Abstract
PfSPZ Vaccine, composed of radiation-attenuated, aseptic, purified, cryopreserved Plasmodium falciparum sporozoites, is administered by direct venous inoculation (DVI) for maximal efficacy against malaria. A critical issue for advancing vaccines that are administered intravenously is the ability to efficiently administer them across multiple age groups. As part of a pediatric safety, immunogenicity, and efficacy trial in western Kenya, we evaluated the feasibility and tolerability of DVI, including ease of venous access, injection time, and crying during the procedure across age groups. Part 1 was an age de-escalation, dose escalation trial in children age 13 months-5 years and infants age 5-12 months; part 2 was a vaccine efficacy trial including only infants, using the most skilled injectors from part 1. Injectors could use a vein viewer, if needed. A total of 1,222 injections (target 0.5mL) were initiated by DVI in 511 participants (36 were 5-9-year-olds, 65 were 1-5-year-olds, and 410 infants). The complete volume was injected in 1,185/1,222 (97.0%) vaccinations, 1,083/1,185 (91.4%) achieved with the first DVI. 474/511 (92.8%) participants received only complete injections, 27/511 (5.3%) received at least one partial injection (<0.5ml), and in 10/511 (2.0%) venous access was not obtained. The rate of complete injections by single DVI for infants improved from 77.1% in part 1 to 92.8% in part 2. No crying occurred in 51/59 (86.4%) vaccinations in 5-9-year-olds, 25/86 (29.1%) vaccinations in 13-59-month-olds and 172/1067 (16.1%) vaccinations in infants. Mean administration time ranged from 2.6 to 4.6 minutes and was longer for younger age groups.
These data show that vaccination by DVI was feasible and well tolerated in infants and children in this rural hospital in western Kenya, when performed by skilled injectors. We also report that shipping and storage in liquid nitrogen vapor phase was simple and efficient. (Clinicaltrials.gov NCT02687373)
Keywords: Direct venous inoculation, Plasmodium falciparum whole sporozoite Vaccine, infants, malaria vaccine, feasibility, Africa
Background:
All currently licensed vaccinations against viral or bacterial infections are administered intradermally, subcutaneously, orally, intranasally or intramuscularly [1]. For these vaccines, protection is primarily through antibody responses. However, there are no highly effective vaccines against infections such as malaria and tuberculosis, as durable immunity requires T cells at tissue sites. Thus, new approaches including an alternative route of vaccination may be critical for inducing protective T cell responses at tissue sites. Accordingly, intravenous administration of a vaccine can lead to substantially increased T cell responses and protection compared to the same vaccine given by conventional intradermal or subcutaneous routes [2, 3]. To advance this concept, it is critical to demonstrate the safety and feasibility of intravenous vaccination across all ages, especially in infants and young children where this may be more challenging.
A promising approach for eliciting protective T cell immunity against malaria infection is vaccination with radiation-attenuated, aseptic, purified whole Plasmodium falciparum (Pf) sporozoites (SPZ) using PfSPZ Vaccine (Sanaria Inc, Rockville, MD), in which the sporozoites remain metabolically active, important for priming T cells, but are unable to develop into a blood-stage infection. Because PfSPZ Vaccine contains a whole cell organism (eukaryotic cell), it needs to be cryopreserved and stored in liquid nitrogen vapour phase (LNVP) at −150° to −196°C to maintain viability.
In the first clinical trial in malaria-naive adults in the US, PfSPZ Vaccine administered intradermally or subcutaneously was found to be safe and well tolerated. However, it was poorly immunogenic and induced very limited protection [4]. Comparing the same dosage and vaccine regimen in non-human primates (NHPs) and mice, vaccination by intravenous (IV) administration induced far more potent and durable PfSPZ-specific T cell response in peripheral blood and most notably in the liver in NHPs, the likely site of immune protection, and protection against malaria in mice. Together, these data suggested that the IV route would lead to higher efficacy in humans [4, 5]. A subsequent clinical study confirmed that IV administration of PfSPZ Vaccine substantially improved efficacy, protecting 6/6 malaria naive adults against controlled human malaria infection (CHMI) 3 weeks after the last PfSPZ Vaccine [5]. Superiority of IV compared with intramuscular administration was further shown by Ishizuka et al. in 2016, whereby PfSPZ Vaccine administered by intramuscular injection, even at an 8.1-fold higher dosage, was less efficient in inducing protection than when administered by IV administration [6].
Following the initial proof of concept trials using IV administration in malaria-naive individuals in the US, the feasibility and efficacy of PfSPZ Vaccine administered by direct venous inoculation (DVI) of the 0.5 mL volume through a 25-gauge needle was tested in malaria-exposed adults in Tanzania and Mali. The DVI procedure was completed with a single needlestick in 98.7% and 99.8% of vaccinations in Tanzania and Mali respectively; 97.0% of injections were considered painless by Tanzanian volunteers [7, 8].
To test this vaccine in the primary target population of African children, who suffer the highest burden of malaria morbidity and mortality, in late 2015, an age de-escalation phase 1 trial to evaluate safety, tolerability and immunogenicity of PfSPZ vaccine administered by DVI was initiated in Tanzanians aged 6 months to 45 years in a low-transmission setting [9]. As initial results did not show any safety signals, a similar study design that included higher dosages was used to evaluate safety, tolerability and feasibility in children living in western Kenya in an area of high malaria transmission. The results from this age de-escalation and dose escalation trial were used to inform and prepare the first phase 2 trial to evaluate safety and efficacy of PfSPZ Vaccine in infants in western Kenya.
We report here on the feasibility and tolerability of DVI and logistics of using a vaccine stored in LNVP during the trial in western Kenya.
Methods:
Study design and participants:
Between July 2016 and August 2018, we conducted a single center, double-blind, randomized, placebo-controlled trial in two parts in Siaya County, western Kenya, where malaria transmission is perennial and high (27% by microscopy in 2015 among children 6 months to 14 years in the lake region)[10]. In part 1, an age- de-escalation, dose escalation design, we evaluated safety, tolerability and feasibility of PfSPZ Vaccine administered by DVI in healthy children from 5 months to 9 years inclusive. In part 2, the three highest safe dosages tested in part 1 were administered to infants 5 to 12 months of age in three vaccinations, 8 weeks apart, and evaluated for safety, tolerability and feasibility. Efficacy was evaluated through monthly active and passive malaria surveillance during 12 months after vaccination 3.
After community sensitization, participants who lived within a 10-km radius of Siaya County Referral Hospital (parts 1 and 2) or within 10-km radius of Wagai Health Centre (part 2 only) were recruited and parents/legal guardians were consented at a central point in their villages or at the study clinic. During screening, children were evaluated for exclusion criteria, which included chronic illnesses, HIV exposure, and clinically significant abnormalities in electrocardiogram or laboratory parameters.
Intervention
PfSPZ Vaccine is produced by Sanaria Inc., Rockville MD in compliance with current Good Manufacturing Practice (cGMPs) and meets all regulatory standards. PfSPZ Vaccine was shipped from the manufacturer’s site in the United States in containers filled with LNVP under strict temperature control. During storage at the clinical trial site, the LNVP containers were refilled weekly. The vaccine was delivered in cryovials containing either 1.5x105 PfSPZ for the two lowest dosages in part 1 or 4.5x105 PfSPZ and was thawed and diluted in phosphate buffered saline (PBS) with human serum albumin (HSA). The final injectable volume for each vaccine as well as normal saline placebo was 0.5mL drawn into a 1mL syringe. After thawing, the PfSPZ Vaccine was administered within 30 minutes or suitably disposed. Dilution and syringe preparation were performed under aseptic conditions in a biological safety cabinet by an unblinded pharmacy team. The preparation time within the pharmacy was recorded for each syringe. Diluted PfSPZ Vaccine is indistinguishable from normal saline. Before the pharmacy team was given the signal to start thawing a vaccine vial, the blinded vaccination team had examined the child and identified suitable veins for injection. The time for this evaluation was not measured. The appropriate syringe for the study subject was handed to the vaccination team through a window connecting the vaccination and vaccine preparation rooms. The time from handover of the filled syringe until completed administration was recorded for each vaccination.
PfSPZ Vaccine or normal saline placebo was administered by DVI using a 25-gauge needle. We restricted injection sites to veins in the hand, arm, foot, and ankle; selected sites needed to be free of skin lesions or scars. In infants and young children, there was an option to administer the vaccine through a 24-Gauge intravenous cannula; this option was used when difficult venous access was experienced during the screening blood draw or when the first attempt at venous access was challenging. If the intravenous cannula was used, a pre-vaccination flush of 0.5–1 mL normal saline was given prior to injection of the study product (vaccine or placebo), followed by a post-vaccination flush with 3 mL normal saline to assure no study product was left in the cannula.
A vaccination was defined as complete if all volume was injected intravenously (either by DVI or by intravenous cannula), as successful injection if all volume was administered intravenously by 1 DVI (with no use of intravenous cannula), and as partial injection if less than 0.5ml was given or part of the volume was injected paravenously. As the cannula was frequently used in combination with attempted DVI, the total number of attempts needed, including both DVI and cannula placement, was defined as ‘needlesticks’. Failed injection was defined as having attempted the allowed number of needlesticks (see below) with no study product/placebo injected at all.
Children’s arms were washed with water and soap by a nurse before entering the vaccination room. The vaccination team consisted of an injector and an assistant, and one quality assurance officer to record the timing of hand over of the vaccine and the time of DVI completion. The injector used sterile gloves and standard precautions to prevent infection. Before inoculation of the investigational product, a small amount of blood was aspirated to ensure intravenous placement.
In cases where veins could not be visualized easily, a portable vein viewer (Vein Viewer® Flex, Christie Medical) was used to help identify veins of a sufficient calibre. The injector also palpated the identified area to assess the depth of the vein visualized with the help of the vein viewer.
Part 1
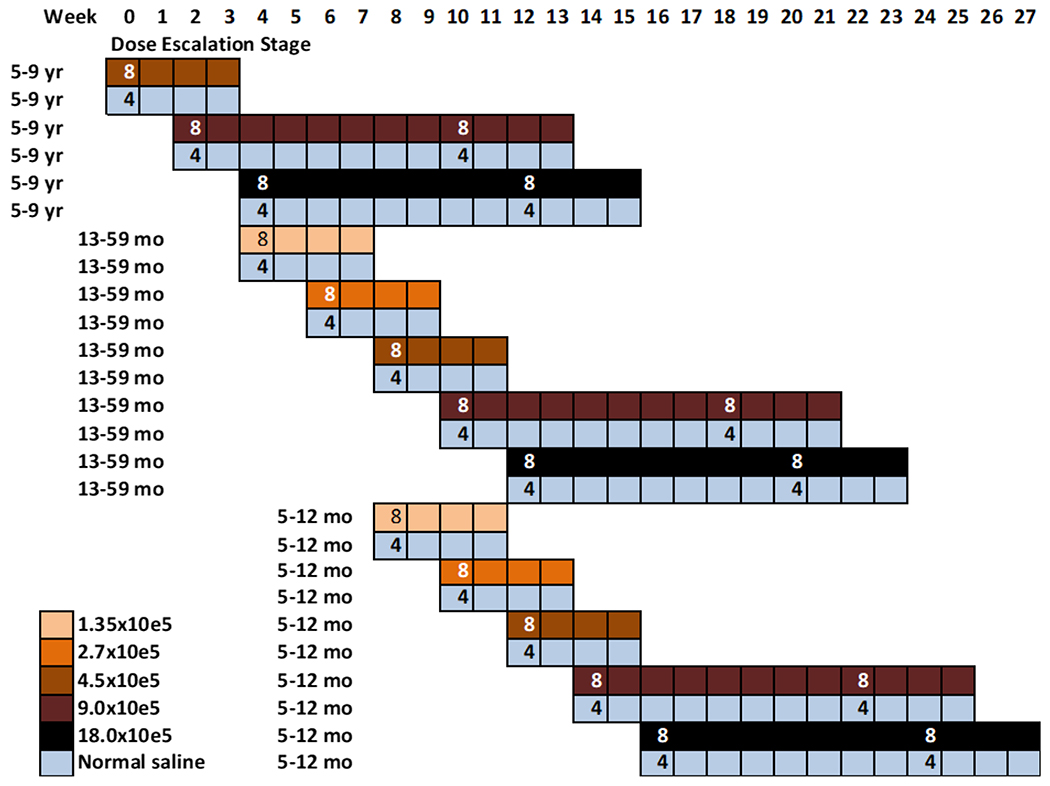
156 participants in groups of 12 were randomly allocated to vaccine (n=8) or placebo (n=4) using a staggered dose-escalation, age de-escalation approach (Figure 1). The sample size was meant to detect serious common safety concerns. Vaccinations began in the 5–9-year age group; once a given dosage was shown to be well tolerated and without safety concerns in this age group, children 13–59 months were vaccinated with the lowest dosage and finally children 5–12 months old. We started at a dosage of 4.5 x 105 PfSPZ in the 5–9 year olds, and increased to 9 x 105 PfSPZ, and finally to 1.8 x 106 PfSPZ. In the younger children, we successively tested two lower dosages (1.35 x 105 and 2.7 x 105 PfSPZ) before moving to the three higher dosages. The two highest dosages were given twice, 8 weeks apart, in each age group, while the lower dosages were given once. In part 1, a child who received a partial injection during the first vaccination was replaced by a backup participant, but continued follow up for 28 days. Injectors were allowed a maximum of three needlesticks in part 1.
Figure 1 -.
Timeline of age de-escalation and dose escalation for Part 1
Part 2
Altogether 337 infants between 5 and 12 months old inclusive at vaccination 1 were randomized 1:1:1:1 to receive PfSPZ Vaccine at dosages of 4.5 x 105, 9.0 x 105, and 1.8 x 106 PfSPZ or normal saline placebo. The sample size was calculated assuming 30% incidence of malaria in the control arm and at least 60% vaccine efficacy in the PfSPZ Vaccine dose arm when compared with placebo, which led to a requirement of 77 participants in each study arm (PfSPZ vs NS) to provide 80% power. All groups received three vaccinations or placebo by DVI, administered at 8-week intervals. In part 2, if DVI was not successful after three attempts, mothers were asked if they were willing to bring their children back on another day for another DVI attempt (again with up to three needlesticks).
We attempted to administer as much of the study product intravenously as possible. If, during the first attempt, swelling was observed, the injector tried to administer the remaining volume at another injection site with a maximum of 3 needlesticks.
Tolerability assessment
After each vaccination (including placebo injections) in part 1 and part 2, study staff recorded how many needlesticks were required, whether any swelling was visible during the injection, suggestive of paravenous injection, and whether the child cried during the procedure. In part 2 we specified whether the crying was observed before, during or after the procedure. If any product was injected paravenously, we estimated the amount of the paravenous injection and declared this DVI to be a partial injection. One hour after vaccination, the injection site was examined for local swelling or other injection site reactions (pain, presence of erythema or bruising); the same observations were repeated again two hours after vaccination in part 1 only. In the 5-9 year olds the mother and /or child were asked by a staff member who was not part of the vaccination team how they rated the child’s pain experience (painless, mild, moderate, or severe pain).
Injectors
All injectors were clinical officers (8) (comparable to physician assitants) and one medical officer trained locally, and all had experience in pediatric phlebotomy. The PI, a German trained paediatrician, supervised the training and acted initially also as injector. Training for DVI took place during study preparation and consisted of one two-hour session where the direct venous inoculation of an injectate (normal saline) by insulin syringe was performed once by each trainee on another adult study staff member and instructions were provided on the timing of syringe preparation and the procedures for injection and study documentation. Performance was assessed during vaccination of 13–59-month-olds and infants in part 1, and only those who achieved more than 80% success with DVI on first attempt continued vaccinating in part 2.
Statistical analysis
Frequencies and percentages of binary and categorical variables were summarized; chi-squared or Fischer’s Exact tests were used to assess statistical significance. Continuous variables were summarized using means and standard deviations, medians, and ranges. Statistical analyses were done in SAS V9.3 (SAS Inc., Cary, NC).
As feasibility of DVI is not dependent on whether vaccine or placebo are injected, nor on vaccine dosage, we stratified our analyses by age group, but not by study arm, and feasibility was evaluated in relation to the increasing experience of injectors over time.
Results
Study population
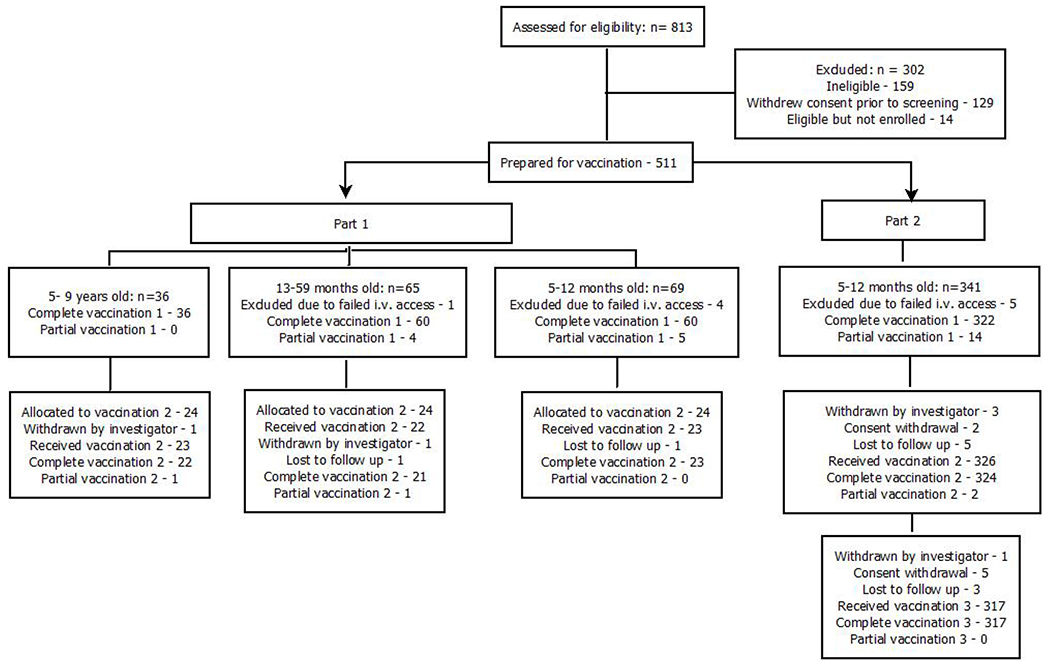
Across parts 1 and 2, a total of 511 infants and children met eligibility criteria and were prepared for DVI. Of these, 36 were 5–9 years old, 65 were 13–59 months old, and 410 were infants 5–12 months of age: 69 in part 1 and 341 in part 2 (Figure 2; Table 1).
Figure 2 -.
Consort diagram of participant enrolment and vaccinations
Table 1:
Baseline Characteristics of all eligible children by age
| 5-9 years | 13-59 months | 5-12 months | |||
|---|---|---|---|---|---|
| Part 1 | Part 1 | Part 1 | Part 2 | ||
| N | 36 | 65 | 69 | 341 | |
| Age (months) | Mean / Median | 88.4/90.5 | 31.2/31 | 8.2/8 | 8.3/8 |
| (Min,Max) | (60,115) | (13,58) | (5,12) | (5,12) | |
| Gender | Male | 47.2% | 43.1% | 42.9% | 53.7% |
Vaccine storage and preparation
The usual shipment time from the manufacturer in the US to the study site was 5 to 7 days, with no refill of liquid nitrogen necessary. There was one temperature deviation due to customs delay, and the contents of that shipment were discarded. During storage at the clinical trial site, no temperature deviations occurred.
The preparation time for the dosage of 1.8 x 106 PfSPZ was the longest, and it was monitored to assess the performance of the pharmacy team. In August 2016, the average syringe preparation time from thawing to handover of the vaccine to the injector was 11 minutes, and declined steadily to 5 minutes in July 2017.
Injection time from syringe handover from the pharmacy to completed injection was shortest in 5–9 year old children, with a mean of 2.6 minutes (range: 1, 9 minutes), then increased to 3.2 minutes (range: 1,13) and 3.6 minutes (range: 1,16) in 13–59-month-olds and infants, respectively, in part 1. In part 2, mean injection time at vaccination 1 was 4.6 minutes (range: 2,16), likely due to a more rigorous documentation process developed during the trial, and decreased to 3.9 minutes (range: 2, 10) by vaccination 3 (Table 2).
Table 2:
Time from syringe handoff from pharmacy to completed vaccine injection (Minutes)
| Part 1 | Part 2** | |||||
|---|---|---|---|---|---|---|
| 5–9 years | 13–59 months | 5–12 months | Vacc 1 | Vacc 2 | Vacc 3 | |
| Number of vaccinations* | 59 | 86 | 88 | 336 | 326 | 317 |
| Mean time, minutes (SD) | 2.6 (1.8) | 3.2 (2.3) | 3.6 (2.6) | 4.6 (2.0) | 4.1 (1.5) | 3.9 (1.3) |
| Min, Max | 1, 9 | 1, 13 | 1, 16 | 2, 16 | <1, 14 | 2, 10 |
Includes partial and complete vaccinations
Part 2 included only infants 5-12 months
Feasibility of DVI
Injectors’ performance
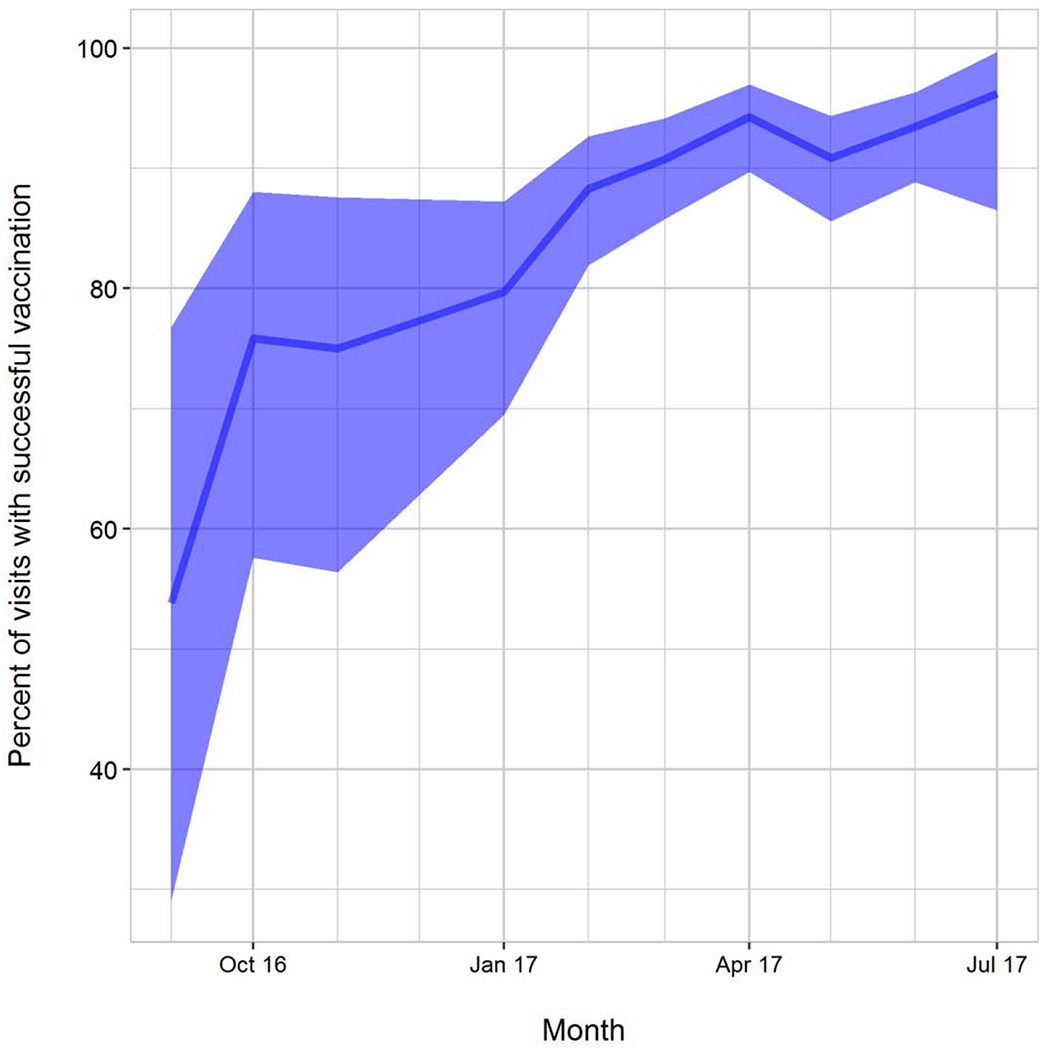
Injectors’ performance was monitored during part 1. All managed to gain venous access in 5-9 year old children successfully. In the younger age groups, however, 4 of the 10 injectors were not as successful and towards the end of part 1, the six clinical officers with the best performance record were chosen to continue DVI in part 2. The majority of injections in part 2 were performed by two of these individuals. Considering a complete vaccination by one single DVI as success, the success rate at the beginning of vaccinations in infants in part 1 averaged 54%, with constant and significant improvement of the injecting team over time to a success rate of 96% at the end of the trial (Figure 3). Table 3 shows that it was a combination of improving overall team performance and improving performance of the two primary injectors which led to the high success rate towards the end of the trial. Injector Number 6 had a very high success rate but was transferred to a non-vaccinating clinic site, because of his good phlebotomy skills, which were needed there.
Figure 3 -.
Percent of Complete Vaccinations with 1 DVI in 5-12-Month-Olds, Over Time
P<0.001 for Fisher’s Exact Test for trend over time.
Note: Figure includes performance of all injectors; 10 injectors worked from September 2016 – January 2017 in Part 1 , but only 6 continued in January 2017 with injections for Part 2 infants.
Table 3:
Individual injector’s performance in part 1 and part 2
| Injector Number | Part 1 | Part 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of total vaccinations | % successful at 1st DVI | Number of infant vaccinations | % infant vaccinations successful at 1st DVI | Number of Vacc 1 | % successful at 1st DVI | Number of Vacc 2 | % successful at 1st DVI | Number of Vacc 3 | % successful at 1st DVI | Total % successful at 1st DVI | |
| 1 | 65 | 92 | 34 | 94 | 153 | 90 | 162 | 98 | 80 | 94 | 94 |
| 2 | 50 | 96 | 19 | 95 | 146 | 88 | 141 | 93 | 227 | 93 | 91 |
| 3 | 23 | 91 | 9 | 89 | 24 | 83 | 15 | 87 | 4 | 50 | 81 |
| 4 | 26 | 89 | 10 | 90 | 6 | 50 | 5 | 80 | 0 | N/A | 64 |
| 5 | 22 | 86 | 6 | 50 | 6 | 83 | 3 | 67 | 6 | 100 | 87 |
| 6 | 12 | 92 | 3 | 100 | 1 | 100 | 0 | N/A | 0 | N/A | 100 |
| 7 | 9 | 67 | 1 | 0 |
Did not continue to do injections |
||||||
| 8 | 11 | 46 | 5 | 20 | |||||||
| 9 | 1 | 100 | 0 | N/A | |||||||
| 10 | 14 | 79 | 1 | 100 | |||||||
PART 1
Among all 170 children and infants enrolled, we were able to vaccinate 165 with any study product/placebo; of these 156 had complete first vaccinations (Table 4).
Table 4:
All vaccination attempts in parts 1 and 2
| Part 1 | Part 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-9 years (N=36) | 13-59 months (N=65) | 5-12 months (N=69) | 5-12 months (N=341) | ||||||||||
| Vac 1 | Vac2 | All Vac | Vac1 | Vac 2 | All Vac | Vac 1 | Vac 2 | All Vac | Vac 1 | Vac 2 | Vac 3 | All Vac | |
| Total number of vaccinations attempted | 36 | 23** | 59 | 65 | 22** | 87 | 69 | 23** | 92 | 341 | 326 | 317 | 984 |
| Failed venous access, n (%) | 0 | 0 | 0 | 1 (1.5) | 0 | 1 (1.1) | 4 (5.8) | 0 | 4 (4.3) | 5 (1.5) | 0 | 0 | 5 (0.5) |
| Partial injection, n (%) | 0 | 1 (4.3) | 1 (1.7) | 4 (6.2) | 1 (4.5) | 5 (5.7) | 5 (7.2) | 0 | 5 (5.4) | 14 (4.2) | 2 (0.6) | 0 | 16 (1.6%) |
| Complete vaccinations (V) (DVI or cannula used), n (% of total attempted) | 36 (100) | 22 (95.7) | 58 (98.3) | 60 (92.3) | 21 (95.5) | 81 (93.1) | 60 (87.0) | 23 (100) | 83 (90.2) | 322 (94.4) | 324 (99.4) | 317 (100) | 963 (97.9) |
| Complete Vaccination by DVI only, n (%)* | 36 (100) | 22 (95.7) | 58 (98.3) | 56 (93.3) | 21 (95.5) | 77 (95.1) | 51 (85.0) | 23 (100) | 74 (89.2) | 318 (98.8) | 324 (100) | 317 (100) | 959 (99.6) |
| 1 DVI, n (%)* | 34 (94.4) | 20 (91.0) | 54 (93.1) | 51 (85) | 20 (95.2) | 71 (87.7) | 44 (73.3) | 20 (87.0) | 64 (77.1) | 291 (90.4) | 309 (95.4) | 294 (92.7) | 894 (92.8) |
| 2 DVI, n (%)* | 2 (5.6) | 1 (4.5) | 3 (5.2) | 3 (5.0) | 0 | 3 (3.7) | 6 (10) | 2 (8.7) | 8 (9.6) | 21 (6.5) | 15 (4.6) | 22 (6.9) | 58 (6.0) |
| 3 DVI, n (%)* | 0 | 1 (4.5) | 1 (1.7) | 2 (3.3) | 1 (4.8) | 3 (3.7) | 1 (1.7) | 1 (4.3) | 2 (2.4) | 4 (1.2) | 0 | 1 (0.3) | 5 (0.5) |
| > 3 DVI, n (%)* | NA | NA | NA | NA | NA | NA | NA | NA | NA | 2 (0.6) | 0 | 0 | 2 (0.2) |
| Cannula used at any attempt***, n (%)* | 0 | 0 | 0 | 4 (6.7) | 0 | 4 (4.9) | 9 (15.0) | 0 | 9 (10.8) | 4 (1.2) | 0 | 0 | 4 (0.4) |
| 1 needlestick | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 6 | 3 | 0 | 0 | 3 |
| 2 needlesticks | 0 | 0 | 0 | 2 | 0 | 2 | 3 | 0 | 3 | 0 | 0 | 0 | 0 |
| 3 needlesticks | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| > 3 needlesticks | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 1 | 0 | 0 | 1 |
N=number of participants per age group; n= number of vaccinations; %= n/N
Denominator used was the number of complete vaccinations (V)
Total number of 24 not achieved because of withdrawal by investigator (n=2) or loss to follow up (n=2)
No partial injections by intravenous cannula
In 5–9 year-olds, all 36 children received a complete first vaccination, 22 of 23 (95.7%) received a complete and one (4.3%) a partial second vaccination. Of 58 completed vaccinations 54 (93.1%) were achieved with a single DVI, 3 (5.2%) required two DVI, and 1 (1.7%) required three DVI.
Among the 13–59-month-olds, 60/65 (92.3%) received a complete first vaccination, in one child of 2 years (1.5%) we could not obtain venous access, and four (6.2%) children received a partial first vaccination. Vaccination 2 was given to 22 children with one (4.5%) partial injection. Of 81 completed vaccinations, 71 (87.7%) were completed with a single DVI, 3 (3.7%) required two DVIs, and 3 (3.7%) required three DVIs. In four of 60 (6.7%) children at vaccination 1, the study product was completely administered through an intravenous cannula (Table 4). In two of these, the cannula was placed after 2 unsuccessful DVI attempts. In the other two children, the cannula was the first choice due to difficulties at screening blood draw. At vaccination 2 no intravenous cannula was used.
Among the 69 infants in part 1, 60 (87.0%) had a complete first vaccination, four (5.8%) had failed venous access, and five (7.2%) received only a partial first vaccination . Vaccination 2 was given to 23 infants. Of the 83 completed vaccinations, 64 (77.1%) were given by a single DVI, 8 (9.6%) required two DVIs, and 2 (2.4%) required three DVIs. In nine of 60 (15%) children an intravenous cannula was used during vaccination 1; in three children this occurred after one unsuccessful DVI, and in six the cannula was the first choice due to difficulties at screening blood draw.
The vein viewer was used in 1/59 (1.7%) of vaccinations in 5–9-year-olds, in 10/87 (11.5%) of 13–59-month olds and in 29/92 (31.5%) of infants.
In all nine partial injections at vaccination 1, the injection was stopped because a paravenous swelling was observed after less than 0.4mL of the dosage had been injected. In the 2 partial injections at vaccination 2, between 0.4mL to 0.5mL were administered.
Post-vaccination observations in part 1 revealed injection site reactions ( pain, redness, swelling or induration) in 23 of 156 (14.7%) participants who had received the complete dosage at vaccination 1, while two of nine (22.2%) participants with partial injections experienced an injection site reaction (OR=0.66, 95%CI 0.131, 7.637, p=0.23). All injection site reactions were rated as mild (under 10mm swelling/ redness/ induration) or moderate (10-25mm swelling/ redness/ induration). Additional information on safety and tolerability is provided in Steinhardt et al. [11]
PART 2
Venous access was unsuccessful in 5 of 341 infants (1.5%) (Table 4). One of these children was randomized to receive vaccine/placebo; after three unsuccessful attempts at intravenous access, the mother withdrew consent. In the other four infants, venous access had been difficult during the screening blood draw, and investigators decided to insert an intravenous cannula before randomization. These attempts at cannulation were unsuccessful, and the infants were not randomized and were considered screening failures because of failed venous access. Vaccination 1 was administered to 336 infants; 14 (4.2%) received only a partial injection. Among 326 infants who presented for vaccination 2, two (0.6%) participants received only partial vaccination; all 317 infants who presented for vaccination 3 were successfully vaccinated. Of 963 complete vaccinations in part 2, 894 (92.8%) were successful with one single DVI, 58 (6%) needed 2 DVIs, and 5 (0.5%) needed 3 DVIs (Table 4). One infant required a total of 4 needlesticks, 3 on day 1 and 1 on another appointment. Another child received a total of 6 needlesticks, 3 on each of 2 different days. In 13 of 16 partial injections in part 2, due to the option to administer remaining syringe content at another injection site if swelling was observed, a volume of 0.4mL or more (but less than 0.5mL) was injected intravenously.
Eight infants required more than one attempt at DVI for two different vaccinations, but none needed more than one DVI attempt at all 3 vaccinations. Of the 57 participants where more than one DVI was needed to administer vaccination 1 or 2, one parent withdrew consent before vaccination 3 citing religious beliefs; no other parent withdrew consent before completing the 3 vaccinations.
In part 2 an intravenous cannula was inserted in 4/336 (1.2%) infants at vaccination 1. In 3 of these the cannula option was chosen because of difficult screening blood draw and vaccination was successful with one needlestick. In the fourth child, 6 attempts at administering vaccine 1 (3 on each of 2 different days) were unsuccessful, but two months later she was vaccinated successfully at the fourth attempt, with an intravenous cannula. This child developed a grade 3 swelling post vaccination, possibly due to the many failed attempts, and was withdrawn from further vaccinations by the investigators. Cannulas were not used during vaccinations 2 or 3. The use of the vein viewer decreased slightly over time from 41% in vaccination 1 to 35% in vaccination 3.
Post-vaccination observations in part 2 revealed local injection site reactions (pain, swelling, bruising or induration) in 44/336 participants (13.1%) across all vaccinations, rated as mild in 42/336 participants, but 2 infants experienced swelling of more than 25 mm in diameter (considered severe). After vaccination 1, 21/322 (6.5%) participants experienced injection site reactions after a complete injection of study product and 5/14 (35.7%) after a partial injection (p=0.002). At vaccination 2, injection site reactions were experienced by 14/324 (4.3%) participants after complete vaccination compared to 0/2 (0%) partial injections. There were no partial injections at vaccination 3.
Location of administration
Most injections in the 5–9-year-olds were given in the antecubital fossa, but the back of the hand was more commonly used in the two younger age groups. This may have been due to the use of the vein viewer, which did not visualize the blood vessels in the antecubital fossa as well as those on the back of the hand. The wrist and feet were only used if no suitable veins were found at other sites (Table 5).
Table 5:
Successful injection sites (only 1 DVI needed to complete vaccination)
| PART 1 | PART 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-9 years | 13-59 months | 5-12 months | 5-12 months | ||||||
| Injection site | Vac 1 | Vac 2 | Vac 1 | Vac 2 | Vac 1 | Vac 2 | Vac 1 | Vac 2 | Vac 3 |
| N=34 | N=20 | N=51 | N=20 | N=44 | N=20 | N=291 | N=309 | N=294 | |
| Antecubital fossa n(%) | 32 (94.1) | 19 (95.0) | 17 (33.3) | 7 (35.0) | 6 (13.6) | 3 (15) | 174 (59.2) | 139 (45.0) | 140 (47.6) |
| Back of hand n(%) | 2 (5.9) | 1 (5.0) | 31 (60.8) | 13 (65.0) | 36 (81.8) | 16 (80) | 111 (38.1) | 165 (53.4) | 147 (50.0) |
| Wrist n(%) | 0 (0) | 0 (0) | 3 (5.9) | 0 (0) | 2 (4) | 0 (0) | 5 (1.7) | 5 (1.6) | 6 (2.0) |
| Foot n(%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 1 (0.3) | 0 (0) | 1 (0.3) |
Pain perception
Children 5–9 years of age cried during 13.6% (8/59) of all DVI procedures, and they and/or their mothers rated 78.0% of procedures as painless, and associated with mild pain in the remaining 22.0%. In 13–59-month-olds, crying was observed during 70.9% (61/86) of all vaccinations. Infants cried during 83.9% (895/1,067) of vaccinations in parts 1 and 2. In 172/1,067 (16.1%) vaccinations, infants did not cry and some slept or breastfed during the procedure. In part 2, of the infants who cried during 821 injections, the crying started before the injection in 304 (37.0%) vaccinations, and in 810 (98.7%) of the vaccinations it was recorded during the procedure.
Discussion
Intravenous vaccination by DVI is a novel concept in the field of vaccinology based on its potential to elicit significantly higher T cell responses in tissues that may be critical for mediating protection against malaria, TB, and potentially other infectious agents. This has been demonstrated in humans for both the radiation-attenuated PfSPZ Vaccine [4, 5] and chemo-attenuated PfSPZ-CVac [12]. For TB, intravenous administration of BCG has now been shown to be superior to other routes in non human primates [13].
A major potential issue with advancing this concept is the feasibility of DVI across multiple age groups, especially in resource-poor settings. This trial demonstrated in the largest number of subjects to date (n=511) that administering the vaccine by DVI to young children and infants can be done successfully when performed by trained phlebotomists. In this study, out of 10 injectors who initially injected older children, 6 were selected to inject infants, in order to minimize unsuccessful venous access. DVI was most efficiently performed and well tolerated in 5–9-year-olds, with 93.1% of vaccinations completed with a single needlestick and rated as painless by 78.0% of mothers and/or participants. In 13–59-month-olds, 87.7% were injected once only, and in infants, once the team gained experience, and the injector team was reduced to the best injectors, the success rate of DVI (considering only complete vaccinations) increased to 92.8%, and the option of using an intravenous cannula was no longer used in vaccinations 2 and 3 of part 2. These results are comparable to the findings from a phase 1 trial of the PfSPZ Vaccine in Tanzania [9], where 9 of 21 (43%) DVI procedures in infants were successful with one injection at first vaccination, compared to an 83% (15 of 18) success rate at third vaccination, and a decrease in use of intravenous cannula over time.
The injectors in this study had access to a vein viewer when needed, as was the case for the youngest age groups. This device is not currently available and affordable in resource limited settings, an obstacle that would have to be overcome if vaccines needing to be given by DVI were to be introduced into routine vaccination programs for young infants. However, provision of a vein viewer could be considered for use during campaigns, where specialised and experienced teams could administer the vaccines to infants, and would not be needed for administration to older children and adults.
Training physicians, nurses or phlebotomists in DVI is straightforward. We employed one two-hour didactic session (including practicum) for the entire team. Ifvaccines prove to be highly effective with intravenous administration, there would be a major effort for training to ensure that there are a large number of experienced personnel to mitigate effects of staff turnover.
Injections for vaccinations are the most common source of iatrogenic pain in childhood and a source of distress for children, their parents, and those administering the injection [14]. It is estimated that up to 25% of adults have a fear of needles, with most fears developing in childhood [15]. All the vaccinations evaluated so far have been given intramuscularly, subcutaneously, or intradermally and among methods to reduce the pain during injection, breastfeeding during vaccination is considered efficient [16]. In our study, the majority of school-aged children did not cry during the procedure.; moreover 78% of school-aged children and/or their mothers assessed the procedure as painless. In contrast, the majority of younger children did cry during the procedure, though this may also have been related to the positioning and restriction of movement, which was uncomfortable and frightening for some children. We collected data on onset of crying only for the infants vaccinated in part 2, and more than one third of them started crying before the injection, suggesting that other factors like anxiety of being touched by strangers and the positioning while searching for a vein also played a role in crying behaviour. While the proportion was small, the fact that infants did not cry at all during 172 (16.1%) vaccinations, and either slept or breastfed throughout the procedure suggests that the injection itself was sometimes not painful for these infants. In a meta-analysis of several randomized controlled trials involving intramuscular or subcutaneous vaccinations in India, Turkey, Iran and Canada, researchers found significant reductions in crying time (in seconds) in the breastfeeding groups compared to the control groups (holding the child, applying local pain-reducing substances) [17]. We encouraged mothers to breastfeed during the procedure, but we did not collect data on duration of crying and whether an infant was breastfeeding during the procedure; therefore the pain experience cannot be directly compared between intravenous and intramuscular or subcutaneous vaccinations.
While the majority of currently used vaccines require storage between +2 to +8° Celsius, PfSPZ Vaccine must be maintained at −150° to −196°C, which requires storage in LNVP. The standard cold chain is dependent on electricity or gas, which poses a challenge in many low-resource settings where electricity fluctuations make it difficult to keep vaccines at a constant temperature. In our trial, we stored PfSPZ Vaccine in LNVP containers at the clinical trial site in Siaya for months, bringing liquid nitrogene every week from Kisumu, approximaley one hour away, to top up the tanks. In contrast, when we have conducted trials at the same site with vaccines that required storage at 2-8 degrees C, we have had to store the vaccine in refrigerators with generator back up in Kisumu, and transport the vaccine to Siaya daily for administration. As liquid nitrogen is widely available across Africa and other tropical areas for veterinary use, LNVP storage can be advantageous, enhancing delivery to areas like Siaya, since the vaccine can remain stable for weeks to months in a free-standing container [18].
Conclusion
Our study showed that PfSPZ Vaccine storage, handling, and preparation is feasible in resource-limited settings. Administration by DVI is feasible in infants and young children with a phlebotomy team composed of skilled injectors. Ultimately, more than 90% of vaccinations could be administered to infants with a single injection. DVI should therefore be considered an acceptable route for vaccination if it provides a higher level of protection than more standard routes of administration.
Acknowledgements
We sincerely thank all study staff, participants and caregivers who made this study possible. We are very grateful to the DSMB members for their careful review of safety data and to the Director of KEMRI for facilitating the study. Special thanks to Helen Wong and Prof. Feiko ter Kuile from Liverpool School of Tropical Medicine for their tireless support. We thank the Sanaria Manufacturing and Quality Teams for providing the PfSPZ Vaccine for this study and the Sanaria Regulatory, Clinical and Pharmaceutical Operations teams for supporting this trial.
Funding
This work was supported by the National Institutes of Health Vaccine Research Center. Manufacturing and quality control release and stability assays for PfSPZ Vaccine were supported in part by National Institutes of Allergy and Infectious Diseases, National Institutes of Health SBIR grants 5R44AI055229-09A1 and 2R44AI058375-06A1, awarded to SLH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval and consent to participate
Written informed consent was obtained from a parent/guardian of each child. The protocol was approved by institutional review boards (IRBs) of the Kenya Medical Research Institute (KEMRI/SERU/ CGHR/017/3129) and the Centers for Disease Control and Prevention (CDC IRB Protocol #6787), as well as by the Kenya Pharmacy and Poisons Board (Ref. No. PPB/ECCT/16/02/05), and registered at Clinicaltrials.gov (NCT02687373).
Availability of data and material
The dataset used in this study is available from the corresponding author on request.
Declaration of Competing Interest
Potential conflicts of interest. All Sanaria co-authors (BKLS, PFB, ERJ, YA, TLR, SLH, WW) are or were employees of Sanaria (Rockville, MD), which manufactures the vaccine tested in this study. All other co-authors declare no conflicts of interest.
Disclosures
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
The following co-authors are or were employees of Sanaria (Rockville, MD), which manufactures the vaccine tested in this study: Abebe Y, Wijayalath W, James ER, Sim BKL, Billingsley PF, Richie TL, Hoffman SL
References
- 1.Zhang L, Wang W, Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev Vaccines. 2015;14(ll):1509–23. doi: 10.86/14760584.2015.1081067. Epub 2015 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sultan H, Kumai T, Nagato T, Wu J, Salazar AM, Celis E. The route of administration dictates the immunogenicity of peptide-based cancer vaccines in mice. Cancer Immunol Immunother. 2019;68(3):455–66. doi: 10.1007/s00262-018-2294-5. Epub 2019 Jan 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barclay WR, Anacker RL, Brehmer W, Leif W, Ribi E. Aerosol-Induced Tuberculosis in Subhuman Primates and the Course of the Disease After Intravenous BCG Vaccination. Infect Immun. 1970;2(5):574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science. 2011;334(6055):475–80. Epub 2011/09/08. doi: 10.1126/science.l211548. [DOI] [PubMed] [Google Scholar]
- 5.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–65. Epub 2013/08/08. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 6.Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med. 2016;22(6):614–23. Epub 2016/05/09. doi: 10.1038/nm.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis. 2017;17(5):498–509. Epub 2017/02/16. doi: 10.1016/S1473-3099(17)30104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jongo SA, Shekalaghe SA, Church LWP, Ruben AJ, Schindler T, Zenklusen I, et al. Safety, Immunogenicity, and Protective Efficacy against Controlled Human Malaria Infection of Plasmodium falciparum Sporozoite Vaccine in Tanzanian Adults. Am J Trop Med Hyg. 2018;99(2):338–49. doi: 10.4269/ajtmh.17-1014. Epub 2018 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jongo SA, Church LWP, Mtoro AT, Chakravarty S, Ruben AJ, Swanson PA, et al. Safety and Differential Antibody and T-Cell Responses to Plasmodium falciparum Sporozoite Vaccine by Age in Tanzanian Adults, Adolescents, Children, and Infants. The American journal of tropical medicine and hygiene. 2019;15(10):18–0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Malaria Control Programme (NMCP) KNBoSK, and ICF, 2016 I Kenya Malaria Indicator Survey 2015. 2016.
- 11.Steinhardt LC, Richie TL, Yego R, Akach D, Hamel MJ, Gutman JR, et al. Safety, tolerability, and immunogenicity of PfSPZ Vaccine administered by direct venous inoculation to infants and young children: findings from an age de-escalation, dose-escalation double-blinded randomized, controlled study in western Kenya. Clin Infect Dis. 2019;26(5573993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mordmuller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017;542(7642):445–9. doi: 10.1038/nature21060. Epub 2017 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharpe S, White A, Sarfas C, Sibley L, Gleeson F, McIntyre A, et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: Protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinb). 2016;101:174–190.(doi): 10.1016/j.tube.2016.09.004. Epub Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schechter NL, Zempsky WT, Cohen LL, McGrath PJ, McMurtry CM, Bright NS. Pain reduction during pediatric immunizations: evidence-based review and recommendations. Pediatrics. 2007;119(5):e1184–98. doi: 10.542/peds.2006-1107. [DOI] [PubMed] [Google Scholar]
- 15.Paediatrics and Child Health Division TRACoP. Management of procedure-related pain in children and adolescents. Journal of Paediatrics and Child Health 2006;42(Suppl 1):S1–S29. [DOI] [PubMed] [Google Scholar]
- 16.Taddio A, Appleton M, Bortolussi R, Chambers C, Dubey V, Halperin S, et al. Reducing the pain of childhood vaccination: an evidence-based clinical practice guideline. CMAJ. 2010;182(18):E843–55. doi: 10.1503/cmaj.101720. Epub 2010 Nov 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison D, Reszel J, Bueno M, Sampson M, Shah VS, Taddio A, et al. Breastfeeding for procedural pain in infants beyond the neonatal period. Cochrane Database Syst Rev. 2016;10:CD011248.(doi): 10.1002/14651858.CD011248.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richie TL, Billingsley PF, Sim BK, James ER, Chakravarty S, Epstein JE, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine. 2015;33(52):7452–61. Epub 2015/11/27. doi: 10.1016/j.vaccine.2015.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]