfunction, the most common being late diastolic pulmonary artery forward flow (PAFF) [4–8]. The correlation of these noninvasive indices with invasive hemodynamic data has not been investigated in a robust way. The purpose of this study was to determine the prevalence and clinical implications of RV diastolic dysfunction in TOF patients.
2. Methods
2.1. Patient selection
We reviewed the MACHD (Mayo Adult Congenital Heart Disease) Registry and identified all adults (age ≥ 18 years) with repaired TOF that underwent right heart catheterization at Mayo Clinic Rochester, Minnesota from January 1, 1990 through December 31, 2017. From this cohort we excluded patients without digital echocardiographic images, patients with tricuspid valve prostheses, and patients who had atrial arrhythmia at the time of echocardiogram (Supplementary Fig. 1). The Mayo Clinic Institutional Review Board approved this study and waived informed consent for patients that provided research authorization.
2.2. Study endpoints and definitions
The primary objective was to assess the ability of echocardiographic indices to detect severe RV diastolic dysfunction as measured by the gold standard of invasive hemodynamic assessment. The secondary objectives were to determine the effect of severe RV diastolic dysfunction on RV adaptation to volume overload due to severe pulmonary regurgitation, and to determine the impact of severe RV diastolic dysfunction on transplant-free survival.
There are no published clinically significant cut-off points for RV diastolic dysfunction based on invasive hemodynamics in the TOF population. As a result, we created RV diastolic function profiles using the median values of right ventricular end-diastolic pressure (RVEDP) and right atrial pressure (RAP) of our cohort. The patients were dichotomized into normal vs high RVEDP using the median RVEDP, and into normal vs high RAP using the median RAP. Based on the median RVEDP and RAP values, we created 4 RV diastolic function profiles: normal RVEDP and normal RAP [NRVEDP/NRAP]; normal RVEDP and high RAP [NRVEDP/HRAP]; high RVEDP and normal RAP [HRVEDP/NRAP]; and high RVEDP and high RAP [HRVEDP/HRAP].
The early stage of diastolic dysfunction is characterized by abnormal (delayed) relaxation during which the RV filling pressure is typically normal. This is followed by a more advanced stage of diastolic dysfunction that is characterized by abnormal ventricular compliance and elevation of RV filling pressures [9]. Based on this conceptual framework, we considered NRVEDP/NRAP as the ‘normal’ group, and defined mild/moderate RV diastolic dysfunction as groups NRVEDP/HRAP and HRVEDP/NRAP, and severe RV diastolic dysfunction as group HRVEDP/HRAP.
2.3. Cardiac catheterization
Cardiac catheterization was performed in all patients while on chronic medications in the fasting state and mild sedation using 7 Fr fluid-filled catheters. Fluoroscopy, characteristic pressure waveforms, and oximetry we used to confirm catheter positions. Pressure measurements were recorded at end expiration and represent an average of 3 beats for patients in sinus rhythm and 5 beats for patients in atrial fibrillation [10]. Cardiac output was determined by the Fick technique using assumed O2 consumption and directly measured O2 contents in the pulmonary and systemic circulations [11]. Hemodynamic pressure tracings were recorded, digitized (240 Hz), and stored for offline analysis. Offline review of hemodynamic tracings, angiographic images and cardiac catheterization reports were performed in all patients.
2.4. Echocardiography
Two-dimensional, M-mode and Doppler echocardiography were performed according to standard American Society of Echocardiography guidelines [3,12], and only echocardiograms performed within 7 days from the time of cardiac catheterization were analyzed for this study. The severity of tricuspid regurgitation, pulmonary regurgitation, RV enlargement, and RV systolic dysfunction were graded as none/trivial, mild, mild-moderate, moderate, moderate-severe, and severe based on standard assessment by comprehensive echocardiogram [13].
We selected echocardiographic indices as the predictive variables for this study based the indices of diastolic function assessment endorsed by the American Society of Echocardiography [3], and other indices described in studies of diastolic function in the TOF population [4–8]. (1) tricuspid inflow early diastolic velocity/tricuspid inflow late diastolic velocity (E/A); (2) tricuspid inflow early diastolic velocity/tricuspid annular tissue Doppler early systolic velocity (E/e’); (3) tricuspid inflow deceleration time (DT); (4) hepatic vein diastolic flow reversal (HVDFR); (5) late diastolic PAFF; (6) inferior vena cava (IVC) size index to normal body surface area of 1.72.
Offline measurements of tricuspid inflow and tissue Doppler indices (apical view), IVC size (subcostal short axis), hepatic vein pulse wave Doppler (subcostal short axis), and pulmonary artery pulse wave Doppler (parasternal long or short axis) were performed in all patients by an experienced sonographer (R.P), Fig. 1. In order to mitigate the effect of respirophasic variation, HVDFR and PAFF were considered to be present if identified in 3 consecutive cardiac cycles, and IVC size was assessed as the average of the largest and smallest dimensions during the respiratory cycle. Dilated IVC was defined as IVC > 21 mm or 12 mm per 1.73 [3]. A random sample of 50 patients was reviewed by one of the investigators (A.C.E) who was blinded to the initial measurement performed by the sonographer.
Fig. 1.
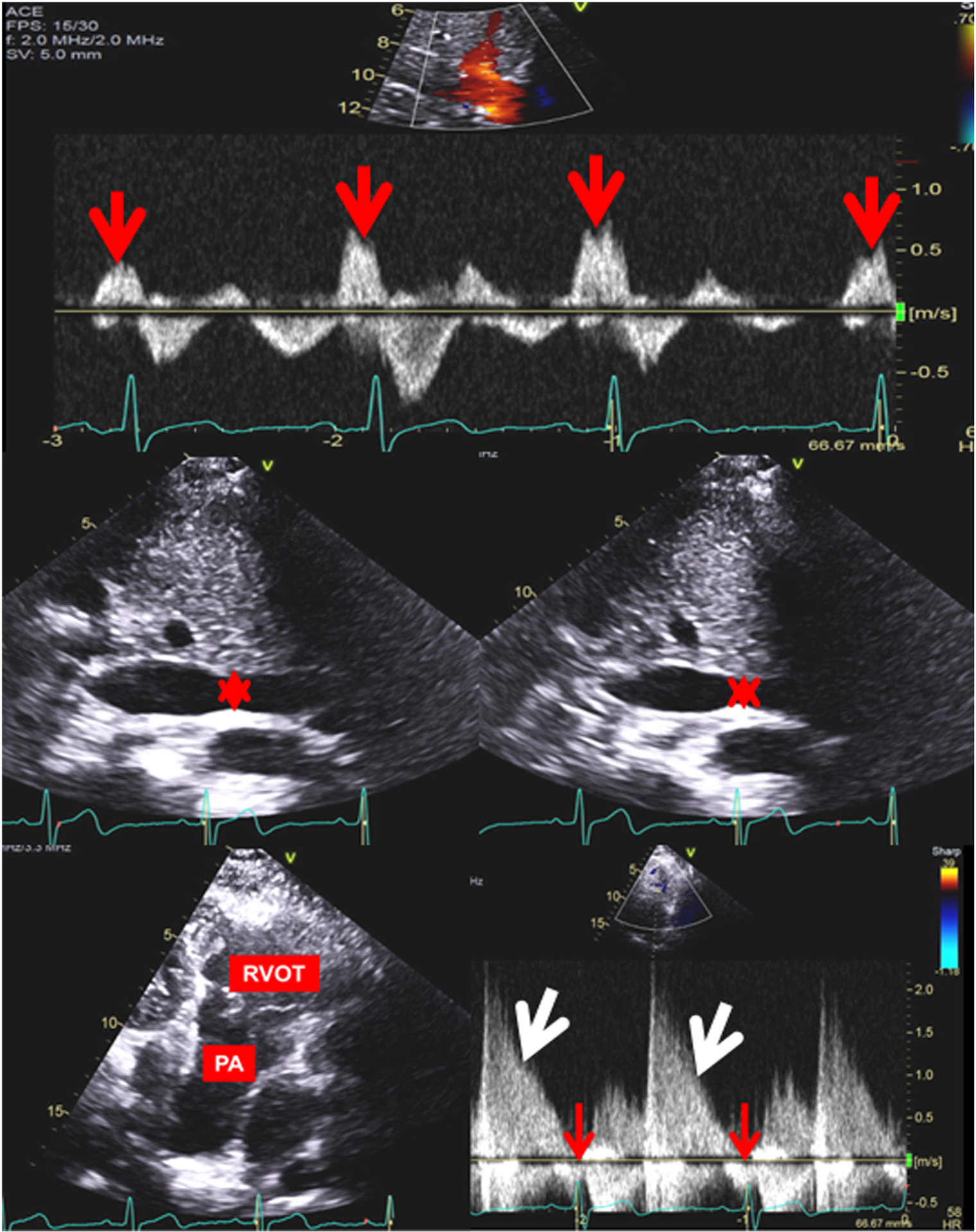
Top: Hepatic vein pulse wave Doppler showing diastolic flow reversal (red arrows) in 3 consecutive cardiac cycles Middle: Two-dimensional image of inferior vena cava showing measurements (red asterisk) at the largest dimension/inspiration on the left and at the smallest dimension/expiration on the right Bottom: Two-dimensional image of the right ventricular outflow tract (RVOT) and pulmonary artery (PA) on the left. Pulse wave Doppler of the main PA showing pulmonary regurgitation (white arrow) and late diastolic pulmonary artery forward flow (red arrow).
In order to assess the effect of severe RV diastolic dysfunction on RV adaptation to volume overload, we performed a subgroup analysis in the subset of patients with severe pulmonary regurgitation. These patients were considered to have severe pulmonary regurgitation if they had at least one of the criteria for severe pulmonary regurgitation stipulated in the guideline [13]. For the secondary study objective, we compared CMRI-derived RV volumetric indices and peak oxygen consumption among patients with severe pulmonary regurgitation with and without severe RV diastolic dysfunction. Peak oxygen consumption was assessed using symptom limited treadmill cardiopulmonary exercise test with respiratory quotient of >1.1 as previously described [14].
2.5. Cardiac magnetic resonance imaging
The protocol for volumetric assessment using CMRI at this institution has been previously described [15]. All CMRI studies were performed on a 1.5-T system (Signa; GE Healthcare, Waukesha, WI) using an eight-element phased-array cardiac coil. RV end-diastolic volume (RVEDV) and RV end-systolic volume (RVESV) were obtained by manual tracing of endocardial borders from axial images at end-diastole and endsystole respective, and RVEF was calculated from these volumes.
2.6. Outcomes assessment
The occurrence of heart transplant was ascertained by review of clinical notes, and all-cause mortality was ascertained using Mayo Clinic registration database and Accurint, an institutionally-approved location service. Vital status was ascertained in 100% of the patients as of December 31, 2017.
2.7. Statistical analysis
Data were presented as mean ± standard deviation, median (interquartile range), number (%), or statistic (95% confidence interval). Between-group comparisons were performed using Fisher’s exact test, t-test, analysis of variance or Kruskal Wallis test as appropriate. The interobserver agreement between observer #1 (R.P) and observer #2 (A.C.E) was assessed using kappa coefficient (k) and intraclass correlation (ICC) as appropriate. Logistics regression analyses were used to test the ability of the pre-defined echocardiographic indices to detect severe RV diastolic dysfunction, and receiver operator characteristic curve was used to determine the optimal cut-off point for continuous predictors. We assessed the ability of each of the echocardiographic indices to detect RV diastolic dysfunction using the area under the curve (AUC). Exploratory analyses were performed to determine if a combination of multiple echocardiographic indices resulted in an improvement in AUC. IVC size and HVDFR had to best prediction, and we tested this by combining both variables. If a patient had both dilated IVC and HVDFR, we coded the patient as positive (1) and patients that do not have both indices were coded as negative (zero) in the logistic regression model. Kaplan Meier analysis and Cox aggression analysis were used to assess the relationship between the RV diastolic function profiles and transplant-free survival, and the time of cardiac catheterization was used as the baseline for time-to-event analyses. A p < 0.050 was considered statistically significant. All statistical analyses were performed with JMP software (version 13.0; SAS Institute Inc., Cary NC).
3. Results
Among the 173 patients that met the inclusion criteria for the study, the mean age at the time of echocardiogram was 40 ± 13, mean age at the time of TOF repair was 6 (3–14) years and 74 (43%) had prior palliative shunts. Supplementary Table 1 compares the baseline characteristics of the study cohort compared to other patients that did not meet the inclusion criteria for the study.
3.1. RV diastolic function profiles
The age at the time of cardiac catheterization was 40 ± 13 years, and the indications for cardiac catheterization were preoperative evaluation (n = 84, 49%), congestive heart failure (n = 36, 21%), and arrhythmia (n = 65, 38%). The mean and median RVEDP were 14.6 ± 5.6 mmHg and 14 (11–17) mmHg respectively. The mean and median RAP were 10.9 ± 5.6 mmHg and 10 (7–14) mmHg respectively. Based on pre-defined cut-off points, we divided the cohort into normal RV diastolic function defined as NRVEDP/NRAP (RVEDP ≤14 mmHg and RAP ≤10 mmHg); mild/moderate RV diastolic dysfunction defined as NRVEDP/HRAP (RVEDP ≤14 mmHg and RAP>10 mmHg) and HRVEDP/NRAP (RVEDP>14 mmHg and RAP ≤10 mmHg); and severe RV diastolic dysfunction defined as HRVEDP/HRAP (RVEDP>14 mmHg and RAP>10 mmHg), Table 1.
Table 1.
Invasive and noninvasive hemodynamic data of different RV diastolic function profiles.
| Reference | Mild/Moderate | Severe | |||
|---|---|---|---|---|---|
| NRVEDP/NRAP (n = 68) | NRVEDP/HRAP (n = 10) | HRVEDP/NRAP (n = 27) | HRVEDP/HRAP (n = 69) | p | |
| Echocardiography | |||||
| ≥Moderate tricuspid regurgitationa | 8(12%) | 1(10%) | 4(15%) | 25(37%) | 0.003 |
| ≥Moderate pulmonary regurgitationa | 39(59%) | 5(50%) | 15(60%) | 40(60%) | 0.857 |
| ≥Moderate RV enlargementa | 43(63%) | 5(50%) | 20(74%) | 58(87%) | 0.005 |
| ≥Moderate RV systolic dysfunctiona | 13(19%) | 5(50%) | 5(19%) | 34(52%) | <0.001 |
| RVSP, mmHg | 56 ± 22 | 657 ± 32 | 69 ± 28 | 68 ± 23 | 0.098 |
| Tricuspid regurgitation velocity, m/s | 3.5 ± 0.8 | 3.8 ± 0.7 | 3.7 ± 1.0 | 3.7 ± 0.8 | 0.366 |
| Assumed RA pressure, mmHg | 8 ± 3 | 10 ± 3 | 11 ± 4 | 13 ± 2 | 0.006 |
| Pulmonary valve peak velocity, m/s | 2.7 ± 0.9 | 3.1 ± 0.9 | 3.0 ± 1.1 | 2.8 ± 1.0 | 0.552 |
| RA volume index, ml/m2 | 38 ± 11 | 54 ± 15 | 57 ± 16 | 73 ± 231 | 0.012 |
| ≥Moderate RA enlargementa | 31(46%) | 6(60%) | 19 (70%) | 49(71%) | 0.019 |
| LA volume index, ml/m2 | 22 ± 8 | 32 ± 7 | 26 ± 13 | 34 ± 13 | 0.008 |
| ≥Moderate LA enlargementa | 3(7%) | 2(29%) | 6(24%) | 19 (37%) | 0.004 |
| Medial E/e’ | 10 ± 5 | 11 ± 3 | 11 ± 4 | 11 ± 5 | 0.197 |
| Lateral E/e’ | 6 ± 4 | 9±3 | 6±2 | 7±3 | 0.062 |
| LV ejection fraction, % | 56 ± 10 | 59 ± 7 | 60 ± 7 | 56 ± 9 | 0.231 |
| Catheterization | |||||
| RA pressure, mmHg | 7±2 | 12 ± 1 | 8±2 | 16 ± 4 | <0.001 |
| RVEDP, mmHg | 10(8–12) | 13 (12–14) | 16(15–17) | 17(16–22) | <0.001 |
| RV systolic pressure, mmHg | 54(43–64) | 68(60–89) | 70(60–94) | 69(65–93) | <0.001 |
| PA systolic pressure, mmHg | 33(26–43) | 42(29–42) | 43(33–62) | 48(40–63) | <0.001 |
| PA diastolic pressure, mmHg | 7(6–10) | 14(9–18) | 8(6–15) | 14(11–18) | <0.001 |
| Mean PA pressure, mmHg | 18(14–23) | 23(20–33) | 24(17–33) | 28(23–33) | <0.001 |
| PAWP, mmHg | 11 ± 4 | 14 ± 3 | 12 ± 4 | 17 ± 5 | <0.001 |
| PVR, index, WU*m2 | 3.2(21.8–4.2) | 4.3(2.5–9.4) | 4.4(3.0–7.1) | 5.7(2.9–8.8) | <0.001 |
| Cardiac index, L/min*m2 | 2.5 ± 0.7 | 2.1 ± 0.5 | 2.1 ± 0.6 | 2.0 ± 0.5 | <0.001 |
| Mean arterial pressure, mmHg | 84 ± 14 | 91 ± 12 | 89 ± 15 | 87 ± 15 | 0.089 |
| Mixed venous saturation, % | 72 ± 6 | 69 ± 8 | 71 ± 7 | 65 ± 10 | <0.001 |
| Aortic saturation, % | 97(95–99) | 97(94–99) | 97(93–99) | 95(92–98) | 0.004 |
RV: right ventricle; LV: left ventricle; RA: Right atrium; LA: Left atrium; RVSP: right ventricular systolic pressure; E: mitral inflow early velocity; e’: tissue Doppler early velocity; RVEDP: right ventricular end-diastolic pressure; LV: left ventricle; PA: pulmonary artery; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance. Data were presented as mean ± standard deviation, median (interquartile range) or number (%).
Qualitative echocardiographic assessment.
3.2. Echocardiographic predictors of RV diastolic dysfunction
The interval between cardiac catheterization and echocardiogram was 1.2 ± 0.9 days, and 97% had echocardiograms within 48 h prior to cardiac catheterization. Table 2 shows the echocardiographic indices of RV diastolic function for the cohort. There was excellent interobserver agreement for the binary variables: dilated IVC (k 0.95, 0.94–0.99, p < 0.001), HVDFR (k 0.93, 0.87–0.98, p < 0.001), and PAFF (k 0.91, 0.85–0.96, p < 0.001). Similarly, there was good interobserver agreement for the continuous variables: E velocity (ICC 0.90, 0.82–0.97, p = 0.001), A velocity (ICC 0.84, 0.72–0.91, p = 0.008), DT (ICC 0.78, 0.65–0.89, p = 0.027), and e’ velocity (ICC 0.94, 95% CI 0.90–0.97, p < 0.001).
Table 2.
Echocardiographic Indices of right ventricular diastolic function.
| Indices | N | Value |
|---|---|---|
| PAFF | 138 | 32 (23%) |
| Tricuspid E, cm/s | 116 | 59 ± 22 |
| Tricuspid A, cm/s | 86 | 36 ± 14 |
| Tricuspid DT, ms | 102 | 153 ± 49 |
| Tricuspid E/A | 86 | 1.7 ± 0.6 |
| Tricuspid e’, cm/s | 129 | 9 ± 7 |
| Tricuspid E/e’ | 107 | 6 ± 3 |
| Dilated IVC | 165 | 103 (62%) |
| HVDFR | 154 | 63 (41%) |
N: Number of patients with variable assessed.
PAFF: Pulmonary artery forward flow; HVDFR: Hepatic vein diastolic flow reversal; IVC: Inferior vena cava; RV: Right ventricle; E: tricuspid inflow early velocity; A: tricuspid inflow late or atrial velocity; e’: tricuspid annular tissue Doppler early velocity; DT: Deceleration time.
Supplementary Table 2 shows the sensitivity and specificity of the different echocardiographic indices for detecting severe RV diastolic dysfunction. The combination of HVDFR and dilated IVC provided the best detection of severe RV diastolic dysfunction with sensitivity of 66%, specificity of 92% and AUC of 0.831. With regards to individual indices, dilated IVC had the best sensitivity of 96% (AUC 0.703) while HVDFR had the best specificity of 69% (AUC 0.648). In order to control for the effect of significant tricuspid regurgitation, separate predictive models were created using data from the 135 patients that had bmoderate tricuspid regurgitation. Again, the combination of HVDFR and dilated IVC provided the best detection of severe RV diastolic dysfunction with sensitivity of 59%, specificity of 94% and AUC of 0.833.
3.3. RV adaptation to volume overload in severe RV diastolic dysfunction
There were 68 (39%) patients with severe pulmonary regurgitation, and among these patients we compared RV size and function between patients with vs without severe RV diastolic dysfunction. The patients with severe RV diastolic dysfunction had more tricuspid regurgitation and more RV systolic dysfunction by qualitative echocardiographic assessment. Cardiac magnetic resonance imaging derived indices showed that the patient with severe RV systolic dysfunction had smaller RV volumes, and RV stroke volume despite having more tricuspid regurgitation. Similarly, the severe RV systolic dysfunction group also had smaller left ventricle stroke volume likely due to reduced left ventricular preload. These differences did not reach statistical significance likely due to small sample size (Supplementary Table 3). Additionally the group with severe RV diastolic dysfunction had lower peak oxygen consumption (18 ± 4 vs 22 ± 5 ml/kg/min, p = 0.029) and percent predicted peak oxygen consumption (55 ± 10 vs 63 ± 13%, p = 0.049).
3.4. Severe RV diastolic dysfunction and transplant-free survival
The mean follow-up from the time of cardiac catheterization was 6.2 ± 4.7 years, yielding a total follow-up of 1142 patient-years. During this period, 27 (16%) patients died and 3 (2%) patients underwent heart transplant. The cause of death was end-stage heart failure (n = 16), ar-rhythmic death (n = 5), postoperative death after cardiac surgery (n = 2), bleeding/stroke related death (n = 1), sepsis (n = 1) and unknown (n = 2).
The 10-year transplant-free survival was 84% for the entire cohort. Using the patient with normal RV diastolic function as the reference, there was no significant difference in the 10-year transplant-free survival between the reference group and mild/moderate RV diastolic dysfunction group (91% vs 85%, p = 0.354). However, the 10-year transplant-free survival was significantly lower in the group with severe RV diastolic dysfunction (91% vs 72%, p = 0.012), and also different between the mild/moderate vs severe RV diastolic dysfunction groups (85% vs 72%, p = 0.038) Fig. 2. In comparison to patients with normal RV diastolic function (reference group), severe RV diastolic dysfunction was an independent predictor of death and/or heart transplant (hazard ratio 2.83, 1.32–6.75, p = 0.009), Table 3. LV ejection faction was also an independent predictor of death and/or heart transplant (hazard ratio 0.82, 0.59–0.95, p = 0.036), Table 3.
Fig. 2.
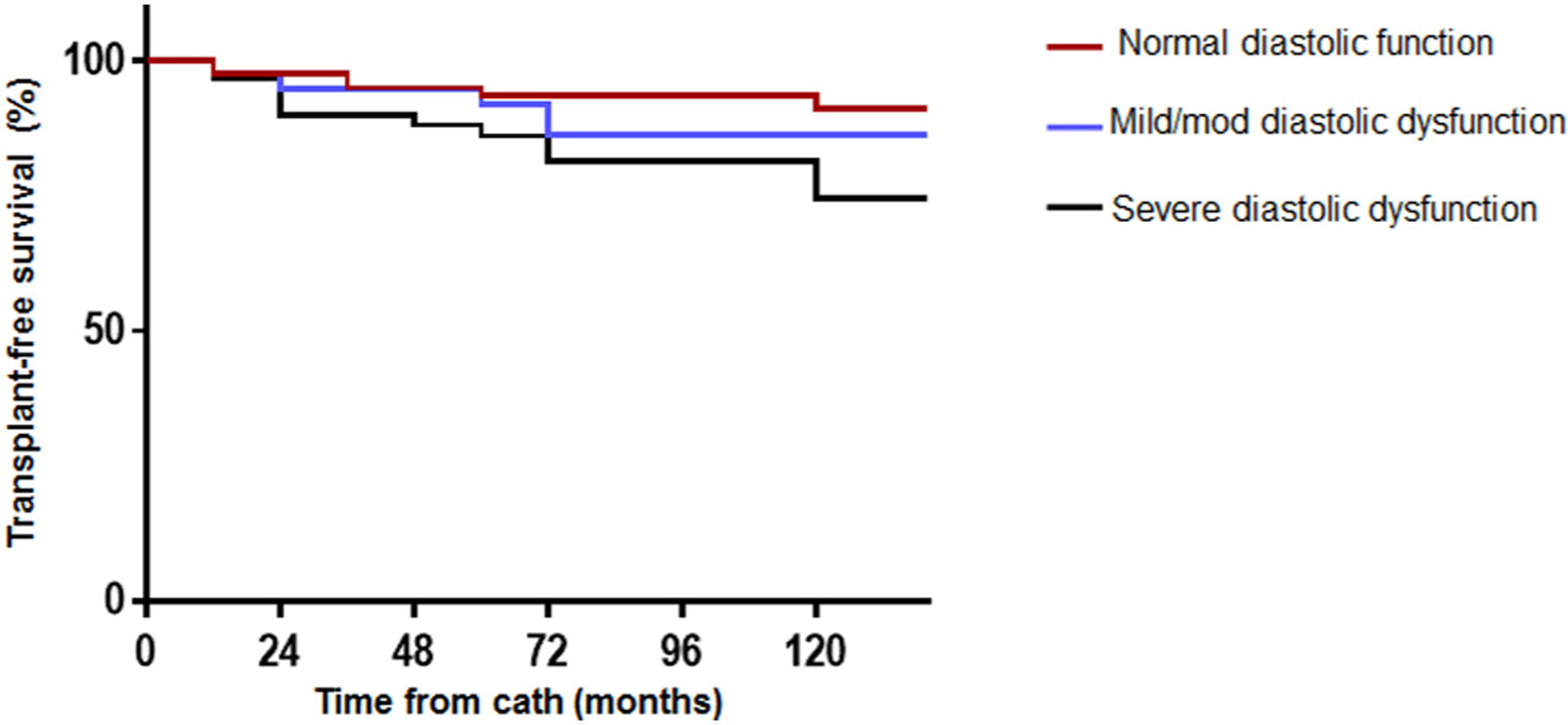
Kaplan Meier analysis comparing transplant-free survival between patients with normal right ventricular (RV) diastolic function (red), mild/moderate RV diastolic dysfunction (blue) and severe RV diastolic dysfunction (black).
Table 3.
Multivariate predictors of death/transplant.
| Full model | Final model | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Normal RV diastolic function | Reference | Reference | ||
| Mild/moderate diastolic dysfunction | 2.23 (0.74–6.68) | 0.265 | 1.68 (0.51–5.47) | 0.218 |
| Severe diastolic dysfunction | 2.92 (1.23–9.44) | 0.016 | 2.83 (1.32–6.75) | 0.009 |
| Age at cardiac cath (per 1 year) | 1.06 (0.94–1.28) | 0.175 | ||
| ≥Moderate RV systolic dysfunctiona | 2.08 (0.98–4.22) | 0.052 | 2.37 (1.09–4.27) | 0.029 |
| ≥Moderate RV dilationa | 1.43 (0.74–2.55) | 0.214 | ||
| ≥Moderate tricuspid regurgitationa | 1.66 (0.82–4.05) | 0.166 | ||
| ≥Moderate pulmonary regurgitationa | 1.09 (0.84–2.43) | 0.169 | ||
| LV ejection fraction, % | 0.84 (0.56–0.97) | 0.039 | 0.82 (0.59–0.95) | 0.036 |
| LV end-diastolic dimension, mm | 1.22 (0.32–3.66) | 0.612 | ||
RV: Right ventricle; LV: Left ventricle; HR: Hazard ratio; CI: Confidence interval.
Denotes echocardiographic data obtained by qualitative assessment.
In order to assess the performance of echocardiographic predictors of RV diastolic dysfunction in predicting transplant-free survival, we created a different regression model using echocardiographic indices instead of invasive hemodynamic indices for categorization of RV diastolic dysfunction. We defined RV diastolic dysfunction based on IVC size and HVDFR (because this combination had the highest AUC). The patients were categorized as ‘normal’ if there was no IVC dilation and no HVDFR, ‘mild/moderate’ if they had either IVC dilation or HVDFR, and ‘severe’ RV diastolic dysfunction if they had both IVC dilation and HVDFR. The presence of both IVC dilation and HVDFR (severe RV diastolic dysfunction by echocardiography) was predictor of transplant-free survival (hazard ratio 1.73, 1.08–3.74, p = 0.022), Supplementary Table 4.
4. Discussion
The prevalence and spectrum of RV diastolic dysfunction have not been systematically studied in patients with TOF. In the absence of consensus criteria and clinically meaningful cut-off points for diastolic dysfunction in this population, we defined RV diastolic dysfunction based on an arbitrary cut-off point using the median values of RVEDP and RAP for the purpose of this study. Based in these criteria, mild/moderate RV diastolic dysfunction and severe RV diastolic dysfunction were present in 21% and 40% of our cohort respectively.
The American Society of Echocardiography recommends the use of tricuspid Doppler echocardiographic indices for the assessment of RV diastolic function [3]. Of the 6 echocardiographic indices assessed in this study, dilated IVC had the best sensitivity of 94% while HVDFR had the best specificity of 69% for detecting severe RV diastolic dysfunction. The combination of HVDFR and dilated IVC provided the best discrimination with optimal balance of 59% sensitivity and 91% specificity (AUC of 0.804) for detecting severe RV diastolic dysfunction. Compared to PAFF, which is the most commonly used marker of restrictive RV physiology, dilated IVC and HVDFR had superior performance in detecting severe RV diastolic dysfunction.
A multicenter study of 556 TOF patients report RV diastolic dysfunction in 52% of the patients based on these guideline criteria [4]. However, the ability of tricuspid Doppler indices and PAFF to identify patients with severe RV diastolic dysfunction (abnormal RV compliance with high filling pressures) has not been previously studied hence the novelty of this study. Of the 3 tricuspid Doppler indices assess, only tricuspid E/e’ was able to detect severe RV diastolic dysfunction, but the performance was inferior to dilated IVC and HVDFR.
Additionally, we noted that patients with severe RV diastolic dysfunction had smaller RV end-diastolic, end systolic, and stroke volumes even though this group had more tricuspid regurgitation. We speculate that there is a disproportional rise in RV filling pressures for a given regurgitant volume because of increased RV stiffness in the group with severe RV diastolic dysfunction, and this limits the amount of blood that the RV can accept in end-diastole. This may explain the lower exercise capacity in the group with severe RV diastolic dysfunction because of inability to augment left ventricular preload necessary to sustain increased cardiac output during exercise. The previous studies that looked at RV dilatation in the setting of restrictive physiology, as defined by the presence of PAFF, have reported conflicting results [7,8,16–19]. Some of the studies reported smaller RV volume in the setting of restrictive RV physiology [16], some reported larger RV volume in the setting of restrictive RV physiology [7,8,17,18] while others reported no difference in RV size based on restrictive physiology [19,20]. It is worthwhile to note that in all the studies that reported larger RV volume in the setting of restrictive RV physiology [8,17,18], the restrictive physiology group had more pulmonary regurgitation thereby limiting the inference that can be drawn about the relationship between restrictive physiology and RV dilation based on these studies. We controlled for this confounder, by limiting the analysis to only patients with severe pulmonary regurgitation in both arms.
The group with severe RV diastolic dysfunction had lower transplant-free survival compared to the rest of the cohort, and severe RV diastolic dysfunction was an independent predictor of mortality after multivariate adjustments. This is novel because the relationship between RV diastolic function and survival in patients with TOF has not been described because the available literature comprise of cross-sectional studies or cohort studies with limited follow-up [4,5,7,8,16–19,21]. Perhaps TOF with severe RV diastolic dysfunction represents a different disease phenotype since they respond differently to volume overload with less RV dilation, and also have a different mortality risk. Maybe these patients may benefit from more aggressive therapies including an earlier RVOT intervention rather waiting for them to reach the recommend RV volume threshold for pulmonary valve replacement. This is purely speculative because we do not have any post intervention outcome data.
4.1. Limitations
Transthoracic echocardiogram was not performed simultaneously at the time of cardiac catheterization and therefore temporal difference in loading conditions is a potential confounder. The study was based on a selected (sicker) cohort of patients that underwent cardiac catheterization which limits generalizability of the results. However the core message of the study is that severe RV diastolic dysfunction is associated with poor prognosis, and echocardiographic indices can identify these high risk patients, and this is very clinically relevant when dealing with symptomatic patients. Some of the patients had missing data especially tricuspid Doppler indices, and tricuspid inflow indices were based on continuous wave Doppler, and these may limit the accuracy of the predictive models reported in this study. We did not account for respirophasic changes in IVC which may have confounded the results. We did not have quantitative echocardiographic assessment of RV size and function. Finally, the definition and classification of diastolic dysfunction used in this study was arbitrary. As a result, further studies are required to validate prognostic importance of these cut-off points.
4.2. Conclusions
Severe RV diastolic dysfunction, based on an arbitrary definition used in this study, was associated with poor prognosis. Echocardiographic indices can identify these high-risk patients, and hence can improve risk stratification in clinical practice. Patients with severe RV diastolic dysfunction had lower RV volumes and stroke volume as well as lower peak oxygen consumption for the same degree of pulmonary regurgitation. These findings suggest a TOF patient with severe RV diastolic dysfunction may represent a different disease phenotype that perhaps may benefit from a different set of criteria for intervention. There is need for prospective studies with simultaneous invasive and noninvasive correlation of diastolic indices to validate the findings of the study. Further studies also required to better understand the chain-of-causality of RV diastolic dysfunction, and strategies for targeting these risk factors in order to prevent RV diastolic dysfunction and potentially improve survival.
Supplementary Material
Acknowledgement
Rae Parker for performing offline analysis of echocardiographic data.
Funding
Dr. Egbe is supported by National Heart, Lung, and Blood Institute (NHLBI) grant K23 HL141448-01. Dr. Borlaug is supported by RO1 HL128526 and U10 HL110262. Dr. Obokata is supported by a research fellowship from the Uehara Memorial Foundation, Japan.
Abbreviations:
- TOF
Tetralogy of Fallot
- RAP: RV
Right ventricle
- RVEDP
Right ventricular end-diastolic pressure
- RAP
right atrial pressure
- E
tricuspid inflow early diastolic velocity
- A
tricuspid inflow late diastolic velocity
- DT
tricuspid inflow deceleration time
- e’
tricuspid annular tissue Doppler early systolic velocity
- HVDFR
hepatic vein diastolic flow reversal
- PAFF
pulmonary artery forward flow
- IVC
inferior vena cava
- ICC
intraclass coefficient
- AUC
Area under the curve
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2020.02.067.
Declaration of competing interest
The authors report no relationships that could be construed as a conflict of interest.
References
- [1].Munkhammar P, Cullen S, Jogi P, de Leval M, Elliott M, Norgard G, Early age at repair prevents restrictive right ventricular (RV) physiology after surgery for tetralogy of Fallot (TOF): diastolic RV function after TOF repair in infancy, J. Am. Coll. Cardiol 32 (1998) 1083–1087. [DOI] [PubMed] [Google Scholar]
- [2].Davlouros PA, Kilner PJ, Hornung TS, Li W, Francis JM, Moon JC, Smith GC, Tat T, Pennell DJ, Gatzoulis MA, Right ventricular function in adults with repaired tetralogy of Fallot assessed with cardiovascular magnetic resonance imaging: detrimental role of right ventricular outflow aneurysms or akinesia and adverse right-to-left ventricular interaction, J. Am. Coll. Cardiol 40 (2002) 2044–2052. [DOI] [PubMed] [Google Scholar]
- [3].Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB, Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography, Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography 23 (2010) 685–713(quiz 786–8). [DOI] [PubMed] [Google Scholar]
- [4].Aboulhosn JA, Lluri G, Gurvitz MZ, Khairy P, Mongeon FP, Kay J, Valente AM, Earing MG, Opotowsky AR, Lui G, Gersony DR, Cook S, Child J, Ting J, Webb G, Landzberg M, Broberg CS and Alliance for adult research in congenital C. Left and right ventricular diastolic function in adults with surgically repaired tetralogy of Fallot: a multi-institutional study, The Canadian Journal of Cardiology. 29 (2013) 866–872. [DOI] [PubMed] [Google Scholar]
- [5].Gatzoulis MA, Clark AL, Cullen S, Newman CG, Redington AN, Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot. Restrictive physiology predicts superior exercise performance, Circulation. 91 (1995) 1775–1781. [DOI] [PubMed] [Google Scholar]
- [6].Cullen S, Shore D, Redington A, Characterization of right ventricular diastolic performance after complete repair of tetralogy of Fallot. Restrictive physiology predicts slow postoperative recovery, Circulation. 91 (1995) 1782–1789. [DOI] [PubMed] [Google Scholar]
- [7].Lu JC, Cotts TB, Agarwal PP, Attili AK, Dorfman AL, Relation of right ventricular dilation, age of repair, and restrictive right ventricular physiology with patient-reported quality of life in adolescents and adults with repaired tetralogy of fallot, Am. J. Cardiol 106 (2010) 1798–1802. [DOI] [PubMed] [Google Scholar]
- [8].Samyn MM, Kwon EN, Gorentz JS, Yan K, Danduran MJ, Cava JR, Simpson PM, Frommelt PC, Tweddell JS, Restrictive versus nonrestrictive physiology following repair of tetralogy of Fallot: is there a difference? Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography. 26 (2013) 746–755. [DOI] [PubMed] [Google Scholar]
- [9].Mottram PM, Marwick TH, Assessment of diastolic function: what the general cardiologist needs to know, Heart. 91 (2005) 681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Miranda WR, Borlaug BA, Hagler DJ, Connolly HM, Egbe AC, Haemodynamic profiles in adult Fontan patients: associated haemodynamics and prognosis, Eur. J. Heart Fail 21 (6) (2019. June) 803–809, 10.1002/ejhf.1365 (Epub 2019 Jan 23). [DOI] [PubMed] [Google Scholar]
- [11].LaFarge CG, Miettinen OS, The estimation of oxygen consumption, Cardiovasc. Res 4 (1970) 23–30. [DOI] [PubMed] [Google Scholar]
- [12].Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU, Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging, Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography 28 (2015) 1–39 e14. [DOI] [PubMed] [Google Scholar]
- [13].Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ, Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the society for cardiovascular magnetic resonance, Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography 30 (2017) 303–371. [DOI] [PubMed] [Google Scholar]
- [14].Egbe AC, Connolly HM, Dearani JA, Bonnichsen CR, Niaz T, Allison TG, Johnson JN, Poterucha JT, Said SM, Ammash NM, When is the right time for Fontan conversion? The role of cardiopulmonary exercise test, Int. J. Cardiol 220 (2016) 564–568. [DOI] [PubMed] [Google Scholar]
- [15].El-Harasis MA, Connolly HM, Miranda WR, Qureshi MY, Sharma N, Al-Otaibi M, DeSimone CV, Egbe A, Progressive right ventricular enlargement due to pulmonary regurgitation: clinical characteristics of a “low-risk” group, Am. Heart J 201 (2018) 136–140. [DOI] [PubMed] [Google Scholar]
- [16].Apitz C, Latus H, Binder W, Uebing A, Seeger A, Bretschneider C, Sieverding L, Hofbeck M, Impact of restrictive physiology on intrinsic diastolic right ventricular function and lusitropy in children and adolescents after repair of tetralogy of Fallot, Heart. 96 (2010) 1837–1841. [DOI] [PubMed] [Google Scholar]
- [17].Kutty S, Valente AM, White MT, Hickey K, Danford DA, Powell AJ, Geva T, Usefulness of pulmonary arterial end-diastolic forward flow late after tetralogy of Fallot repair to predict a “restrictive” right ventricle, Am. J. Cardiol 121 (2018) 1380–1386. [DOI] [PubMed] [Google Scholar]
- [18].Munkhammar P, Carlsson M, Arheden H, Pesonen E, Restrictive right ventricular physiology after tetralogy of Fallot repair is associated with fibrosis of the right ventricular outflow tract visualized on cardiac magnetic resonance imaging, Eur Heart J-Card Img. 14 (2013) 978–985. [DOI] [PubMed] [Google Scholar]
- [19].Helbing WA, Niezen RA, Le Cessie S, van der Geest RJ, Ottenkamp J, de Roos A, Right ventricular diastolic function in children with pulmonary regurgitation after repair of tetralogy of Fallot: volumetric evaluation by magnetic resonance velocity mapping, J. Am. Coll. Cardiol 28 (1996) 1827–1835. [DOI] [PubMed] [Google Scholar]
- [20].Lee W, Yoo SJ, Roche SL, Kantor P, van Arsdell G, Park EA, Redington A, Grosse-Wortmann L, Determinants and functional impact of restrictive physiology after repair of tetralogy of Fallot: new insights from magnetic resonance imaging, Int. J. Cardiol 167 (2013) 1347–1353. [DOI] [PubMed] [Google Scholar]
- [21].van den Berg J, Wielopolski PA, Meijboom FJ, Witsenburg M, Bogers AJ, Pattynama PM, Helbing WA, Diastolic function in repaired tetralogy of Fallot at rest and during stress: assessment with MR imaging, Radiology. 243 (2007) 212–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


